Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
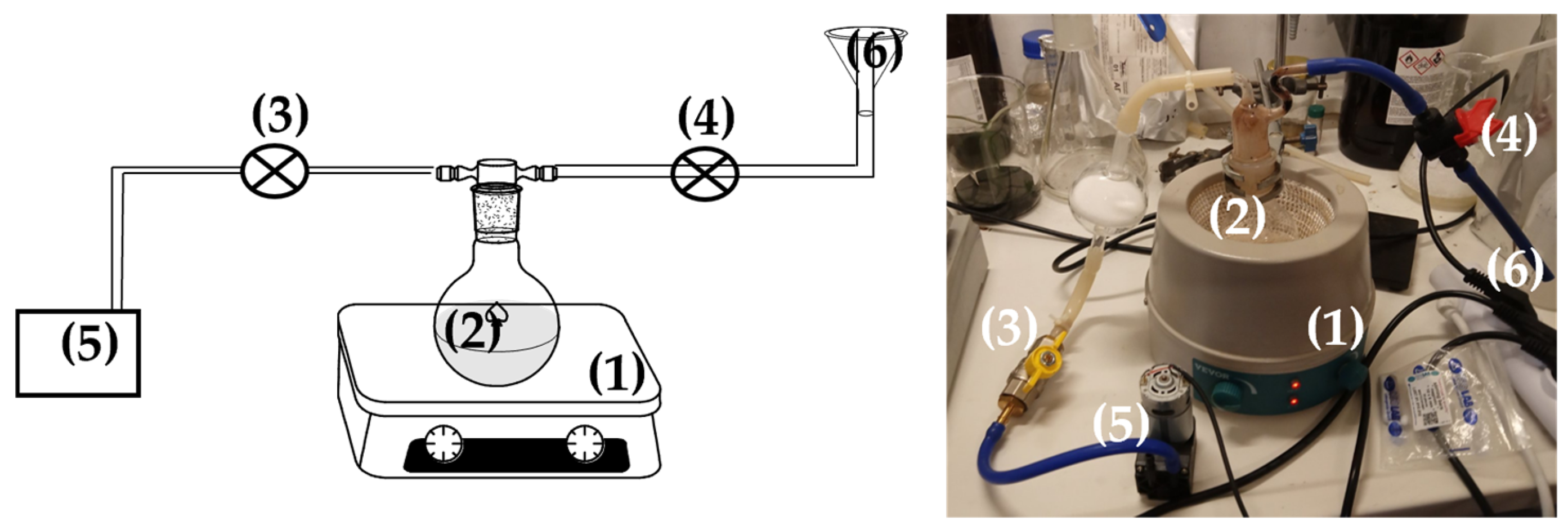
2.2. Preparation of EG@Lap and EG@Mt Nanohybrids
2.3. Preparation of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.4. Physicochemical Characterization of EG@Lap and EG@Mt Nanohybrids and LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.5. EG Release Kinetic Studies of EG@Lap and EG@Mt Nanohybrids
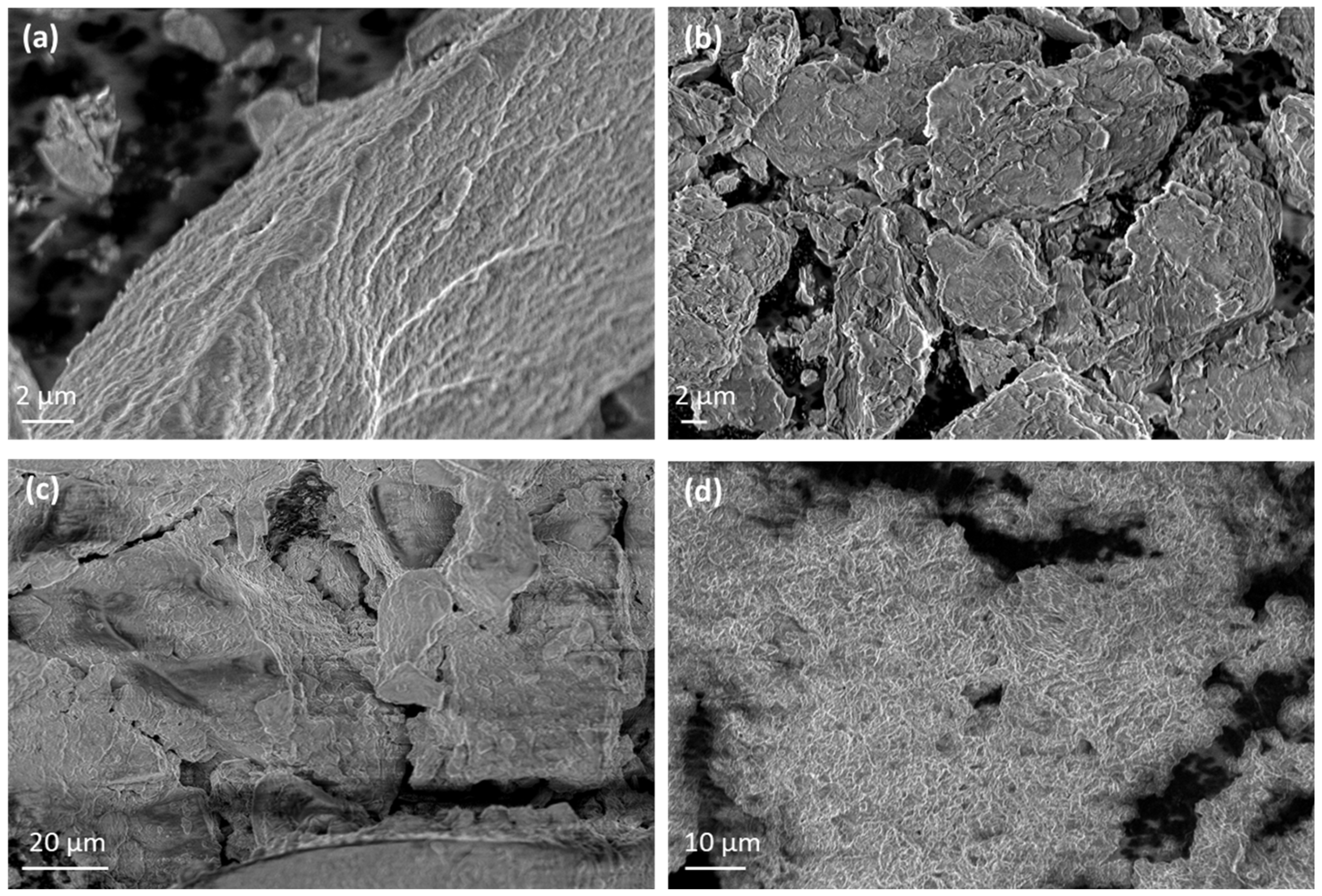
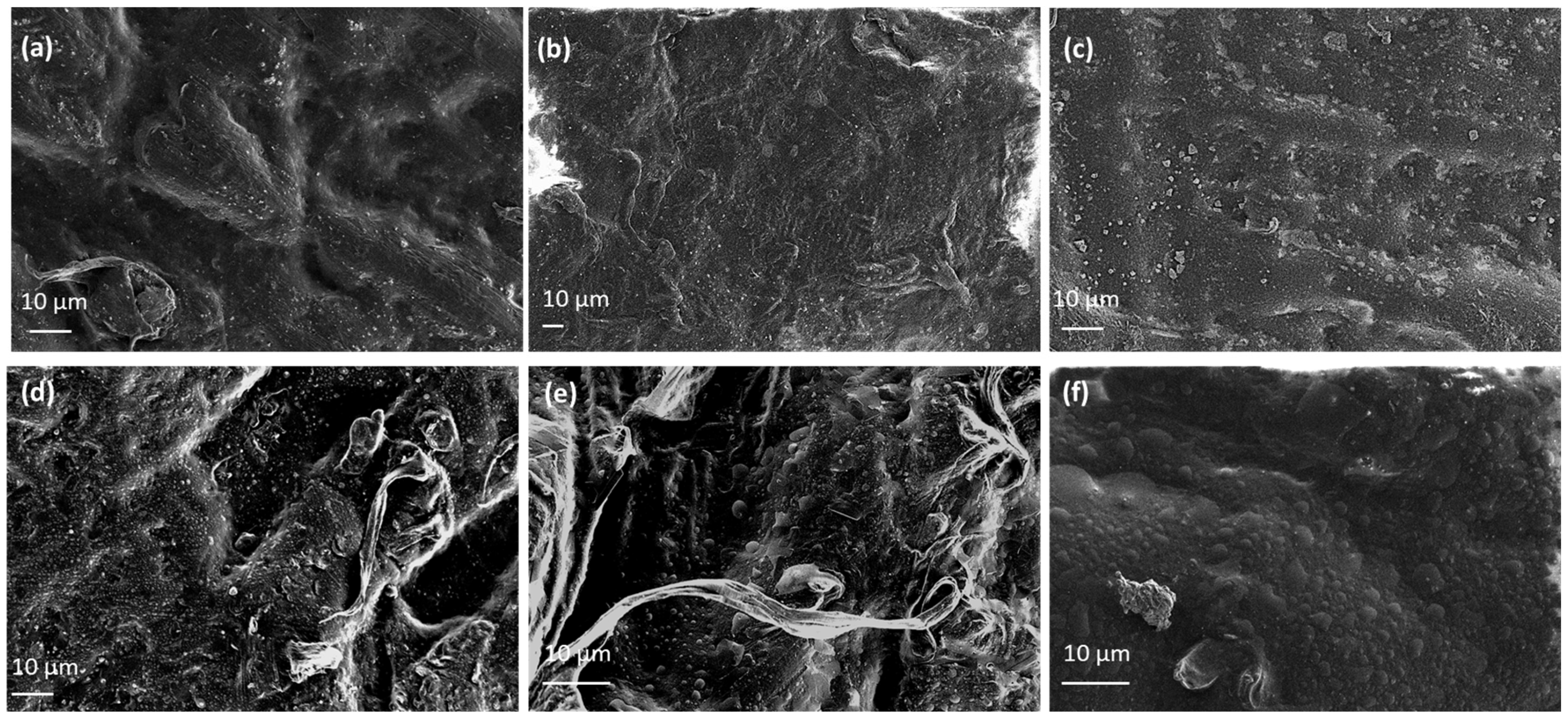
2.6. Morphological Characterization of EG@Lap and EG@Mt Nanohybrids and LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.7. Tensile Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.8. Water/Oxygen Barrier Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.8.1. Water Barrier Properties
2.8.2. Oxygen Barrier Properties
2.9. In Vitro Antioxidant Activity Determination of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.10. EG Release Kinetics of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.11. Packaging Preservation Test of Fresh Minced Pork Wrapped with LDPE/15EG@Lap and LDPE/15EG@Mt Active Films and Pure LDPE Film
2.11.1. Packaging Preservation Test of Minced Pork Meat
2.11.2. Total Viable Count (TVC) of Minced Pork Meat
2.11.3. Sensory Analysis of Minced Pork Meat
2.11.4. pH Analysis of Minced Pork Meat
2.11.5. Lab* Analysis
2.12. Statistical Analysis
3. Results
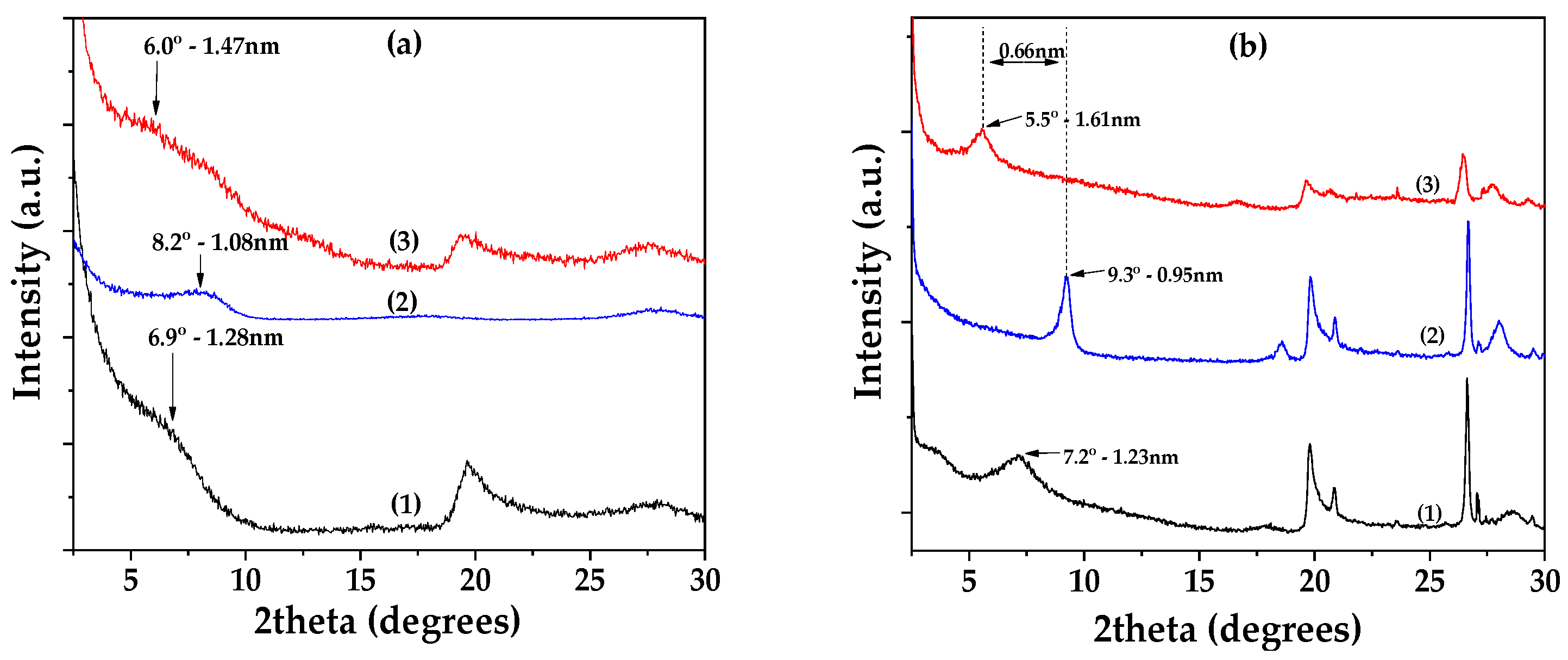
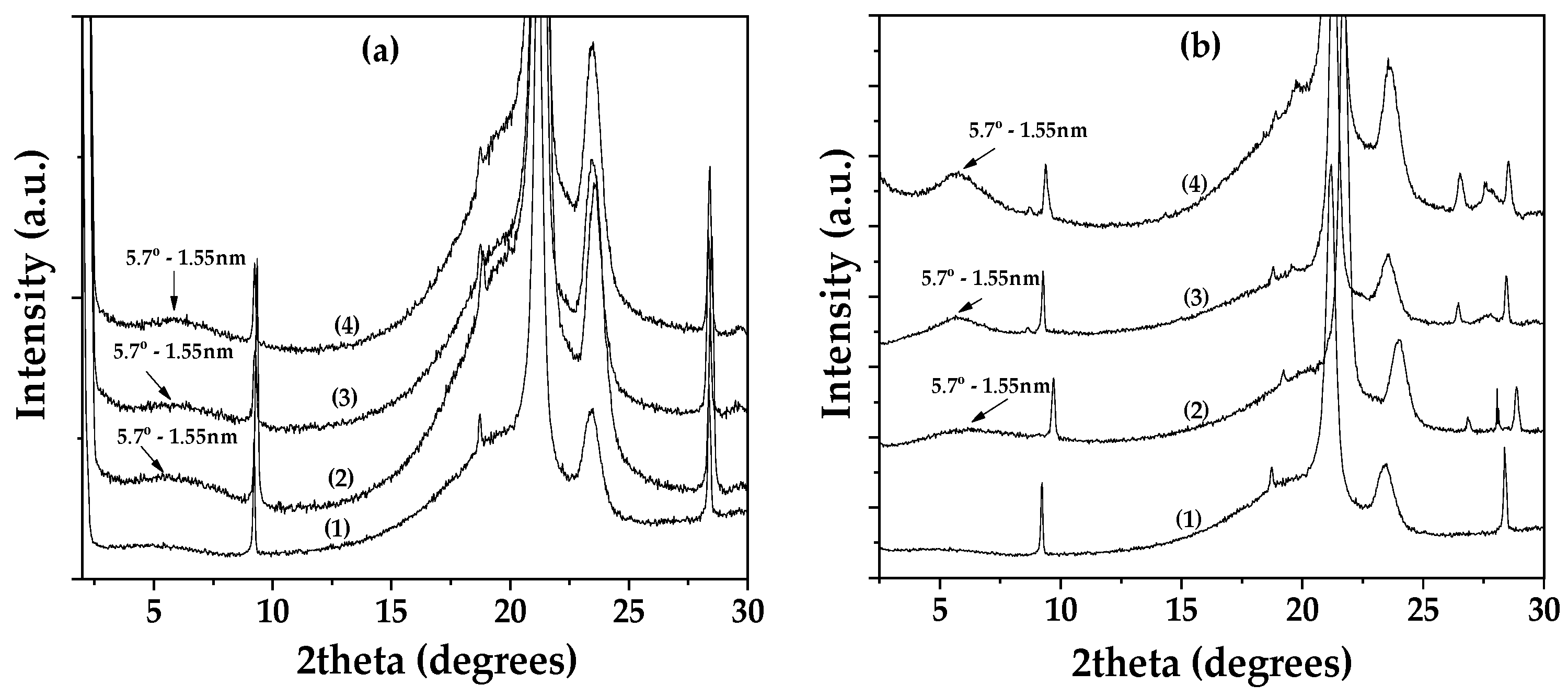
3.1. Physicochemical Characterization of EG@Lap and EG@Mt Nanohybrids
3.2. Physicochemical Characterization of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
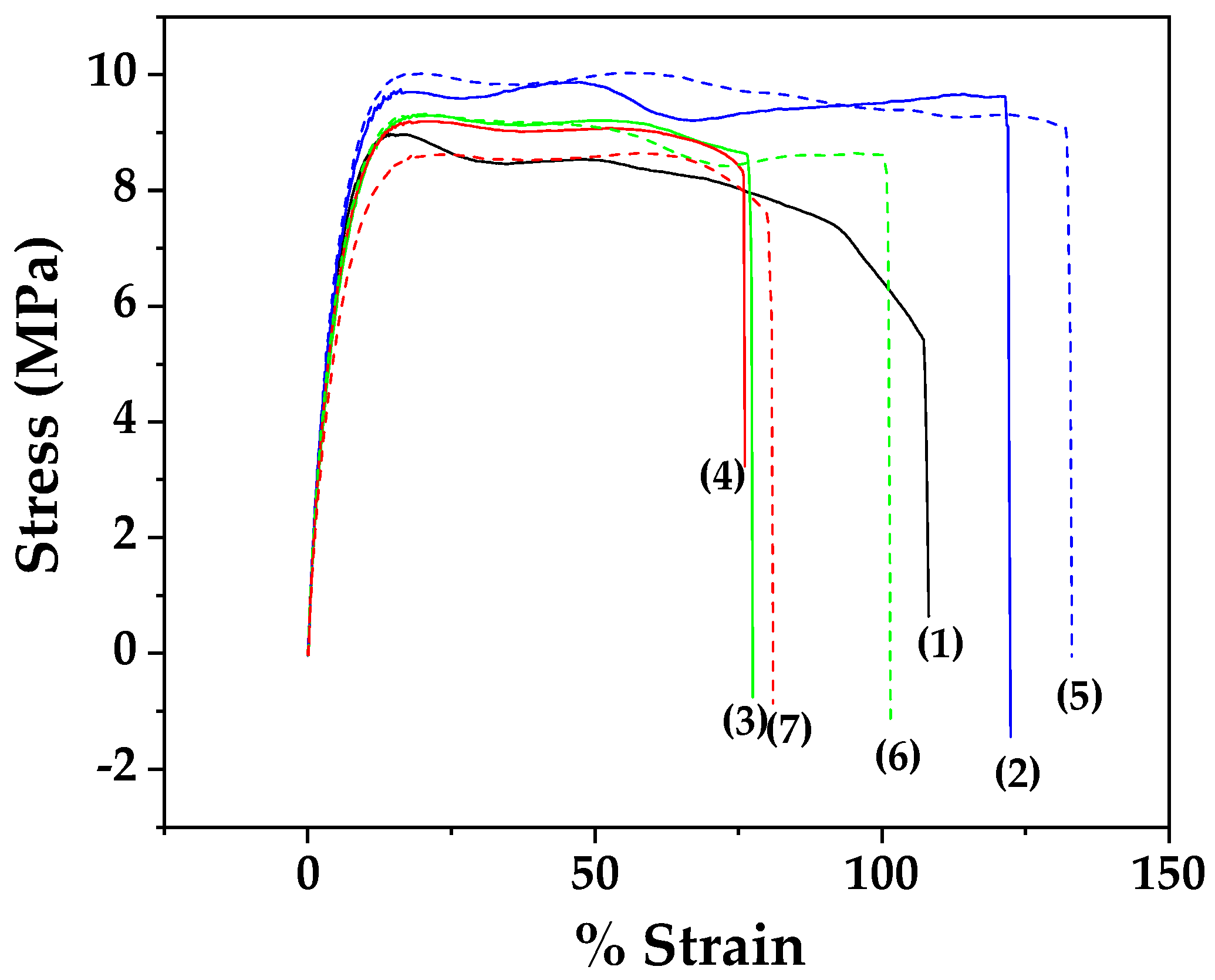
