Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications
Abstract
:1. Introduction
2. Optical Properties of Metal-Embedded Silica Nanoparticles
2.1. Monometal-Embedded Silica NPs
2.1.1. Au-Embedded Silica NPs
2.1.2. Ag-Embedded Silica NPs
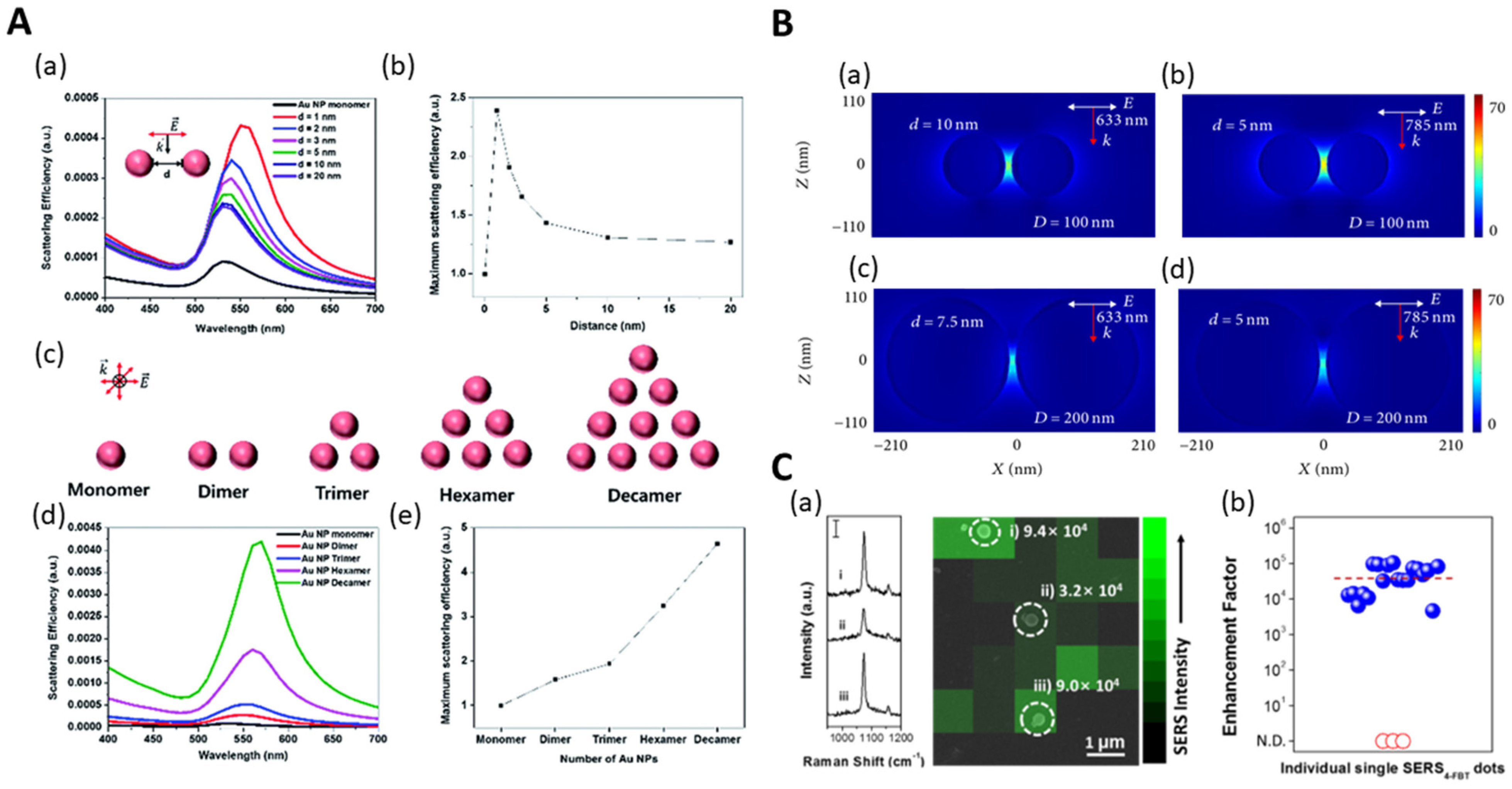
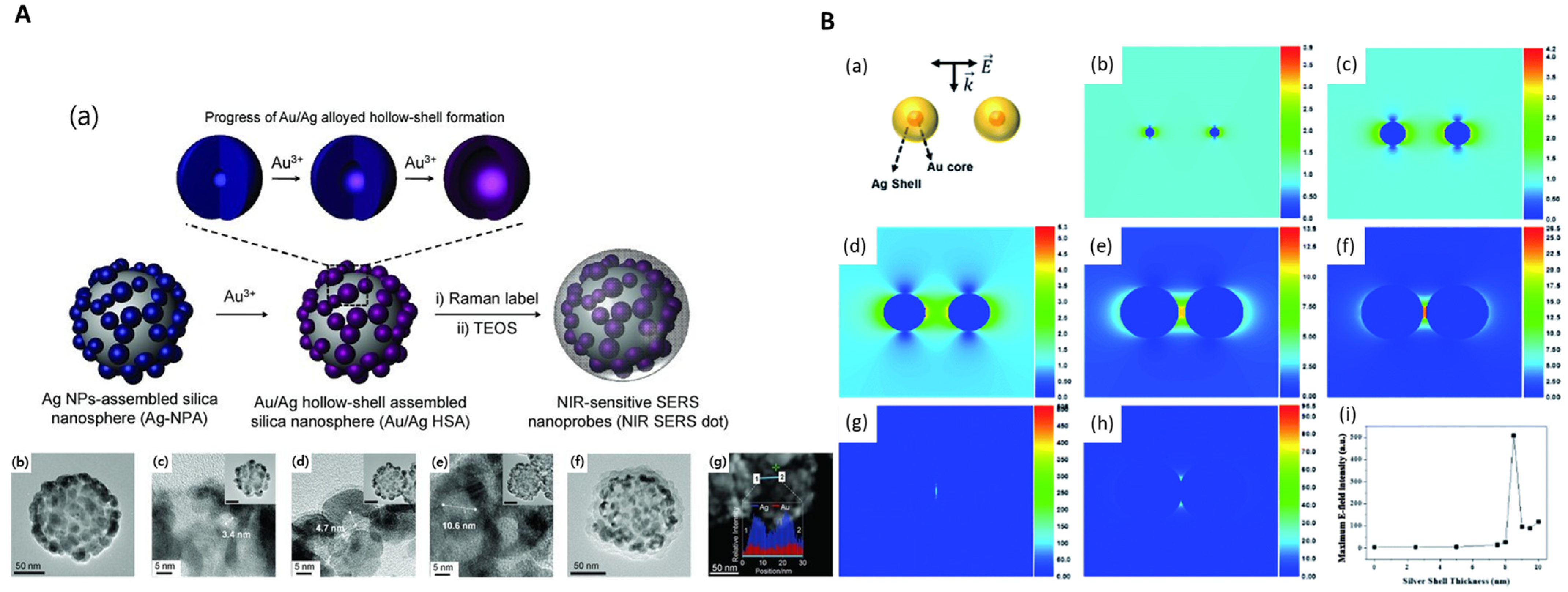
2.2. Bimetal-Embedded Silica NPs
| Metal-Embedded Silica NPs | Composition | Preparation | Optical Properties | Applications | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silica Core (Size) | Metal NP (Size) | Methods | Functionalization | Growth Solution | Reducing Agent | Capping Agent | Absorbance | SERS Enhancement Factor | ||||
| Au-Embedded Silica NPs | SiO2@Au | Silica NPs (227 nm) | Au NPs (14 nm) | direct deposition | APTS | TurkevichAu NPs | - | - | ~540 nm | - | method development | |
| Au NPs | direct reduction | APTS | trisodium citrate-HAuCl4 solution | trisodium citrate | trisodium citrate | ~538 nm(pH = 3.85–5.38) | - | [29] | ||||
| Au NPs | seed-mediated growth | APTS | TurkevichAu NPs | trisodium citrate | trisodium citrate | ~551 nm | - | |||||
| SiO2@Au | Silica NPs (600 nm) | Au NPs (10 nm) | direct deposition | APTS | Au NPs * | - | - | - | - | nitrogen adsorption | ||
| Au NPs (50 nm) | direct reduction | APTS | K2CO3–HAuCl4 solution | formaldehyde | PVP | - | - | [30] | ||||
| SiO2@Au | Silica NPs (100 nm) | Au NPs (1.5–40 nm) | direct reduction | amine-grafted Silica NPs * | K2CO3–HAuCl4 solution | NaBH4 | sodiumcitrate dihydrate | 518–634 nm(pH = 3.09–10.60) | - | photothermal conversion | [27] | |
| SiO2@Au | Silica NPs (132 nm) | Au NPs (5 nm) | seed-mediated growth | APTS | K2CO3–HAuCl4 solution | NaBH4 | sodiumcitrate dihydrate | - | - | photothermal conversion | [28] | |
| SiO2@Au@Au | Silica NPs (150 nm) | Au NPs (3 nm) | seed-mediated growth | APTS | THPC Au NPs | ascorbic acid | PVP(Mw 40,000) | 3.8 × 106 | SERS imaging | [33] | ||
| SiO2@Au | Silica NPs (160 nm) | Au NPs (1–15 nm) | direct reduction | APTS | HAuCl4 solution | ascorbic acid | PVP(Mw 40,000) | 543–632 nm | - | nanozyme | [34] | |
| SiO2@Au | Silica NPs (670 nm) | Au NPs (16–20 nm) | direct deposition | APTS | TurkevichAu NPs | - | - | ~550 nm | - | method development | ||
| direct reduction | APTS | HAuCl4 solution | trisodium citrate | trisodium citrate | - | - | [35] | |||||
| SiO2@Au | Silica NPs (120 nm) | Au NPs (21–39 nm) | direct reduction | APTS | trisodium citrate-HAuCl4 solution | NaBH4 | trisodium citrate | 631–784 nm | 2.0 × 105 | SERS probe development | [36] | |
| SiO2@Au@GO | Silica NPs (220 nm) | Au NPs (1–5 nm) | direct deposition | APTS | THPC Au NPs | - | - | ~562 nm | - | photothermal therapy | [38] | |
| SiO2@Au | Silica NPs (400 nm) | Au NPs (15 nm) | direct deposition | APTS | TurkevichAu NPs | - | - | ~523 nm | - | photothermal therapy | [40] | |
| Ag-Embedded Silica NPs | SiO2@Ag | Silica NPs (150 nm) | Ag shell thickness (32–76 nm) | direct reduction | MPTS | AgNO3 solution | octylamine | PVP(Mw 40,000) | 560–1000 nm | 6.4 × 105 | NIR-SERS probe | [43] |
| SiO2@AgRLC-Ag | Silica NPs (150 nm) | Ag NPs (9–15 nm) | direct reduction | MPTS | AgNO3 solution | octylamine | PVP(Mw 40,000) | 400–800 nm | 1.7 × 107 | detection of cancer biomarker | [44] | |
| SiO2@Ag | Silica NPs (300 nm) | Ag NPs | seed-mediated growth | APTS and GA | AgNO3 solution | triethanolamine | - | 410 nm | - | electrically conductive adhesives | [45] | |
| SiO2@Ag | Silica NPs (670 nm) | Ag NPs (10–61 nm) | powderization and heat treatment | - | AgNO3– NH4OHsolution | - | - | 403–410 nm | - | method development | [46] | |
| SiO2@Ag | Silica NPs (182 nm) | Ag shell thickness (215–363 nm) | seed-mediated growth | - | [Ag(NH3)2]+solution | - | PVP(Mw 40,000) | 436–443 nm | - | detection of drug and metabolite | [47] | |
