Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Formulation and Characterization
2.2. Cell Culture
2.3. Nanoparticle Cellular Uptake
2.4. Animal Model
2.5. Echocardiogram
2.6. Tissue Preservation and Processing
2.7. Splenocyte Isolation, Subpopulation Analysis, and Stimulation Evaluation
2.8. Single-Dose Pharmacokinetic Study
2.9. LC-MS/MS Assay of Rapamycin in Blood and Tissues
2.9.1. Sample Processing
2.9.2. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.9.3. Calibration and Quantification
2.10. Pharmacokinetic Analysis
2.11. Immunofluorescence Staining and Imaging
2.12. Statistics
3. Results
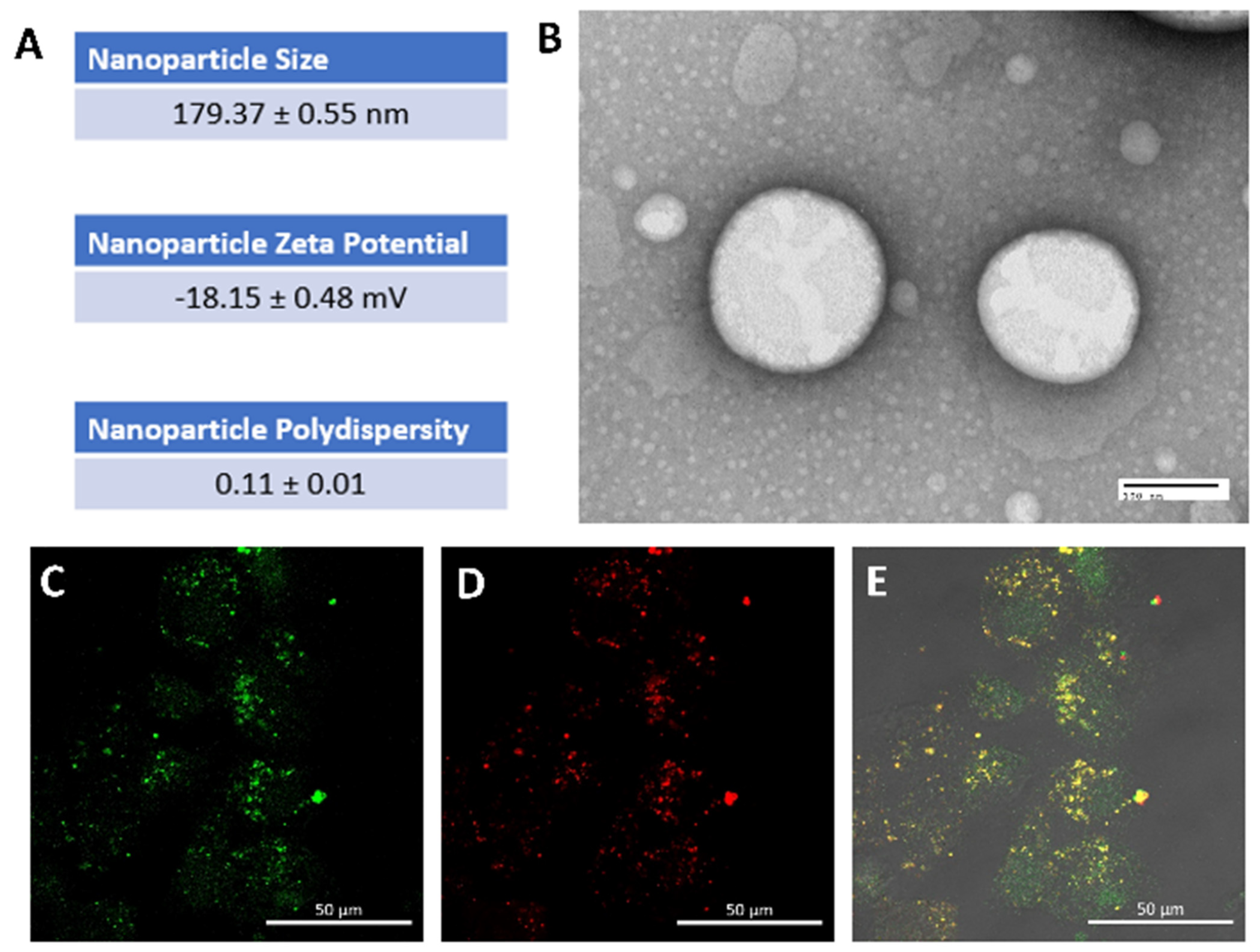
3.1. Rapamycin PFC Nanoparticles Characterization and Cellular Uptake
3.2. Effects of Rapamycin PFC Nanoparticles Treatment on Vital Organs
3.2.1. Cardiac Function
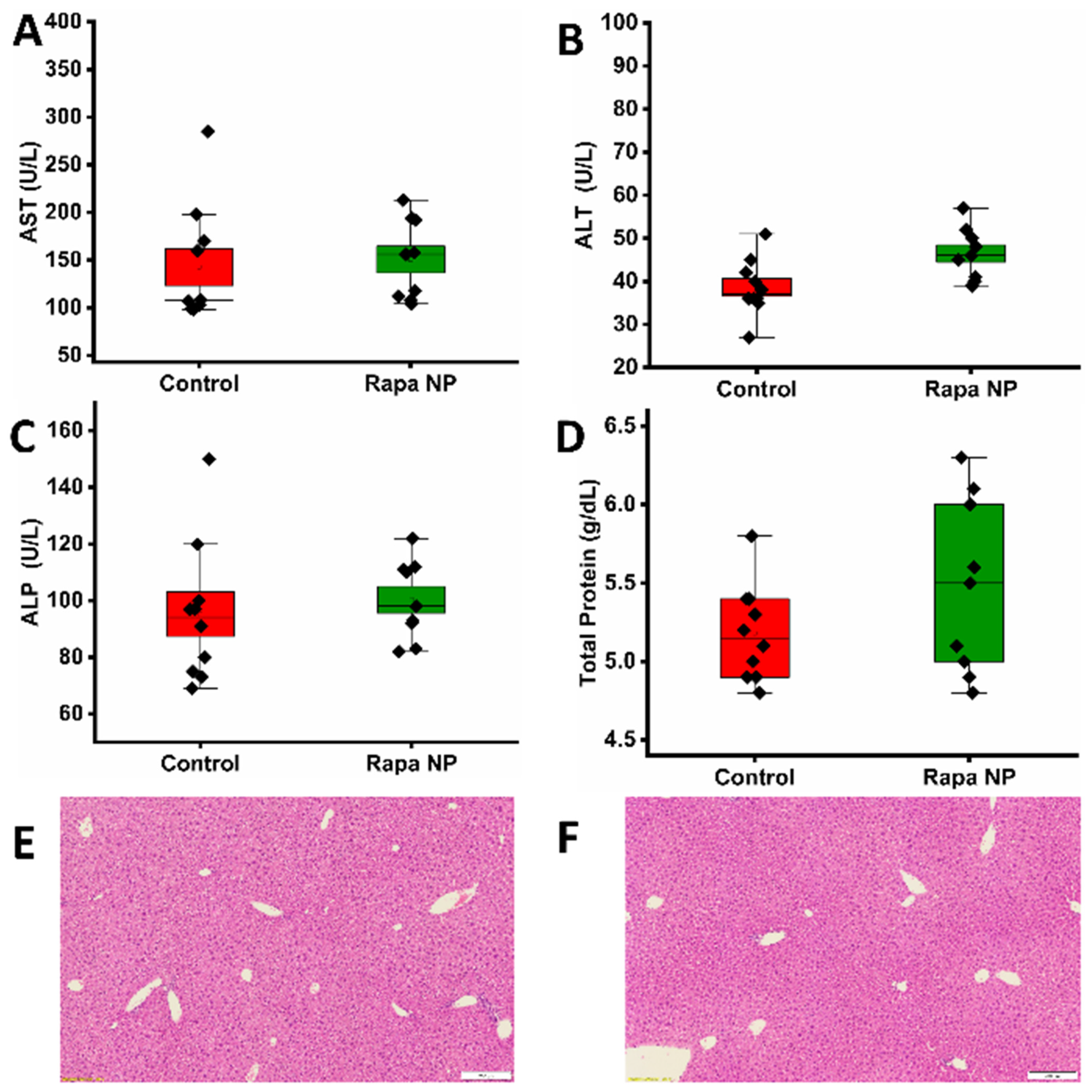
3.2.2. Hepatic Function
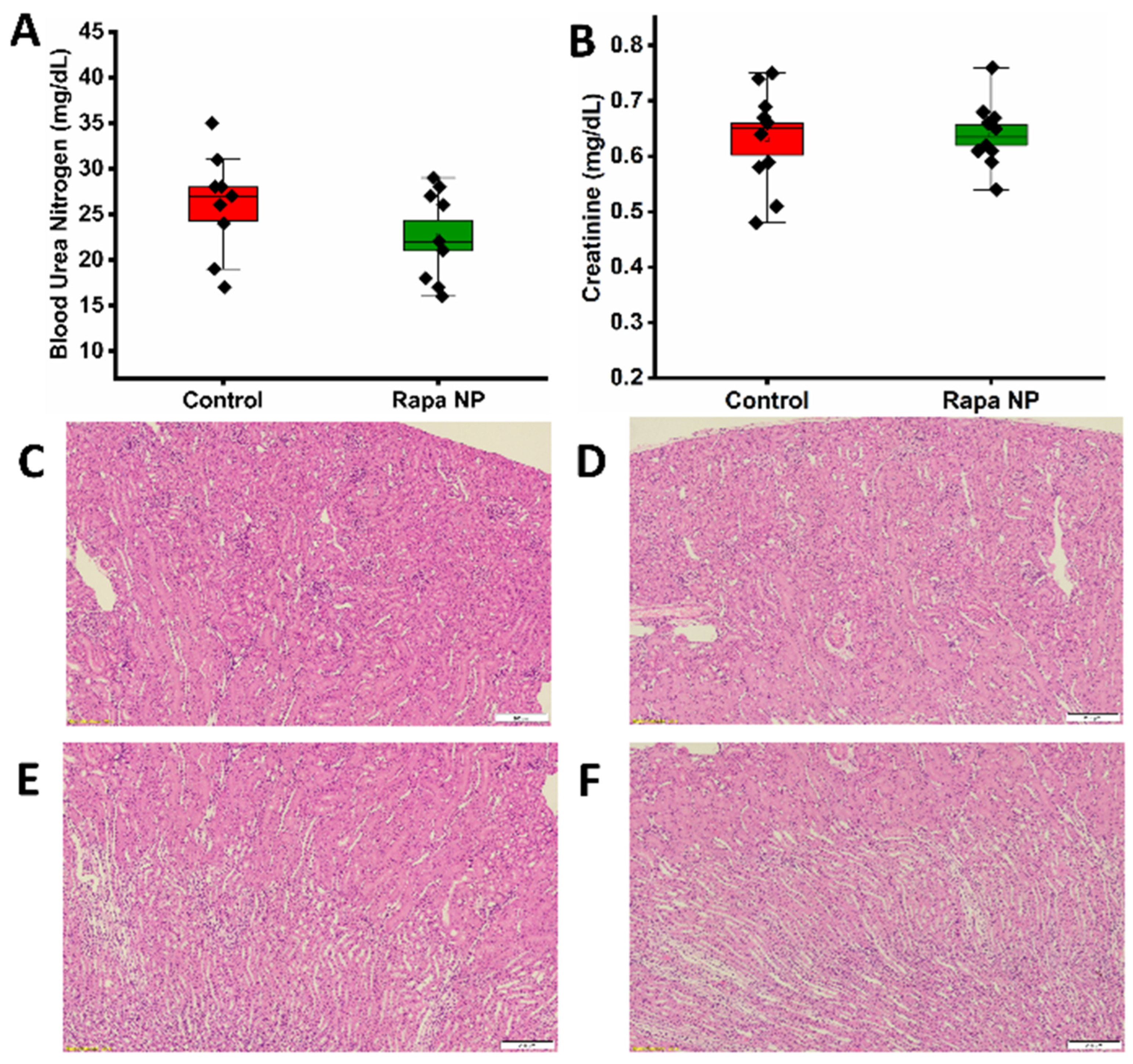
3.2.3. Renal Function
3.3. Effects of Rapamycin PFC Nanoparticles Treatment on Splenocytes
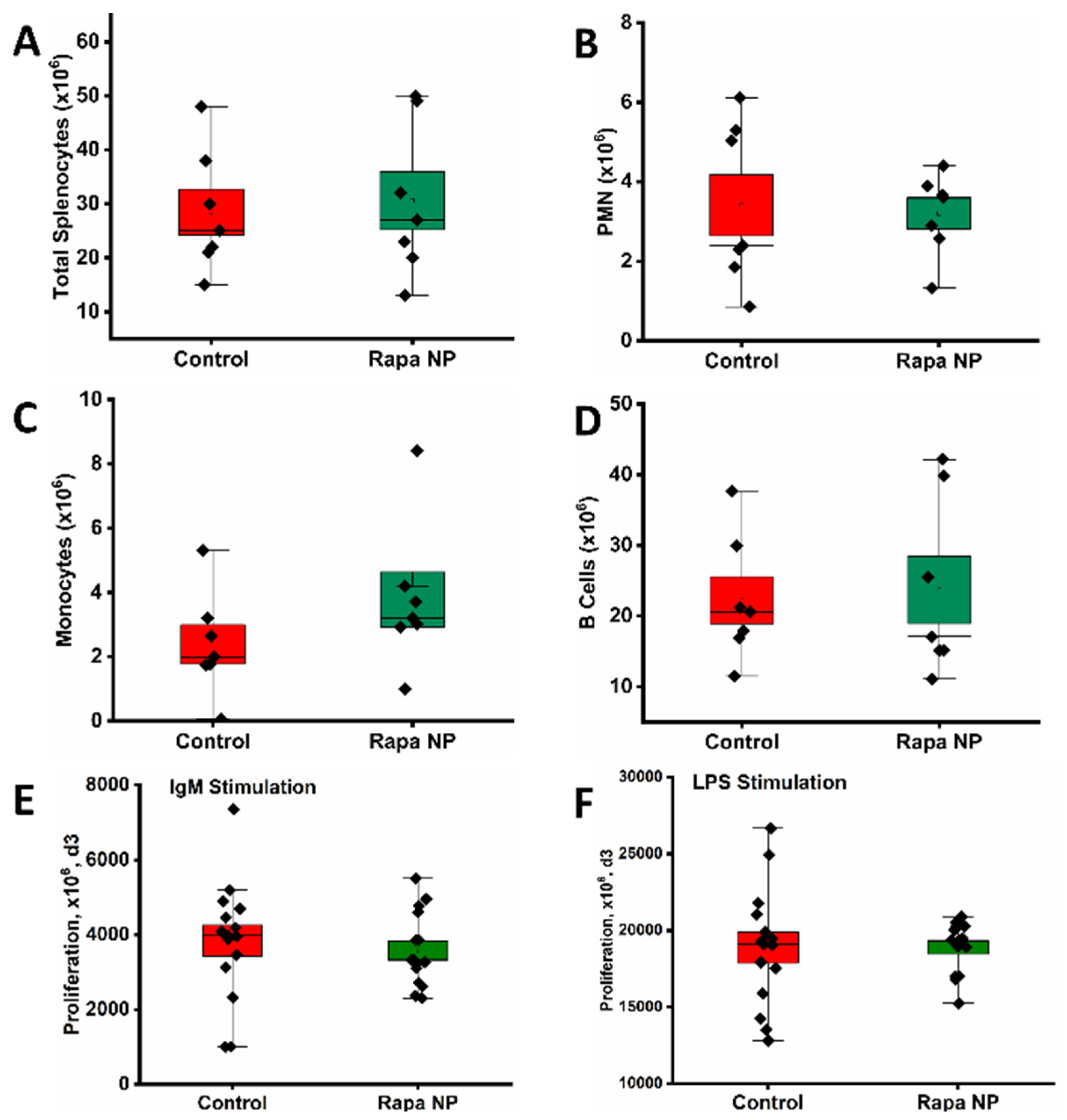
3.3.1. The Number of Total and Subpopulation of Splenocytes as Well as Splenocyte Proliferation Under Different Stimulations
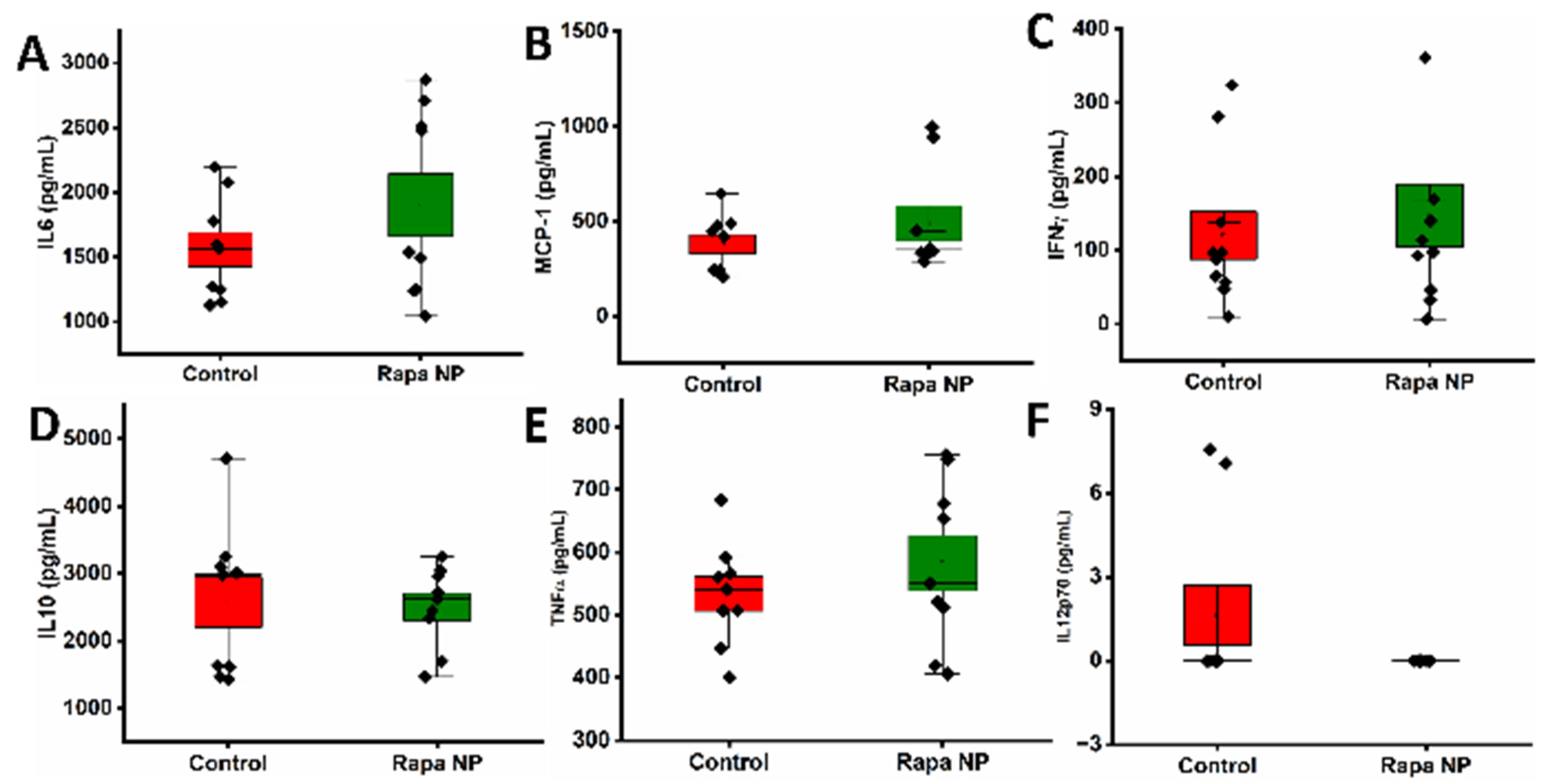
3.3.2. The Splenocyte Cytokine Productions Under Different Stimulations
3.4. Pharmcokenitics and Biodistribution
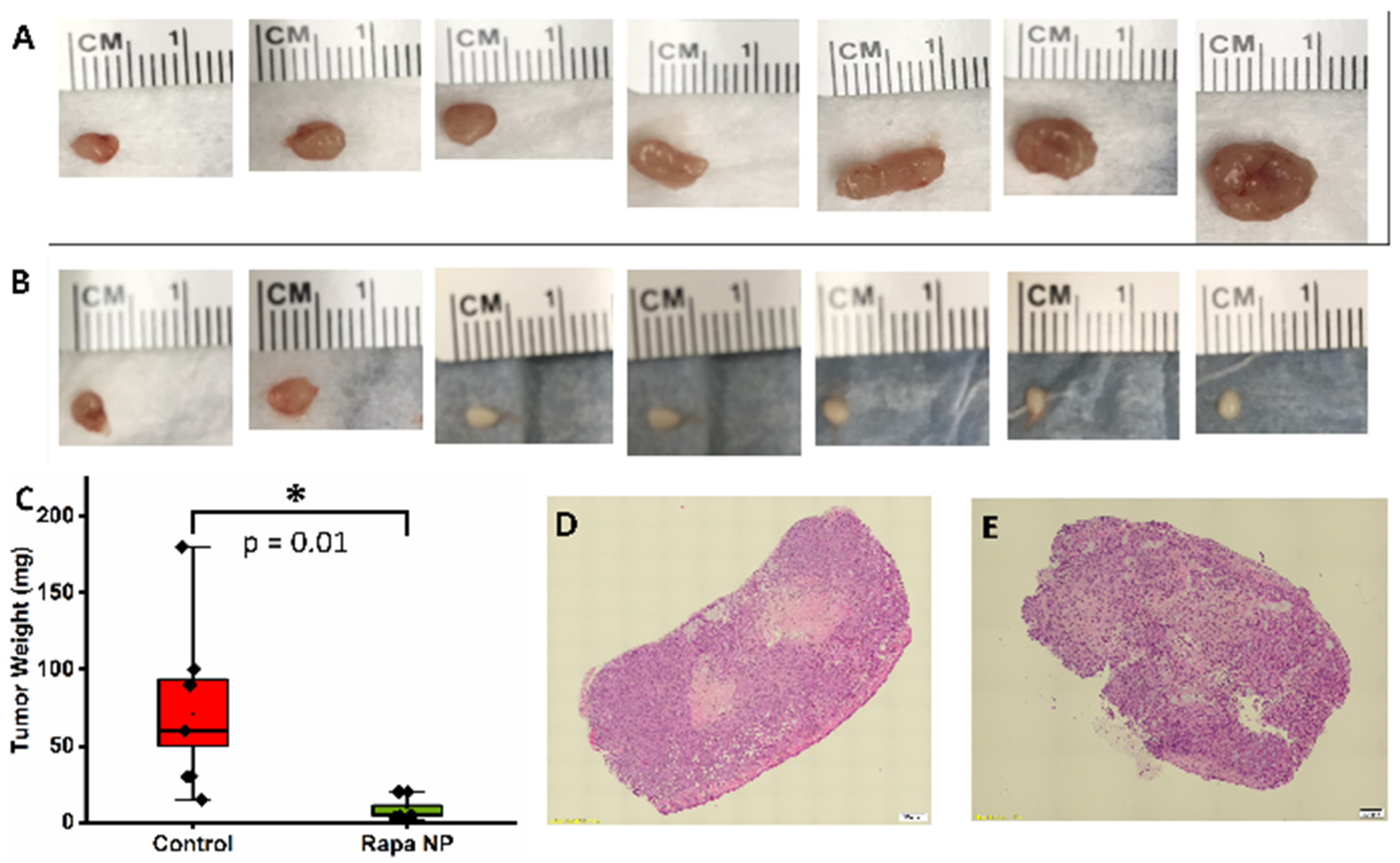
3.5. Rapamycin PFC Nanoparticles Treatment Inhibited Ovarian Tumor Growth
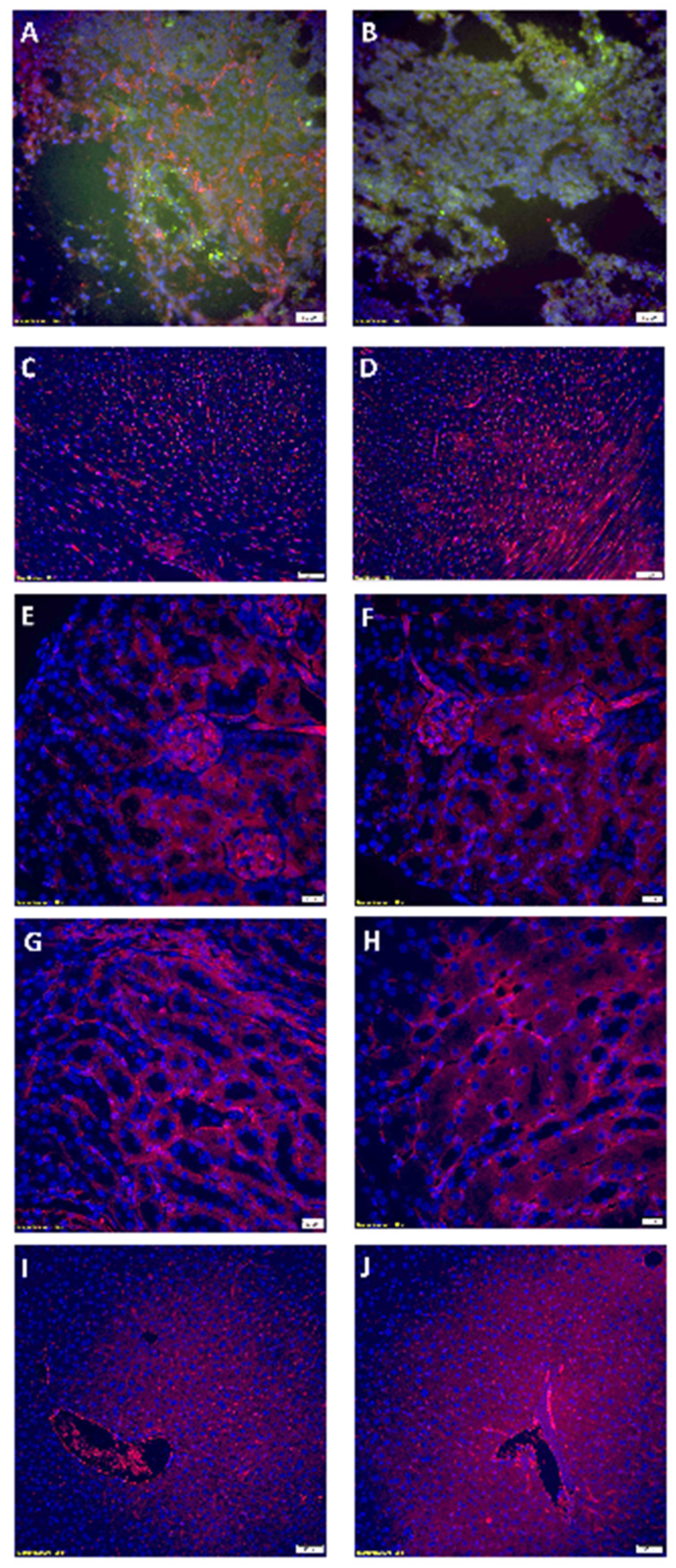