3.3. Tensile Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
3.4. Water/Oxygen Barrier Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
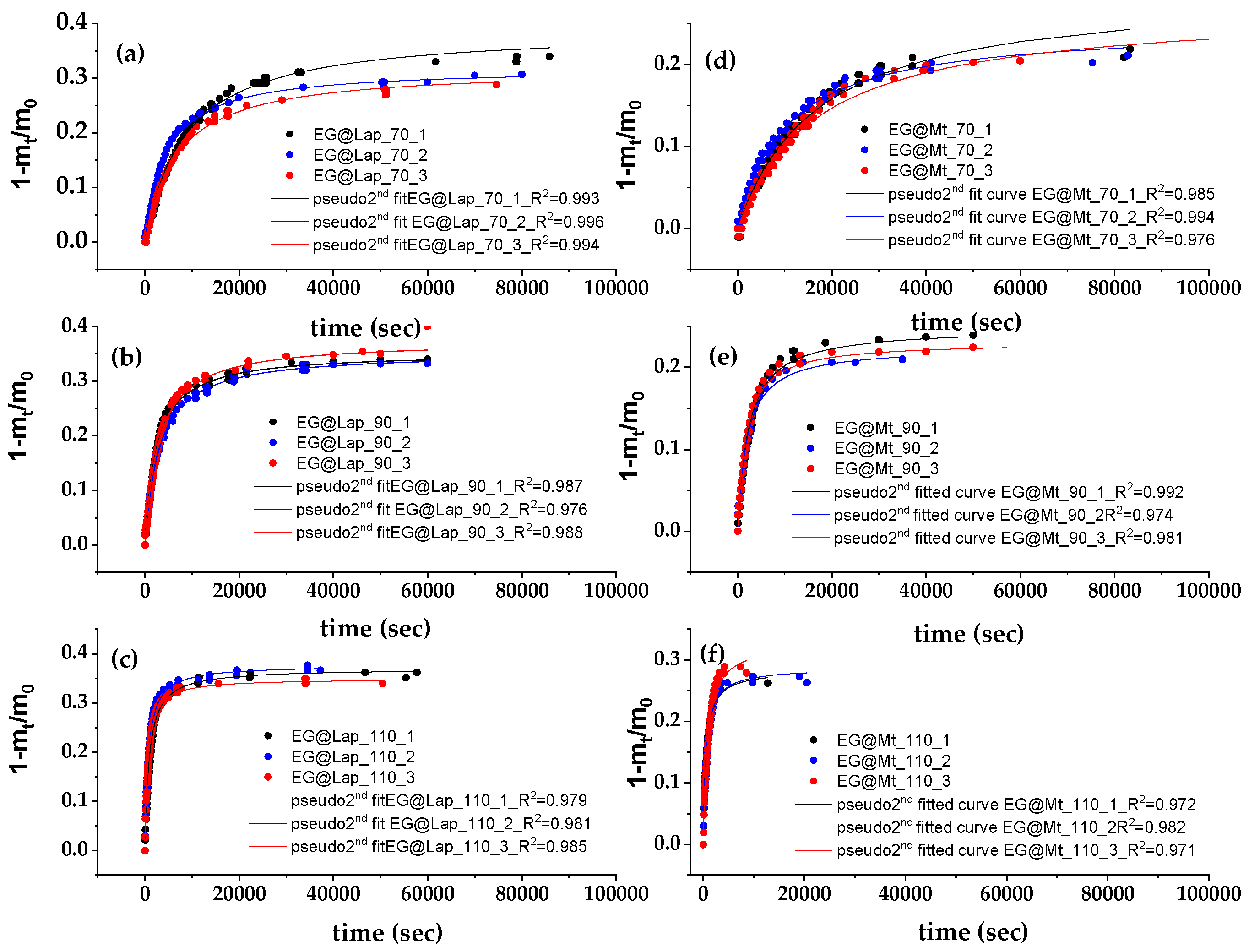
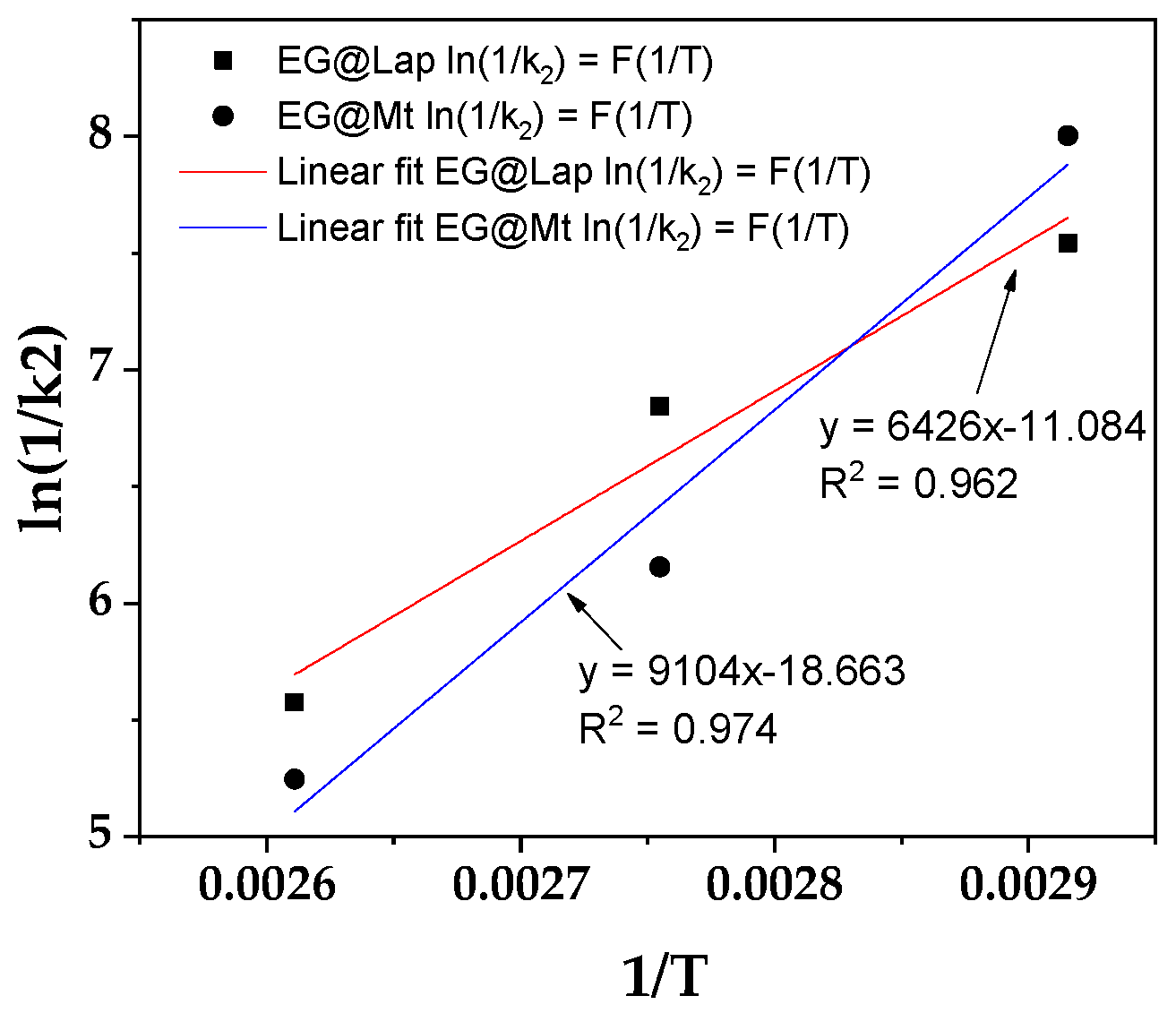
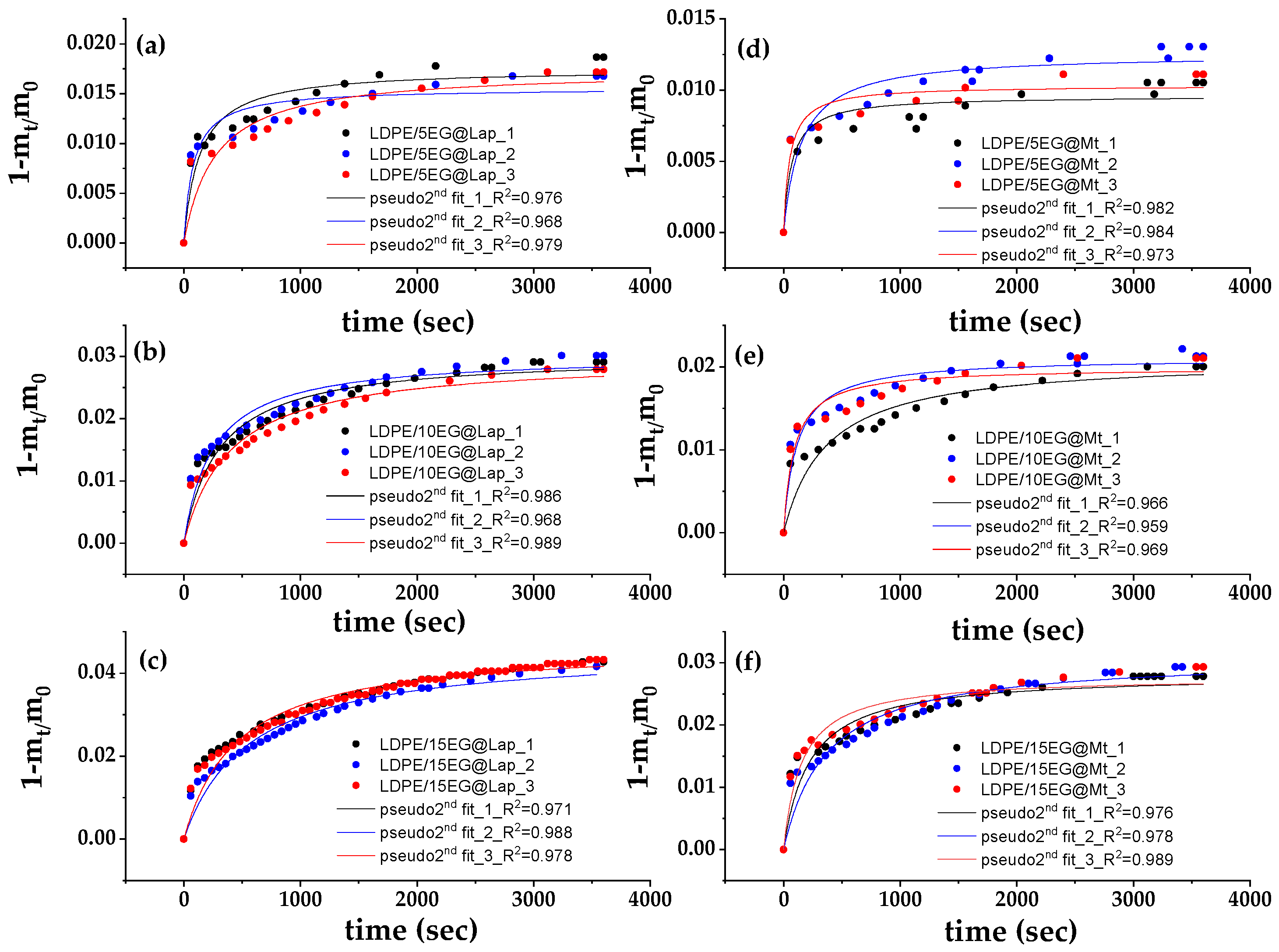
3.5. EG Release Kinetics from LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
3.6. Antioxidant Activity of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
3.7. Packaging Preservation Test of Fresh Minced Pork Wrapped with LDPE/15EG@Lap and LDPE/15EG@Mt Active Films and Pure LDPE Film
3.7.1. Total Viable Counts (TVCs)
3.7.2. Sensory Analysis Results
3.7.3. pH Analysis
3.7.4. Lab* Colorimetry Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Donovan, J.; Topple, C. Achieving Sustainability in Food Manufacturing Operations and Their Supply Chains: Key Insights from a Systematic Literature Review. Sustain. Prod. Consum. 2021, 28, 1491–1499. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.M.E. Sustainable Food Waste Management Model for Bangladesh. Sustain. Prod. Consum. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Takacs, B.; Borrion, A. The Use of Life Cycle-Based Approaches in the Food Service Sector to Improve Sustainability: A Systematic Review. Sustainability 2020, 12, 3504. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Ares, G.; Thøgersen, J.; Monteleone, E. A Sense of Sustainability?—How Sensory Consumer Science Can Contribute to Sustainable Development of the Food Sector. Trends Food Sci. Technol. 2019, 90, 180–186. [Google Scholar] [CrossRef]
