The Role of Oxygen Vacancies in Phase Transition and the Optical Absorption Properties within Nanocrystalline ZrO2
Abstract
1. Introduction
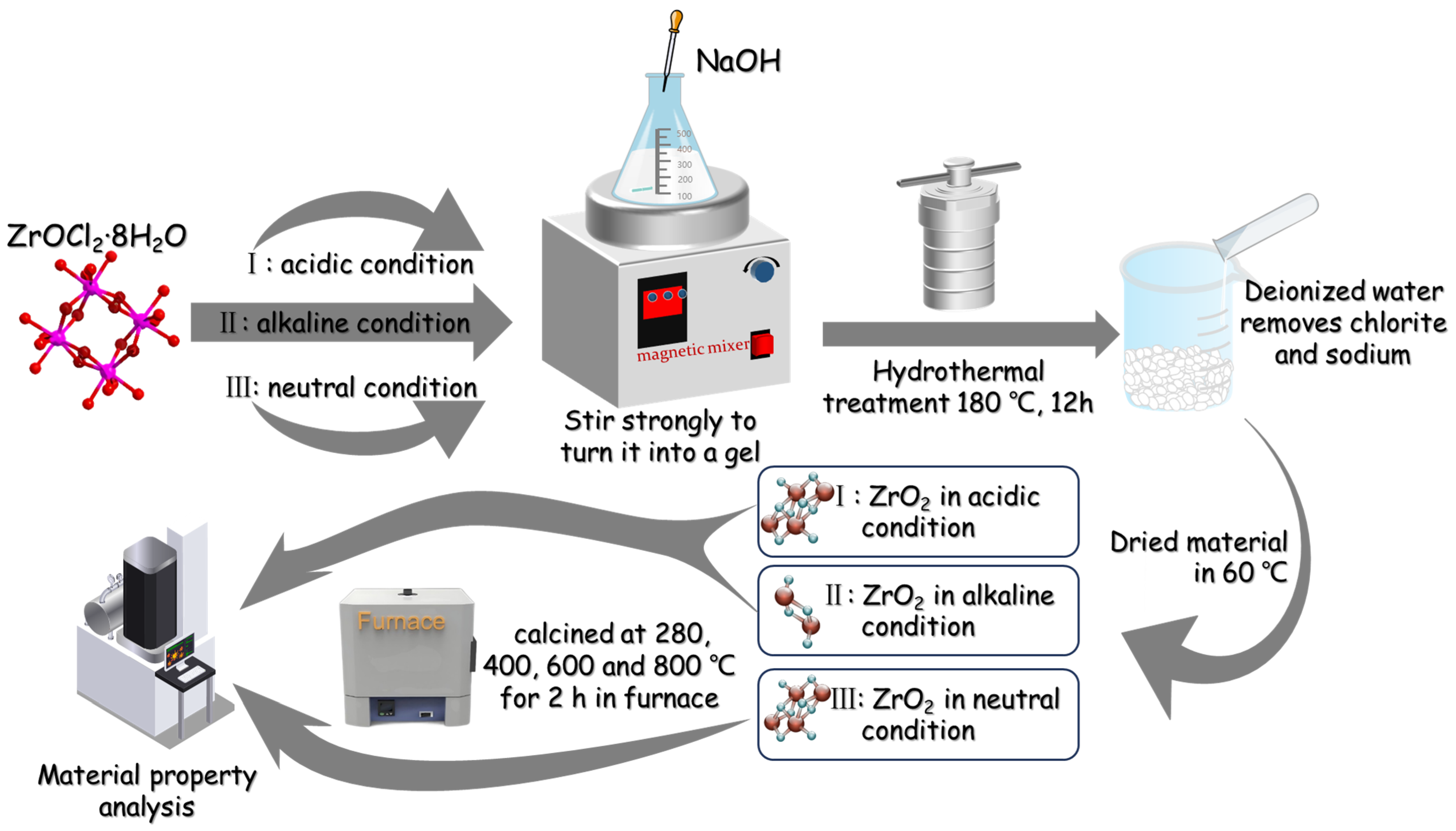
2. Materials and Methods
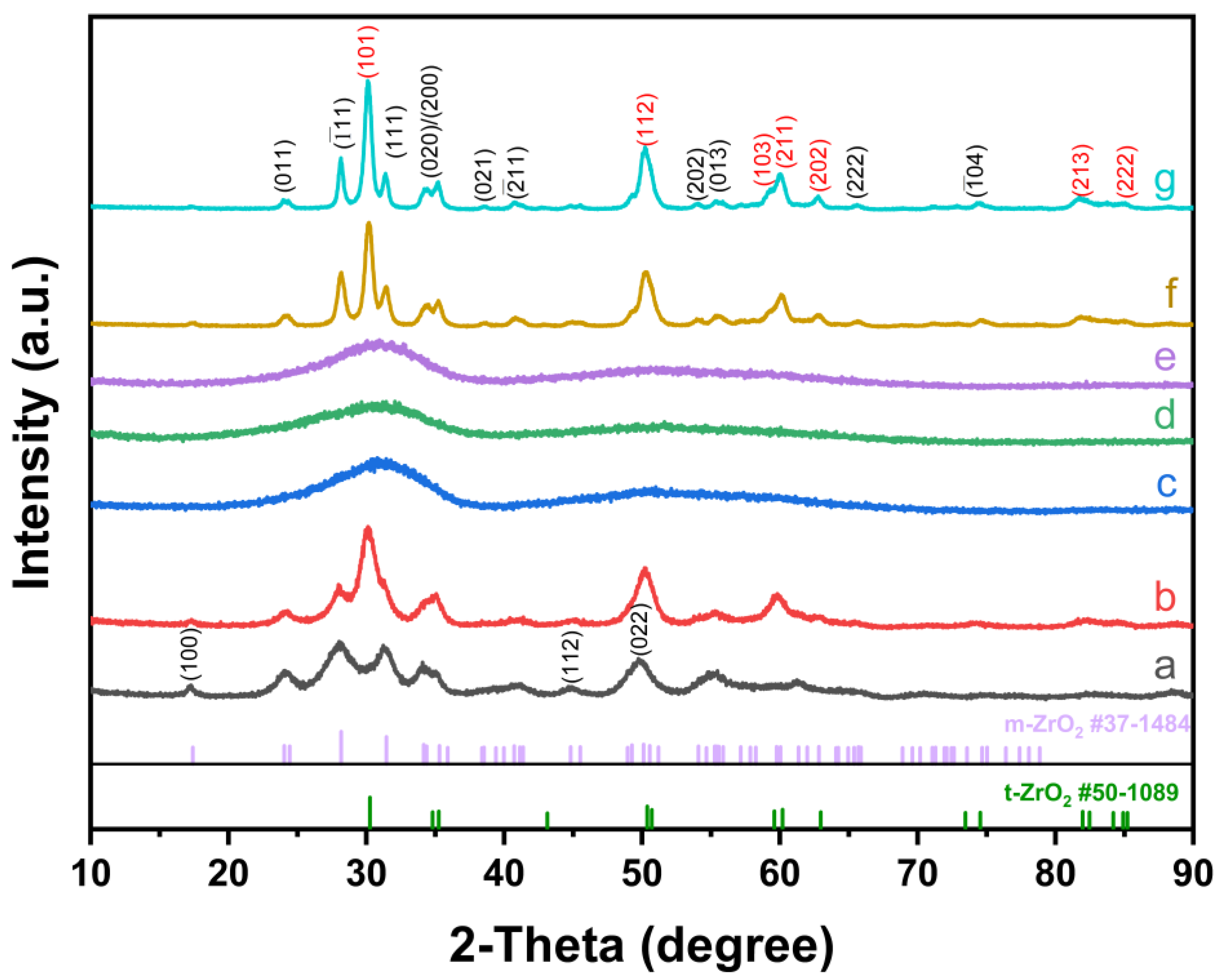
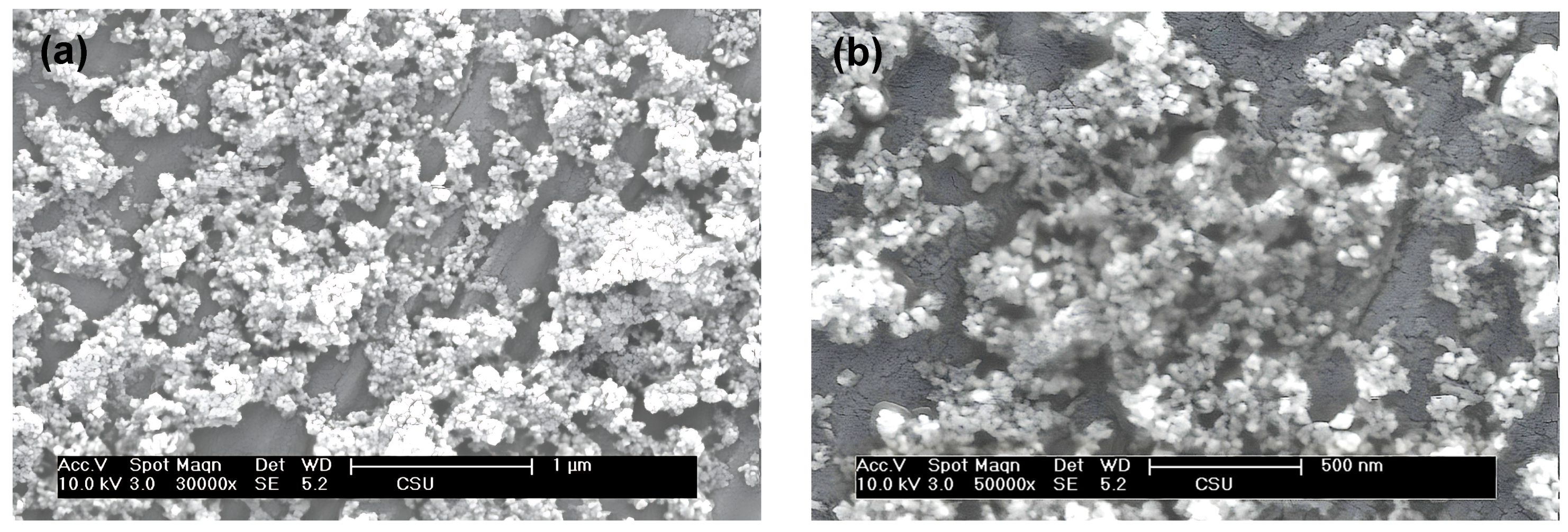
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leib, E.W.; Vainio, U.; Pasquarelli, R.M.; Kus, J.; Czaschke, C.; Walter, N.; Janssen, R.; Müller, M.; Schreyer, A.; Weller, H.; et al. Synthesis and thermal stability of zirconia and yttria-stabilized zirconia microspheres. J. Colloid Interface Sci. 2015, 448, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Pakseresht, A.; Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Nafchi, M.R.; Bazli, M.; Kirubaharan, K. Failure mechanisms and structure tailoring of YSZ and new candidates for thermal barrier coatings: A systematic review. Mater. Des. 2022, 222, 111044. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Borik, M.A.; Volkova, T.V.; Kuritsyna, I.E.; Lomonova, E.E.; Myzina, V.A.; Ryabochkina, P.A.; Tabachkova, N.Y. Features of the local structure and transport properties of ZrO2-Y2O3-Eu2O3 solid solutions. J. Alloys Compd. 2019, 770, 320–326. [Google Scholar] [CrossRef]
- Chen, S.; Miao, C.; Liang, L.; Ouyang, J. Oxygen Vacancies-Mediated CO2 Methanation Over Ni/CeO2–ZrO2 Solid Solutions Assembled on Clay Minerals. Energy Fuels 2022, 36, 8340–8350. [Google Scholar] [CrossRef]
- Liu, R.; Wang, C.A. Synthesis of aluminum-doped mesoporous zirconia with improved thermal stability. Microporous Mesoporous Mater. 2014, 186, 1–6. [Google Scholar] [CrossRef]
