From Structure to Sensing: Molecular Mechanistic Insights into Plant-Derived Carbon Dots for Heavy Metal Ion Detection
Abstract
:1. Introduction
2. Synthesis and Structural Properties of P-CDs
3. Application of P-CDs for the Detection of Heavy Metal Ions
3.1. Iron (Fe3+, Fe2+)
3.2. Mercury (Hg2+)
3.3. Copper (Cu2+, Cu+)
3.4. Chromium
3.5. Co(II)
3.6. Cd(II)
3.7. Ag(I)
3.8. Zn2+, Al3+, Au3+, V5+, and Ru3+
4. Industrial Applications of Plant-Derived Carbon Dots for Heavy Metal Ion Detection
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhariwal, J.; Rao, G.K.; Vaya, D. Recent advancements towards the green synthesis of carbon quantum dots as an innovative and eco-friendly solution for metal ion sensing and monitoring. RSC Sustain. 2023, 2, 11–36. [Google Scholar] [CrossRef]
- He, H.Z.; Leung, K.H.; Yang, H.; Shiu-Hin Chan, D.; Leung, C.H.; Zhou, J.; Bourdoncle, A.; Mergny, J.L.; Ma, D.L. Label-free detection of sub-nanomolar lead(II) ions in aqueous solution using a metal-based luminescent switch-on probe. Biosens. Bioelectron. 2013, 41, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, J.; Wu, X.; Hu, Y.; Yu, W.; Wang, J.; Dong, J.; Li, M.; Liang, S.; Hu, J. A critical review on secondary lead recycling technology and its prospect. Renew. Sustain. Energy Rev. 2016, 61, 108–122. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Meyer, P.A.; Brown, M.J.; Falk, H. Global approach to reducing lead exposure and poisoning. Mutat. Res. Mutat. Res. 2008, 659, 166–175. [Google Scholar] [CrossRef]
- Scoullos, M. Mercury—Cadmium—Lead Handbook for Sustainable Heavy Metals Policy and Regulation: Handbook for Sustainable Heavy Metals Policy and Regulation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 31, ISBN 1402002246. [Google Scholar]
- Lin, Y.; Wang, S.; Wu, Q.; Larssen, T. Material flow for the intentional use of mercury in China. Environ. Sci. Technol. 2016, 50, 2337–2344. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Shah, M.T.; Suleman, M.; Abdul Baqi, S.; Sattar, A.; Khan, N.; Rehman, A.U. Determination of heavy metals in drinking water and their adverse effects on human health. A review. Pure Appl. Biol. 2020, 9, 96–104. [Google Scholar] [CrossRef]
- Jamshaid, M.; Khan, A.A.; Ahmed, K.; Saleem, M. Heavy metal in drinking water its effect on human health and its treatment techniques-a review. Int. J. Biosci. 2018, 12, 223–240. [Google Scholar]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Yu, J.; Song, N.; Zhang, Y.K.; Zhong, S.X.; Wang, A.J.; Chen, J. Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sensors Actuators B Chem. 2015, 214, 29–35. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, G.; Ran, L.; Liu, M.; Geng, P.; Yi, W. Green Synthesis of Biomass-Derived Porous Carbon for Electrochemical Detection of Heavy Metal Ions: Methods, Properties, and Applications. J. Environ. Chem. Eng. 2024, 12, 113903. [Google Scholar] [CrossRef]
- Khan, A.U.; Liu, Y.; Wang, S.; Ullah, M.W.; Chen, Q.; Zhang, D.; Kang, Z.; Mao, B. Advancements in the green synthesis of carbon dots for sustainable development. Sustain. Mater. Technol. 2024, 41, e01004. [Google Scholar] [CrossRef]
- Singh, P.; Arpita, N.; Kumar, S.; Kumar, P.; Kataria, N.; Bhankar, V.; Kumar, K.; Kumar, R.; Hsieh, C.-T.; Khoo, K.S. Assessment of biomass-derived carbon dots as highly sensitive and selective templates for the sensing of hazardous ions. Nanoscale 2023, 15, 16241–16267. [Google Scholar] [CrossRef]