3.6. Rapamycin PFC Nanoparticles Treatment Inhibited Tumoral Vessel Establishement Without Impairing Blood Vessels in the Vital Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baert, T.; Ferrero, A.; Sehouli, J.; O’Donnell, D.M.; Gonzalez-Martin, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.S.P.; Querleu, D.; et al. The systemic treatment of recurrent ovarian cancer revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Cheng, M.; Lee, N.R.; Huang, H.Y.; Lee, W.L.; Chang, W.H.; Wang, P.H. Comparing Paclitaxel-Carboplatin with Paclitaxel-Cisplatin as the Front-Line Chemotherapy for Patients with FIGO IIIC Serous-Type Tubo-Ovarian Cancer. Int. J. Environ. Res. Public Health 2020, 17, 2213. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.H.; Lee, M.W.; Ryu, H.; Song, I.C.; Yun, H.J.; Jo, D.Y.; Ko, Y.B.; Lee, H.J. Efficacy of cisplatin combined with vinorelbine as second- or higher-line palliative chemotherapy in patients with advanced ovarian cancer. Medicine 2023, 102, e33271. [Google Scholar] [CrossRef] [PubMed]
- Sin, E.I.; Chia, C.S.; Tan, G.H.C.; Soo, K.C.; Teo, M.C. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int. J. Hyperth. 2017, 33, 690–695. [Google Scholar] [CrossRef]
- Angeles, M.A.; Quenet, F.; Vieille, P.; Gladieff, L.; Ruiz, J.; Picard, M.; Migliorelli, F.; Chaltiel, L.; Martinez-Gomez, C.; Martinez, A.; et al. Predictive risk factors of acute kidney injury after cytoreductive surgery and cisplatin-based hyperthermic intra-peritoneal chemotherapy for ovarian peritoneal carcinomatosis. Int. J. Gynecol. Cancer 2019, 29, 382–391. [Google Scholar] [CrossRef] [PubMed]