- Yang, H.; Ouyang, J.; Zhang, X.; Wang, N.; Du, C. Synthesis and optical properties of yttria-doped ZrO2 nanopowders. J. Alloys Compd. 2008, 458, 474–478. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Su, D.; Ji, H.; Zhang, X.; Zhang, W. Super-hydrophobic hexamethyl-disilazane modified ZrO2–SiO2 aerogels with excellent thermal stability. J. Mater. Chem. A 2016, 4, 5632–5638. [Google Scholar] [CrossRef]
- Smits, K.; Olsteins, D.; Zolotarjovs, A.; Laganovska, K.; Millers, D.; Ignatans, R.; Grabis, J. Doped zirconia phase and luminescence dependence on the nature of charge compensation. Sci. Rep. 2017, 7, 44453. [Google Scholar] [CrossRef]
- Chen, S.; Ma, F.; Xu, A.; Wang, L.; Chen, F.; Lu, W. Study on the structure, acidic properties of V–Zr nanocrystal catalysts in oxidative dehydrogenation of propane. Appl. Surf. Sci. 2014, 289, 316–325. [Google Scholar] [CrossRef]
- Chen, D.; Cao, L.; Hanley, T.L.; Caruso, R.A. Facile synthesis of monodisperse mesoporous zirconium titanium oxide microspheres with varying compositions and high surface areas for heavy metal ion sequestration. Adv. Funct. Mater. 2012, 22, 1966–1971. [Google Scholar] [CrossRef]
- Kim, M.R.; Park, S.Y.; Jang, D.J. Composition variation and thermal treatment of ZnxCd1−xS alloy nanoparticles to exhibit controlled and efficient luminescence. J. Phys. Chem. C 2010, 114, 6452–6457. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Yang, H.; Tang, A. Phase and optical properties of solvothermal prepared Sm2O3 doped ZrO2 nanoparticles: The effect of oxygen vacancy. J. Alloys Compd. 2016, 682, 654–662. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kleitz, F.; Fontaine, F.G. Lewis acidity quantification and catalytic activity of Ti, Zr and Al-supported mesoporous silica. Dalton Trans. 