Additive Manufacturing of Binary and Ternary Oxide Systems Using Two-Photon Polymerization and Low-Temperature Sintering
Abstract
:1. Introduction
2. Materials and Methods
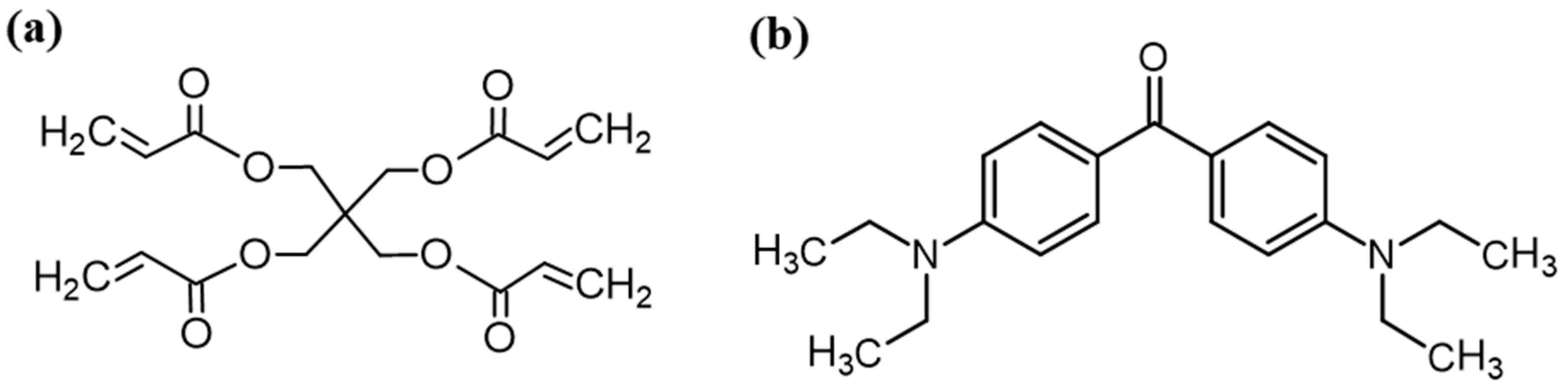
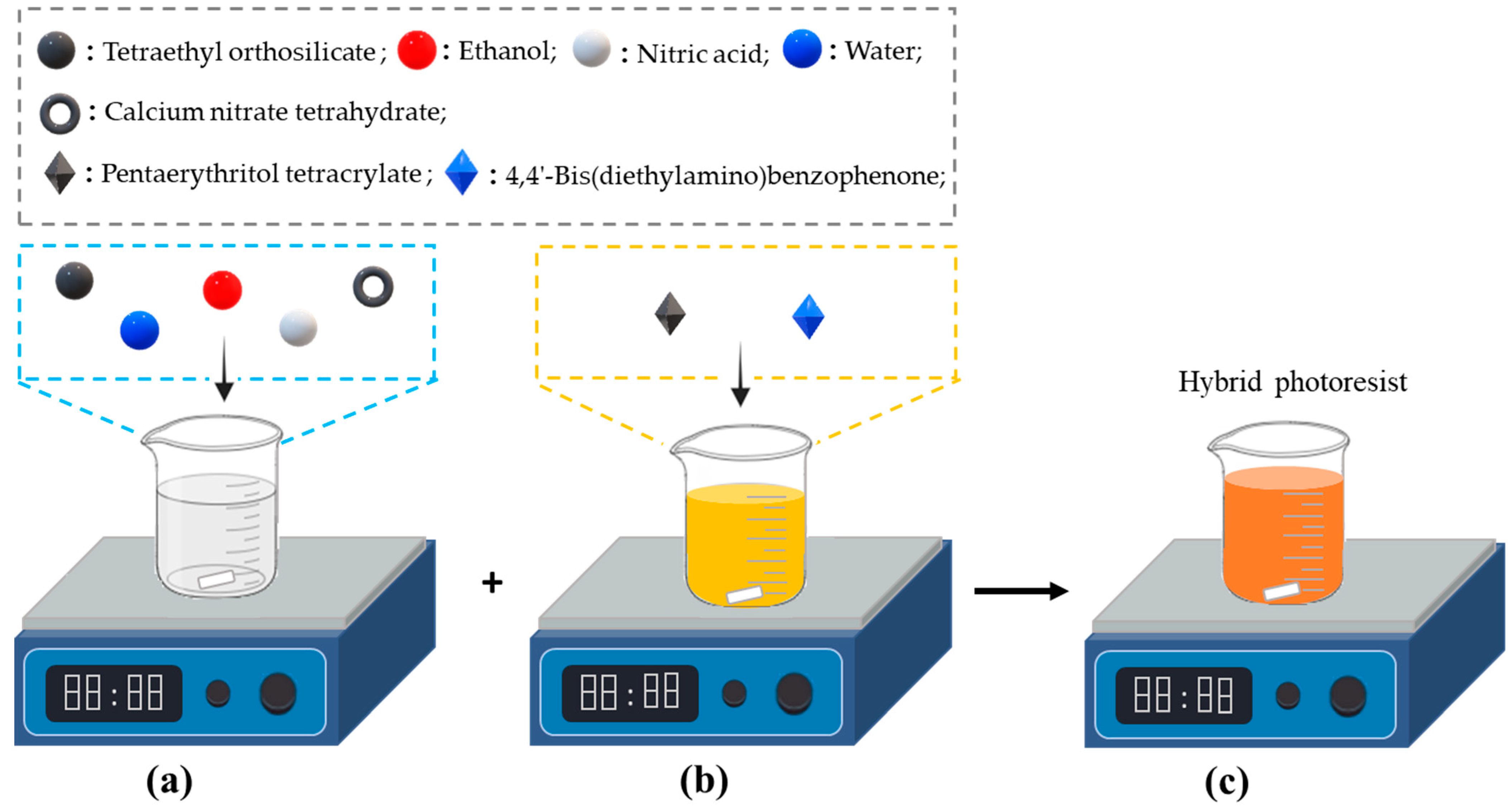
2.1. Syntheses of Organic Resin and Hybrid Photoresists
2.2. Two-Photon Polymerization 3D Printing
2.3. Heat Treatment Process
2.4. Structural and Morphological Characterizations
3. Results and Discussion
3.1. 3D Printed Microstructures
3.2. Morphological and Structural Analyses
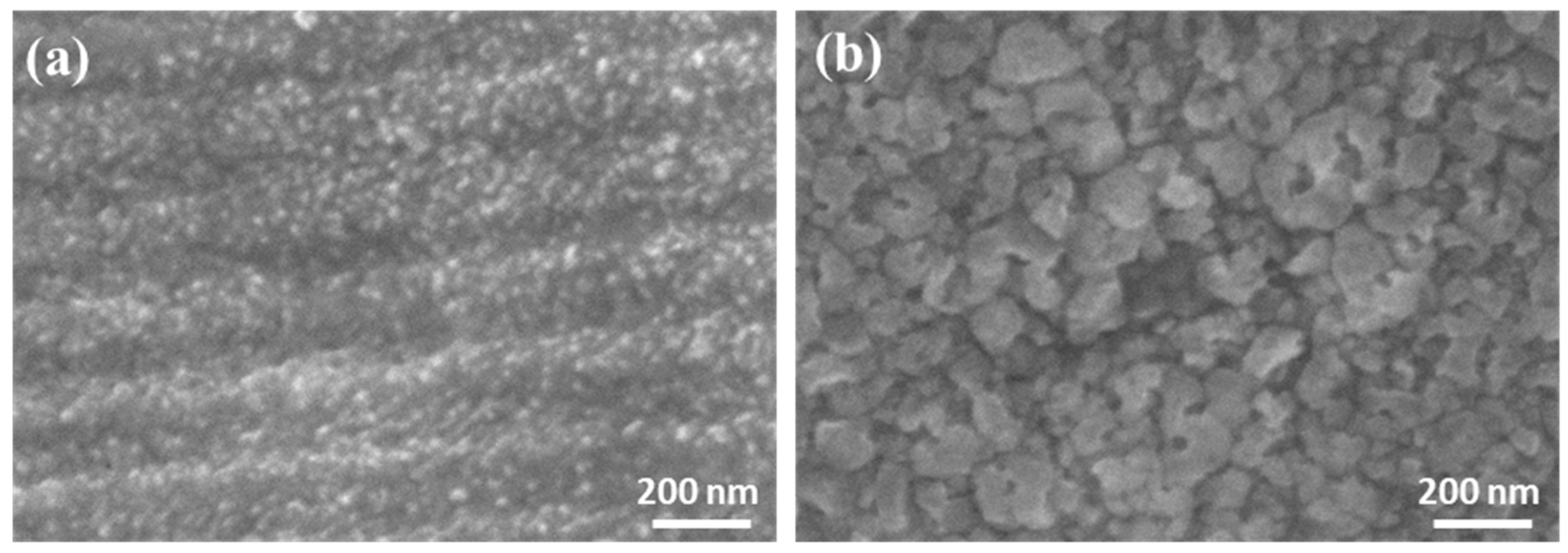
3.2.1. Morphological Analysis
3.2.2. FTIR Analysis
3.3. TGA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, C.; Li, Z.; Hao, L.; Li, Y. A Comprehensive Review on Additive Manufacturing of Glass: Recent Progress and Future Outlook. Mater. Des. 2023, 227, 111736. [Google Scholar] [CrossRef]
- Doualle, T.; André, J.-C.; Gallais, L. 3D Printing of Silica Glass through a Multiphoton Polymerization Process. Opt. Lett. 2021, 46, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, J.; Xu, Q.; Li, Y. Two-Photon Polymerization of Silica Glass Diffractive Micro-Optics with Minimal Lateral Shrinkage. Opt. Express 2023, 31, 36037–36047. [Google Scholar] [CrossRef] [PubMed]
- El Aadad, H.; El Hamzaoui, H.; Brévalle-Wasilewski, G.; Bernard, R.; Kinowski, C.; Quiquempois, Y.; Douay, M. Solmers: Versatile Hybrid Resins for Nanometric 3D Printing of Silica-Based Photonic Components. Mater. Today Adv. 2024, 22, 100500. [Google Scholar] [CrossRef]
- Ye, P.; Hong, Z.; Loy, D.A.; Liang, R. Solvent-Free Silsesquioxane Self-Welding for 3D Printing Multi-Refractive Index Glass Objects. Adv. Opt. Mater. 2024, 12, 2400783. [Google Scholar] [CrossRef]
- Lai, L.-L.; Huang, P.-H.; Stemme, G.; Niklaus, F.; Gylfason, K.B. 3D Printing of Glass Micro-Optics with Subwavelength Features on Optical Fiber Tips. ACS Nano 2024, 18, 10788–10797. [Google Scholar] [CrossRef]