- Saah, F.K.; Gbawoquiya, F.; Chaudhary, R.; Nagpal, G.; Saah, F.K.; Gbawoquiya, F.L. Green Synthesis of Carbon Quantum Dots for Detecting Heavy Metal Ionsin Environmental Samples. High Technol. Lett. 2023, 29, 174–205. [Google Scholar] [CrossRef]
- Prasasti, R.I.; Jannah, R.; Wati, A.N.B.; Kusumandari, K.; Isnaeni, I. Synthesis of carbon dots based on corn cobs as heavy metal ion sensors using the microwave method. J. Phys. Theor. Appl. 2022, 6, 97. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Carbon dots: Preparation, properties, and application. In Nanocarbon and Its Composites: Preparation, Properties and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 651–676. ISBN 9780081025093. [Google Scholar]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Frey, P.A.; Reed, G.H. The ubiquity of iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef]
- Taylor, K.G.; Konhauser, K.O. Iron in earth surface systems: A major player in chemical and biological processes. Elements 2011, 7, 83–88. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Chen, M.; Wu, X.; Yang, Y.; Wang, D.; Zuo, S.; Zeng, Z.; Xiong, W.; Guo, C. Green Synthesis of Nitrogen–Doped Carbon Dots from Fresh Tea Leaves for Selective Fe3+ Ions Detection and Cellular Imaging. Nanomaterials 2022, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, S.; Lee, J.; Mehta, A.; Kumar, S.; Kim, K.-H.; Basu, S.; Rawat, M. Highly fluorescent carbon dots derived from Mangifera indica leaves for selective detection of metal ions. Sci. Total Environ. 2020, 720, 137604. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Li, T.; Zhang, R.; Kang, Y.; Liu, W.; Cui, Y.; Wei, S.; Wang, N.; Li, L.; Wang, H.; et al. Facile and green synthesis of fluorescent carbon dots with tunable emission for sensors and cells imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sailaja Prasannakumaran Nair, S.; Kottam, N.; SG, P.K. Green Synthesized Luminescent Carbon Nanodots for the Sensing Application of Fe3+ Ions. J. Fluoresc. 2020, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Kalanidhi, K.; Nagaraaj, P. Facile and Green synthesis of fluorescent N-doped carbon dots from betel leaves for sensitive detection of Picric acid and Iron ion. J. Photochem. Photobiol. A Chem. 2021, 418, 113369. [Google Scholar] [CrossRef]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Senol, A.M.; Bozkurt, E. Facile green and one-pot synthesis of seville orange derived carbon dots as a fluorescent sensor for Fe3+ ions. Microchem. J. 2020, 159, 105357. [Google Scholar] [CrossRef]
- Achadu, O.J.; Elizur, G.L.; Boye, T.G.E.; Park, E.Y. Green synthesis of carbon dots using expired agar for a label-free fluorescence signal-amplified detection of ferric ion utilizing oxalate functionalization. Mater. Adv. 2022, 3, 6307–6315. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, J.; Yang, S.; Zheng, M.; Wang, Y.; Ma, S.; Zheng, H. Green synthesis of carbon dots originated from Lycii Fructus for effective fluorescent sensing of ferric ion and multicolor cell imaging. J. Photochem. Photobiol. B Biol. 2017, 175, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Polatoğlu, B.; Bozkurt, E. Green synthesis of fluorescent carbon dots from Kumquat (Fortunella margarita) for detection of Fe3+ ions in aqueous solution. Res. Chem. Intermed. 2021, 47, 1865–1881. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Latief, U.; ul Islam, S.; Khan, Z.M.S.H.; Khan, M.S. A facile green synthesis of functionalized carbon quantum dots as fluorescent probes for a highly selective and sensitive detection of Fe3+ ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120132. [Google Scholar] [CrossRef]