2017, 46, 3864–3876. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: The influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008, 9, 2422–2427. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A. A review on self-modification of zirconium dioxide nanocatalysts with enhanced visible-light-driven photodegradation of organic pollutants. J. Hazard. Mater. 2022, 423, 126996. [Google Scholar] [CrossRef]
- Knez, M.; Nielsch, K.; Niinistö, L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Qi, B.; Liang, S.; Li, Y.; Zhou, C.; Yu, H.; Li, J. ZrO2 Matrix Toughened Ceramic Material-Strength and Toughness. Adv. Eng. Mater. 2022, 24, 2101278. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-based nanomaterials: Recent developments in synthesis and applications. Nanoscale Adv. 2022, 4, 4210–4236. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.H.; Huang, K.W.; Lin, H.C.; Chen, M.J. Low-temperature crystallization and paraelectric–ferroelectric phase transformation in nanoscale ZrO2 thin films induced by atomic layer plasma treatment. J. Mater. Chem. C 2020, 8, 3669–3677. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Krautgasser, C.; Danzer, R.; Supancic, P.; Bermejo, R. Influence of temperature and humidity on the strength of low temperature co-fired ceramics. J. Eur. Ceram. Soc. 2015, 35, 1823–1830. [Google Scholar] [CrossRef]
