Mechanisms of Chromium Removal from Water and Soil Using Bioleached Nano Zero-Valent Iron-Mediated Biochar via Co-Pyrolysis
Abstract
1. Introduction
2. Material and Methods
2.1. Material Preparation
2.2. Material Analysis
2.3. Research Content
2.4. Experimental Methods
3. Results
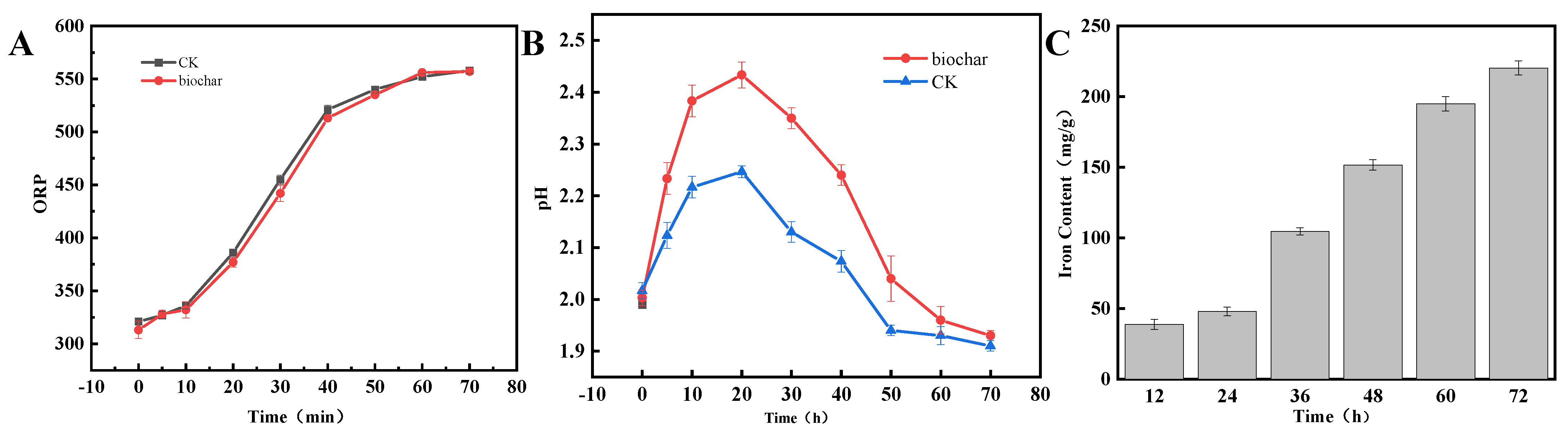
3.1. Bioleaching Process
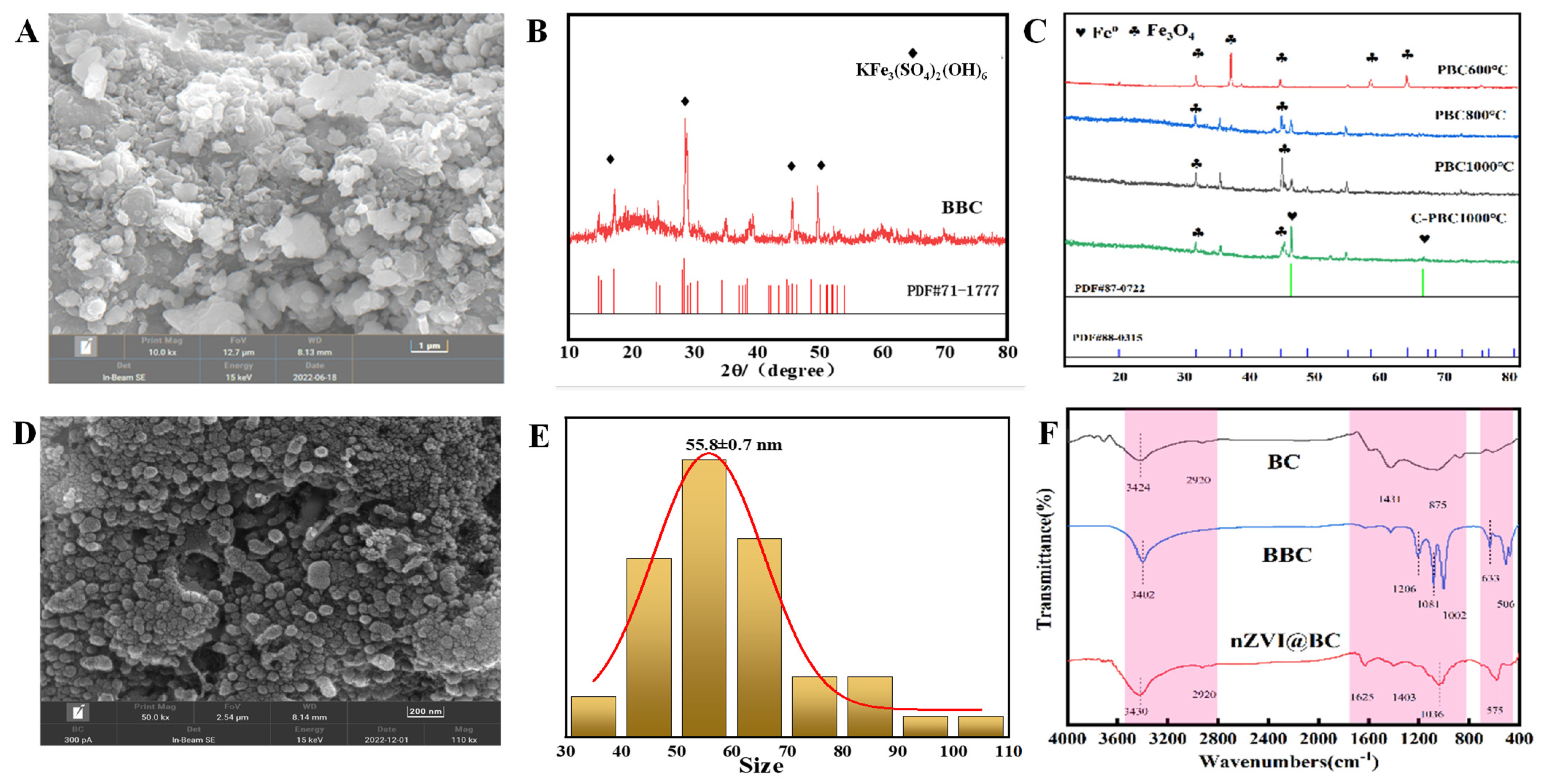
3.2. Characterization
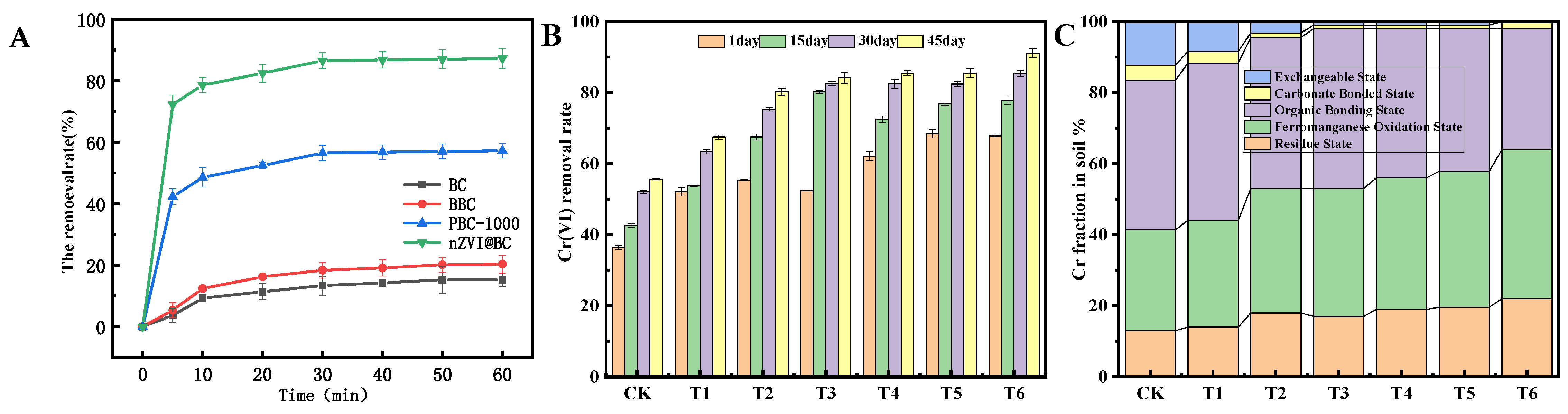
3.3. Results of Batch Experiments
3.4. The nZVI@BC Remediation of Chromium-Contaminated Soil
4. Discussion
4.1. Preparation Process and Reaction Mechanism of nZVI@BC
4.2. Physicochemical Factors in the Removal of Hexavalent Chromium
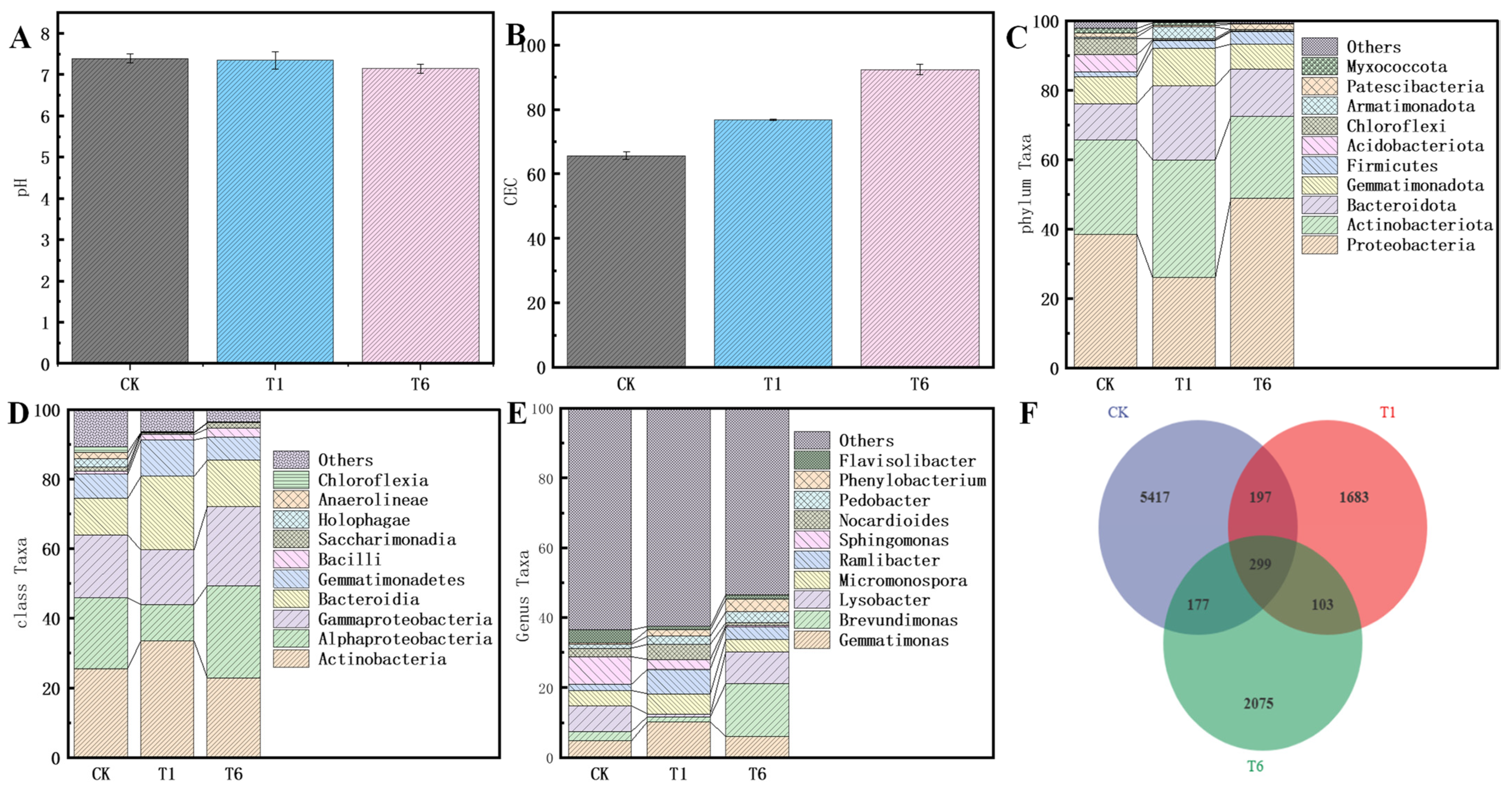
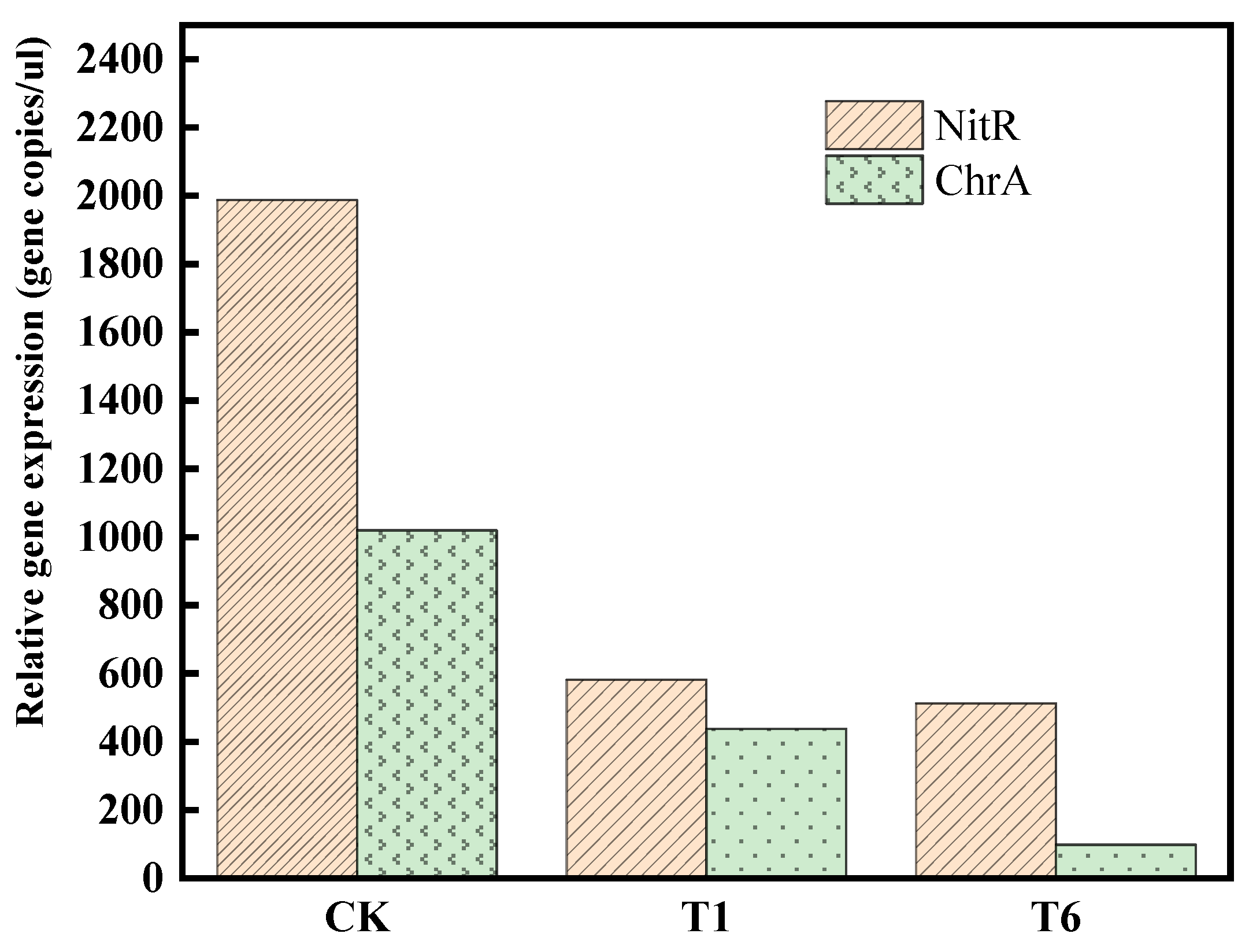
4.3. Effect of nZVI@BC on Cr(VI) Removal and Microbial Abundance in Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef] [PubMed]
- Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere 2019, 244, 125474. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, G.; Elango, L. Chromium and fluoride contamination in groundwater around leather tanning industries in southern India: Implications from stable isotopes δ53Cr/δ52Cr, geochemical and geostatistical modelling. Chemosphere 2018, 220, 943–953. [Google Scholar] [CrossRef]