- Ran, Y.; Wang, S.; Yin, Q.; Wen, A.; Peng, X.; Long, Y.; Chen, S. Green synthesis of fluorescent carbon dots using chloroplast dispersions as precursors and application for Fe3+ ion sensing. Luminescence 2020, 35, 870–876. [Google Scholar] [CrossRef]
- Sindhuja, H.; Selvaraj, H.; Ilangovan, A.; Chandrasekaran, K.; Rajesh, V.; Parthipan, P. Green synthesis of biomass derived carbon dots via microwave-assisted method for selective detection of Fe3+ ions in an aqueous medium. Inorg. Chem. Commun. 2023, 157, 111348. [Google Scholar] [CrossRef]
- Devi, P.; Thakur, A.; Bhardwaj, S.K.; Saini, S.; Rajput, P.; Kumar, P. Metal ion sensing and light activated antimicrobial activity of aloe-vera derived carbon dots. J. Mater. Sci. Mater. Electron. 2018, 29, 17254–17261. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Guo, T.; Yang, T.; Chen, M.; Wang, J. Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens. Bioelectron. 2016, 85, 68–75. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Zhang, Y.; Zhou, K.; Yuan, L.; Shi, R.; Zhang, K.; Fu, Q. Sustainable and green synthesis of waste-biomass-derived carbon dots for parallel and semi-quantitative visual detection of Cr (VI) and Fe3+. Molecules 2022, 27, 1258. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Atchudan, R.; Perumal, S.; Salama, E.S.; Lee, Y.R.; Jeon, B.H. Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 2022, 286, 131764. [Google Scholar] [CrossRef] [PubMed]
- Shanmuga Priya, S.; Suseem, S. Sustainable carbon dots from Borreria hispida: Enhanced colorimetric sensing of Fe3+ ions and biological applications in live cell imaging. RSC Adv. 2024, 14, 17471–17479. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Liu, H.; Ding, L.; Chen, L.; Chen, Y.; Zhou, T.; Li, H.; Xu, Y.; Zhao, L.; Huang, N. A facile, green synthesis of biomass carbon dots coupled with molecularly imprinted polymers for highly selective detection of oxytetracycline. J. Ind. Eng. Chem. 2019, 69, 455–463. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Vasudevan, D.; Narayan, R.; Raju, K.V.S.N. Controllable synthesis of biosourced blue-green fluorescent carbon dots from camphor for the detection of heavy metal ions in water. RSC Adv. 2014, 4, 57137–57143. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.J.; Zhou, D.L.; Bao, N.; Xu, Y.; Wang, A.J.; Feng, J.J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- Kasinathan, K.; Samayanan, S.; Marimuthu, K.; Yim, J.H. Green synthesis of multicolour fluorescence carbon quantum dots from sugarcane waste: Investigation of mercury (II) ion sensing, and bio-imaging applications. Appl. Surf. Sci. 2022, 601, 154266. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Fu, N.; Yang, Y.; Liu, X.; Sun, W.; Gong, L.; Li, W.; Han, A. Green preparation of carbon dots for hg2+ detection and cell imaging. Mater. Express 2020, 10, 1777–1787. [Google Scholar] [CrossRef]
- Askari, F.; Rahdar, A.; Dashti, M.; Trant, J.F. Detecting Mercury (II) and Thiocyanate Using “Turn-on” Fluorescence of Graphene Quantum Dots. J. Fluoresc. 2020, 30, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ning, Y.; Pang, J.; Chen, L.; Zhang, F.; Chai, F. Green and facile synthesis of silicon-doped carbon dots and their use in detection of Hg2+ and visualization of latent fingerprints. New J. Chem. 2023, 47, 147–155. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A. V Essential trace elements in human health: A physician’s view. Tomsk. Publ. House Tomsk. State Univ. 2018, 224, 1–222. [Google Scholar]