- Prasad, K.; Pinjari, D.V.; Pandit, A.B.; Mhaske, S.T. Synthesis of zirconium dioxide by ultrasound assisted precipitation: Effect of calcination temperature. Ultrason. Sonochemistry 2011, 18, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- La Ore, M.S.; Wijaya, K.; Trisunaryanti, W.; Saputri, W.D.; Heraldy, E.; Yuwana, N.W.; Hariani, P.L.; Budiman, A.; Sudiono, S. The synthesis of SO4/ZrO2 and Zr/CaO catalysts via hydrothermal treatment and their application for conversion of low-grade coconut oil into biodiesel. J. Environ. Chem. Eng. 2020, 8, 104205. [Google Scholar] [CrossRef]
- Darbar, D.; Muralidharan, N.; Hermann, R.P.; Nanda, J.; Bhattacharya, I. Evaluation of electrochemical performance and redox activity of Fe in Ti doped layered P2-Na0.67Mn0.5Fe0.5O2 cathode for sodium ion batteries. Electrochim. Acta 2021, 380, 138156. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, Y.; Wang, W.; Zhang, S.; Zhang, Y. A benzene-thermal synthetic route to nanocrystalline GaN. Science 1996, 272, 1926–1927. [Google Scholar] [CrossRef]
- Imparato, C.; Fantauzzi, M.; Passiu, C.; Rea, I.; Ricca, C.; Aschauer, U.; Sannino, F.; D’Errico, G.; De Stefano, L.; Rossi, A.; et al. Unraveling the charge state of oxygen vacancies in ZrO2–x on the basis of synergistic computational and experimental evidence. J. Phys. Chem. C 2019, 123, 11581–11590. [Google Scholar] [CrossRef]
- Qi, F.; Yang, Z.; Zhang, J.; Wang, Y.; Qiu, Q.; Li, H. Interfacial reaction-induced defect engineering: Enhanced visible and near-infrared absorption of wide band gap metal oxides with abundant oxygen vacancies. ACS Appl. Mater. Interfaces 2020, 12, 55417–55425. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.; Grigorjeva, L.; Millers, D.; Sarakovskis, A.; Grabis, J.; Lojkowski, W. Intrinsic defect related luminescence in ZrO2. J. Lumin. 2011, 131, 2058–2062. [Google Scholar] [CrossRef]