- Branca, G.; Lipper, L.; McCarthy, N.; Jolejole, M.C. Food Security, Climate Change, and Sustainable Land Management. A Review. Agron. Sustain. Dev. 2013, 33, 635–650. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Jain, P.C. Greenhouse Effect and Climate Change: Scientific Basis and Overview. Renew. Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-Thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
- Rahman, M.S. Food Preservation: An Overview. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-09148-3. [Google Scholar]
- En, H.; Gupta, R.K.; Lou, F.; Moon, S.H. Role of Microbes in Environmental Sustainability and Food Preservation. In Microbes for Sustainable Development and Bioremediation; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-27587-6. [Google Scholar]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Giannakas, A.E. Plant Extracts-Based Food Packaging Films. In Natural Materials for Food Packaging Application; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 23–49. ISBN 978-3-527-83730-4. [Google Scholar]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of Essential Oils as Preservatives in Food Systems: Challenges and Future Prospectives—A Review. Phytochem. Rev. 2022, 21, 1209–1246. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Pezantes-Orellana, C.; German Bermúdez, F.; Matías De la Cruz, C.; Montalvo, J.L.; Orellana-Manzano, A. Essential Oils: A Systematic Review on Revolutionizing Health, Nutrition, and Omics for Optimal Well-Being. Front. Med. 2024, 11, 1337785. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Chacha, J.S.; Ofoedu, C.E.; Xiao, K. Essential Oil-Based Active Polymer-Based Packaging System: A Review of Its Effect on the Antimicrobial, Antioxidant, and Sensory Properties of Beef and Chicken Meat. J. Food Process. Preserv. 