- Wang, H.; Pan, C.-F.; Li, C.; Menghrajani, K.S.; Schmidt, M.A.; Li, A.; Fan, F.; Zhou, Y.; Zhang, W.; Wang, H. Two-Photon Polymerization Lithography for Imaging Optics. Int. J. Extrem. Manuf. 2024, 6, 42002. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Ladika, D.; Yu, H.; Gailevičius, D.; Wang, H.; Pan, C.; Nair, P.N.S.; Ke, Y.; Mori, T. Two-photon Polymerization Lithography for Optics and Photonics: Fundamentals, Materials, Technologies, and Applications. Adv. Funct. Mater. 2023, 33, 2214211. [Google Scholar] [CrossRef]
- Kotz, F.; Quick, A.S.; Risch, P.; Martin, T.; Hoose, T.; Thiel, M.; Helmer, D.; Rapp, B.E. Two-photon Polymerization of Nanocomposites for the Fabrication of Transparent Fused Silica Glass Microstructures. Adv. Mater. 2021, 33, 2006341. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wang, W.; Ye, F.; Yue, S.; Guo, H.; Gao, G.; Zhao, Y.; Fang, Q.; Nguyen, C.; et al. 3D-Printed Silica with Nanoscale Resolution. Nat. Mater. 2021, 20, 1506–1511. [Google Scholar] [CrossRef]
- Hong, Z.; Ye, P.; Loy, D.A.; Liang, R. High-Precision Printing of Complex Glass Imaging Optics with Precondensed Liquid Silica Resin. Adv. Sci. 2022, 9, 2105595. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Crook, C.; Baldacchini, T. A Sinterless, Low-Temperature Route to 3D Print Nanoscale Optical-Grade Glass. Science 2023, 380, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Simorgh, S.; Alasvand, N.; Khodadadi, M.; Ghobadi, F.; Kebria, M.M.; Milan, P.B.; Kargozar, S.; Baino, F.; Mobasheri, A.; Mozafari, M. Additive Manufacturing of Bioactive Glass Biomaterials. Methods 2022, 208, 75–91. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yin, J.; Gao, X. Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering 2023, 10, 672. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Santos, K.W.; Costa, K.; Gonçalves, I.S.; Alves, M.; Lauda, D.P.; Vasconcellos, L.M.R.; Campos, T.M.B.; Oliveira, I.R. Influence of Manufacturing Parameters on Bioactive Glass 45S5: Structural Analysis and Applications in Bone Tissue Engineering. Ceram. Int. 2024, 50, 51043–51054. [Google Scholar] [CrossRef]