- Torres Landa, S.D.; Reddy Bogireddy, N.K.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Sanni, S.O.; Moundzounga, T.H.G.; Oseghe, E.O.; Haneklaus, N.H.; Viljoen, E.L.; Brink, H.G. One-Step Green Synthesis of Water-Soluble Fluorescent Carbon Dots and Its Application in the Detection of Cu2+. Nanomaterials 2022, 12, 958. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.Y.; Lin, Y.C.; Wu, H.F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Gedda, G.; Sankaranarayanan, S.A.; Putta, C.L.; Gudimella, K.K.; Rengan, A.K.; Girma, W.M. Green synthesis of multi-functional carbon dots from medicinal plant leaves for antimicrobial, antioxidant, and bioimaging applications. Sci. Rep. 2023, 13, 6371. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, S.-F.; Zhao, J.-L.; Zhao, L.; Zhang, A.-Z.; Li, M.-Y. Toxic effects of hexavalent chromium (Cr6+) on bioaccumulation, apoptosis, oxidative damage and inflammatory response in Channa asiatica. Environ. Toxicol. Pharmacol. 2021, 87, 103725. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, P. Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): Occurrence, sources and health risks. Expo. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Guarneri, F.; Costa, C.; Cannavò, S.P.; Catania, S.; Bua, G.D.; Fenga, C.; Dugo, G. Release of nickel and chromium in common foods during cooking in 18/10 (grade 316) stainless steel pots. Contact Dermat. 2017, 76, 40–48. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Chen, J.; Zhou, X.; Zhou, L.; Huang, W.; Guan, Q.; Ye, G. Study on the Influence of Metal Materials on the Migration of Heavy Metals in Stainless Steel Kitchenware. IOP Conf. Ser. Mater. Sci. Eng. 2019, 490, 22032. [Google Scholar] [CrossRef]
- Awasthi, Y.; Ratn, A.; Prasad, R.; Kumar, M.; Trivedi, S.P. An in vivo analysis of Cr6+ induced biochemical, genotoxicological and transcriptional profiling of genes related to oxidative stress, DNA damage and apoptosis in liver of fish, Channa punctatus (Bloch, 1793). Aquat. Toxicol. 2018, 200, 158–167. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Li, J.; Lei, M.; Yan, X. Selective and sensitive chemosensor for lead ions using fluorescent carbon dots prepared from chocolate by one-step hydrothermal method. Sens. Actuators B Chem. 2016, 237, 597–604. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Wee, S.S.; Ng, Y.H.; Ng, S.M. Synthesis of fluorescent carbon dots via simple acid hydrolysis of bovine serum albumin and its potential as sensitive sensing probe for lead (II) ions. Talanta 2013, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jie, X.; Xie, F.; Yang, H.; Wei, W.; Xia, Z. Flavonoid moiety-incorporated carbon dots for ultrasensitive and highly selective fluorescence detection and removal of Pb2+. Nano Res. 2018, 11, 3648–3657. [Google Scholar] [CrossRef]
- Bandi, R.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Green synthesis of highly fluorescent nitrogen—Doped carbon dots from Lantana camara berries for effective detection of lead(II) and bioimaging. J. Photochem. Photobiol. B Biol. 2018, 178, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Verma, N.C.; Khan, S.; Tiwari, S.; Chaudhary, A.; Nandi, C.K. Paper strip based and live cell ultrasensitive lead sensor using carbon dots synthesized from biological media. Sens. Actuators B Chem. 2016, 232, 107–114. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Qin, L.; Yue, X.; Zhang, Q.; Wang, L. Green one-step synthesis of boron and nitrogen co-doped carbon dots based on inner filter effect as fluorescent nanosensors for determination of Fe3+. Ceram. Int. 2023, 49, 7546–7555. [Google Scholar] [CrossRef]