| SiO2@Ag | Silica NPs (155 nm) | Ag NPs (9 nm) | direct reduction | - | AgNO3 solution | NaBH4 | PVP | 411 nm | - | heavy metal detection and catalytic activity | [48] | |
| SiO2@Ag | Silica NPs (300 nm) | Ag NPs (40 nm) | direct reduction | - | [Ag(NH3)2]+solution | PVP | PVP | - | 1.63 × 106 | detection of antibiotic residue | [49] | |
| Bimetal-Embedded Silica NPs | SiO2@Au@Ag | Silica NPs (150 nm) | Au@Ag NPs (11–63 nm) | seed-mediated growth | APTS | AgNO3 solution | ascorbic acid | PVP(Mw 40,000) | 400–800 nm | 4.2 × 106 | SERS probe development | [52] |
| SiO2@Au@Pt | Silica NPs (160 nm) | Au@Pt NPs | seed-mediated growth | APTS | AgNO3 solution | ascorbic acid | PVP(Mw 40,000) | 300–800 nm | - | nanozyme | [53] | |
3. Biological Applications of Metal-Embedded Silica Nanoparticles
3.1. Nanozyme
3.2. Sensing and Detection
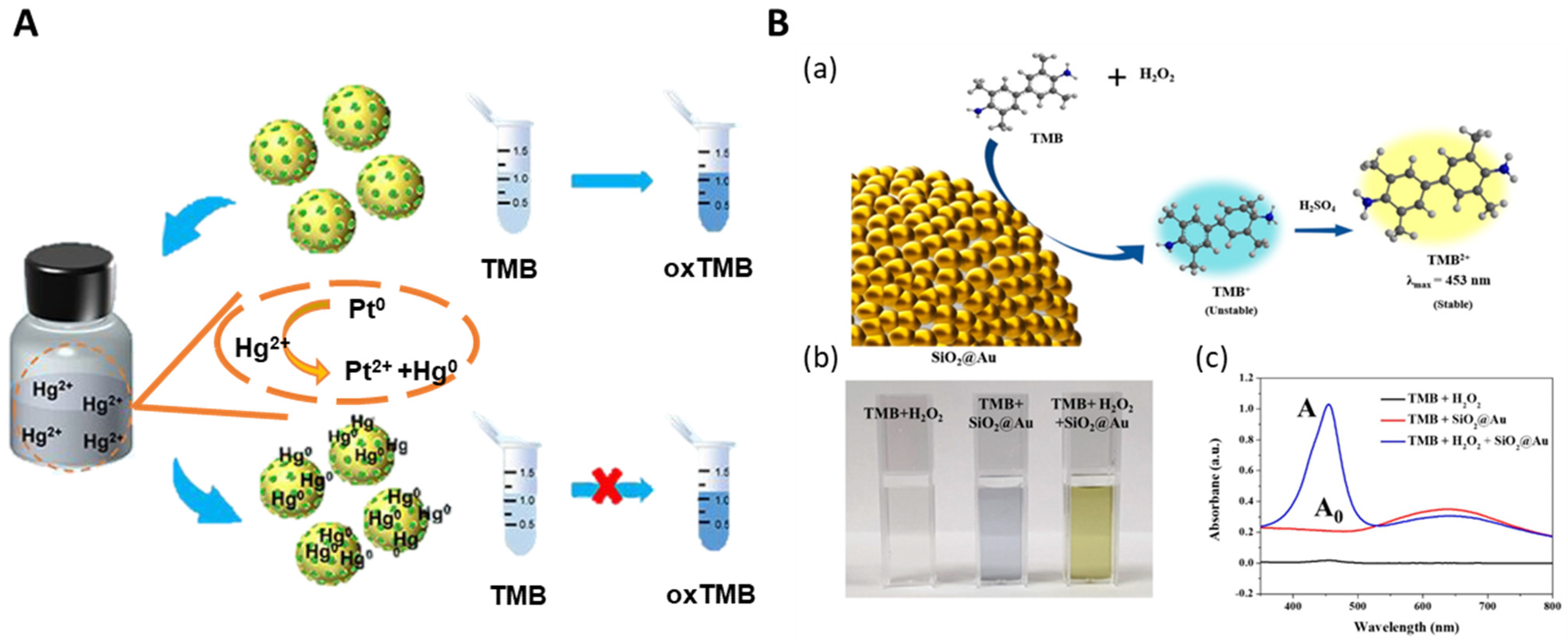
3.2.1. Hazardous Substance Detection
3.2.2. Biomarker Detection
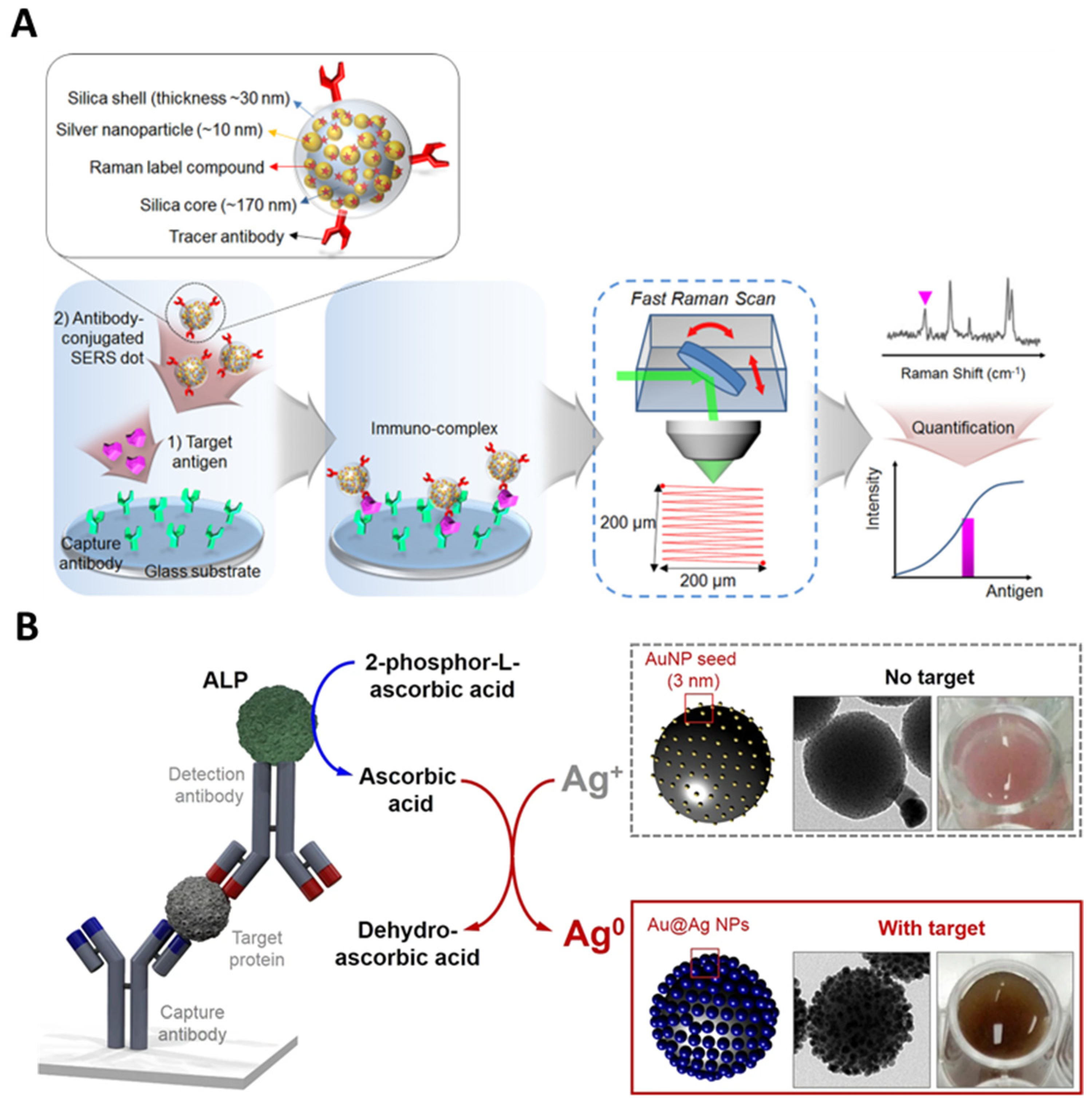
3.3. Bioimaging
3.4. Drug Carriers and Photothermal Therapy
3.5. Molecule Screening
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ringe, E.; Langille, M.R.; Sohn, K.; Zhang, J.; Huang, J.; Mirkin, C.A.; Van Duyne, R.P.; Marks, L.D. Plasmon length: A universal parameter to describe size effects in gold nanoparticles. J. Phys. Chem. Lett. 2012, 3, 1479–1483. [Google Scholar] [CrossRef]
- Biggins, J.S.; Yazdi, S.; Ringe, E. Magnesium nanoparticle plasmonics. Nano Lett. 2018, 18, 3752–3758. [Google Scholar] [CrossRef]
- Asselin, J.R.M.; Boukouvala, C.; Hopper, E.R.; Ramasse, Q.M.; Biggins, J.S.; Ringe, E. Tents, chairs, tacos, kites, and rods: Shapes and plasmonic properties of singly twinned magnesium nanoparticles. ACS Nano 2020, 14, 5968–5980. [Google Scholar] [CrossRef]
- Lee, W.; Kang, B.-H.; Yang, H.; Park, M.; Kwak, J.H.; Chung, T.; Jeong, Y.; Kim, B.K.; Jeong, K.-H. Spread spectrum SERS allows label-free detection of attomolar neurotransmitters. Nat. Commun. 2021, 12, 159. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, Y.; Zhou, C.; Ying, G.; Zhou, X.; Fu, C.; Zhu, Y.; Shen, Y. SERS detection of microRNA biomarkers for cancer diagnosis using gold-coated paramagnetic nanoparticles to capture SERS-active gold nanoparticles. RSC Adv. 2017, 7, 52782–52793. [Google Scholar] [CrossRef]
- Shim, J.-E.; Kim, Y.J.; Choe, J.-H.; Lee, T.G.; You, E.-A. Single-Nanoparticle-Based Digital SERS Sensing Platform for the Accurate Quantitative Detection of SARS-CoV-2. ACS Appl. Mater. Interfaces 2022, 14, 38459–38470. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.; Schädler, V.; Willenbacher, N.; Wagner, N.J. Electrosteric stabilization of colloidal dispersions. Langmuir 2002, 18, 6381–6390. [Google Scholar] [CrossRef]
- Kong, H.J.; Bike, S.G.; Li, V.C. Electrosteric stabilization of concentrated cement suspensions imparted by a strong anionic polyelectrolyte and a non-ionic polymer. Cem. Concr. Res. 2006, 36, 842–850. [Google Scholar] [CrossRef]
- Napper, D.H. Steric stabilization. J. Colloid Interface Sci. 1977, 58, 390–407. [Google Scholar] [CrossRef]
- Lourenco, C.; Teixeira, M.; Simões, S.; Gaspar, R. Steric stabilization of nanoparticles: Size and surface properties. Int. J. Pharm. 1996, 138, 1–12. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.A.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lian, L.; Liu, Y.; Kong, Q.; Wang, L. Controlled synthesis of monodisperse silica particles. Micro Nano Lett. 2016, 11, 532–534. [Google Scholar] [CrossRef]
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Synthesis of nanometric silica particles via a modified Stöber synthesis route. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 559–564. [Google Scholar] [CrossRef]
- Shahabi, S.; Treccani, L.; Dringen, R.; Rezwan, K. Modulation of silica nanoparticle uptake into human osteoblast cells by variation of the ratio of amino and sulfonate surface groups: Effects of serum. ACS Appl. Mater. Interfaces 2015, 7, 13821–13833. [Google Scholar] [CrossRef]