- Gul, H.; Zahid, S.; Kaleem, M. Bioglass, A New Trend Towords Clinical Bone Tissue Engineering. Pak. Oral Dent. J. 2015, 35, 706–712. [Google Scholar]
- Meskher, H.; Sharifianjazi, F.; Tavamaishvili, K.; Irandoost, M.; Nejadkoorki, D.; Makvandi, P. Limitations, Challenges and Prospective Solutions for Bioactive Glasses-Based Nanocomposites for Dental Applications: A Critical Review. J. Dent. 2024, 150, 105331. [Google Scholar] [CrossRef]
- Profeta, A.C.; Prucher, G.M. Bioactive-Glass in Periodontal Surgery and Implant Dentistry. Dent. Mater. J. 2015, 34, 559–571. [Google Scholar] [CrossRef]
- Kowalska, K.J.; Czechowska, J.P.; Zima, A. Novel Phosphate Bioglasses and Bioglass-Ceramics for Bone Regeneration. Ceram. Int. 2024, 50, 45976–45985. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Sockalingum, G.; Michel, J.; Fauré, J.; Banchet, V.; Wortham, L.; Bouthors, S.; Laurent-Maquin, D.; Balossier, G. Synthesis and Characterisation of Sol Gel Derived Bioactive Glass for Biomedical Applications. Mater. Lett. 2006, 60, 3752–3757. [Google Scholar] [CrossRef]
- Shoushtari, M.S.; Hoey, D.; Biak, D.R.A.; Abdullah, N.; Kamarudin, S.; Zainuddin, H.S. Sol–Gel-templated Bioactive Glass Scaffold: A Review. Res. Biomed. Eng. 2024, 40, 281–296. [Google Scholar] [CrossRef]
- Bouhazma, S.; Adouar, I.; Chajri, S.; Herradi, S.; Khaldi, M.; Ouammou, A.; El Bali, B.; Mansori, M.; Lachkar, M. A Sol-Gel Synthesis, Characterization and in Vitro Bioactivity of Binary, Ternary and Quaternary Bioglasses with High Mechanical Strength. Mediterr. J. Chem. 2020, 10, 310–318. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.-M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of Melt-derived 45S5 and Sol-gel–Derived 58S Bioactive Glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Blaker, J.J. Bioactive Composite Materials for Tissue Engineering Scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef]