- Li, M.; Lu, J. Cobalt in lithium-ion batteries. Science 2020, 367, 979–980. [Google Scholar] [CrossRef]
- Hu, G.; Ge, L.; Li, Y.; Mukhtar, M.; Shen, B.; Yang, D.; Li, J. Carbon dots derived from flax straw for highly sensitive and selective detections of cobalt, chromium, and ascorbic acid. J. Colloid Interface Sci. 2020, 579, 96–108. [Google Scholar] [CrossRef]
- Mohammed, E.; Mohammed, K.; Liu, J.; Chen, H.; Xiao, J. Silicon quantum dots-based fluorescent sensor for the detection of cobalt with high sensitivity and selectivity. Chin. Chem. Lett. 2024, 35, 108476. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Yin, J.; Li, H.; Huang, J. Facile ultrasonic synthesized NH2-carbon quantum dots for ultrasensitive Co2+ ion detection and cell imaging. Talanta 2019, 205, 120121. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Cheng, C.; Yang, Y. Green and microwave-assisted synthesis of carbon dots and application for visual detection of cobalt(II) ions and pH sensing. Microchem. J. 2019, 147, 183–190. [Google Scholar] [CrossRef]
- Dutta, A.; Rooj, B.; Mondal, T.; Mukherjee, D.; Mandal, U. Detection of Co2+ via fluorescence resonance energy transfer between synthesized nitrogen-doped carbon quantum dots and Rhodamine 6G. J. Iran. Chem. Soc. 2020, 17, 1695–1704. [Google Scholar] [CrossRef]
- Alkian, I.; Sutanto, H.; Hadiyanto, B.; Prasetio, A.; Aprimanti Utami, B. Facile synthesized carbon dots for simple and selective detection of cobalt ions in aqueous media. Cogent Eng. 2022, 9, 2033467. [Google Scholar] [CrossRef]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental hazards of cadmium: Past, present, and future. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–183. [Google Scholar]
- Pandey, S.C.; Kumar, A.; Sahu, S.K. Single Step Green Synthesis of Carbon Dots from Murraya koenigii leaves; A Unique Turn-off Fluorescent contrivance for Selective Sensing of Cd (II) ion. J. Photochem. Photobiol. A Chem. 2020, 400, 112620. [Google Scholar] [CrossRef]
- Keerthana, P.; Kumar, A.; Bharath, M.; Ghosh, M.; Varghese, A. A ratiometric fluorescent sensor based on dual-emissive carbon dot for the selective detection of Cd2+. J. Environ. Chem. Eng. 2023, 11, 109325. [Google Scholar] [CrossRef]
- Yan, Z.; Yao, W.; Mai, K.; Huang, J.; Wan, Y.; Huang, L.; Cai, B.; Liu, Y. A highly selective and sensitive “on–off” fluorescent probe for detecting cadmium ions and L-cysteine based on nitrogen and boron co-doped carbon quantum dots. RSC Adv. 2022, 12, 8202–8210. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Wang, L.; Zhao, J.; Yang, Q.; Sun, P.; Deng, Y.; Shen, G. One-step synthesis of highly fluorescent carbon dots as fluorescence sensors for the parallel detection of cadmium and mercury ions. Front. Chem. 2022, 10, 1005231. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Akhgari, F.; Farhadi, K.; Samadi, N.; Akhgari, M. Detection of Silver Nanoparticles Using Green Synthesis of Fluorescent Nitrogen-Doped Carbon Dots. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 379–387. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag+ ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef]