- Hasany, M.; Taebnia, N.; Yaghmaei, S.; Shahbazi, M.-A.; Mehrali, M.; Dolatshahi-Pirouz, A.; Arpanaei, A. Silica nanoparticle surface chemistry: An important trait affecting cellular biocompatibility in two and three dimensional culture systems. Colloids Surf. B Biointerfaces 2019, 182, 110353. [Google Scholar] [CrossRef]
- Sadeghi, M.; Moghimifar, Z.; Javadian, H. Fe3O4@ SiO2 nanocomposite immobilized with cellulase enzyme: Stability determination and biological activity. Chem. Phys. Lett. 2023, 811, 140161. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and properties of various magnetic nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 489. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Xu, S.; Li, H.; Lu, Y.; Zhu, C. Recyclable Multifunctional Magnetic Fe3O4@ SiO2@ Au Core/Shell Nanoparticles for SERS Detection of Hg (II). Chemosensors 2023, 11, 347. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, B.; Yang, P.; Li, J.; Xiao, G.; Yao, J.; Gong, X.; Yan, J.; Zhang, H. The Functional Fe3O4@ SiO2@ AuNPs SERS Nanomaterials for Rapid Enrichment and Detection of Mercury Ions in Licorice. Chemosensors 2022, 10, 403. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.-Y.L.; Ong, C.-N.; Ong, W.-Y.; Liya, E.Y. Characterization, purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Whaley Bishnoi, S. Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Z.; Yang, L.; Yang, J.; Jin, M.; Xing, X.; Zhou, G.; Shui, L. Photothermal conversion of SiO 2@ Au nanoparticles mediated by surface morphology of gold cluster layer. RSC Adv. 2020, 10, 33119–33128. [Google Scholar] [CrossRef] [PubMed]
- Ruvalcaba-Ontiveros, R.I.; Murillo-Ramírez, J.G.; Medina-Vázquez, J.A.; Carrasco-Hernández, A.R.; Duarte-Möller, J.A.; Esparza-Ponce, H.E. Synthesis of gold decorated silica nanoparticles and their photothermal properties. Micron 2023, 166, 103415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Y.-G.; Wang, L.-Y.; Zhang, J.-Y.; Chen, M.; Qian, D.-J. Comparative studies between synthetic routes of SiO2@ Au composite nanoparticles. Mater. Res. Bull. 2007, 42, 1457–1467. [Google Scholar] [CrossRef]
- Choma, J.; Dziura, A.; Jamioła, D.; Nyga, P.; Jaroniec, M. Preparation and properties of silica–gold core–shell particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 167–171. [Google Scholar] [CrossRef]
- Lee, M.; Kang, Y.-L.; Rho, W.-Y.; Kyeong, S.; Jeong, S.; Jeong, C.; Chung, W.-J.; Kim, H.-M.; Kang, H.; Lee, Y.-S. Preparation of plasmonic magnetic nanoparticles and their light scattering properties. RSC Adv. 2015, 5, 21050–21053. [Google Scholar] [CrossRef]
- Liu, K.; Xue, X.; Furlani, E.P. Theoretical comparison of optical properties of near-infrared colloidal plasmonic nanoparticles. Sci. Rep. 2016, 6, 34189. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.; Choi, Y.-S.; Kim, M.; Yun, Y.; Pham, X.-H.; Kim, J.; Seong, B.; Kim, W.; Jo, A.; Ham, K.-M. Highly sensitive near-infrared SERS nanoprobes for in vivo imaging using gold-assembled silica nanoparticles with controllable nanogaps. J. Nanobiotechnol. 2022, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Seong, B.; Kim, J.; Kim, W.; Lee, S.H.; Pham, X.-H.; Jun, B.-H. Synthesis of Finely Controllable Sizes of Au Nanoparticles on a Silica Template and Their Nanozyme Properties. Int. J. Mol. Sci. 2021, 22, 10382. [Google Scholar] [CrossRef]
- Dobrowolska, P.; Krajewska, A.; Gajda-Rączka, M.; Bartosewicz, B.; Nyga, P.; Jankiewicz, B.J. Application of Turkevich method for gold nanoparticles synthesis to fabrication of SiO2@ Au and TiO2@ Au core-shell nanostructures. Materials 2015, 8, 2849–2862. [Google Scholar] [CrossRef]
- Wang, R.; Ji, X.; Huang, Z.; Xue, Y.; Wang, D.; Yang, W. Citrate-regulated surface morphology of SiO2@ Au particles to control the surface plasmonic properties. J. Phys. Chem. C 2016, 120, 377–385. [Google Scholar] [CrossRef]