- Martelli, A.; Bellucci, D.; Cannillo, V. Additive Manufacturing of Polymer/Bioactive Glass Scaffolds for Regenerative Medicine: A Review. Polymers 2023, 15, 2473. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, W.; Yang, Z.; He, N.; Chen, F.; Han, X.; Zhou, K. Recent Advances in 3D Printing of Smart Scaffolds for Bone Tissue Engineering and Regeneration. Adv. Mater. 2024, 36, 2403641. [Google Scholar] [CrossRef]
- Baino, F.; Gaido, F.; Gabrieli, R.; Alidoost, D.; Schiavi, A.; Mohammadi, M.; Schwentenwein, M.; Tulyaganov, D.; Verné, E. Vat Photopolymerization of Ultra-Porous Bioactive Glass Foams. Open Ceram. 2024, 20, 100690. [Google Scholar] [CrossRef]
- Diao, Q.; Zeng, Y.; Chen, J. The Applications and Latest Progress of Ceramic 3D Printing. Addit. Manuf. Front. 2024, 3, 200113. [Google Scholar] [CrossRef]
- Aykora, D.; Uzun, M. Bone Tissue Engineering for Osteointegration: Where Are We Now? Polym. Bull. 2024, 81, 8595–8605. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Pullar, R.C. A Comparison of Bioactive Glass Scaffolds Fabricated by Robocasting from Powders Made by Sol-Gel and Melt-Quenching Methods. Processes 2020, 8, 615. [Google Scholar] [CrossRef]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D Printed Scaffolds for Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and in Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]
- Singh, G.; Soundarapandian, S. Effect of Particle Size and Freezing Conditions on Freeze-Casted Scaffold. Ceram. Int. 2019, 45, 11633–11638. [Google Scholar] [CrossRef]
- Zagrajczuk, B.; Dziadek, M.; Olejniczak, Z.; Cholewa-Kowalska, K.; Laczka, M. Structural and Chemical Investigation of the Gel-Derived Bioactive Materials from the SiO2–CaO and SiO2-CaO-P2O5 Systems. Ceram. Int. 2017, 43, 12742–12754. [Google Scholar] [CrossRef]
