pH-Responsive Graphene Oxide-Based 2D/3D Composite for Enhancing Anti-Corrosion Properties of Epoxy Coating
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of ZIF–90
2.3. Synthesis of GO
2.4. Synthesis of ZIF–90/GO
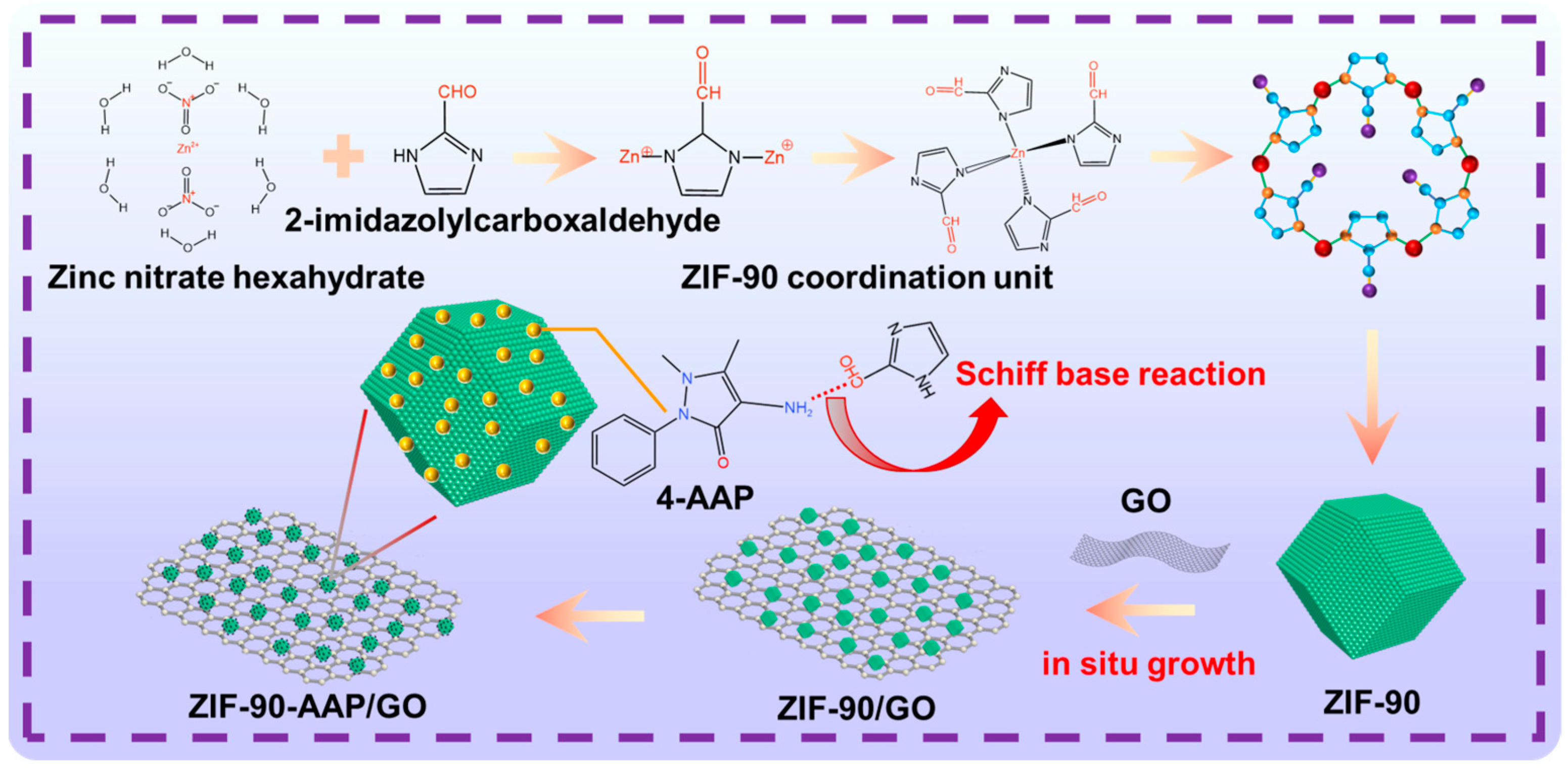
2.5. Preparation of ZIF–90–AAP/GO
2.6. Preparation of ZIF–90–AAP/GO–EP
2.7. Material Characterizations
3. Results and Discussion
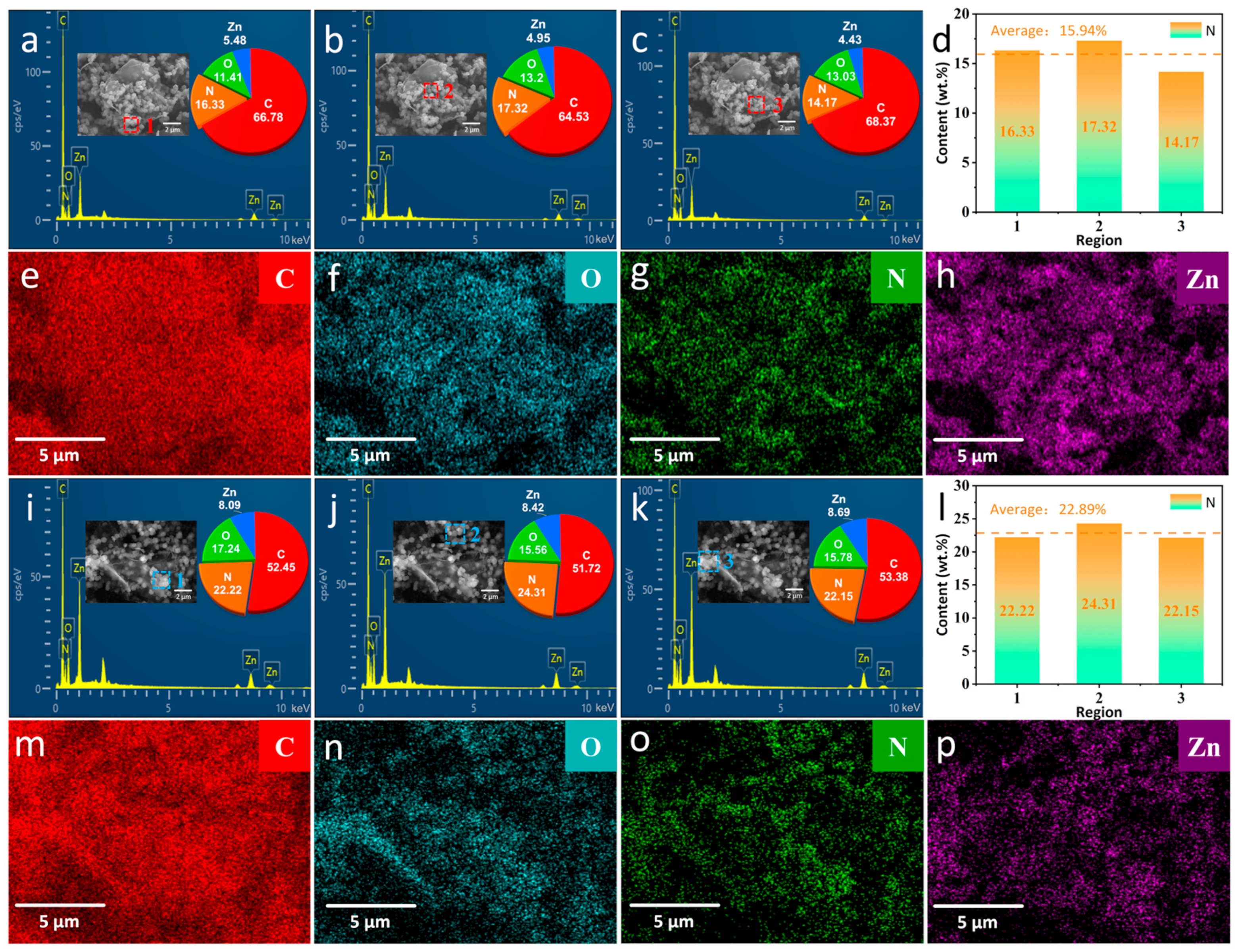
3.1. Characterization of ZIF–90–AAP/GO
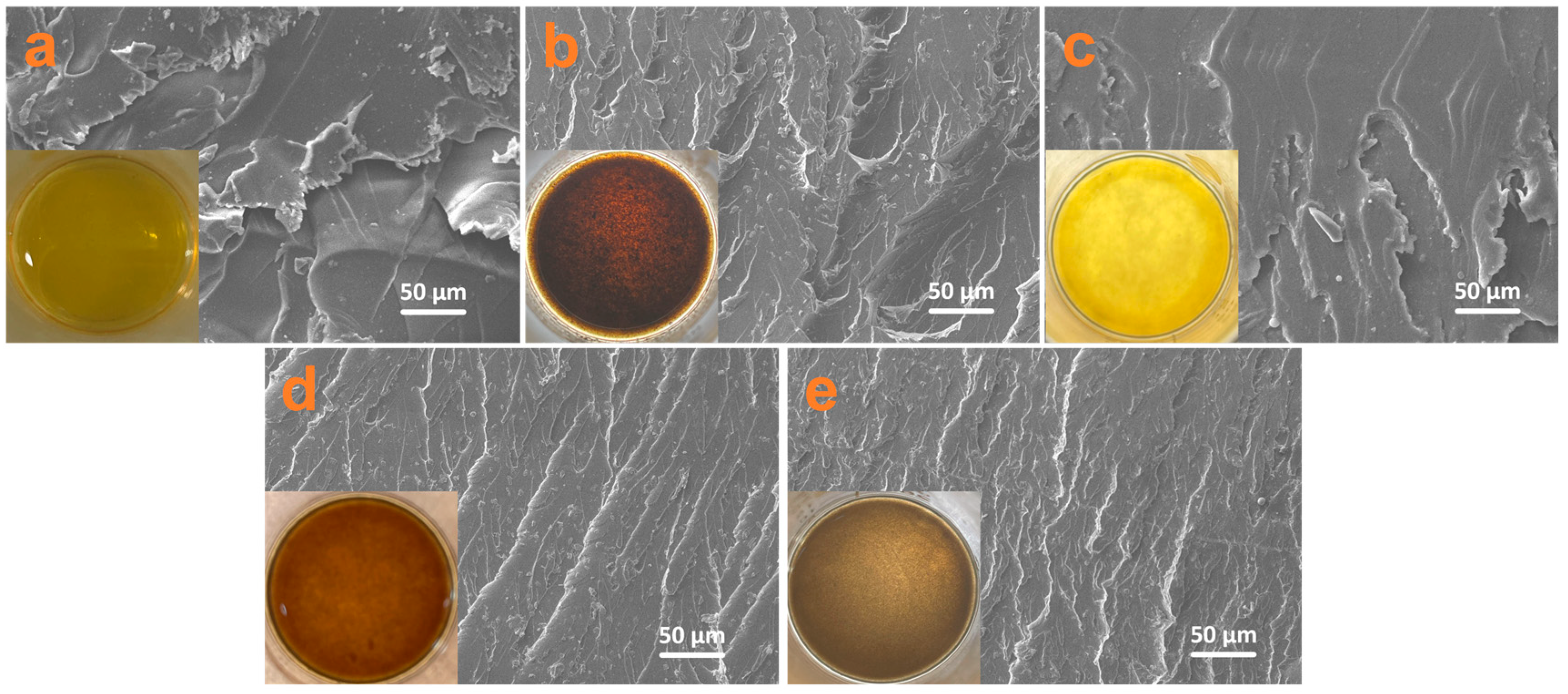
3.2. Dispersion and Compatibility of ZIF–90–AAP/GO in Epoxy Resin
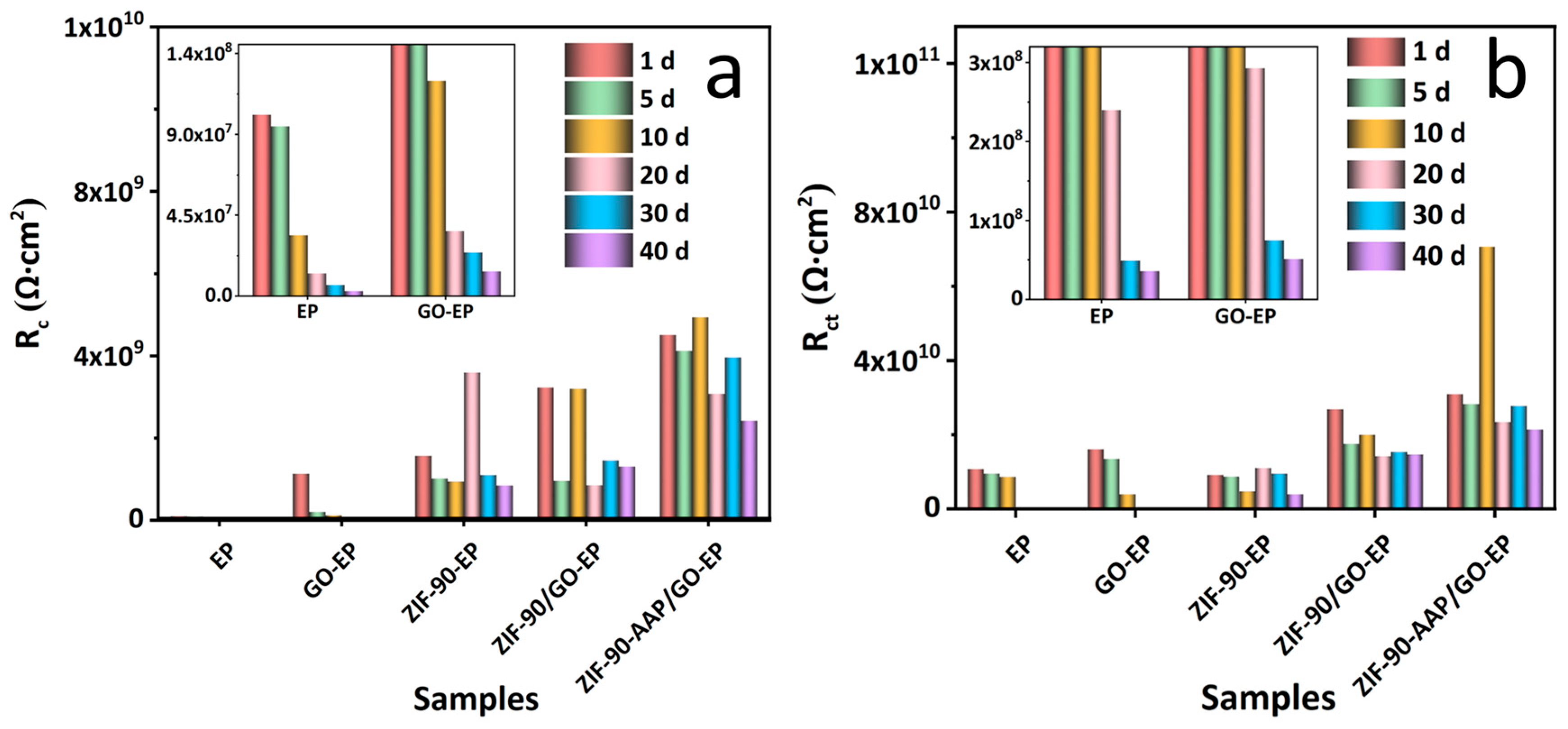
3.3. Anti-Corrosion Properties of Different Coatings
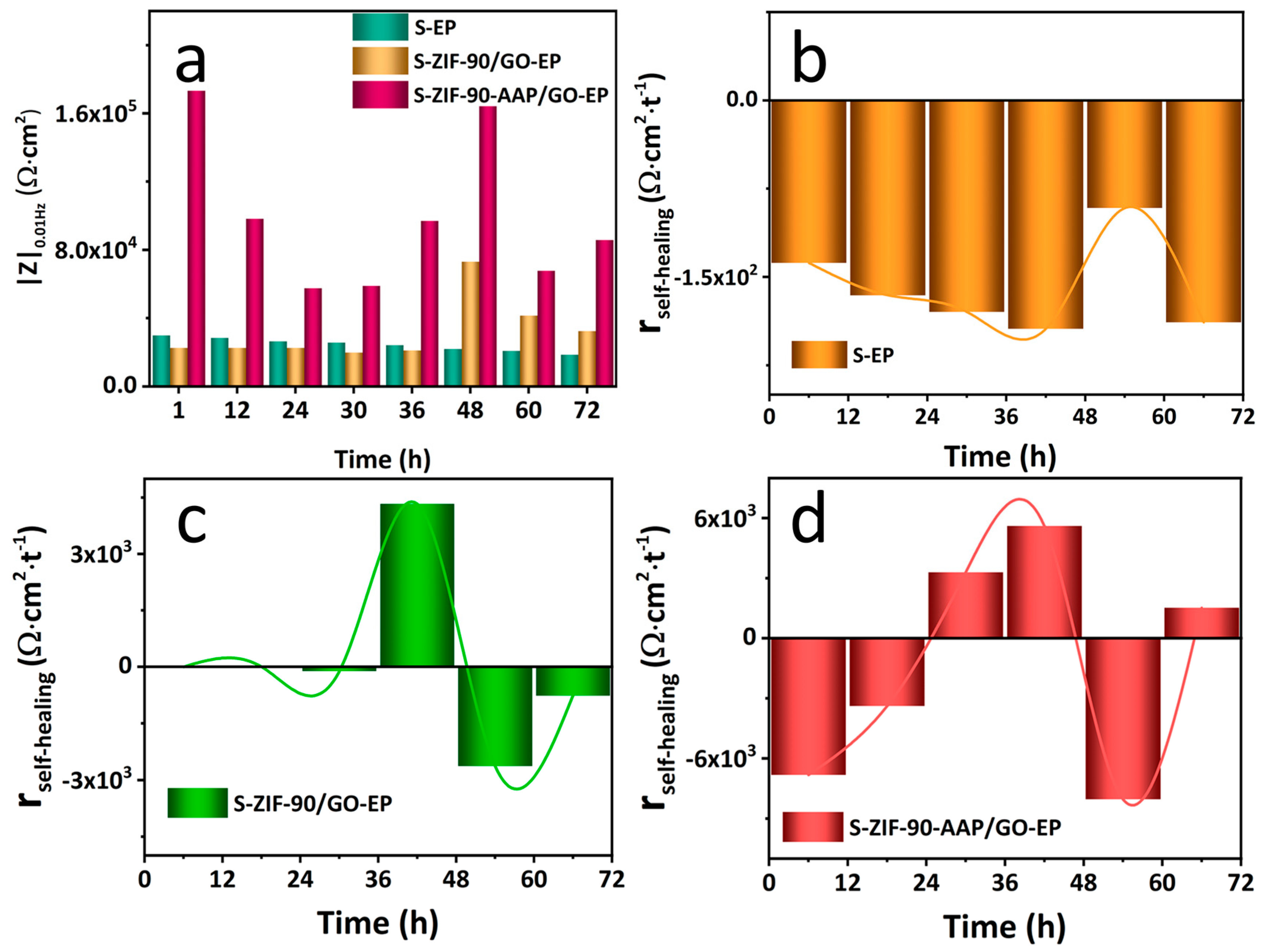
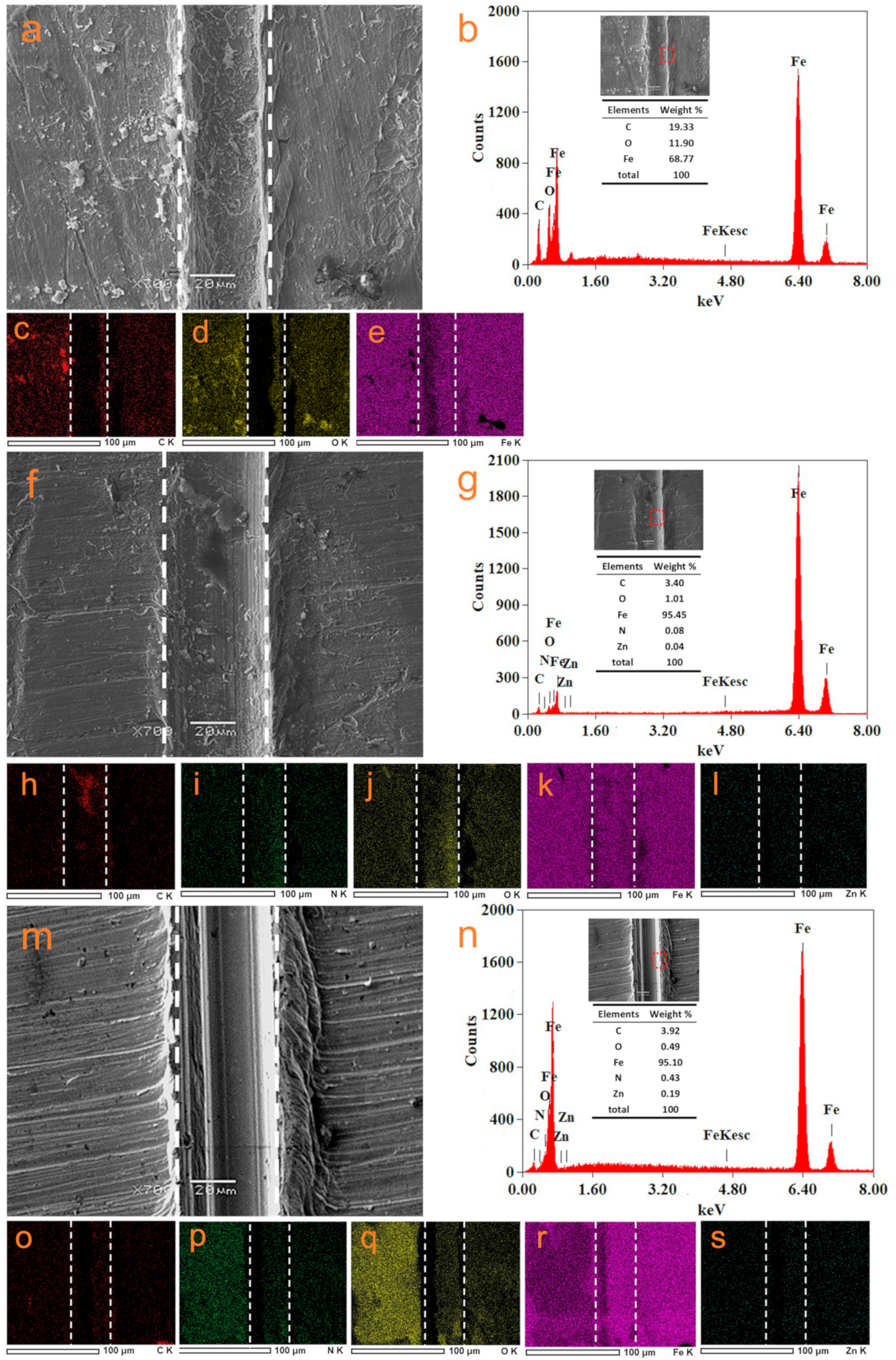
3.4. Self-Healing Properties of Different Coatings
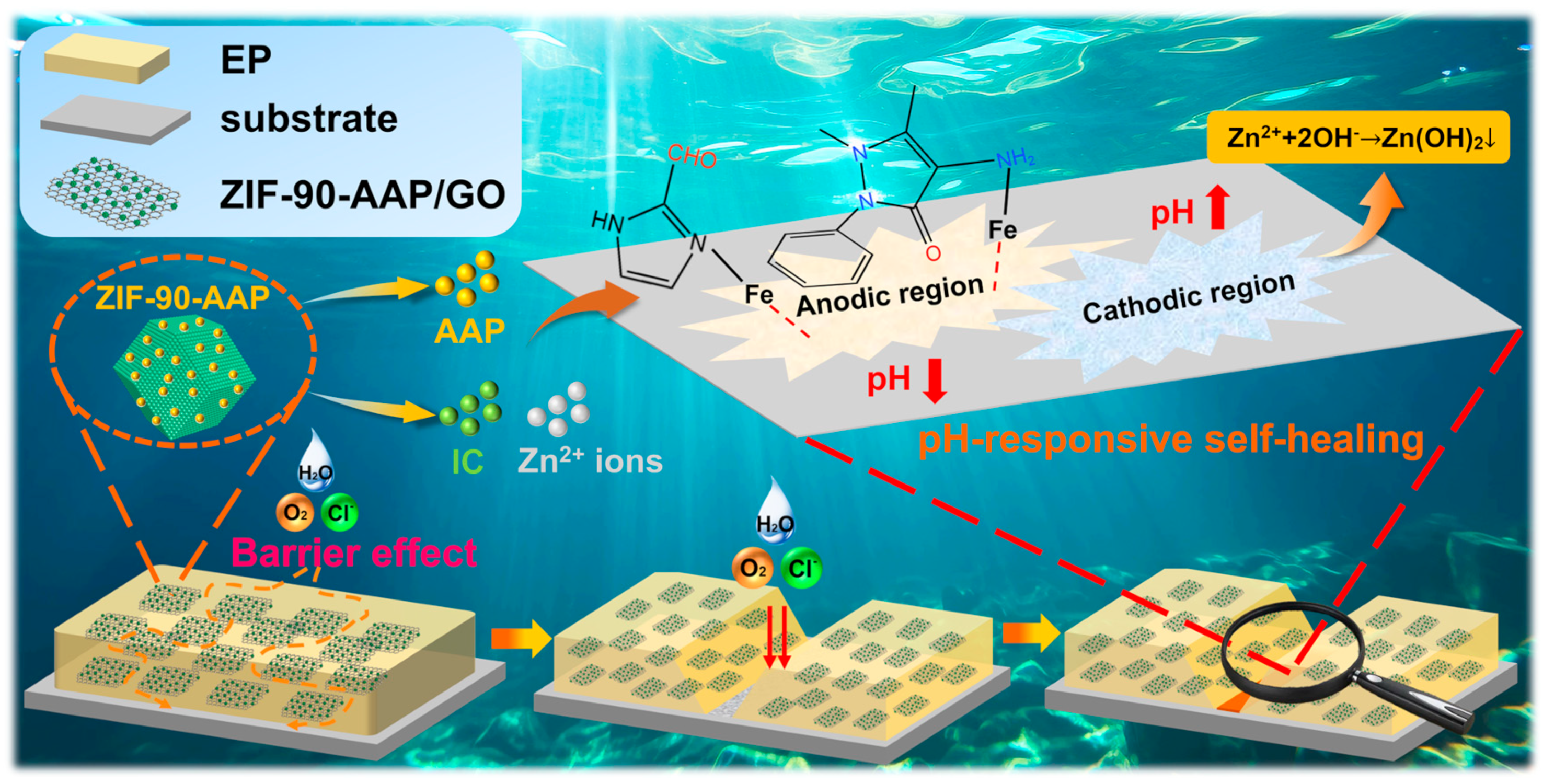
3.5. Anti-Corrosion Mechanism of ZIF–90–AAP/GO–EP
- (i)
- Passive corrosion protection mechanism
- (ii)
- Active corrosion protection mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krishna, P. A Review of Developments in Steel: Implications for Long-Span Structures. Trans. Indian Inst. Met. 2021, 74, 1055–1064. [Google Scholar] [CrossRef]