- Jiang, X.; Qiao, L.; Yang, H.; Li, B.Q.; Ding, S. NIR-triggered Synergetic Photothermal and Chemotherapy Cancer Treatment Based on SiO2@ Au@ SiO2@ QDs-DOX Composite Structural Particles. ChemNanoMat 2023, 9, e202200532. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Hu, N.; Zhang, L.; Zhang, Y.; Yin, L. Docetaxel-loaded SiO2@ Au@ GO core–shell nanoparticles for chemo-photothermal therapy of cancer cells. RSC Adv. 2016, 6, 48379–48386. [Google Scholar] [CrossRef]
- Lim, Z.-Z.J.; Li, J.-E.J.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef]
- Park, J.H.; Choe, H.-S.; Kim, S.-W.; Im, G.-B.; Um, S.H.; Kim, J.-H.; Bhang, S.H. Silica-capped and gold-decorated silica nanoparticles for enhancing effect of gold nanoparticle-based photothermal therapy. Tissue Eng. Regen. Med. 2022, 19, 1161–1168. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Zhao, J.; Dong, X.; Li, C.; Zhu, P.; Yu, X.; Dai, H. Size Effects of Closed Encounter Ag Nanoshell Pairs for SERS Application. J. Nanomater. 2021, 2021, 6657153. [Google Scholar] [CrossRef]
- Kim, H.-M.; Jeong, S.; Hahm, E.; Kim, J.; Cha, M.G.; Kim, K.-M.; Kang, H.; Kyeong, S.; Pham, X.-H.; Lee, Y.-S. Large scale synthesis of surface-enhanced Raman scattering nanoprobes with high reproducibility and long-term stability. J. Ind. Eng. Chem. 2016, 33, 22–27. [Google Scholar] [CrossRef]
- Kang, H.; Yang, J.-K.; Noh, M.S.; Jo, A.; Jeong, S.; Lee, M.; Lee, S.; Chang, H.; Lee, H.; Jeon, S.-J. One-step synthesis of silver nanoshells with bumps for highly sensitive near-IR SERS nanoprobes. J. Mater. Chem. B 2014, 2, 4415–4421. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Yang, J.-K.; Jo, A.; Lee, H.; Heo, E.H.; Jeong, D.H.; Jun, B.-H.; Chang, H.; Lee, Y.-S. Template-assisted plasmonic nanogap shells for highly enhanced detection of cancer biomarkers. Int. J. Mol. Sci. 2021, 22, 1752. [Google Scholar] [CrossRef]
- Xu, C.; Li, W.-J.; Wei, Y.-M.; Cui, X.-Y. Characterization of SiO2/Ag composite particles synthesized by in situ reduction and its application in electrically conductive adhesives. Mater. Des. 2015, 83, 745–752. [Google Scholar] [CrossRef]
- Granbohm, H.; Larismaa, J.; Ali, S.; Johansson, L.-S.; Hannula, S.-P. Control of the size of silver nanoparticles and release of silver in heat treated SiO2-Ag composite powders. Materials 2018, 11, 80. [Google Scholar] [CrossRef]
- Huang, L.; Wan, J.; Wei, X.; Liu, Y.; Huang, J.; Sun, X.; Zhang, R.; Gurav, D.D.; Vedarethinam, V.; Li, Y. Plasmonic silver nanoshells for drug and metabolite detection. Nat. Commun. 2017, 8, 220. [Google Scholar] [CrossRef]
- Khedkar, C.V.; Daware, K.D.; Badgujar, P.S.; Kolekar, Y.D.; Gosavi, S.W.; Patil, S.I. Ag–SiO2 nanocomposite for the optical detection of Hg (II) ions and catalytic reduction of methylene blue. Opt. Mater. 2021, 120, 111426. [Google Scholar] [CrossRef]
- Guo, H.; Ren, X.; Song, X.; Li, X. Preparation of SiO2@ Ag@ molecular imprinted polymers hybrid for sensitive and selective detection of amoxicillin using surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122365. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Sapkota, K.; Chaudhary, P.; Han, S.S. Environmentally sustainable route to SiO 2@ Au–Ag nanocomposites for biomedical and catalytic applications. RSC Adv. 2018, 8, 31311–31321. [Google Scholar] [CrossRef]
- Pham, X.-H.; Lee, M.; Shim, S.; Jeong, S.; Kim, H.-M.; Hahm, E.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Highly sensitive and reliable SERS probes based on nanogap control of a Au–Ag alloy on silica nanoparticles. RSC Adv. 2017, 7, 7015–7021. [Google Scholar] [CrossRef]