2022, 46, e16933. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R. Nanotechnology Applied in Agriculture: Controlled Release of Agrochemicals. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–118. ISBN 978-3-319-14024-7. [Google Scholar]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Zaharioudakis, K.; Kollia, E.; Leontiou, A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Ragkava, E.; Kehayias, G.; Proestos, C.; et al. Carvacrol Microemulsion vs. Nanoemulsion as Novel Pork Minced Meat Active Coatings. Nanomaterials 2023, 13, 3161. [Google Scholar] [CrossRef]
- Zaharioudakis, K.; Salmas, C.E.; Andritsos, N.D.; Kollia, E.; Leontiou, A.; Karabagias, V.K.; Karydis-Messinis, A.; Moschovas, D.; Zafeiropoulos, N.E.; Avgeropoulos, A.; et al. Carvacrol, Citral, Eugenol and Cinnamaldehyde Casein Based Edible Nanoemulsions as Novel Sustainable Active Coatings for Fresh Pork Tenderloin Meat Preservation. Front. Food. Sci. Technol. 2024, 4, 1400224. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Development of Carvacrol@natural Zeolite Nanohybrid and Poly-Lactide Acid/Triethyl Citrate/Carvacrol@natural Zeolite Self-Healable Active Packaging Films for Minced Pork Shelf-Life Extension 2024. Available online: https://www.preprints.org/manuscript/202405.1992/v1 (accessed on 18 November 2024).