- Uemori, R.; Fujioka, M.; Inoue, T.; Minagawa, M.; Shirahata, H.; Nose, T. Steels for Marine Transportation and Construction. Nippon. Steel Tech. Rep. 2012, 101, 37. [Google Scholar]
- Nishioka, K.; Ichikawa, K. Progress in thermomechanical control of steel plates and their commercialization. Sci. Technol. Adv. Mater. 2016, 13, 023001. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, H.; Chapman, J.C. Bi–steel steel–concrete–steel sandwich construction. In Composite Construction in Steel and Concrete IV; ASCE: Reston, VA, USA, 2002; pp. 656–667. [Google Scholar]
- Moynihan, M.C.; Allwood, J.M. The flow of steel into the construction sector. Resour. Conserv. Recycl. 2012, 68, 88–95. [Google Scholar] [CrossRef]
- Kitada, T. Considerations on recent trends in, and future prospects of, steel bridge construction in Japan. J. Constr. Steel Res. 2006, 62, 1192–1198. [Google Scholar] [CrossRef]
- Blekkenhorst, F.; Ferrari, G.; Wekken, C.V.D.; IJsseling, F. Development of high strength low alloy steels for marine applications: Part 1: Results of long term exposure tests on commercially available and experimental steels. Br. Corros. J. 1986, 21, 163–176. [Google Scholar] [CrossRef]