- Pham, X.-H.; Tran, V.-K.; Hahm, E.; Kim, Y.-H.; Kim, J.; Kim, W.; Jun, B.-H. Synthesis of Gold-Platinum Core-Shell Nanoparticles Assembled on a Silica Template and Their Peroxidase Nanozyme Properties. Int. J. Mol. Sci. 2022, 23, 6424. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Ding, Y.; Luo, J.; Gu, M.; Yu, Z. NiCu bimetallic nanoparticles on silica support for catalytic hydrolysis of ammonia borane: Composition-dependent activity and support size effect. ACS Appl. Energy Mater. 2019, 2, 5851–5861. [Google Scholar] [CrossRef]
- Chen, H.Q.; Ze, H.; Yue, M.F.; Wei, D.Y.; Wu, Y.F.; Dong, J.C.; Zhang, Y.J.; Zhang, H.; Tian, Z.Q.; Li, J.F. Unmasking the critical role of the ordering degree of bimetallic nanocatalysts on oxygen reduction reaction by in situ Raman spectroscopy. Angew. Chem. 2022, 134, e202117834. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Park, Y.; Yim, J.; Jun, B.H.; Kyeong, S.; Yang, J.K.; Kim, G.; Hong, S.; Lee, L.P. Near-Infrared SERS Nanoprobes with Plasmonic Au/Ag Hollow-Shell Assemblies for In Vivo Multiplex Detection. Adv. Funct. Mater. 2013, 23, 3719–3727. [Google Scholar] [CrossRef]
- Noh, M.S.; Lee, S.; Kang, H.; Yang, J.-K.; Lee, H.; Hwang, D.; Lee, J.W.; Jeong, S.; Jang, Y.; Jun, B.-H. Target-specific near-IR induced drug release and photothermal therapy with accumulated Au/Ag hollow nanoshells on pulmonary cancer cell membranes. Biomaterials 2015, 45, 81–92. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Gu, C.; Kong, X.; Yan, S.; Gai, P.; Li, F. Glucose dehydrogenase-like nanozyme based on black phosphorus nanosheets for high-performance biofuel cells. ACS Sustain. Chem. Eng. 2020, 8, 16549–16554. [Google Scholar] [CrossRef]
- Mikolajczak, D.J.; Koksch, B. Peptide-Gold Nanoparticle Conjugates as Sequential Cascade Catalysts. ChemCatChem 2018, 10, 4324–4328. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Xu, X.; Xi, J.; Han, J.; Fan, L.; Guo, R. Construction of core-in-shell Au@ N-HCNs nanozymes for tumor therapy. Colloids Surf. B Biointerfaces 2022, 217, 112671. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Nanozymes: Definition, activity, and mechanisms. Adv. Mater. 2023, 2211041. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, W.; Lv, X.; Du, X.; He, J.; Cai, J. Dendritic Silica Nanospheres with Au–Pt Nanoparticles as Nanozymes for Label-Free Colorimetric Hg2+ Detection. ACS Appl. Nano Mater. 2022, 5, 18885–18893. [Google Scholar] [CrossRef]
- Pham, X.-H.; Seong, B.; Bock, S.; Hahm, E.; Huynh, K.-H.; Kim, Y.-H.; Kim, W.; Kim, J.; Kim, D.-E.; Jun, B.-H. Nonenzymatic hydrogen peroxide detection using surface-enhanced Raman scattering of gold–silver core–shell-assembled silica nanostructures. Nanomaterials 2021, 11, 2748. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Hahm, E.; An, J.; Kim, H.-M.; Jo, A.; Seong, B.; Kim, Y.-H.; Son, B.S.; Kim, J. Facile histamine detection by surface-enhanced Raman scattering using SiO2@ Au@ Ag alloy nanoparticles. Int. J. Mol. Sci. 2020, 21, 4048. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Huynh, K.-H.; Kim, H.-M.; Son, B.S.; Jeong, D.H.; Jun, B.-H. Sensitive and selective detection of 4-aminophenol in the presence of acetaminophen using gold–silver core–shell nanoparticles embedded in silica nanostructures. J. Ind. Eng. Chem. 2020, 83, 208–213. [Google Scholar] [CrossRef]
- Yang, J.-K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.-J.; Kim, H.-I.; Lee, H.-Y.; Jeong, D.H.; Kim, J.-H. Single-step and rapid growth of silver nanoshells as SERS-active nanostructures for label-free detection of pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Hahm, E.; Pham, X.-H.; Kang, H.; Jeong, D.-H.; Chang, H.; Jun, B.-H. Mercury Ion-Responsive Coalescence of Silver Nanoparticles on a Silica Nanoparticle Core for Surface-Enhanced Raman Scattering Sensing. ACS Appl. Nano Mater. 2023, 6, 23469–23476. [Google Scholar] [CrossRef]
- Reach, G.; Wilson, G.S. Can continuous glucose monitoring be used for the treatment of diabetes. Anal. Chem. 1992, 64, 381A–386A. [Google Scholar]