- Li, Q.; Bai, Z.; Xi, X.; Guo, Z.; Liu, C.; Liu, X.; Zhao, X.; Li, Z.; Cheng, Y.; Wei, Y. Rapid microwave-assisted green synthesis of guanine-derived carbon dots for highly selective detection of Ag+ in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119208. [Google Scholar] [CrossRef]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef]
- Jayan, S.S.; Jayan, J.S.; Sneha, B.; Abha, K. Facile synthesis of carbon dots using tender coconut water for the fluorescence detection of heavy metal ions. Mater. Today Proc. 2020, 43, 3821–3825. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Facile green synthesis of carbon dots from Pyrus pyrifolia fruit for assaying of Al3+ ion via chelation enhanced fluorescence mechanism. J. Mol. Liq. 2018, 264, 9–16. [Google Scholar] [CrossRef]
- Arumugham, T.; Alagumuthu, M.; Amimodu, R.G.; Munusamy, S.; Iyer, S.K. A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain. Mater. Technol. 2020, 23, e00138. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Dai, L.; Luo, Y.; Fu, Y.; Yang, N.; Wun, J.; Chen, L. High photoluminescent carbon nanodots and quercetin-Al3+ construct a ratiometric fluorescent sensing system. Carbon 2014, 77, 1148–1156. [Google Scholar] [CrossRef]
- Liao, J.; Cheng, Z.; Zhou, L. Nitrogen-Doping Enhanced Fluorescent Carbon Dots: Green Synthesis and Their Applications for Bioimaging and Label-Free Detection of Au3+ Ions. ACS Sustain. Chem. Eng. 2016, 4, 3053–3061. [Google Scholar] [CrossRef]
- Mohandoss, S.; Ahmad, N.; Velu, K.S.; Khan, M.R.; Palanisamy, S.; You, S.G.; Lee, Y.R. Synthesis of Photoluminescent Carbon Dots Using Hibiscus Tea Waste and Heteroatom Doping for Multi-Metal Ion Sensing: Applications in Cell imaging and Environmental Samples. Chemosensors 2023, 11, 474. [Google Scholar] [CrossRef]
- Hoan, B.T.; Thanh, T.T.; Tam, P.D.; Trung, N.N.; Cho, S.; Pham, V.H. A green luminescence of lemon derived carbon quantum dots and their applications for sensing of V5+ ions. Mater. Sci. Eng. B 2019, 251, 114455. [Google Scholar] [CrossRef]
- Hashemi, N.; Mousazadeh, M.H. Green synthesis of photoluminescent carbon dots derived from red beetroot as a selective probe for Pd2+ detection. J. Photochem. Photobiol. A Chem. 2021, 421, 113534. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; Zhai, J.; An, Y.; Li, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Preparation of solvent-free starch-based carbon dots for the selective detection of Ru3+ ions. 2022, 12, 18779–18783. RSC Adv. 2022, 12, 18779–18783. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Ahmadi, E.; Mavaei, M. A novel voltammetric sensor based on graphene quantum dots-thionine/nano-porous glassy carbon electrode for detection of cisplatin as an anti-cancer drug. Sens. Actuators B Chem. 2019, 299, 126975. [Google Scholar] [CrossRef]
- Alshehri, R.F.; Amin, A.S.; Darwish, E.R. Colorimetric probe for the determination of osmium through a novel optical sensor in environmental samples. Talanta Open 2024, 9, 100311. [Google Scholar] [CrossRef]
- Yarur, F.; Macairan, J.R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
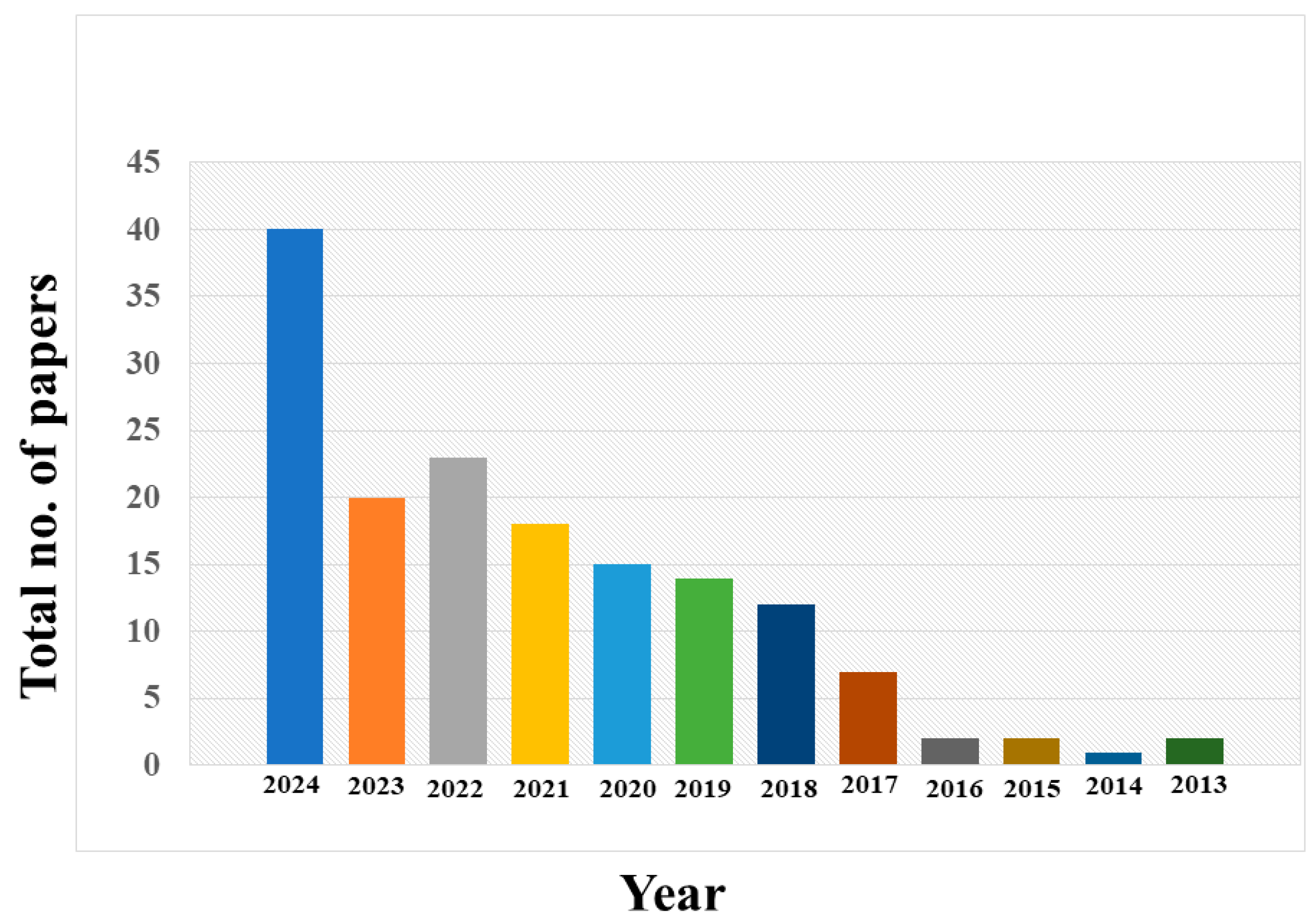
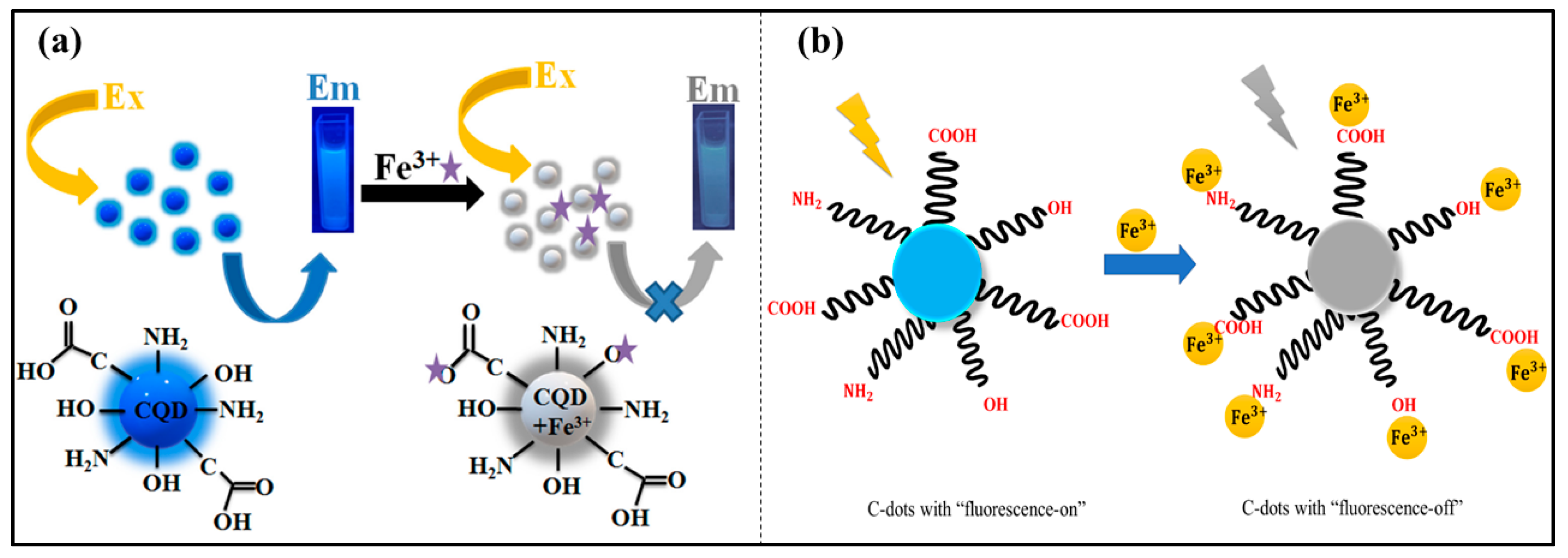
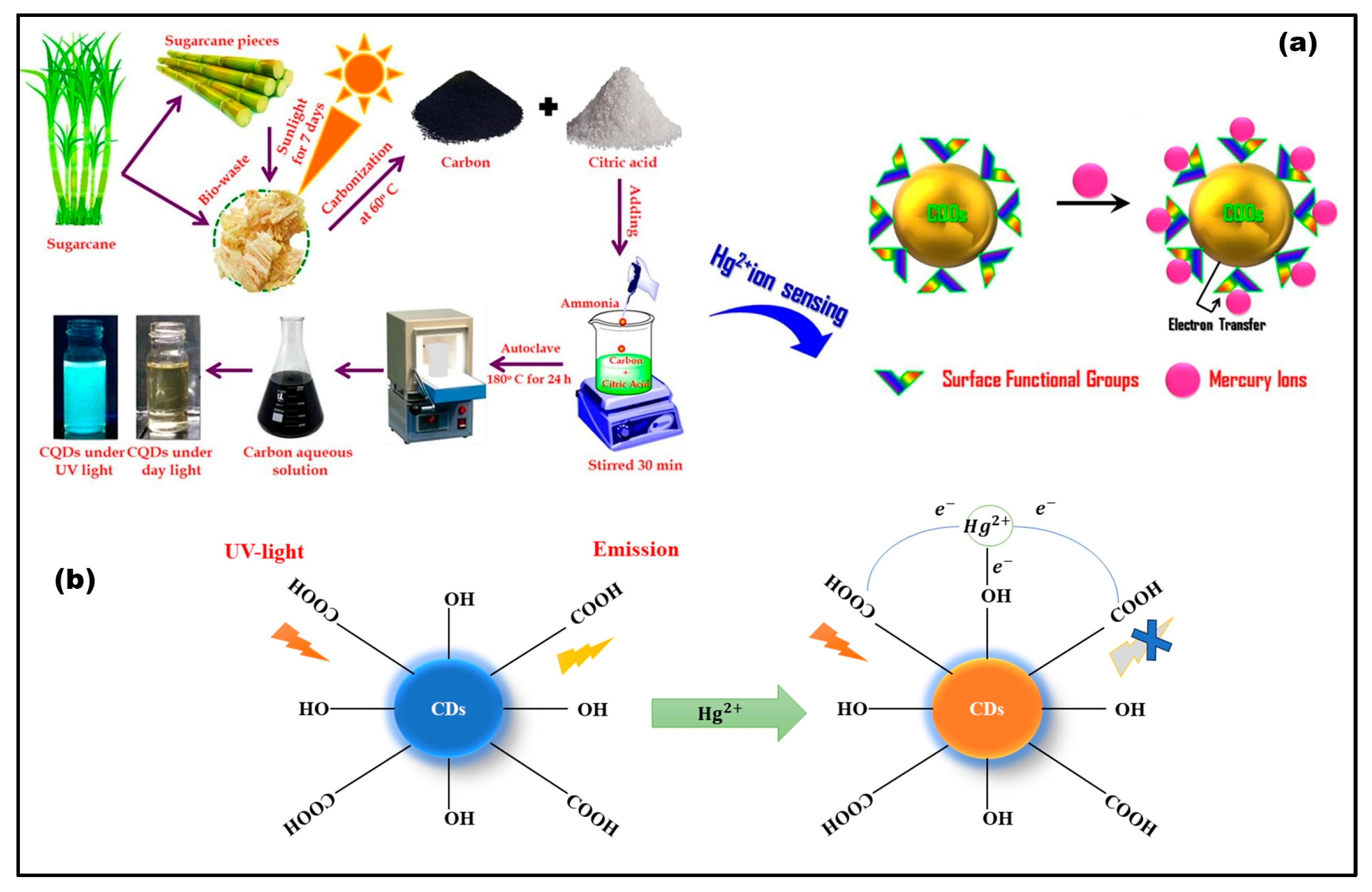



| Sr. No. | Method | Natural Precursor | Detection Limit | Size of P-CDs | Linear Concentration Range | Quantum Yield (%) | Metal Ions | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Pyrolysis | Mangifera indica | 0.62 ppm | 1–5 nm | 18.2% | Fe2+ | [24] | |