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Baikousi, M.; Karabagias, V.K.; Karageorgou, I.; Iordanidis, G.; Emmanouil-Konstantinos, C.; Leontiou, A.; Karydis-Messinis, A.; Zafeiropoulos, N.E.; Kehayias, G.; et al. Low-Density Polyethylene-Based Novel Active Packaging Film for Food Shelf-Life Extension via Thyme-Oil Control Release from SBA-15 Nanocarrier. Nanomaterials 2024, 14, 423. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Activated Carbon for Food Packaging Application: Review. Walailak J. Sci. Technol. (WJST) 2018, 15, 255–271. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7—Bionanocomposites with Hybrid Nanomaterials for Food Packaging Applications. In Advances in Biocomposites and Their Applications; Karak, N., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2024; pp. 201–225. ISBN 978-0-443-19074-2. [Google Scholar]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Saad, H.; Ayed, A.; Srasra, M.; Attia, S.; Srasra, E.; Bouhtoury, F.C.-E.; Tabbene, O.; Saad, H.; Ayed, A.; Srasra, M.; et al. New Trends in Clay-Based Nanohybrid Applications: Essential Oil Encapsulation Strategies to Improve Their Biological Activity. In Nanoclay—Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2022; ISBN 978-1-80356-558-3. [Google Scholar]
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite Nanoclay Based Formulation for Controlled and Selective Release of Volatile Essential Oil Compounds. Mater. Chem. Phys. 2022, 277, 125569. [Google Scholar] [CrossRef]
- Uddin, M.N.; Hossain, M.T.; Mahmud, N.; Alam, S.; Jobaer, M.; Mahedi, S.I.; Ali, A. Research and Applications of Nanoclays: A Review. SPE Polym. 2024, 5, 507–535. [Google Scholar] [CrossRef]
- Frontiers|Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Available online: https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2019.00398/full (accessed on 3 August 2024).