- Carne, G.; Leconte, S.; Sirot, V.; Breysse, N.; Badot, P.-M.; Bispo, A.; Deportes, I.; Dumat, C.; Rivière, G.; Crépet, A. Mass balance approach to assess the impact of cadmium decrease in mineral phosphate fertilizers on health risk: The case-study of French agricultural soils. Sci. Total Environ. 2020, 760, 143374. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.; Ong, H.C.; Mofijur, M. Heavy metal toxicity, sources, and remediation techniques for contaminated water and soil. Environ. Technol. Innov. 2021, 25, 102114. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.; Khan, S.B.; Asiri, A.M. Exploring the potential of surface-modified alginate beads for catalytic removal of environmental pollutants and hydrogen gas generation. Int. J. Biol. Macromol. 2024, 277, 133697. [Google Scholar] [CrossRef]
- Singh, C.; Sahu, J.; Mahalik, K.; Mohanty, C.; Mohan, B.R.; Meikap, B. Studies on the removal of Pb(II) from wastewater by activated carbon developed from Tamarind wood activated with sulphuric acid. J. Hazard. Mater. 2007, 153, 221–228. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Abdollahi, H.; Noaparast, M.; Shafaei, S.Z.; Manafi, Z.; Muñoz, J.A.; Tuovinen, O.H. Silver-catalyzed bioleaching of copper, molybdenum and rhenium from a chalcopyrite–molybdenite concentrate. Int. Biodeterior. Biodegrad. 2015, 104, 194–200. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Yang, W.; Yuan, F.; Wang, B.; Peng, Q. Impregnating biochar with Fe and Cu by bioleaching for fabricating catalyst to activate H2O2. Appl. Microbiol. Biotechnol. 2022, 106, 2249–2262. [Google Scholar] [CrossRef]