- McGinness, J.E.; Proctor, P.H.; Demopoulos, H.B.; Hokanson, J.A.; Kirkpatrick, D.S. Amelioration of cis-platinum nephrotoxicity by orgotein (superoxide dismutase). Physiol. Chem. Phys. 1978, 10, 267–277. [Google Scholar]
- Vogl, S.E.; Zaravinos, T.; Kaplan, B.H.; Wollner, D. Safe and effective two-hour outpatient regimen of hydration and diuresis for the administration of cis-diamminedichloroplatinum (II). Eur. J. Cancer 1981, 17, 345–350. [Google Scholar] [CrossRef]
- Tiseo, M.; Martelli, O.; Mancuso, A.; Sormani, M.P.; Bruzzi, P.; Di Salvia, R.; De Marinis, F.; Ardizzoni, A. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori 2007, 93, 138–144. [Google Scholar] [CrossRef]
- Ouchi, A.; Asano, M.; Aono, K.; Watanabe, T.; Kato, T. Comparison of short and continuous hydration regimen in chemotherapy containing intermediate- to high-dose Cisplatin. J. Oncol. 2014, 2014, 767652. [Google Scholar] [CrossRef]
- Ninomiya, K.; Hotta, K.; Hisamoto-Sato, A.; Ichihara, E.; Gotoda, H.; Morichika, D.; Tamura, T.; Kayatani, H.; Minami, D.; Kubo, T.; et al. Short-term low-volume hydration in cisplatin-based chemotherapy for patients with lung cancer: The second prospective feasibility study in the Okayama Lung Cancer Study Group Trial 1201. Int. J. Clin. Oncol. 2016, 21, 81–87. [Google Scholar] [CrossRef]
- Lavole, A.; Danel, S.; Baudrin, L.; Gounant, V.; Ruppert, A.M.; Epaud, C.; Belmont, L.; Rosencher, L.; Cadranel, J.; Milleron, B. Routine administration of a single dose of cisplatin ≥75 mg/m2 after short hydration in an outpatient lung-cancer clinic. Bull. Cancer 2012, 99, E43–E48. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Takigawa, N.; Hisamoto-Sato, A.; Ichihara, E.; Kudo, K.; Uchida, K.; Yanase-Nakamura, K.; Tanaka, H.; Kato, Y.; Tabata, M.; et al. Reappraisal of short-term low-volume hydration in cisplatin-based chemotherapy: Results of a prospective feasibility study in advanced lung cancer in the Okayama Lung Cancer Study Group Trial 1002. Jpn. J. Clin. Oncol. 2013, 43, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, H.; Kubota, K.; Itani, H.; Taniyama, T.K.; Nakamichi, S.; Wakui, H.; Kanda, S.; Nokihara, H.; Yamamoto, N.; Sekine, I.; et al. Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: A prospective study. Jpn. J. Clin. Oncol. 2013, 43, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Al Bahrani, B.J.; Moylan, E.J.; Forouzesh, B.; Della-Fiorentina, S.A.; Goldrick, A.J. A short outpatient hydration schedule for cisplatin administration. Gulf J. Oncol. 2009, 5, 30–36. [Google Scholar]
- Oka, T.; Kimura, T.; Suzumura, T.; Yoshimoto, N.; Nakai, T.; Yamamoto, N.; Matsuura, K.; Mitsuoka, S.; Yoshimura, N.; Kudoh, S.; et al. Magnesium supplementation and high volume hydration reduce the renal toxicity caused by cisplatin-based chemotherapy in patients with lung cancer: A toxicity study. BMC Pharmacol. Toxicol. 2014, 15, 70. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Watanabe, K.; Tsukiyama, I.; Matsushita, H.; Yabushita, H.; Matsuura, K.; Wakatsuki, A. Nephroprotective effects of hydration with magnesium in patients with cervical cancer receiving cisplatin. Anticancer Res. 2015, 35, 2199–2204. [Google Scholar]