| 2. | Hydrothermal | Betel leaves | 0.135 μM | 3.7 nm | 0.3–3.3 μM | 4.21% | Fe3+ | [28] |
| 3. | Hydrothermal | Honey | 1.7 × 10−9 mol/L | 2 nm | 5.0 × 10−9–1.0 × 10−4 mol/L | 19.8% | Fe3+ | [33] |
| 4. | Hydrothermal | Lycii Fructus | 21 nM | 3.3 nm | 0 to 30 μM | 17.2% | Fe3+ | [34] |
| 5. | Hydrothermal-carbonization | Chionanthus retusus | 70 μM | 5 ± 2 nm | 0–2 μM | 9% | Fe3+ | [27] |
| 6. | Hydrothermal | gelatin | 0.2 µM | 0.5–5 nm | 0–50 µM | 22.7% | Fe3+ | [37] |
| 7. | Hydrothermal | Kumquat | 0.70 µM | 3 nm | 0–40 µM | 8% | Fe3+ | [35] |
| 8. | Pyrolysis | Aloe-Vera extract, | 33 ppb | 6–8 nm | 70 ppb to 100 ppm | 12.37% | Fe3+ | [40] |
| 9. | Hydrothermal | papaya powder | 0.48 μmol L−1 | 3.4 nm | 1–10 μmol L−1 | 18.98% | Fe3+ | [41] |
| 10. | Hydrothermal | coriander leaves | 0.4 µM | 2.387 nm | 0–60 µM | 6.48% | Fe3+ | [32] |
| 11. | Hydrothermal | Jinhua bergamot | 5.5 nM (Hg2+) and 0.075 μM (Fe3+) | 50 nm | Hg2+ = 0.01–100 μM 0.025–100 μM for Fe3+ | 50.78% | Hg2+ and Fe3+ | [12] |
| 12. | Hydrothermal | wintersweet | Cr = 0.07 μM and Fe = 0.15 μM | 9.38 nm | Cr (VI) = 0.1 to 60 μM And Fe3+ = 0.05 to 100 μM | 14.8% | Cr6+ and Fe3+ | [42] |
| 13. | Hydrothermal | Poa Pratensis | Fe2+ = 1.4 and Mn2+ = 1.2 μM | 2 nm | 5.0 to 25 μM | 7% | Fe3+ and Mn2+ | [43] |
| 14. | Hydrothermal | Borreria hispida | 1.2 × 10−6 M | 2 nm | - | 40.8% | Fe3+ | [44] |
| Sr. No. | Method | Natural Precursor | Detection Limit | Size of P-CDs | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Microwave | flour | 0.5 nM | 1–4 nm | 0.0005–0.01 µM | Hg2+ | [52] | |
| 2. | Hydrothermal | Pea | 0.96 µM | 20 nm | 0–200 µM | 12.09% | Hg2+ | [53] |
| 3. | Hydrothermal | Jinhua bergamot | 5.5 nM (Hg2+) and 0.075 μM (Fe3+) | 50 nm | Hg2+ = 0.01–100 μM 0.025–100 μM for Fe3+, | 50.78% | Hg2+ and Fe3+ | [12] |
| Sr. No. | Method | Natural Precursor | Detection Limit | Size of P-CDs | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Pyrolysis | Eleusine coracana | 10 nM | 3–8 nm | 0 to 100 μM | - | Cu2+ | [63] |
| 2. | Green | Prawn shells | 5 nM | 4 nm | 0 to 5 µM | 9% | Cu2+ | [64] |
| 3. | Microwave pyrolysis | Pinecone | 0.005 µg/mL | 15.2 nm and 42.1 nm | 2.5–22.5 µg/mL | 17% | Cu2+ | [61] |
| 4. | Hydrothermal | Bamboo leaves | 115 nM | 3.6 nm | 0.333 to 66.6 µM | 7.1%. | Cu2+ | [60] |
| Sr. No. | Method | Precursor | Detection Limit | Size | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Microwave | Potato dextrose agar | 106–110 pM | 4.3 nm | 0–20 μM | 9.0% | Pb2+ | [76] |
| 2. | Hydrothermal | Ginkgo biloba leaves | 0.1–20.0 nM | 4.18 nm | 0.1–20 × 10−3 | 16.1% | Pb2+ | [77] |
| 3. | Acid hydrolysis | Bovine serum albumin | 5.05 μM | 1–2 nm | 0–6 × 10−3 μM | - | Pb2+ | [73] |
| 4. | Hydrothermal | Chocolate | 12.7 nM | 6.41 nm | 0.033 to 10 μM | - | Pb2+ | [70] |
| 5. | Hydrothermal | Ocimum sanctum | 0.59 nM | 3 nm | 0.01−1.0 μM | 9.3% | Pb2+ | [71] |
| 6. | Hydrothermal | Lantana camara berries | 9.64 nM | 20 nm | 0–200 nM | 33.15% | Pb2+ | [75] |
| Sr. No. | Method | Natural Precursor | Detection Limit | Size of P-CDs | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Microwave | Kelp | 0.39 μmol/L | 3.7 nm | 1–200 μmol/L | 23.5%. | Co2+ | [82] |
| 2. | Hydrothermal | Nerium Oleander L. petals | 6.45 nM | 5–6 nm. | 0–40 μM | 3.5% | Co2+ | [83] |
| 3. | Hydrothermal method | Flax straw | Co2+ = 0.38, Cr6+ = 0.19 μM | 2.2 nm | Co2+ = 0–500, Cr6+ = 0.5–80 μM | 20.7% | Co2+ or Cr6+ | [79] |