- Zhang, X.; Gai, X.; Zhong, Z.; Bian, F.; Yang, C.; Li, Y.; Wen, X. Understanding variations in soil properties and microbial communities in bamboo plantation soils along a chromium pollution gradient. Ecotoxicol. Environ. Saf. 2021, 222, 112507. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O., Jr.; Tuovinen, O.H.; Bigham, J.M. Oxidation of galena by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Can. J. Microbiol. 1995, 41, 508–514. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2014, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, N.; Kargar, M.; Zamin, F.; Rastakhiz, N.; Manafi, Z. A Review on Various Aspects of Jarosite and Its Utilization Potentials. Ann. Chim. Sci. Mater. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Linsong, W.; Peng, Z.; Yu, F.; Sujun, L.; Yue, Y.; Li, W.; Wei, S. Recovery of metals from jarosite of hydrometallurgical nickel production by thermal treatment and leaching. Hydrometallurgy 2020, 198, 105493. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2019, 383, 121240. [Google Scholar] [CrossRef]
- Panahi, H.K.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, F.; Yang, J.; Li, Q.; Tu, B.; Zhao, D. Synthesis of ordered mesoporous alumina with large pore sizes and hierarchical structure. Microporous Mesoporous Mater. 2011, 143, 406–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhang, Y.; Wu, S.; Xin, J. Enhancement of Cr(VI) removal by mechanically activated micron-scale zero-valent aluminum (MA-mZVAl): Performance and mechanism especially at near-neutral pH. Chem. Eng. J. 2018, 353, 760–768. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Kunhikrishnan, A.; Bush, R. Differential effect of biochar upon reduction-induced mobility and bioavailability of arsenate and chromate. Chemosphere 2015, 144, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wei, J.; Hu, Y.; Wei, C. Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr(VI). J. Hazard. Mater. 2018, 363, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, H.; Hao, T. CaCO3 coated nanoscale zero-valent iron (nZVI) for the removal of chromium(VI) in aqueous solution. Sep. Purif. Technol. 2020, 257, 117967. [Google Scholar] [CrossRef]
- Chang, D.; Chen, T.; Liu, H.; Xi, Y.; Qing, C.; Xie, Q.; Frost, R.L. A new approach to prepare ZVI and its application in removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2014, 244, 264–272. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Li, M.; Liu, X. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Li, X.; Liu, L. Recent advances in nanoscale zero-valent iron/oxidant system as a treatment for contaminated water and soil. J. Environ. Chem. Eng. 2021, 9, 106276. [Google Scholar] [CrossRef]