- Yoshida, T.; Niho, S.; Toda, M.; Goto, K.; Yoh, K.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; Ohe, Y. Protective effect of magnesium preloading on cisplatin-induced nephrotoxicity: A retrospective study. Jpn. J. Clin. Oncol. 2014, 44, 346–354. [Google Scholar] [CrossRef]
- Gandara, D.R.; Wiebe, V.J.; Perez, E.A.; Makuch, R.W.; DeGregorio, M.W. Cisplatin rescue therapy: Experience with sodium thiosulfate, WR2721, and diethyldithiocarbamate. Crit. Rev. Oncol. Hematol. 1990, 10, 353–365. [Google Scholar] [CrossRef]
- Kemp, G.; Rose, P.; Lurain, J.; Berman, M.; Manetta, A.; Roullet, B.; Homesley, H.; Belpomme, D.; Glick, J. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: Results of a randomized control trial in patients with advanced ovarian cancer. J. Clin. Oncol. 1996, 14, 2101–2112. [Google Scholar] [CrossRef]
- Zhou, Q.; Quirk, J.D.; Hu, Y.; Yan, H.; Gaut, J.P.; Pham, C.T.N.; Wickline, S.A.; Pan, H. Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 6086. [Google Scholar] [CrossRef]
- Zhou, Q.; Doherty, J.; Akk, A.; Springer, L.E.; Fan, P.; Spasojevic, I.; Halade, G.V.; Yang, H.; Pham, C.T.N.; Wickline, S.A.; et al. Safety Profile of Rapamycin Perfluorocarbon Nanoparticles for Preventing Cisplatin-Induced Kidney Injury. Nanomaterials 2022, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N.; Baker, H.; Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Vezina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.I. Sourcing a chemical succession for cyclosporin from parasites and human pathogens. Drug Discov. Today 2005, 10, 688–691. [Google Scholar] [CrossRef]
- Kuo, C.J.; Chung, J.; Fiorentino, D.F.; Flanagan, W.M.; Blenis, J.; Crabtree, G.R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature 1992, 358, 70–73. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef]