- Pappas, G.S.; Liatsi, P.; Kartsonakis, I.A.; Danilidis, I.; Kordas, G. Synthesis and Characterization of New SiO2–CaO Hollow Nanospheres by Sol–Gel Method: Bioactivity of the New System. J. Non. Cryst. Solids 2008, 354, 755–760. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and Characterization of Bioactive Glass Nanoparticles Prepared by Sol–Gel for Biomedical Applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A Mineralogical Perspective on the Apatite in Bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Kumar, K.C.V.; Subha, T.J.; Ahila, K.G.; Ravindran, B.; Chang, S.W.; Mahmoud, A.H.; Mohammed, O.B.; Rathi, M.A. Spectral Characterization of Hydroxyapatite Extracted from Black Sumatra and Fighting Cock Bone Samples: A Comparative Analysis. Saudi J. Biol. Sci. 2021, 28, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Petkova, V.; Stoyanov, V.; Kostova, B. Phase Transformation in the CaO-SiO2-P2O5 Natural System during High Temperature Heat Treatment. J. Chem. Technol. Metall. 2022, 57, 614–621. [Google Scholar]
- Mihailova, I.; Radev, L.; Aleksandrova, V.; Colova, I.; Salvado, I.M.M.; Fernandes, M.H. V Carbonate-Apatite Forming Ability of Polyphase Glass-Ceramics in the CaO-MgO-SiO. J. Chem. Technol. Metall. 2015, 50, 502–511. [Google Scholar]
- Bertoluzza, A.; Fagnano, C.; Morelli, M.A.; Gottardi, V.; Guglielmi, M. Raman and Infrared Spectra on Silica Gel Evolving toward Glass. J. Non. Cryst. Solids 1982, 48, 117–128. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Doménech-Carbó, A.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Identification of Synthetic Resins Used in Works of Art by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2001, 55, 1590–1602. [Google Scholar] [CrossRef]
- Wen, C.; Bai, N.; Luo, L.; Ye, J.; Zhan, X.; Zhang, Y.; Sa, B. Structural Behavior and in Vitro Bioactivity Evaluation of Hydroxyapatite-like Bioactive Glass Based on the SiO2-CaO-P2O5 System. Ceram. Int. 2021, 47, 18094–18104. [Google Scholar] [CrossRef]