- Wang, F.-H.; Zhao, B.; Zhang, F.; Gao, J.; Hao, H.-T.; Zhang, S. A novel heavy metal chelating agent sixthio guanidine acid for in situ remediation of soils contaminated with multielements: Its synthesis, solidification, biodegradability, and leachability. J. Soils Sediments 2015, 16, 371–381. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Kang, Y.; Tsang, E.P. Immobilization and phytotoxicity of chromium in contaminated soil remediated by CMC-stabilized nZVI. J. Hazard. Mater. 2014, 275, 230–237. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ. Pollut. 2021, 291, 118129. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, X.; Xu, Z.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, M.; Chen, W.; Li, X.; Balseiro-Romero, M. Changes in the integrated functional stability of microbial community under chemical stresses and the impacting factors in field soils. Ecol. Indic. 2019, 110, 105919. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Li, H.; Peng, D.; Zeng, C.; Xu, H. Remediation of hexavalent chromium contaminated soil by nano-FeS coated humic acid complex in combination with Cr-resistant microflora. Chemosphere 2019, 242, 125251. [Google Scholar] [CrossRef]
- Wang, X.; Gao, P.; Li, D.; Liu, J.; Yang, N.; Gu, W.; He, X.; Tang, W. Risk assessment for and microbial community changes in Farmland soil contaminated with heavy metals and metalloids. Ecotoxicol. Environ. Saf. 2019, 185, 109685. [Google Scholar] [CrossRef]
- Peng, Q.-A.; Shaaban, M.; Wu, Y.; Liu, F.; Hu, R.; Wang, B. Nitrate dependent Fe-oxidizing bacterial diversity in subtropical soils of China. CATENA 2019, 176, 181–188. [Google Scholar] [CrossRef]