- Pham, X.-H.; Seong, B.; Hahm, E.; Huynh, K.-H.; Kim, Y.-H.; Kim, J.; Lee, S.H.; Jun, B.-H. Glucose detection of 4-mercaptophenylboronic acid-immobilized gold-silver core-shell assembled silica nanostructure by surface enhanced Raman scattering. Nanomaterials 2021, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Wohlfahrt, G.; Knäblein, J.; Schomburg, D. Aspects of the mechanism of catalysis of glucose oxidase: A docking, molecular mechanics and quantum chemical study. J. Comput. Aided Mol. Des. 1998, 12, 425–440. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wang, M.; Petti, L.; Jiang, T.; Jia, Z.; Xie, S.; Zhou, J. SERS-based multiplex immunoassay of tumor markers using double SiO2@ Ag immune probes and gold-film hemisphere array immune substrate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 48–58. [Google Scholar] [CrossRef]
- Hong, D.; Jo, E.-J.; Jung, C.; Kim, M.-G. Absorption-Modulated SiO2@ Au Core–Satellite Nanoparticles for Highly Sensitive Detection of SARS-CoV-2 Nucleocapsid Protein in Lateral Flow Immunosensors. ACS Appl. Mater. Interfaces 2022, 14, 45189–45200. [Google Scholar] [CrossRef]
- Jia, X.; Wang, K.; Li, X.; Liu, Z.; Liu, Y.; Xiao, R.; Wang, S. Highly sensitive detection of three protein toxins via SERS-lateral flow immunoassay based on SiO2@ Au nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2022, 41, 102522. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Ebeid, N.; El-Shershaby, H.M.; Shafik, H.; Moustafa, K. Establishment of liquid phase double antibody radioimmunoassay system for in-vitro determination of erythropoietin hormone in human serum. J. Radioanal. Nucl. Chem. 2023, 332, 3103–3112. [Google Scholar] [CrossRef]
- Tomás, A.L.; de Almeida, M.P.; Cardoso, F.; Pinto, M.; Pereira, E.; Franco, R.; Matos, O. Development of a gold nanoparticle-based lateral-flow immunoassay for pneumocystis pneumonia serological diagnosis at point-of-care. Front. Microbiol. 2019, 10, 2917. [Google Scholar] [CrossRef]
- Chang, H.; Kang, H.; Ko, E.; Jun, B.-H.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. PSA detection with femtomolar sensitivity and a broad dynamic range using SERS nanoprobes and an area-scanning method. Acs Sens. 2016, 1, 645–649. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Ohori, M.; Wheeler, T.M.; Dunn, J.K.; Stamey, T.A.; Scardino, P.T. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J. Urol. 1994, 152, 1714–1720. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, T.H.; Kim, H.-M.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Adenosine triphosphate-encapsulated liposomes with plasmonic nanoparticles for surface enhanced Raman scattering-based immunoassays. Sensors 2017, 17, 1480. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, T.H.; Kim, H.-M.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Enzyme-catalyzed Ag growth on Au nanoparticle-assembled structure for highly sensitive colorimetric immunoassay. Sci. Rep. 2018, 8, 6290. [Google Scholar] [CrossRef]
- Chen, K.; Ma, B.; Li, J.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.; Zhang, M. A rapid and sensitive europium nanoparticle-based lateral flow immunoassay combined with recombinase polymerase amplification for simultaneous detection of three food-borne pathogens. Int. J. Environ. Res. Public Health 2021, 18, 4574. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Nara, S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015, 170, 470–483. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Ding, S.-N. Simultaneous detection of two ovarian cancer biomarkers in human serums with biotin-enriched dendritic mesoporous silica nanoparticles-labeled multiplex lateral flow immunoassay. Sens. Actuators B Chem. 2022, 371, 132597. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.; An, J.; Bock, S.; Pham, X.-H.; Huynh, K.-H.; Choi, Y.; Hahm, E.; Song, H.; Kim, J.-W. Au–Ag assembled on silica nanoprobes for visual semiquantitative detection of prostate-specific antigen. J. Nanobiotechnol. 2021, 19, 73. [Google Scholar] [CrossRef]