- Barua, S.; Sahu, D.; Shahnaz, N.; Khan, R. Chapter 1—Chemistry of Two-Dimensional Nanomaterials. In Two-Dimensional Nanostructures for Biomedical Technology; Khan, R., Barua, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 978-0-12-817650-4. [Google Scholar]
- Klecandová, L.; Nakonieczny, D.S.; Reli, M.; Simha Martynková, G. Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers. Materials 2023, 16, 5179. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Nanomaterials: A Review about Halloysite Nanotubes, Properties, and Application in the Biological Field. Int. J. Mol. Sci. 2022, 23, 11518. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Wong, L.W.; Baifa, Z.; Sadjadi, S.; Auckloo, S.A.B.; Palaniandy, K.; Pasbakhsh, P.; Tan, J.B.L.; Singh, R.K.R.; Yuan, P. Halloysite Clay Nanotubes: Innovative Applications by Smart Systems. Appl. Clay Sci. 2024, 251, 107319. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Oliveira, L.H.; de Lima, I.S.; da, S. Neta, E.R.; de Lima, S.G.; Trigueiro, P.; Osajima, J.A.; da Silva-Filho, E.C.; Jaber, M.; Fonseca, M.G. Essential Oil in Bentonite: Effect of Organofunctionalization on Antibacterial Activities. Appl. Clay Sci. 2023, 245, 107158. [Google Scholar] [CrossRef]
- Das, S.S.; Neelam; Hussain, K.; Singh, S.; Hussain, A.; Faruk, A.; Tebyetekerwa, M. Laponite-Based Nanomaterials for Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 424–443. [Google Scholar] [CrossRef]
- Ferreira, C.M.; da Silva, G.J. Absorption of Essential Oils in Laponite: Stability Enhancement and Structural Characteristics. Appl. Clay Sci. 2023, 238, 106936. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.-J.; Avery, R.K.; Moghaddam, K.M.; Haghiniaz, R.; Yalcintas, E.P.; Barros, N.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials For Drug Delivery. Adv. Healthc. Mater. 2022, 11, e2102054. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, W.; Zhang, Y.; Wu, X.; Huang, J.; Bo, R.; Liu, M.; Yu, J.; Li, J. Laponite/Lactoferrin Hydrogel Loaded with Eugenol for Methicillin-Resistant Staphylococcus Aureus-Infected Chronic Skin Wound Healing. J. Tissue Viability 2024, 33, 487–503. [Google Scholar] [CrossRef]
- Kinninmonth, M.A.; Liauw, C.M.; Verran, J.; Taylor, R.; Edwards-Jones, V.; Shaw, D.; Webb, M. Investigation into the Suitability of Layered Silicates as Adsorption Media for Essential Oils Using FTIR and GC–MS. Appl. Clay Sci. 2013, 83–84, 415–425. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A Fresh Look at the Laponite Phase Diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Thomas, J., Jr.; Bohor, B.F. Surface Area of Montmorillonite from the Dynamic Sorption of Nitrogen and Carbon Dioxide. Clays Clay Miner. 1968, 16, 83–91. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Damasceno, R.O.S.; Pinheiro, J.L.S.; Rodrigues, L.H.M.; Gomes, R.C.; Duarte, A.B.S.; Emídio, J.J.; Diniz, L.R.L.; de Sousa, D.P. Anti-Inflammatory and Antioxidant Activities of Eugenol: An Update. Pharmaceuticals 2024, 17, 1505. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Available online: https://www.turkjps.org/articles/pharmacological-and-toxicological-properties-of-eugenol/doi/tjps.62207 (accessed on 18 November 2024).
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Silva, M.V.; de Lima, A.; da, C.A.; Silva, M.G.; Caetano, V.F.; de Andrade, M.F.; da Silva, R.G.C.; de Moraes Filho, L.E.P.T.; de Lima Silva, I.D.; Vinhas, G.M. Clove Essential Oil and Eugenol: A Review of Their Significance and Uses. Food Biosci. 2024, 62, 105112. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, K.; Moynihan, M.; Liu, M.; Kim, S. Biologic Properties of Eugenol and Zinc Oxide-Eugenol: A Clinically Oriented Review. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter Pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef]
- Wrona, M.; Manso, S.; Silva, F.; Cardoso, L.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. New Active Packaging Based on Encapsulated Carvacrol, with Emphasis on Its Odour Masking Strategies. Food Packag. Shelf Life 2023, 40, 101177. [Google Scholar] [CrossRef]
- Rojas, A.; Misic, D.; de Dicastillo, C.L.; Zizovic, I.; Velásquez, E.; Gutiérrez, D.; Aguila, G.; Vidal, C.P.; Guarda, A.; Galotto, M.J. A Review on Thymol-Based Bioactive Materials for Food Packaging. Ind. Crops Prod. 2023, 202, 116977. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Alonso, J.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Modifications Induced by the Addition of a Nanoclay in the Functional and Active Properties of an EVOH Film Containing Carvacrol for Food Packaging. J. Membr. Sci. 2012, 423–424, 247–256. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Saadat, S.; Rawtani, D.; Rao, P.K. Antibacterial Activity of Chitosan Film Containing Syzygium Aromaticum (Clove) Oil Encapsulated Halloysite Nanotubes against Foodborne Pathogenic Bacterial Strains. Mater. Today Commun. 2022, 32, 104132. [Google Scholar] [CrossRef]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE Based Active Nanocomposite Films with Nanoclays Impregnated with Volatile Compounds. Food Res. Int. 2018, 107, 337–345. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef] [PubMed]
- Bamps, B.; Buntinx, M.; Peeters, R. Seal Materials in Flexible Plastic Food Packaging: A Review. Packag. Technol. Sci. 2023, 36, 507–532. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Trusca, R.-D.; Ficai, A.; Stelescu, M.D.; Sonmez, M.; Nituica, M.; Mustatea, G.; Holban, A.M. Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene. Int. J. Mol. Sci. 2024, 25, 6073. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7—Extrusion of Biopolymers for Food Applications. In Advances in Biopolymers for Food Science and Technology; Pal, K., Sarkar, P., Cerqueira, M.Â., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 137–169. ISBN 978-0-443-19005-6. [Google Scholar]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Burelo, M.; Hernández-Varela, J.D.; Medina, D.I.; Treviño-Quintanilla, C.D. Recent Developments in Bio-Based Polyethylene: Degradation Studies, Waste Management and Recycling. Heliyon 2023, 9, e21374. [Google Scholar] [CrossRef]
- Saleh, T.A. Chapter 3—Kinetic Models and Thermodynamics of Adsorption Processes: Classification. In Interface Science and Technology; Saleh, T.A., Ed.; Surface Science of Adsorbents and Nanoadsorbents; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 65–97. [Google Scholar]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) Sorption Properties of Activated Carbon Produced via Pyrolysis of the “Posidonia oceanica” Seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef]
- Frenkel, J. Theorie der Adsorption und verwandter Erscheinungen. Z. Phys. 1924, 26, 117–138. [Google Scholar] [CrossRef]
- Knopf, D.A.; Ammann, M. Technical Note: Adsorption and Desorption Equilibria from Statistical Thermodynamics and Rates from Transition State Theory. Atmos. Chem. Phys. 2021, 21, 15725–15753. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Für Phys. Chem. 1889, 4U, 96–116. [Google Scholar] [CrossRef]
- ISO 11035:1994(En); Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11035:ed-1:v1:en (accessed on 6 June 2024).