- Li, D.; Li, G.; Zhang, D. Field-scale studies on the change of soil microbial community structure and functions after stabilization at a chromium-contaminated site. J. Hazard. Mater. 2021, 415, 125727. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhou, S.; Cai, Y.; Zhang, X.; Shaaban, M.; Peng, Q.-a.; Cai, Y. Mechanisms of Chromium Removal from Water and Soil Using Bioleached Nano Zero-Valent Iron-Mediated Biochar via Co-Pyrolysis. Nanomaterials 2024, 14, 1895. https://doi.org/10.3390/nano14231895
Liu Z, Zhou S, Cai Y, Zhang X, Shaaban M, Peng Q-a, Cai Y. Mechanisms of Chromium Removal from Water and Soil Using Bioleached Nano Zero-Valent Iron-Mediated Biochar via Co-Pyrolysis. Nanomaterials. 2024; 14(23):1895. https://doi.org/10.3390/nano14231895
Chicago/Turabian StyleLiu, Zhiyi, Shuhong Zhou, Yubing Cai, Xuehai Zhang, Muhammad Shaaban, Qi-an Peng, and Yajun Cai. 2024. "Mechanisms of Chromium Removal from Water and Soil Using Bioleached Nano Zero-Valent Iron-Mediated Biochar via Co-Pyrolysis" Nanomaterials 14, no. 23: 1895. https://doi.org/10.3390/nano14231895
APA StyleLiu, Z., Zhou, S., Cai, Y., Zhang, X., Shaaban, M., Peng, Q.-a., & Cai, Y. (2024). Mechanisms of Chromium Removal from Water and Soil Using Bioleached Nano Zero-Valent Iron-Mediated Biochar via Co-Pyrolysis. Nanomaterials, 14(23), 1895. https://doi.org/10.3390/nano14231895