- Yang, Q.; Inoki, K.; Ikenoue, T.; Guan, K.L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006, 20, 2820–2832. [Google Scholar] [CrossRef]
- Ravichandran, K.; Wang, Q.; Ozkok, A.; Jani, A.; Li, H.; He, Z.; Ljubanovic, D.; Weiser-Evans, M.C.; Nemenoff, R.A.; Edelstein, C.L. CD4 T cell knockout does not protect against kidney injury and worsens cancer. J. Mol. Med. 2016, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gartenhaus, R.B.; Lapidus, R.G.; Hussain, A.; Zhang, Y.; Wang, X.; Dan, H.C. Differential IKK/NF-kappaB Activity Is Mediated by TSC2 through mTORC1 in PTEN-Null Prostate Cancer and Tuberous Sclerosis Complex Tumor Cells. Mol. Cancer Res. 2015, 13, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Shigemitsu, K.; Tsujishita, Y.; Hara, K.; Nanahoshi, M.; Avruch, J.; Yonezawa, K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J. Biol. Chem. 1999, 274, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ho, D.; Vatner, D.E.; Vatner, S.F. Echocardiography in Mice. Curr. Protoc. Mouse Biol. 2011, 1, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Bibee, K.P.; Cheng, Y.J.; Ching, J.K.; Marsh, J.N.; Li, A.J.; Keeling, R.M.; Connolly, A.M.; Golumbek, P.T.; Myerson, J.W.; Hu, G.; et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014, 28, 2047–2061. [Google Scholar] [CrossRef]
- Quarles, E.; Basisty, N.; Chiao, Y.A.; Merrihew, G.; Gu, H.; Sweetwyne, M.T.; Fredrickson, J.; Nguyen, N.H.; Razumova, M.; Kooiker, K.; et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 2020, 19, e13086. [Google Scholar] [CrossRef]