- Meiszterics, A.; Sinkó, K. Sol–Gel Derived Calcium Silicate Ceramics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 143–148. [Google Scholar] [CrossRef]


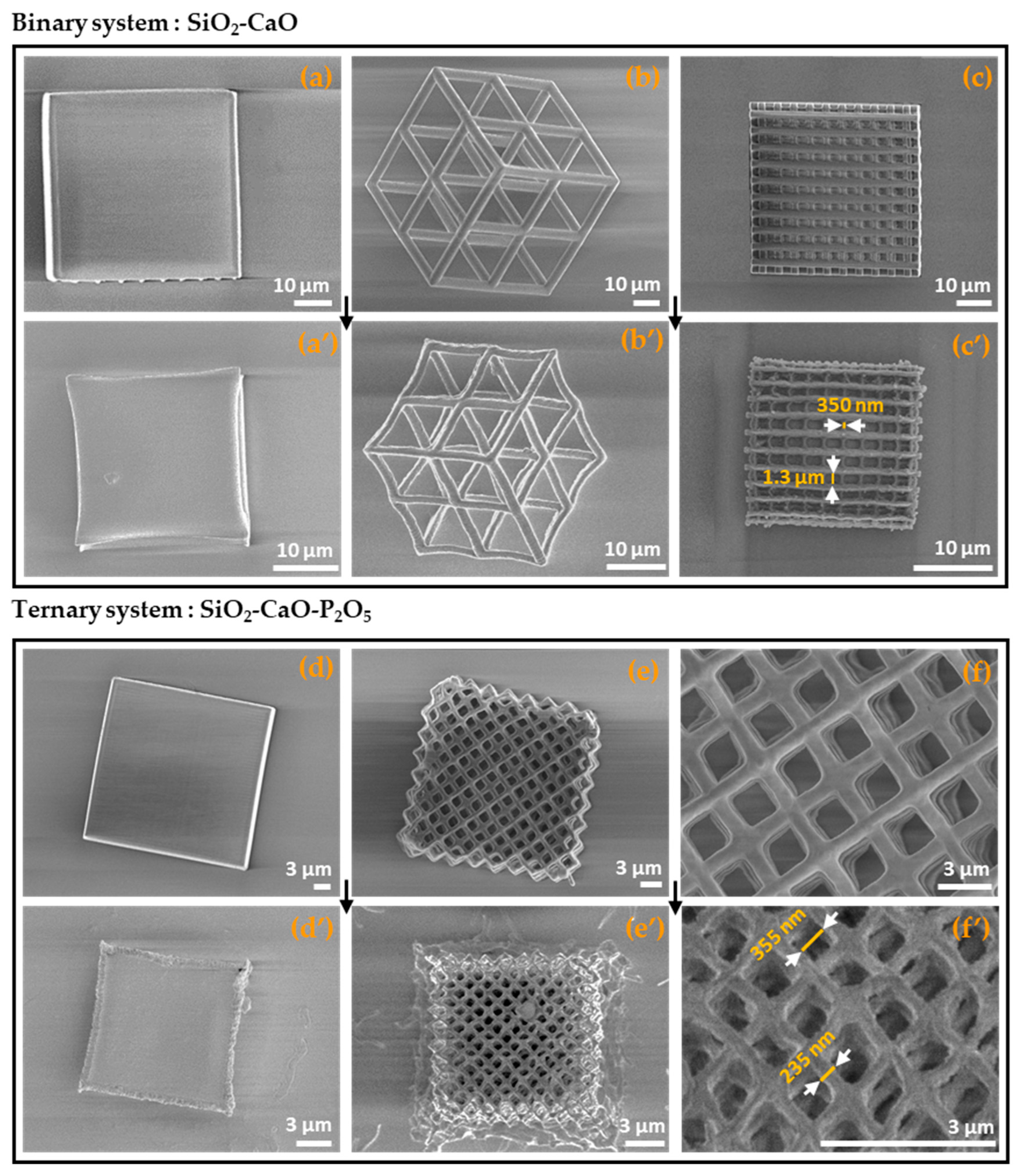


| System | Side Length (RT) | Side Length (700 °C) | Shrinkage Rate (%) |
|---|---|---|---|
| Binary system | 30.2 µm | 13.0 µm | 57 |
| Ternary system | 30.5 µm | 13.7 µm | 55 |
| Binary System (cm−1) | Ternary System (cm−1) | Attribution |
|---|---|---|
| 2964 | 2964 | ν (C-H) |
| 2900 | 2902 | ν (C-H) |
| 1722 | 1722 | ν (C=O) |
| 1634 | 1634 | ν(C=C) |
| 1635 | 1635 | ν (C=C) |
| 1619 | 1918 | ν (C=C) |
| 1472 | 1470 | δ (C-H) |
| 1408 | 1407 | δ (C-H) |
| 1296 | 1294 | γtwisting (CH2) |
| 1263 | 1263 | ν (C-O-CH3)ester |
| 1169 | 1169 | ν (C-C) |
| 1059 | 1059 | ν (C-O) |
| 982 | 981 | ν (Si-OH) |
| 806 | 806 | δ (=C-H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Aadad, H.; El Hamzaoui, H.; Quiquempois, Y.; Douay, M. Additive Manufacturing of Binary and Ternary Oxide Systems Using Two-Photon Polymerization and Low-Temperature Sintering. Nanomaterials 2024, 14, 1977. https://doi.org/10.3390/nano14231977
El Aadad H, El Hamzaoui H, Quiquempois Y, Douay M. Additive Manufacturing of Binary and Ternary Oxide Systems Using Two-Photon Polymerization and Low-Temperature Sintering. Nanomaterials. 2024; 14(23):1977. https://doi.org/10.3390/nano14231977
Chicago/Turabian StyleEl Aadad, Halima, Hicham El Hamzaoui, Yves Quiquempois, and Marc Douay. 2024. "Additive Manufacturing of Binary and Ternary Oxide Systems Using Two-Photon Polymerization and Low-Temperature Sintering" Nanomaterials 14, no. 23: 1977. https://doi.org/10.3390/nano14231977
APA StyleEl Aadad, H., El Hamzaoui, H., Quiquempois, Y., & Douay, M. (2024). Additive Manufacturing of Binary and Ternary Oxide Systems Using Two-Photon Polymerization and Low-Temperature Sintering. Nanomaterials, 14(23), 1977. https://doi.org/10.3390/nano14231977