- He, J.; Zhu, S.; Zhou, J.; Jiang, W.; Yin, L.; Su, L.; Zhang, X.; Chen, Q.; Li, X. Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay. Front. Bioeng. Biotechnol. 2023, 10, 1090281. [Google Scholar] [CrossRef]
- Yu, K.N.; Lee, S.-M.; Han, J.Y.; Park, H.; Woo, M.-A.; Noh, M.S.; Hwang, S.-K.; Kwon, J.-T.; Jin, H.; Kim, Y.-K. Multiplex targeting, tracking, and imaging of apoptosis by fluorescent surface enhanced Raman spectroscopic dots. Bioconjugate Chem. 2007, 18, 1155–1162. [Google Scholar] [CrossRef]
- Lee, S.; Chon, H.; Yoon, S.-Y.; Lee, E.K.; Chang, S.-I.; Lim, D.W.; Choo, J. Fabrication of SERS-fluorescence dual modal nanoprobes and application to multiplex cancer cell imaging. Nanoscale 2012, 4, 124–129. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Garai, E.; Liu, J.T.; Sensarn, S.; Mandella, M.J.; Van de Sompel, D.; Friedland, S.; Van Dam, J.; Contag, C.H.; Gambhir, S.S. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc. Natl. Acad. Sci. USA 2013, 110, E2288–E2297. [Google Scholar] [CrossRef]
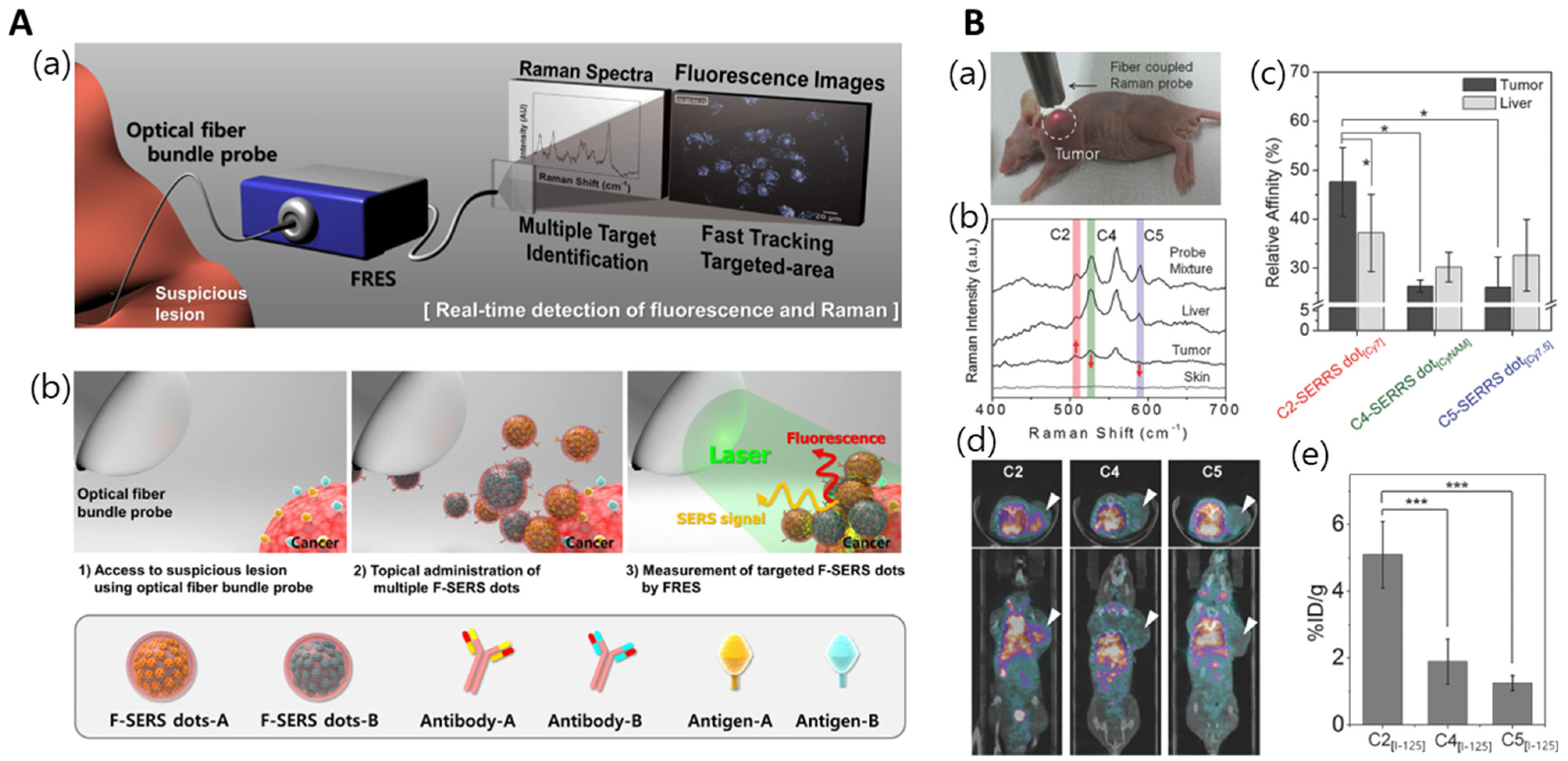
- Jeong, S.; Kim, Y.-i.; Kang, H.; Kim, G.; Cha, M.G.; Chang, H.; Jung, K.O.; Kim, Y.-H.; Jun, B.-H.; Hwang, D.W. Fluorescence-Raman dual modal endoscopic system for multiplexed molecular diagnostics. Sci. Rep. 2015, 5, 9455. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Jo, A.; Chang, H.; Yang, J.K.; Jeong, C.; Kyeong, S.; Lee, Y.W.; Samanta, A.; Maiti, K.K. Ultrasensitive NIR-SERRS Probes with Multiplexed Ratiometric Quantification for In Vivo Antibody Leads Validation. Adv. Healthc. Mater. 2018, 7, 1700870. [Google Scholar] [CrossRef]
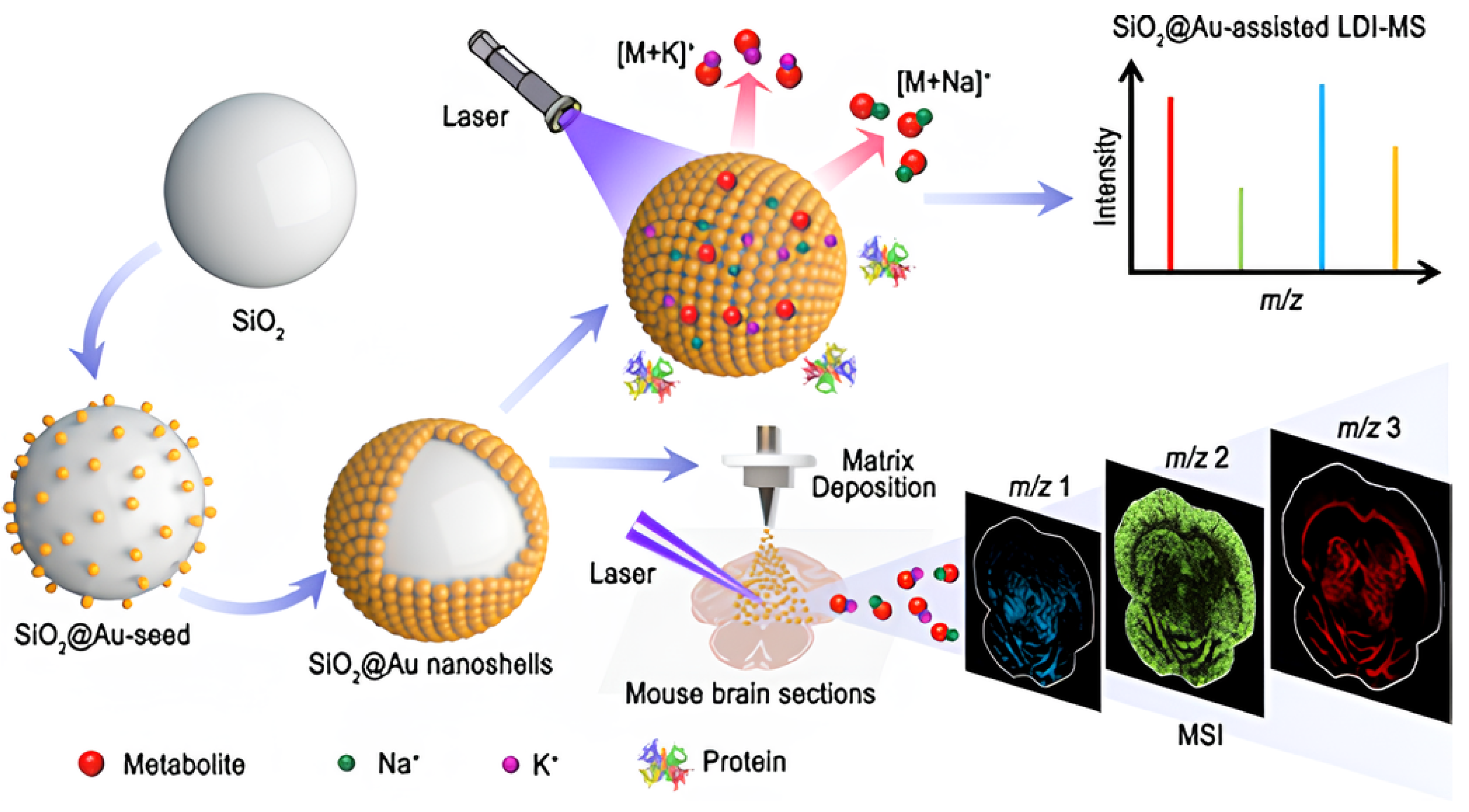
- Du, M.; Chen, D.; Chen, Y.; Huang, Y.; Ma, L.; Xie, Q.; Xu, Y.; Zhu, X.; Chen, Z.; Yin, Z. Plasmonic gold nanoshell-assisted laser desorption/ionization mass spectrometry for small-biomolecule analysis and tissue imaging. ACS Appl. Nano Mater. 2022, 5, 9633–9645. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, D.; Yin, X.; Jin, X.; Zhang, X.; Zheng, S.; Wang, C.; Liu, Y. Intracellular pH-responsive and rituximab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J. Exp. Clin. Cancer Res. 2017, 36, 24. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S. Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- He, M.; Qin, Z.; Liang, X.; He, X.; Zhu, B.; Lu, Z.; Wei, Q.; Zheng, L. A pH-responsive mesoporous silica nanoparticles-based drug delivery system with controlled release of andrographolide for OA treatment. Regen. Biomater. 2021, 8, rbab020. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S.; Song, B.; Guo, D.; Wang, J.; Hu, G.; Su, Y.; He, Y. Fluorescent silicon nanorods-based nanotheranostic agents for multimodal imaging-guided photothermal therapy. Nano-Micro Lett. 2019, 11, 73. [Google Scholar] [CrossRef]
- Tang, H.; Chen, C.-J.; Huang, Z.; Bright, J.; Meng, G.; Liu, R.-S.; Wu, N. Plasmonic hot electrons for sensing, photodetection, and solar energy applications: A perspective. J. Chem. Phys. 2020, 152, 220901. [Google Scholar] [CrossRef]
- Kang, E.J.; Baek, Y.M.; Hahm, E.; Lee, S.H.; Pham, X.-H.; Noh, M.S.; Kim, D.-E.; Jun, B.-H. Functionalized β-cyclodextrin immobilized on Ag-embedded silica nanoparticles as a drug carrier. Int. J. Mol. Sci. 2019, 20, 315. [Google Scholar] [CrossRef]
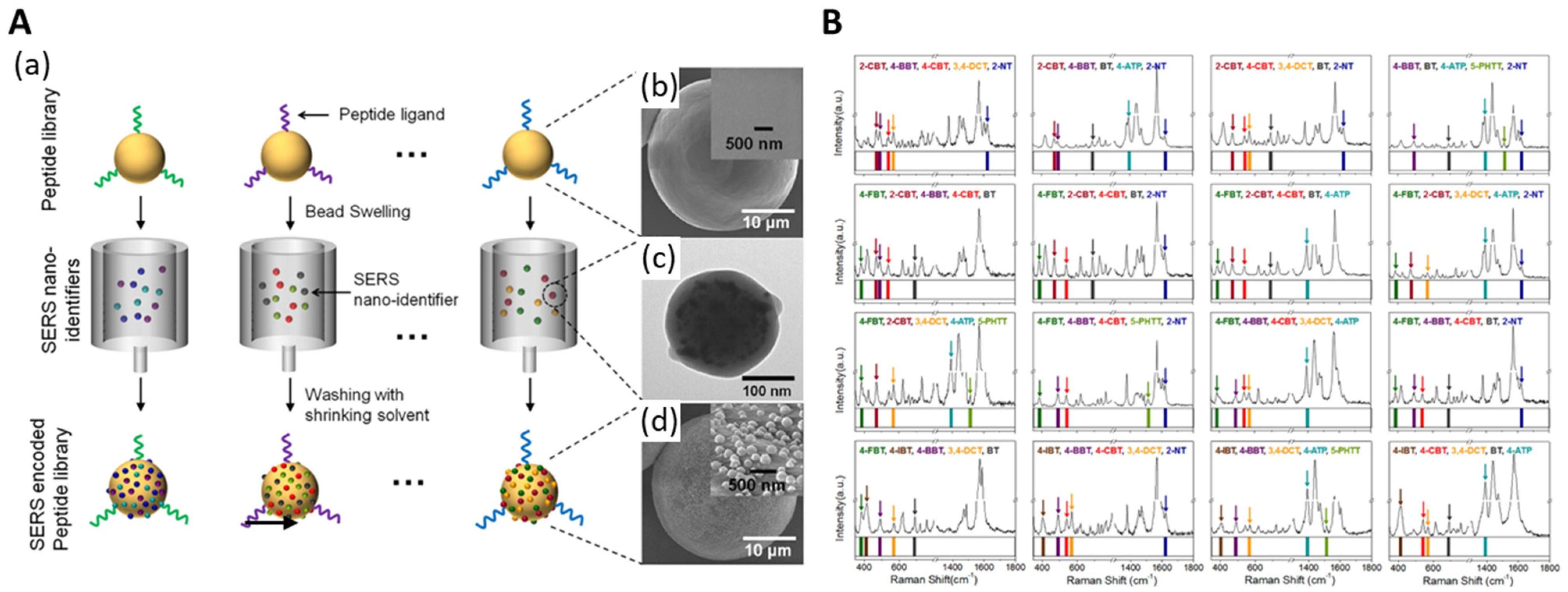
- Kang, H.; Jeong, S.; Koh, Y.; Geun Cha, M.; Yang, J.-K.; Kyeong, S.; Kim, J.; Kwak, S.-Y.; Chang, H.-J.; Lee, H. Direct identification of on-bead peptides using surface-enhanced Raman spectroscopic barcoding system for high-throughput bioanalysis. Sci. Rep. 2015, 5, 10144. [Google Scholar] [CrossRef]