- Lee, D.J.W.; Hodzic Kuerec, A.; Maier, A.B. Targeting ageing with rapamycin and its derivatives in humans: A systematic review. Lancet Healthy Longev. 2024, 5, e152–e162. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef]
- Chaveroux, C.; Eichner, L.J.; Dufour, C.R.; Shatnawi, A.; Khoutorsky, A.; Bourque, G.; Sonenberg, N.; Giguere, V. Molecular and genetic crosstalks between mTOR and ERRalpha are key determinants of rapamycin-induced nonalcoholic fatty liver. Cell Metab. 2013, 17, 586–598. [Google Scholar] [CrossRef]
- Ge, C.; Ma, C.; Cui, J.; Dong, X.; Sun, L.; Li, Y.; Yu, A. Rapamycin suppresses inflammation and increases the interaction between p65 and IkappaBalpha in rapamycin-induced fatty livers. PLoS ONE 2023, 18, e0281888. [Google Scholar] [CrossRef]
- Suzuki, O.; Koura, M.; Uchio-Yamada, K.; Sasaki, M. Urinary protein analysis in mice lacking major urinary proteins. Exp. Anim. 2021, 70, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Paluri, R.K.; Sonpavde, G.; Morgan, C.; Rojymon, J.; Mar, A.H.; Gangaraju, R. Renal toxicity with mammalian target of rapamycin inhibitors: A meta-analysis of randomized clinical trials. Oncol. Rev. 2019, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Caruthers, S.D.; Hughes, M.; Marsh, J.N.; Cyrus, T.; Winter, P.M.; Neubauer, A.M.; Wickline, S.A.; Lanza, G.M. Clinical applications of perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics. Int. J. Nanomed. 2007, 2, 515–526. [Google Scholar]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002, 8, 128–135. [Google Scholar] [CrossRef]