- Wang, D.D.; Qi, N.; Jiang, M.; Chen, Z.Q. Defects versus grain size effects on the ferromagnetism of ZrO2 nanocrystals clarified by positron annihilation. Appl. Phys. Lett. 2013, 102, 042407. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Liu, J. Influence of oxygen vacancy compensation on the structure, electronic and mechanical properties of yttrium stabilized tetragonal zirconia. Mater. Sci. Semicond. Process. 2021, 135, 106082. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Cong, Y.; Li, B.; Yue, S.; Fan, D.; Wang, X.J. Effect of oxygen vacancy on phase transition and photoluminescence properties of nanocrystalline zirconia synthesized by the one-pot reaction. J. Phys. Chem. C 2009, 113, 13974–13978. [Google Scholar] [CrossRef]
- Ji, S.; Kim, S.K.; Choi, S.H.; Byun, J.S.; Kim, D.K.; Lee, H.J.; Choi, H.M.; Song, W.; Myung, S.; Suk, J.; et al. Yttria-Stabilized Zirconia Nanoparticles—Carbon Nanotube Composite as a Polysulfide-Capturing Lithium–Sulfur Battery Separator. ACS Appl. Energy Mater. 2022, 5, 12196–12205. [Google Scholar] [CrossRef]
- Thakur, S.; Sareen, S.; Verma, M.; Kaur, K.; Mutreja, V. Synthesis of elusive monoclinic ZrO2 nanostructures via hydrothermal treatment. J. Inorg. Organomet. Polym. Mater. 2024, 34, 61–78. [Google Scholar] [CrossRef]
- Qing, S.; Chen, H.; Han, L.J.; Ye, Z.; Shi, L.; Shu, Z.; Chen, L.; Xu, L.; Xu, Q. Photocatalytic activity investigation of α-zirconium phosphate nanoparticles compositing with C3N4 under ultraviolet light. ACS Omega 2020, 5, 27873–27879. [Google Scholar] [CrossRef]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Wu, X.; Tan, M.; Xu, B.; Zhao, S.; Ma, Q.; He, Y.; Zeng, C.; Yang, G.; Tsubaki, N.; Tan, Y. Tuning