| 4. | Microwave | Orange | 1.63 μM | 8.82 nm | 0–200 μM | 49.42% | Co2+ | [84] |
| Sr. No. | Method | Precursor | Detection Limit | Size | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Hydrothermal | Murraya koenigii | 0.29 nM | 2–8 nm | 0.01–8 μM | 5.4% | Cd2+ | [86] |
| 2. | Hydrothermal | L-arginine | 0.20 μM | 2.68 ± 0.67 nm | 0–26.8 μM | 71.6% | Cd2+ | [89] |
| Sr. No. | Method | Precursor | Detection Limit | Size | Linear Concentration Range | Quantum Yield | Metal Ion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Hydrothermal | Broccoli | 0.5 µM | 2–6 nm | 0 to 600 µM | - | Ag+ | [90] |
| 2. | Hydrothermal | Pomegranate juice | 3.8 × 10−10 M | 2–5 nm | 8.3 × 10−10–3.3 × 10−8 M | - | Ag+ | [91] |
| 3. | Microwave | Guanine | 90 nM | 3.75 nm | 0–80 µM | 54% | Ag+ | [93] |
| Sr. No. | Method | Precursor | Detection Limit (μM) | Size of P-CDs | Linear Concentration (μM) | Quantum Yield (%) | Metal Ions | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Hydrothermal | Lemon juice | 32 μM | 4–5 nm | 21% | V5+ | [101] | |
| 2. | Hydrothermal | Pyrus pyrifolia | 0.0025 μM | 2.0 ± 1.0 nm | 0.005–50 μM | 10.8% | Al3+ | [96] |
| 3. | Robust method | Camphor | - | 1–4 nm | 21.16% | Cd2+ and Hg2+ | [49] | |
| 4. | Hydrothermal | Red beetroot | 0.033 μM | 5–7 nm | 3 to 43 µM | 27.6% | Pd2+ | [102] |
| 5. | Hydrothermal carbonization | Peach gum | 0.64 µM | 2–5 nm | 0–50 µM | 28.46% | Au3+ | [99] |
| 6. | Hydrothermal | Citric acid and diethylenetriamine | - | 2.51 nm | 0.558 μM | 86% | Al3+ | [98] |
| 7. | Hydrothermal | Poa Pratensis | Fe3+ = 1.4 and Mn2+ = 1.2 μM | 9 nm | 5.0 to 25 μM | 7% | Fe3+ and Mn2+ | [43] |
| 8. | Hydrothermal | Hibiscus Tea Waste | Ag+ = 0.0445 μM, Cd2+ = 0.1644 μM, and Cr3+ = 0.0546 μM | 6.2 ± 0.5 nm | 0–10 μM | 9.2% | Ag+, Cd2+, and Cr3+ ions | [100] |
| 9. | Hydrothermal carbonization | Catharanthus roseus | Al3+ = 0.5 μM; Fe3+ = 0.3 μM | 5 nm | Al3+ = 0–6 μM | 28.2% | Al3+ and Fe3+ | [97] |
| 10. | Microwave | Formamide and L-glutathione | Co2+ = 0.0968 μM, Fe3+ = 0.0617 μM, Hg2+ = 0.0395 μM, Pb2+ = 0.0371 μM | 7.2 ± 1.2 nm | 0.961 μM | 6.49% | Co2+, Fe3+, Hg2+, and Pb2+ | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, H.; Jain, V.; Ballal, S.; Ariffin, I.A.; Chahar, M.; Saini, S.; Bhattu, M.; Singh, H.; Bechelany, M.; Singh, J. From Structure to Sensing: Molecular Mechanistic Insights into Plant-Derived Carbon Dots for Heavy Metal Ion Detection. Nanomaterials 2024, 14, 1766. https://doi.org/10.3390/nano14211766
Soni H, Jain V, Ballal S, Ariffin IA, Chahar M, Saini S, Bhattu M, Singh H, Bechelany M, Singh J. From Structure to Sensing: Molecular Mechanistic Insights into Plant-Derived Carbon Dots for Heavy Metal Ion Detection. Nanomaterials. 2024; 14(21):1766. https://doi.org/10.3390/nano14211766
Chicago/Turabian StyleSoni, Himanshi, Vicky Jain, Suhas Ballal, Indang Ariati Ariffin, Mamata Chahar, Suman Saini, Monika Bhattu, Harbinder Singh, Mikhael Bechelany, and Jagpreet Singh. 2024. "From Structure to Sensing: Molecular Mechanistic Insights into Plant-Derived Carbon Dots for Heavy Metal Ion Detection" Nanomaterials 14, no. 21: 1766. https://doi.org/10.3390/nano14211766
APA StyleSoni, H., Jain, V., Ballal, S., Ariffin, I. A., Chahar, M., Saini, S., Bhattu, M., Singh, H., Bechelany, M., & Singh, J. (2024). From Structure to Sensing: Molecular Mechanistic Insights into Plant-Derived Carbon Dots for Heavy Metal Ion Detection. Nanomaterials, 14(21), 1766. https://doi.org/10.3390/nano14211766