- Heymann, H.; Machado, B.; Torri, L.; Robinson, A.L. How Many Judges Should One Use for Sensory Descriptive Analysis? J. Sens. Stud. 2012, 27, 111–122. [Google Scholar] [CrossRef]
- Assanti, E.; Karabagias, V.K.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G. Shelf Life Evaluation of Fresh Chicken Burgers Based on the Combination of Chitosan Dip and Vacuum Packaging under Refrigerated Storage. J. Food Sci. Technol. 2021, 58, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Kouloumpis, A.; Zygouri, P.; Karouta, N.; Spyrou, K.; Stathi, P.; Tsoufis, T.; Georgakilas, V.; Gournis, D.; Rudolf, P. Layer-by-Layer Assembly of Clay–Carbon Nanotube Hybrid Superstructures. ACS Omega 2019, 4, 18100–18107. [Google Scholar] [CrossRef]
- Enotiadis, A.; Tsokaridou, M.; Chalmpes, N.; Sakavitsi, V.; Spyrou, K.; Gournis, D. Synthesis and Characterization of Porous Clay-Organic Heterostructures. J. Sol-Gel Sci. Technol. 2019, 91, 295–301. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef]
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The Barrier Properties of Sustainable Multiphase and Multicomponent Packaging Materials: A Review. Prog. Mater. Sci. 2023, 133, 101071. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in Barrier Coatings and Film Technologies for Achieving Sustainable Packaging of Food Products—A Review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Zhong, G.-J.; Olah, A.; Li, Z.-M.; Baer, E.; Zhu, L. Promising Strategies and New Opportunities for High Barrier Polymer Packaging Films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite Nanotubes for Food Packaging Application: A Review. Appl. Clay Sci. 2023, 234, 106856. [Google Scholar] [CrossRef]
- Giannakas, A. Giannakas Na-Montmorillonite Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
| Sample Name | LDPE (g) | EG@Lap (g-wt.%) | EG@Mt (g-wt.%) | Twin-Screw Extruder Operating Conditions | ||
|---|---|---|---|---|---|---|
| T (°C) | Speed (rpm) | Time (min) | ||||
| LDPE | 5 | - | - | 200 | 100 | 3 |
| LDPE/5EG@Lap | 4.75 | 0.25–5 | - | 200 | 100 | 3 |
| LDPE/10EG@Lap | 4.50 | 0.50–10 | - | 200 | 100 | 3 |
| LDPE/15EG@Lap | 4.25 | 0.75–15 | - | 200 | 100 | 3 |
| LDPE/5EG@Mt | 4.75 | - | 0.25–5 | 200 | 100 | 3 |
| LDPE/10EG@Mt | 4.50 | - | 0.50–10 | 200 | 100 | 3 |
| LDPE/15EG@Mt | 4.25 | - | 0.75–15 | 200 | 100 | 3 |
| 70 °C | EG@Lap | EG@Mt |
|---|---|---|
| k2 (s−1) | 5.29 × 10−4 ± 0.24 × 10−4 | 3.34 × 10−4 ± 1.7 × 10−4 |
| qe | 0.338 ± 0.053 | 0.235 ± 0.031 |
| 90 °C | EG@Lap | EG@Mt |
| k2 (s−1) | 1.07 × 10−3 ± 0.12 × 10−3 | 2.12 × 10−3 ± 0.31 × 10−3 |
| qe | 0.356 ± 0.013 | 0.264 ± 0.012 |
| 110 °C | EG@Lap | EG@Mt |
| k2 (s−1) | 3.79 × 10−3 ± 0.8 × 10−3 | 5.27 × 10−3 ± 1.47 × 10−3 |
| qe | 0.365 ± 0.014 | 0.299 ± 0.025 |
| Sample Name | E (Mpa) | σuts (MPa) | %ε |
|---|---|---|---|
| LDPE | 206.8 ± 16.2 a | 9.5 ± 1.0 a,b | 106.5 ± 3.7 a,b |
| LDPE/5EG@Lap | 186.7 ± 13.9 a,b | 10.2 ± 0.4 a | 122.5 ± 4.6 a,d |
| LDPE/10EG@Lap | 162.9 ± 13.4 b,c | 9.5 ± 0.3 a,b | 76.2 ± 3.4 b,c |
| LDPE/15EG@Lap | 140.4 ± 3.2 c | 8.7 ± 0.2 b | 74.8 ± 3.0 c |
| LDPE/5EG@Mt | 182.8 ± 4.6 a,b | 10.0 ± 0.4 a | 132.6 ± 4.7 a |
| LDPE/10EG@Mt | 161.6 ± 3.1 b,c | 9.5 ± 0.4 a.b | 100.6 ± 4.5 a,c |
| LDPE/15EG@Mt | 159.5 ± 2.0 b,c | 9.4 ± 0.4 a,b | 85.0 ± 4.3 b,c,d |
| Dwv (cm2·s−1) × 10−5 | PeO2 (cm2·s−1) × 10−8 | |
|---|---|---|
| LDPE | 48.7 ± 9.5 a | 2.61 ± 0.013 a |
| LDPE/5EG@Lap | 9.3 ± 3.4 b | 1.77 ± 0.343 b |
| LDPE/10EG@Lap | 11.1 ± 6.8 b | 2.34 ± 0.082 a,c |
| LDPE/15EG@Lap | 11.7 ± 4.4 a,b | 2.03 ± 0.136 b,c,d |
| LDPE/5EG@Mt | 12.9 ± 6.2 a,b | 1.84 ± 0.026 b,d |
| LDPE/10EG@Mt | 8.8 ± 2.1 b | 2.08 ± 0.012 b,c |
| LDPE/15EG@Mt | 6.4 ± 4.1 b | 2.52 ± 0.265 a,c |
| k2 (s−1) | qe | EC50,DPPH (mg/L) | |
|---|---|---|---|
| LDPE | - | - | n.d. |
| LDPE/5EG@Lap | 0.480 ± 0.090 | 0.017 ± 0.001 | 12.1 ± 0.3 |
| LDPE/10EG@Lap | 0.110 ± 0.023 | 0.030 ± 0.002 | 0.33 ± 0.02 |
| LDPE/15EG@Lap | 0.040 ± 0.004 | 0.046 ± 0.003 | 0.11 ± 0.01 |
| LDPE/5EG@Mt | 1.280 ± 0.271 | 0.011 ± 0.002 | 18.14 ± 2.3 |
| LDPE/10EG@Mt | 0.370 ± 0.016 | 0.021 ± 0.001 | 3.57 ± 0.54 |
| LDPE/15EG@Mt | 0.160 ± 0.072 | 0.029 ± 0.001 | 1.29 ± 0.23 |
| Samples | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|
| LDPE | 4.68 Aa | 6.20 Bb | 6.44 Bc | 7.14 Cd | 8.13 De | 8.27 De |
| LDPE/15EG@Lap | 4.68 Aa | 5.49 Bb | 5.7 Bc | 6.08 Cd | 7.09 Ce | 7.78 Df |
| LDPE/15EG@Mt | 4.68 Aa | 6.09 Bb | 5.72 Bc | 6.30 Cd | 7.54 Ce | 8.09 Df |
| Parameters | Sample | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|---|
| LDPE | 5.00 Aa | 3.97 Bb | 3.40 Cc | 3.27 Cd | 2.77 De | 2.10 Ef | |