the crystallite size of monoclinic ZrO2 to reveal critical roles of surface defects on m–ZrO2 catalyst for direct synthesis of isobutene from syngas. Chin. J. Chem. Eng. 2021, 35, 211–219. [Google Scholar] [CrossRef]
- Teeparthi, S.R.; Awin, E.W.; Kumar, R. Dominating role of crystal structure over defect chemistry in black and white zirconia on visible light photocatalytic activity. Sci. Rep. 2018, 8, 5541. [Google Scholar] [CrossRef] [PubMed]
- Garcilaso, V.; Barrientos, J.; Bobadilla, L.F.; Laguna, O.H.; Boutonnet, M.; Centeno, M.A.; Odriozola, J.A. Promoting effect of CeO2, ZrO2 and Ce/Zr mixed oxides on Co/γ-Al2O3 catalyst for Fischer-Tropsch synthesis. Renew. Energy 2019, 132, 1141–1150. [Google Scholar] [CrossRef]
- Dow, W.P.; Wang, Y.P.; Huang, T.J. Yttria-stabilized zirconia supported copper oxide catalyst: I. Effect of oxygen vacancy of support on copper oxide reduction. J. Catal. 1996, 160, 155–170. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Burgess, R.; Di Valentin, C.; Pacchioni, G. Paramagnetic defects in polycrystalline zirconia: An EPR and DFT study. Chem. Mater. 2013, 25, 2243–2253. [Google Scholar] [CrossRef]
- Pezzotti, G.; Munisso, M.C.; Porporati, A.A.; Lessnau, K. On the role of oxygen vacancies and lattice strain in the tetragonal to monoclinic transformation in alumina/zirconia composites and improved environmental stability. Biomaterials 2010, 31, 6901–6908. [Google Scholar] [CrossRef]
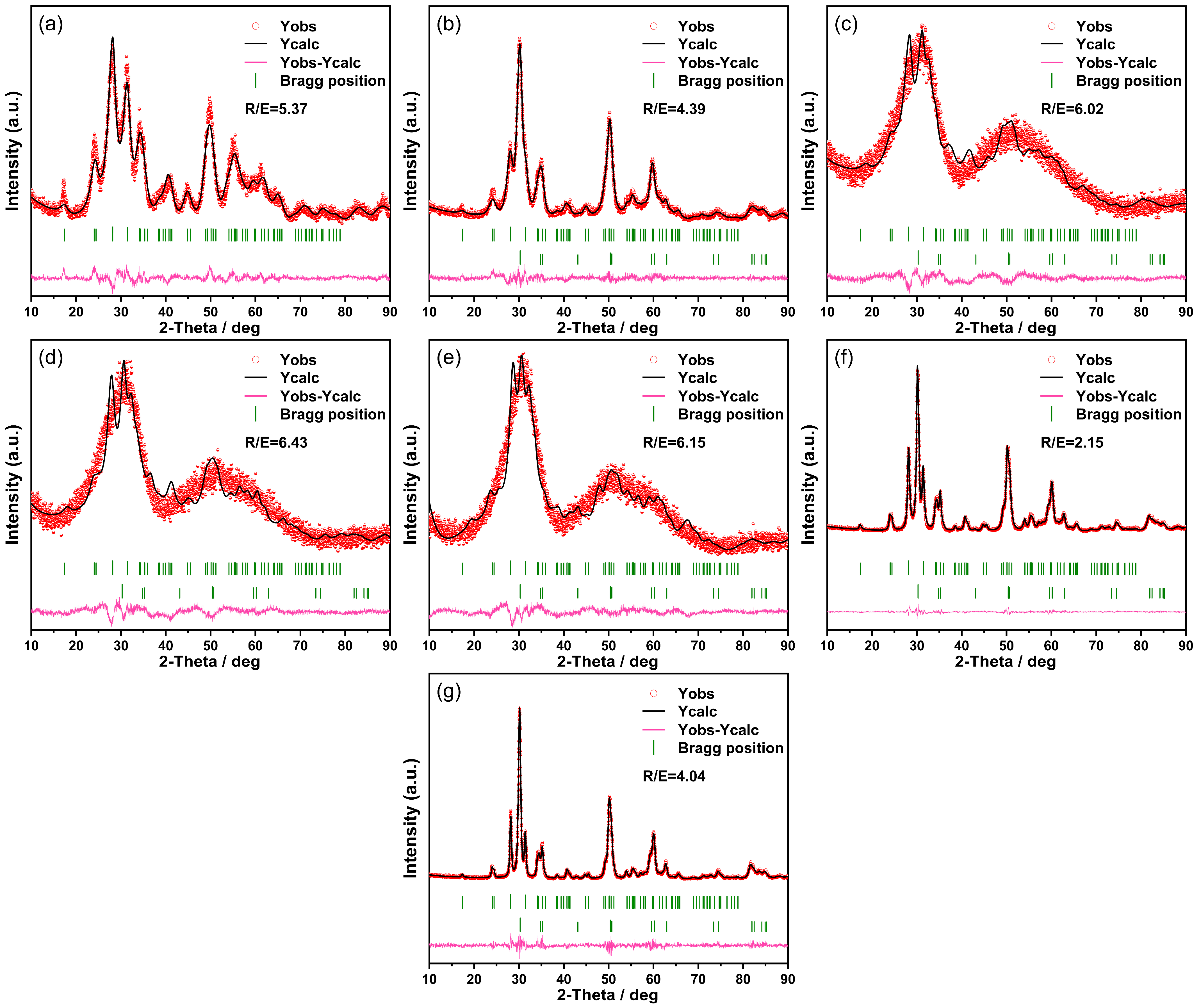
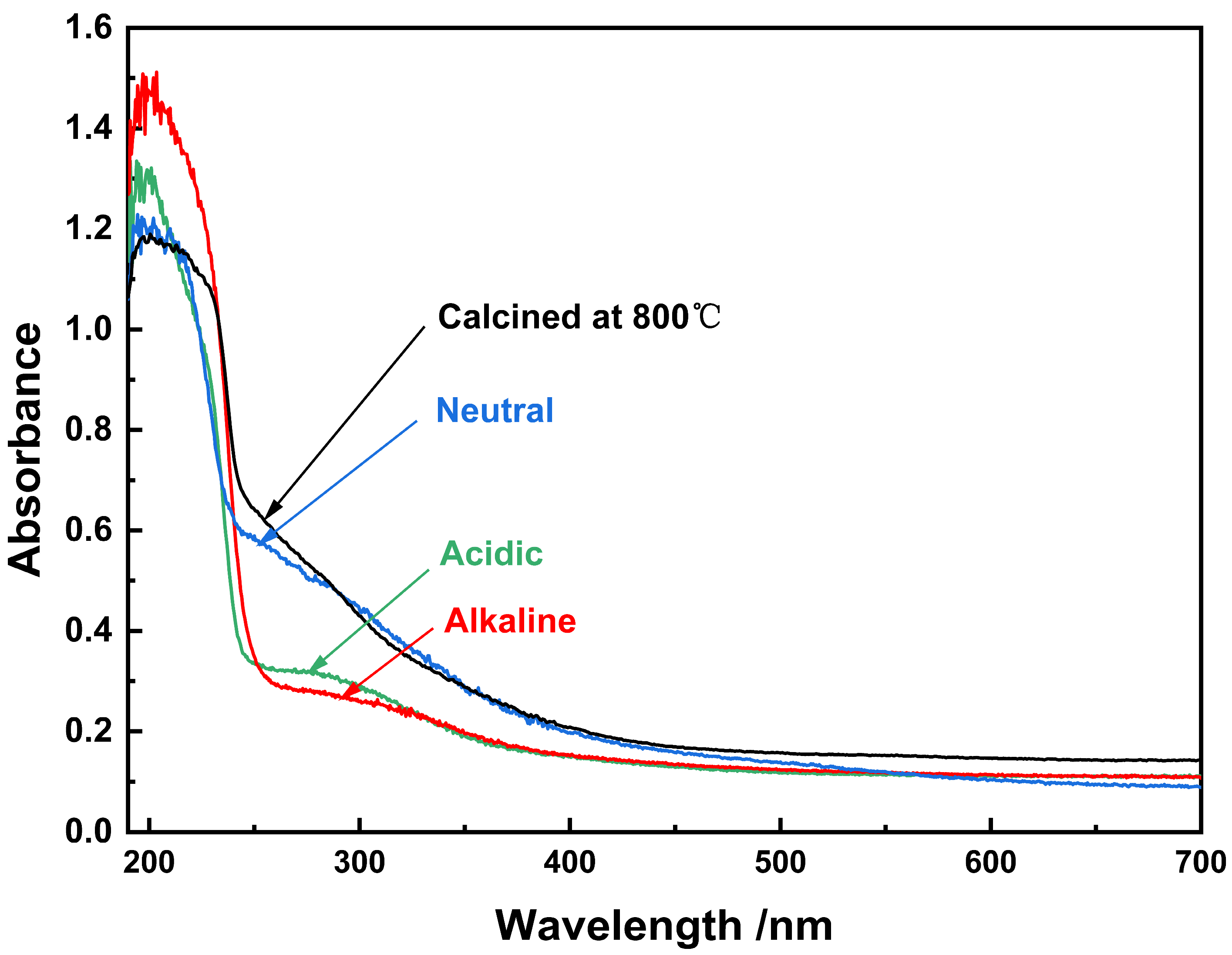



| Sample | Phase | Space Group | Phase Fraction/% | Lattice Parameters/nm | Residual | |||