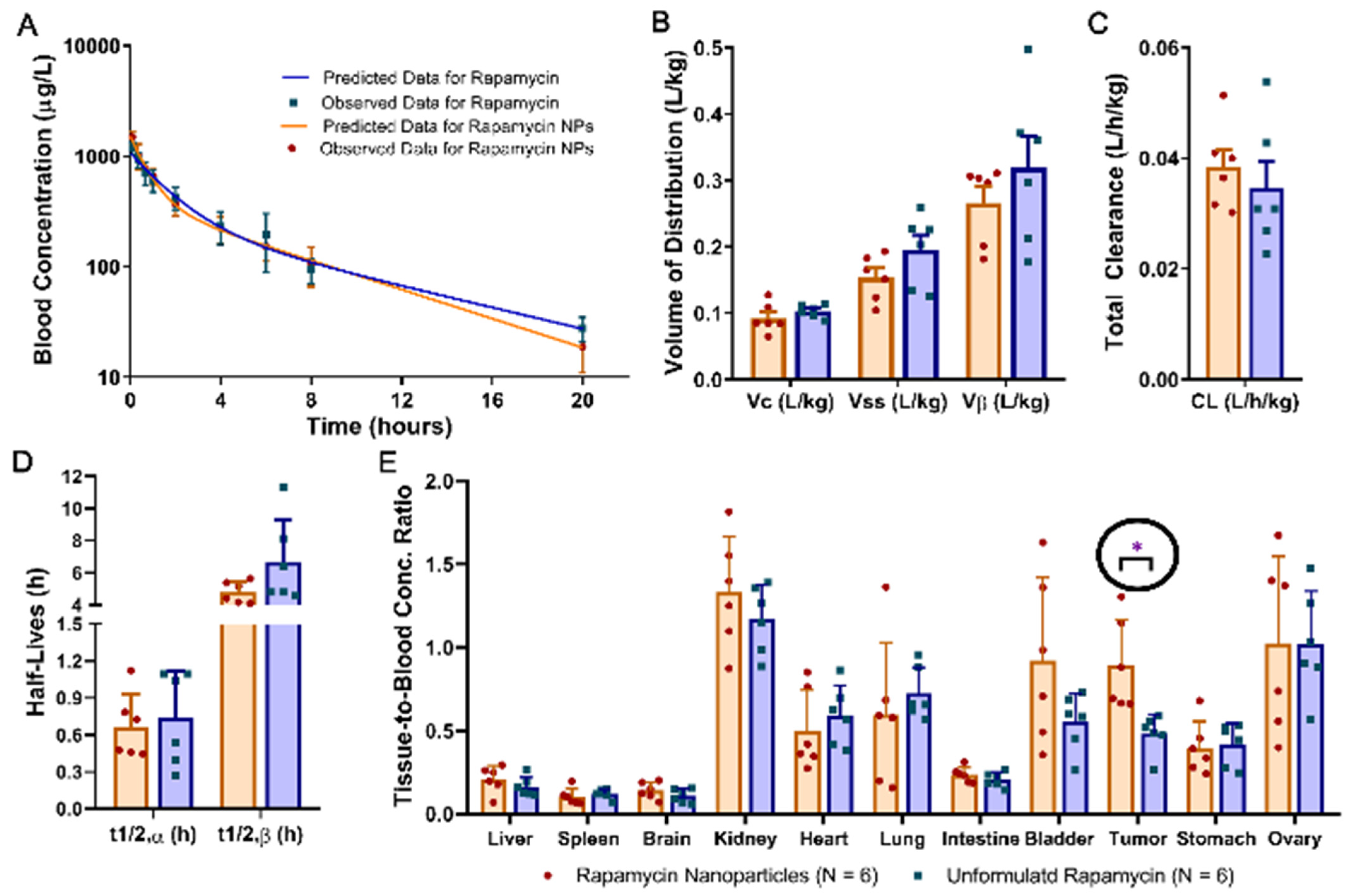
| Unformulated Rapamycin (N = 6) | Rapamycin Nanoparticles (N = 6) | |
|---|---|---|
| AUC (μg·h/L) | 3877 ± 1131 | 3419 ± 639 |
| t1/2,α (h) | 0.737 ± 0.382 | 0.668 ± 0.266 |
| t1/2,β (h) | 6.67 ± 2.67 | 4.81 ± 0.67 |
| VC (L/kg) | 0.103 ± 0.010 | 0.092 ± 0.022 |
| Vss (L/kg) | 0.195 ± 0.054 | 0.153 ± 0.034 |
| Vβ (L/kg) | 0.319 ± 0.117 | 0.266 ± 0.059 |
| CL (L/h/kg) | 0.035 ± 0.012 | 0.038 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Harding, J.C.; Fan, P.; Spasojevic, I.; Kovacs, A.; Akk, A.; Mitchell, A.; Springer, L.E.; Gaut, J.P.; Rauch, D.A.; et al. Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice. Nanomaterials 2024, 14, 1752. https://doi.org/10.3390/nano14211752
Zhou Q, Harding JC, Fan P, Spasojevic I, Kovacs A, Akk A, Mitchell A, Springer LE, Gaut JP, Rauch DA, et al. Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice. Nanomaterials. 2024; 14(21):1752. https://doi.org/10.3390/nano14211752
Chicago/Turabian StyleZhou, Qingyu, John C. Harding, Ping Fan, Ivan Spasojevic, Attila Kovacs, Antonina Akk, Adam Mitchell, Luke E. Springer, Joseph P. Gaut, Daniel A. Rauch, and et al. 2024. "Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice" Nanomaterials 14, no. 21: 1752. https://doi.org/10.3390/nano14211752
APA StyleZhou, Q., Harding, J. C., Fan, P., Spasojevic, I., Kovacs, A., Akk, A., Mitchell, A., Springer, L. E., Gaut, J. P., Rauch, D. A., Wickline, S. A., Pham, C. T. N., Fuh, K., & Pan, H. (2024). Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice. Nanomaterials, 14(21), 1752. https://doi.org/10.3390/nano14211752