- Bhat, R.S.; Munjunatha, K.B.; Bhat, S.I.; Venkatakrishna, K.; Hegde, A.C. Electrochemical Studies of Zn–Ni–Fe Alloy Coatings for Better Corrosion Resistance Applications. J. Mater. Eng. Perform. 2022, 31, 6819–6826. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Moga, S.; Negrea, D. Preparation and characterization of anticorrosive layers deposited by micro-arc oxidation on low carbon steel. Surf. Coat. Technol. 2014, 253, 194–198. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Chen, C.-W.; Tsai, F.-T.; Lin, C.-K.; Chen, C.-C. Hard Anodization Film on Carbon Steel Surface by Thermal Spray and Anodization Methods. Materials 2021, 14, 3580. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Aljibori, H.; Alamiery, A.; Kadhum, A.A. Advances in corrosion protection coatings: A comprehensive review. Int. J. Corros. Scale Inhib. 2023, 12, 1476–1520. [Google Scholar]
- Li, H.; Zhang, Q.-H.; Meng, X.-Z.; Yan, H.-J.; Hu, H.-S.; Wu, L.-K.; Cao, F.-H. Active/passive protection and anti-UV waterborne epoxy coatings based on low defect functionalized PDA–GO–CeO2 material for excellent corrosion control. Chem. Eng. J. 2024, 479, 147859. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Dong, J.; Zhou, K.; Lu, G.; Pu, J. Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios. Prog. Org. Coat. 2019, 127, 70–79. [Google Scholar] [CrossRef]
- He, P.; Wang, J.; Lu, F.; Ma, Q.; Wang, Z. Synergistic effect of polyaniline grafted basalt plates for enhanced corrosion protective performance of epoxy coatings. Prog. Org. Coat. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, G.; Dong, Y.; Zhang, Y. Anticorrosive epoxy coatings based on polydopamine modified molybdenum disulfide. Prog. Org. Coat. 2019, 133, 154–160. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Liu, P.; Yu, H. Boron nitride–epoxy inverse “nacre-like” nanocomposite coatings with superior anticorrosion performance. Corros. Sci. 2021, 183, 109333. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Y.; Zhu, Y. Towards industrialization of graphene oxide. Sci. China Mater. 2020, 63, 1861–1869. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide–years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Del Giudice, F.; Shen, A.Q. Shear rheology of graphene oxide dispersions. Curr. Opin. Chem. Eng. 2017, 16, 23–30. [Google Scholar] [CrossRef]
- Nemati, F.; Rezaie, M.; Tabesh, H.; Eid, K.; Xu, G.; Ganjali, M.R.; Hosseini, M.; Karaman, C.; Erk, N.; Show, P.L.; et al. Cerium functionalized graphene nano-structures and their applications: A review. Environ. Res. 2022, 208, 112685. [Google Scholar] [CrossRef]
- Kumar, S.S.A.; Bashir, S.; Ramesh, K.; Ramesh, S. New perspectives on Graphene/Graphene oxide based polymer nanocomposites for corrosion applications: The relevance of the Graphene/Polymer barrier coatings. Prog. Org. Coat. 2021, 154, 106215. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, C.; Wang, A.; Zhou, X.; Xing, X.; Liu, J.; Li, Z.; Chen, Q.; Lu, Q. Research progress on self-healing polymer/graphene anticorrosion coatings. Prog. Org. Coat. 2021, 155, 106231. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. 2–Mercaptobenzimidazole–inbuilt metal-organic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating. Corros. Sci. 2021, 191, 109715. [Google Scholar] [CrossRef]