|---|---|---|---|---|---|---|---|---|
| a | b | c | Rwp/% | R/E | ||||
| ZrO2 in acidic condition | Monoclinic ZrO2 | mP21/c (14) | 100 | 5.2281 | 5.2566 | 5.3646 | 10.56% | 5.37 |
| ZrO2 in alkaline condition | Monoclinic ZrO2 | mP21/c (14) | 52.8 | 5.1752 | 5.2158 | 5.3365 | 8.83% | 4.39 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 47.2 | 3.6297 | 3.6297 | 5.1171 | |||
| ZrO2 in neutral condition | Monoclinic ZrO2 | mP21/c (14) | 83.3 | 4.9119 | 5.2679 | 5.4161 | 11.47 | 6.02 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 16.7 | 3.4629 | 3.4629 | 5.3115 | |||
| ZrO2 (neutral) calcined at 280 °C | Monoclinic ZrO2 | mP21/c (14) | 83.3 | 4.9593 | 5.1612 | 5.4228 | 13.13 | 6.43 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 16.7 | 3.4618 | 3.4618 | 5.2946 | |||
| ZrO2 (neutral) calcined at 400 °C | Monoclinic ZrO2 | mP21/c (14) | 83.6 | 4.7394 | 5.3893 | 5.477 | 12.72 | 6.15 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 16.4 | 3.5745 | 3.5745 | 5.2084 | |||
| ZrO2 (neutral) calcined at 600 °C | Monoclinic ZrO2 | mP21/c (14) | 48.6 | 5.1471 | 5.2005 | 5.314 | 7.99 | 2.15 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 51.4 | 3.596 | 3.596 | 5.1825 | |||
| ZrO2 (neutral) calcined at 800 °C | Monoclinic ZrO2 | mP21/c (14) | 38.5 | 5.1474 | 5.2013 | 5.315 | 8.51 | 4.04 |
| Tetragonal ZrO2 | tP42/nmc2 (137) | 61.5 | 3.6016 | 3.6016 | 5.1835 | |||
| Labels in Figure 6 | A | B | C | D |
|---|---|---|---|---|
| Indirect band gap | 4.81 eV | 4.70 eV | 4.59 eV | 4.34 eV |
| Peak positions (nm) and energy state | 199.1948 (6.23 eV) | 200.2167 (6.20 eV) | 199.1706 (6.22 eV) | 199.8592 (6.21 eV) |