| Metal-Embedded Silica NP | Catalyst | Optimization of Catalytic Performance | Results | Application | Reference |
|---|---|---|---|---|---|
| SiO2@Au@Au | Peroxidase | 1. TMB conc. 2. H2O2 conc. 3. pH solution 4. NP amount 5. Reaction time 6. Termination time | 1. 0.8 mM 2. 200 mM 3. pH 4 4. 20, 25 mg 5. 25 min 6. 5 min | - | [34] |
| SiO2@Au@Pt | Peroxidase | 1. NP amount 2. TMB conc. 3. Incubation time 4. pH solution | 1. 5 μg 2. 0.5 mM 3. 15 min 4. pH 4 | - | [53] |
| AuPt@DSN | Peroxidase | 1. TMB conc. 2. H2O2 conc. | 1. 0.4 mM 2. 4 mM | Hg+ detection | [64] |
| SiO2@Au@Ag | Peroxidase | 1. TMB conc. 2. Incubation time 3. NP amount 4. pH solution | 1. 0.8 mM 2. 15 min 3. 20 μg 4. pH 6 | H2O2 detection | [65] |
| Metal-Embedded Silica NP | Modification of NPS | Detection Method | Target Material | LOD | Reference |
|---|---|---|---|---|---|
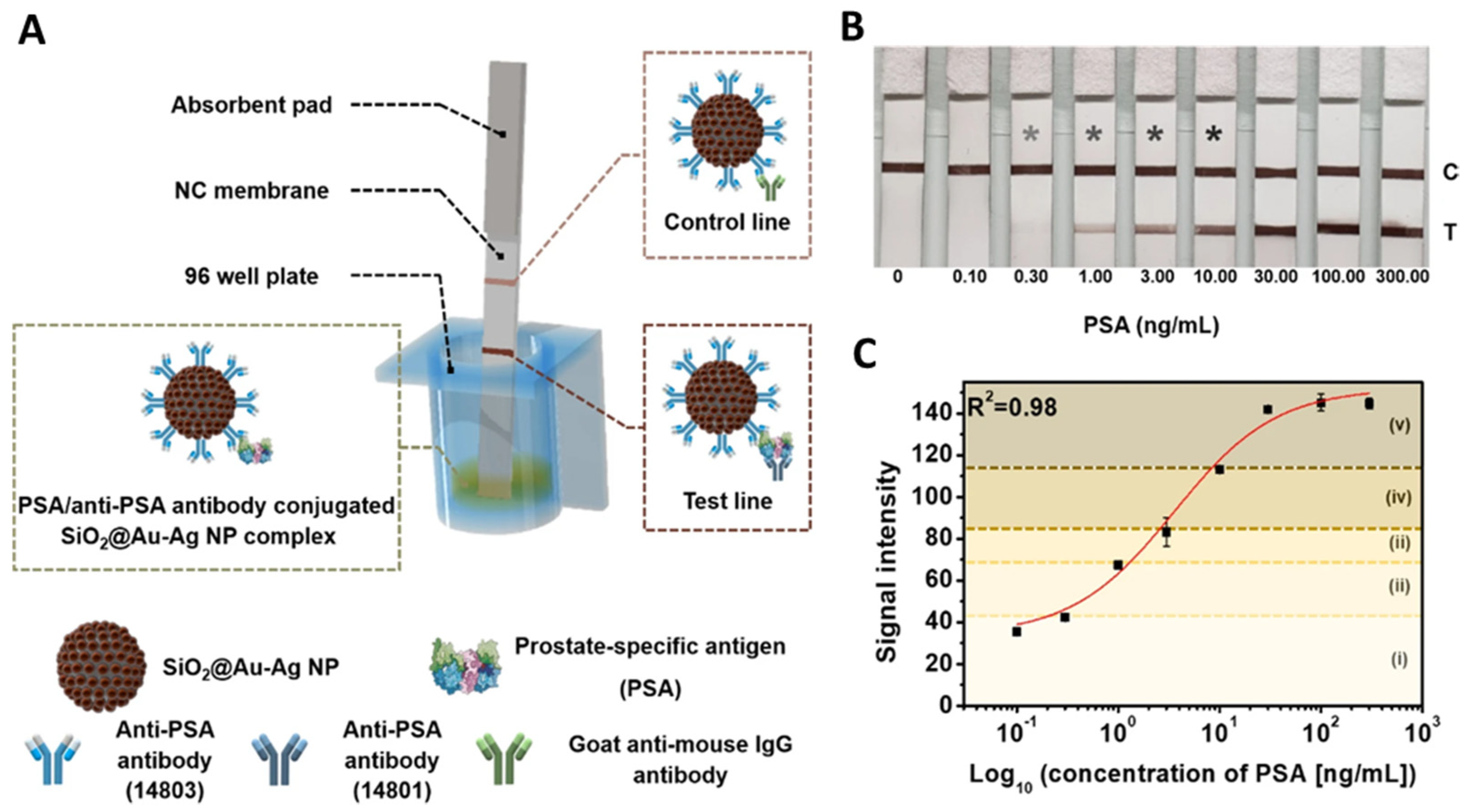
| SiO2@Ag | Anti-PSA antibody | SIA | PSA | 2.0 pg/mL | [44] |
| SiO2@Ag | Molecular imprinted polymers | SERS | ofloxacin | 2.7 × 10−9 M | [49] |
| SiO2@Au@Ag | - | SERS | Histamine | 3.698 ppm | [66] |
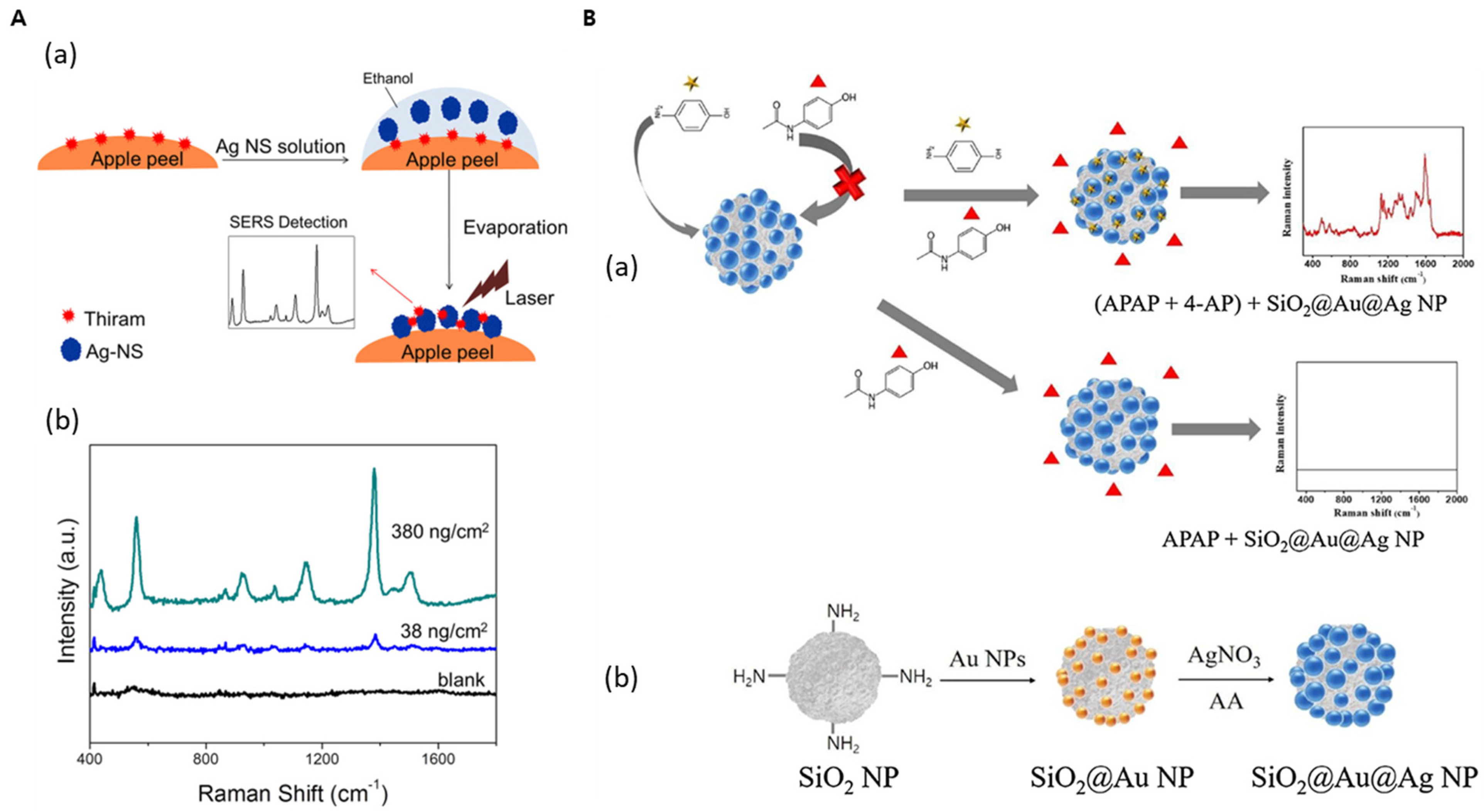
| SiO2@Au@Ag | - | SERS | 4-AP | 3.5 ppm | [67] |
| Ag NS | - | SERS | Thiram | 38 ng/cm2 | [68] |
| SiO2@Ag4-FBT | - | SERS | Hg+ | 0.819 μM | [69] |
| SiO2@Au@Ag | 4-MPBA | SERS | Glucose | 1. 0.15 mM | [71] |
| SiO2@Au CSNP | SARS-CoV-2 nucleocapsid protein antibody | LFIA | SARS-CoV-2 nucleocapsid protein | 0.24 pg/mL | [74] |
| SiO2@AgSiO2 | PSA capture antibody | SIA | PSA | 0.11 pg/mL | [79] |
| SiO2@Au@Ag | - | Liposome decomposition SIA | 4-ATP | 1.3 × 10−17 mol | [82] |
| SiO2@Au seed | - | Colorimetric immunoassay | IgG | 0.021 ng/mL | [85] |
| SiO2@Au@Ag | Anti-PSA antibody | LFIA | PSA | 0.2 ng/mL | [89] |
| Metal-Embedded Silica NP | a. RLC b. Fluorescence Dye | Ligand | Specific Target | Imaging Method | Reference |
|---|---|---|---|---|---|
| SiO2@Ag | a. 4-ATP, 4-MT b. FITC, AF647 | Annexin V | phosphatidylserine | Fluorescence SERS | [91] |
| SiO2@Ag | a. RITC, FITC b. AF610 | Anti-HER2 Anti-EGFR | MDA-MB-231/HER2 breast cancer cell | Fluorescence SERS | [94] |
| Au-Ag hollow shell | a. Cy7LA a. CyNAMLA a. Cy7.5LA | C2 antibody | TSPAN8 | NIR-SERRS | [95] |
| SiO2@Au | - | - | Strawberry zebrafish, honeybee, mouse brain tissues | LDI-MS | [96] |
| Metal-Embedded Silica NP | Ligand | Cancer Therapy Method | Specific Target | Cell Viability | Reference |
|---|---|---|---|---|---|
| SiO2@Au@GO | - | Photothermal effect Docetaxel | DU145 cells | 37% | [38] |
| SGS | - | Photothermal effect | hMSC | - | [40] |
| AuHNs | Anti-EGFR | Photothermal effect Doxorubicin | A549 cells | 35% | [57] |
| SiO2@Ag | Cysteinyl-β-CD EDA-β-CD | Doxorubicin | MCF-7 cells | 60% | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-S.; Noh, M.S.; Kim, Y.-H.; Namgung, J.; Yoo, K.; Shin, M.-S.; Yang, C.-H.; Kim, Y.J.; Yu, S.-J.; Chang, H.; et al. Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications. Nanomaterials 2024, 14, 268. https://doi.org/10.3390/nano14030268
Cho H-S, Noh MS, Kim Y-H, Namgung J, Yoo K, Shin M-S, Yang C-H, Kim YJ, Yu S-J, Chang H, et al. Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications. Nanomaterials. 2024; 14(3):268. https://doi.org/10.3390/nano14030268
Chicago/Turabian StyleCho, Hye-Seong, Mi Suk Noh, Yoon-Hee Kim, Jayoung Namgung, Kwanghee Yoo, Min-Sup Shin, Cho-Hee Yang, Young Jun Kim, Seung-Ju Yu, Hyejin Chang, and et al. 2024. "Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications" Nanomaterials 14, no. 3: 268. https://doi.org/10.3390/nano14030268
APA StyleCho, H.-S., Noh, M. S., Kim, Y.-H., Namgung, J., Yoo, K., Shin, M.-S., Yang, C.-H., Kim, Y. J., Yu, S.-J., Chang, H., Rho, W. Y., & Jun, B.-H. (2024). Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications. Nanomaterials, 14(3), 268. https://doi.org/10.3390/nano14030268