- Liu, T.; Wei, J.; Ma, L.; Liu, S.; Zhang, D.; Zhao, H. Effect of polyaniline-based plate on the anticorrosion performance of epoxy coating. Prog. Org. Coat. 2021, 151, 106109. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Cai, G.; Liao, B.; Guo, X. Functionalization of h–BN by the exfoliation and modification of carbon dots for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106757. [Google Scholar] [CrossRef]
- Habibiyan, A.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G.; Kasaeian, M. Rational assembly of mussel-inspired polydopamine (PDA)–Zn (II) complex nanospheres on graphene oxide framework tailored for robust self-healing anti-corrosion coatings application. Chem. Eng. J. 2020, 391, 123630. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, G.; He, Y.; Zhong, F.; Li, H.; Wu, Y.; Chen, J. Bio-inspired superior barrier self-healing coating: Self-assemble of graphene oxide and polydopamine-coated halloysite nanotubes for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 139, 105402. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Sun, Y.; Chen, K. Dual Self-Healing Anticorrosion Coatings Based on Multibranched Waterborne Polyurethane and TiO2 Nanocapsule-Loaded Graphene Oxide. Adv. Mater. Interfaces 2022, 9, 2102001. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Application of nanoporous cobalt-based ZIF–67 metal-organic framework (MOF) for construction of an epoxy-composite coating with superior anti-corrosion properties. Corros. Sci. 2021, 178, 109099. [Google Scholar] [CrossRef]
- Alipanah, N.; Dehghani, A.; Abdolmaleki, M.; Bahlakeh, G.; Ramezanzadeh, B. Designing environmentally-friendly pH-responsive self-redox polyaniline grafted graphene oxide nano-platform decorated by zeolite imidazole ZIF–9 MOF for achieving smart functional epoxy-based anti-corrosion coating. J. Environ. Chem. Eng. 2023, 11, 109048. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.; Xu, J.; Khalid, N.; Elhissi, A.; Ahmed, W. A facile one-step approach to synthesizing ZnO/graphene composites for enhanced degradation of methylene blue under visible light. Appl. Surf. Sci. 2013, 274, 273–281. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Jiang, H.; Sheng, A.; Li, H.; Jia, H.; Yun, Z.; Wei, Z.; Wang, H. (3–Aminopropyl) Triethoxysilane-Modified ZIF–90 Nanoparticle/Polydimethylsiloxane Mixed Matrix Membranes for Ethanol Recovery via Pervaporation. ACS Appl. Nano Mater. 2021, 5, 183–194. [Google Scholar] [CrossRef]