| 227.0601 (5.46 eV) | 228.4576 (5.43 eV) | 222.0763 (5.58 eV) | 228.6299 (5.43 eV) | |
| - | 264.767 (4.68 eV) | 252.5948 (4.91 eV) | 251.3968 (4.93 eV) | |
| 270.3473 (4.59 eV) | 269.7686 (4.60 eV) | 270.0315 (4.59 eV) | 269.5722 (4.60 eV) |
| Deconvolution | Zr3d5/2 | Zr3d3/2 | OLattice | ODefect | OOH | C-C | Zr-O-C | C=O |
|---|---|---|---|---|---|---|---|---|
| Binding energy/eV | 182.10 | 184.44 | 530.76 | 533.03 | 535.02 | 284.99 | 286.47 | 288.80 |
| Area/% | 52.36 | 47.64 | 31.58 | 58.48 | 9.94 | 44.28 | 12.75 | 5.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Peng, Y.; Zhou, W.; Liang, X.; Wang, G.; Zhang, Q.; Yuan, B. The Role of Oxygen Vacancies in Phase Transition and the Optical Absorption Properties within Nanocrystalline ZrO2. Nanomaterials 2024, 14, 967. https://doi.org/10.3390/nano14110967
Ouyang J, Peng Y, Zhou W, Liang X, Wang G, Zhang Q, Yuan B. The Role of Oxygen Vacancies in Phase Transition and the Optical Absorption Properties within Nanocrystalline ZrO2. Nanomaterials. 2024; 14(11):967. https://doi.org/10.3390/nano14110967
Chicago/Turabian StyleOuyang, Jing, Yonghui Peng, Wentao Zhou, Xianfeng Liang, Gang Wang, Qi Zhang, and Bo Yuan. 2024. "The Role of Oxygen Vacancies in Phase Transition and the Optical Absorption Properties within Nanocrystalline ZrO2" Nanomaterials 14, no. 11: 967. https://doi.org/10.3390/nano14110967
APA StyleOuyang, J., Peng, Y., Zhou, W., Liang, X., Wang, G., Zhang, Q., & Yuan, B. (2024). The Role of Oxygen Vacancies in Phase Transition and the Optical Absorption Properties within Nanocrystalline ZrO2. Nanomaterials, 14(11), 967. https://doi.org/10.3390/nano14110967