| Aroma | LDPE/15EG@Mt | 5.00 Aa | 4.53 Bb | 3.87 Bc | 3.50 Cd | 3.30 Ce | 2.53 Df |
| LDPE/15EG@Lap | 5.00 Aa | 4.47 Bb | 3.93 Bc | 3.40 Cd | 3.33 Ce | 2.73 Df | |
| Color | LDPE | 5.00 Aa | 4.10 Bb | 4.10 Bb | 3.63 Cc | 3.53 Cd | 2.17 Df |
| LDPE/15EG@Mt | 5.00 Aa | 4.37 Bb | 3.80 Bc | 3.43 Cd | 3.23 Ce | 2.43 Df | |
| LDPE/15EG@Lap | 5.00 Aa | 4.57 Bb | 3.90 Bc | 3.43 Cd | 3.27 Ce | 2.57 Df | |
| Texture | LDPE | 5.00 Aa | 4.03 Bb | 3.73 Cc | 3.80 Bd | 3.67 Be | 2.57 Cf |
| LDPE/15EG@Mt | 5.00 Aa | 4.57 Bb | 3.87 Bc | 3.63 Bd | 3.57 Be | 2.73 Cf | |
| LDPE/15EG@Lap | 5.00 Aa | 4.57 Bb | 3.93 Bc | 3.87 Bc | 3.77 Bd | 2.87 Ce |
| Samples | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|
| LDPE | 5.66 (±0.04) Aa | 5.57 (±0.04) Aa | 5.47 (±0.04) Bb | 5.50 (±0.04) Ab | 5.46 (±0.04) Ab | 5.37 (±0.04) Ac |
| LDPE/15EG@Mt | 5.66 (±0.04) Aa | 5.53 (±0.04) Aa | 5.54 (±0.04) Ab | 5.58 (±0.04) Bc | 5.52 (±0.04) Ac | 5.44 (±0.04) Bd |
| LDPE/15EG@Lap | 5.66 (±0.04) Aa | 5.53 (±0.04) Aa | 5.52 (±0.04) Ab | 5.55 (±0.04) Ab | 5.52 (±0.04) Bc | 5.39 (±0.04) Ad |
| Sample | Days | L* | a* | b* | ΔL (±std) | Δa (±std) | Δb (±std) | ΔE (±std) |
|---|---|---|---|---|---|---|---|---|
| LDPE | Day 0 | 56.27 (±0.52) Aa | 16.96 (±0.64) Aa | 10.37 (±0.19) Aa | — | — | — | — |
| LDPE/15EG@Mt | Day 0 | 56.27 (±0.52) Aa | 16.96 (±0.64) Aa | 10.37 (±0.19) Aa | — | — | — | — |
| LDPE/15EG@Lap | Day 0 | 56.27 (±0.52) Aa | 16.96 (±0.64) Aa | 10.37 (±0.19) Aa | — | — | — | — |
| LDPE | Day 2 | 53.34 (±0.38) Ba | 12.49 (±0.53) Bb | 12.19 (±0.86) Ba | −2.93 (±0.38) | −4.47 (±0.53) | 1.82 (±0.86) | 5.65 (±0.38) |
| LDPE/15EG@Mt | Day 2 | 55.34 (±0.39) Aa | 16.33 (±0.35) Aa | 10.53 (±0.45) Aa | −0.93 (±0.39) | −0.63 (±0.35) | 0.16 (±0.45) | 1.13 (±0.39) |
| LDPE/15EG@Lap | Day 2 | 56.09 (±0.86) Aa | 16.06 (±0.26) Aa | 10.41 (±0.36) Aa | −0.18 (±0.86) | −0.9 (±0.26) | 0.04 (±0.36) | 0.92 (±0.86) |
| LDPE | Day 4 | 50.75 (±0.47) Ca | 10.24 (±0.68) Cb | 12.92 (±0.91) Bb | −2.59 (±0.47) | −2.25 (±0.68) | 0.73 (±0.91) | 3.51 (±0.47) |
| LDPE/15EG@Mt | Day 4 | 53.18 (±0.54) Ba | 14.19 (±0.76) Ba | 12.03 (±0.41) Ab | −2.16 (±0.54) | −2.14 (±0.76) | 1.5 (±0.41) | 3.39 (±0.54) |
| LDPE/15EG@Lap | Day 4 | 53.00 (±0.53) Ba | 14.75 (±0.48) Bb | 11.86 (±0.45) Ab | −3.09 (±0.53) | −1.31 (±0.48) | 1.45 (±0.45) | 3.66 (±0.53) |
| LDPE | Day 6 | 47.23 (±0.46) Db | 13.35 (±0.59) Db | 13.79 (±1.04) Bb | −3.52 (±0.46) | 3.11 (±0.59) | 0.87 (±1.04) | 4.78 (±0.46) |
| LDPE/15EG@Mt | Day 6 | 51.05 (±0.52) Ca | 13.62 (±0.73) Ca | 12.51 (±0.42) Ab | −2.13 (±0.52) | −0.57 (±0.73) | 0.48 (±0.42) | 2.26 (±0.52) |
| LDPE/15EG@Lap | Day 6 | 50.35 (±0.50) Ca | 13.87 (±0.45) Cb | 12.57 (±0.48) Ab | −2.65 (±0.50) | −0.88 (±0.45) | 0.71 (±0.48) | 2.88 (±0.50) |
| LDPE | Day 8 | 41.06 (±0.38) Ec | 11.48 (±0.61) Ec | 15.62 (±1.10) Cc | −6.17 (±0.38) | −1.87 (±0.61) | 1.83 (±1.10) | 6.7 (±0.38) |
| LDPE/15EG@Mt | Day 8 | 49.01 (±0.50) Cb | 13.08 (±0.70) Ca | 13.01 (±0.44) Ab | −2.04 (±0.50) | −0.54 (±0.70) | 0.5 (±0.44) | 2.17 (±0.50) |
| LDPE/15EG@Lap | Day 8 | 47.83 (±0.47) Db | 13.03 (±0.43) Cb | 13.33 (±0.51) Ab | −2.52 (±0.47) | −0.84 (±0.43) | 0.76 (±0.51) | 2.76 (±0.47) |
| LDPE | Day 10 | 35.72 (±0.33) Fd | 9.99 (±0.53) Fd | 17.65 (±1.24) Cd | −5.34 (±0.33) | −1.49 (±0.53) | 2.03 (±1.24) | 5.9 (±0.33) |
| LDPE/15EG@Mt | Day 10 | 47.05 (±0.48) Cb | 12.55 (±0.67) Ca | 13.53 (±0.46) Ab | −1.96 (±0.48) | −0.53 (±0.67) | 0.52 (±0.46) | 2.1 (±0.48) |
| LDPE/15EG@Lap | Day 10 | 45.44 (±0.45) Db | 12.25 (±0.40) Cb | 14.13 (±0.54) Bd | −2.39 (±0.45) | −0.78 (±0.40) | 0.8 (±0.54) | 2.64 (±0.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kechagias, A.; Salmas, C.E.; Chalmpes, N.; Leontiou, A.A.; Karakassides, M.A.; Giannelis, E.P.; Giannakas, A.E. Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials 2024, 14, 1938. https://doi.org/10.3390/nano14231938
Kechagias A, Salmas CE, Chalmpes N, Leontiou AA, Karakassides MA, Giannelis EP, Giannakas AE. Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials. 2024; 14(23):1938. https://doi.org/10.3390/nano14231938
Chicago/Turabian StyleKechagias, Achilleas, Constantinos E. Salmas, Nikolaos Chalmpes, Areti A. Leontiou, Michael A. Karakassides, Emmanuel P. Giannelis, and Aris E. Giannakas. 2024. "Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films" Nanomaterials 14, no. 23: 1938. https://doi.org/10.3390/nano14231938
APA StyleKechagias, A., Salmas, C. E., Chalmpes, N., Leontiou, A. A., Karakassides, M. A., Giannelis, E. P., & Giannakas, A. E. (2024). Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials, 14(23), 1938. https://doi.org/10.3390/nano14231938