- Hwang, M.-J.; Kim, M.-G.; Kim, S.; Kim, Y.C.; Seo, H.W.; Cho, J.K.; Park, I.-K.; Suhr, J.; Moon, H.; Koo, J.C. Cathodic electrophoretic deposition (EPD) of phenylenediamine-modified graphene oxide (GO) for anti-corrosion protection of metal surfaces. Carbon 2019, 142, 68–77. [Google Scholar] [CrossRef]
- Abubakar, A.; Abdulmalek, E.; Norhamidah Wan Ibrahim, W.; Cordova, K.E.; Abdul Rahman, M.B.A. ZIF–90 nanoparticles modified with a homing peptide for targeted delivery of cisplatin. Front. Chem. 2022, 10, 1076350. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ji, L.; Hu, Y.; Gu, J.; Wang, L.; Lu, W.; Meng, J.; Du, Y.; Huang, L.; Nie, D.; et al. Dual-responsive zeolitic imidazolate framework–90 for the combined detection and intracellular imaging of ATP and ROS. Sens. Actuators B Chem. 2022, 363, 131848. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Zheng, Q.; Luo, P.; Yan, W.; Huang, S.; Cheng, D.; Xu, H.H.; Zhang, Z. A β–cyclodextrin–functionalized metal–organic framework enhances the insecticidal activity of indoxacarb by affecting amino acid metabolism in red imported fire ants. Chem. Eng. J. 2023, 458, 141417. [Google Scholar] [CrossRef]
- Zhan, G.; Zeng, H.C. A Synthetic Protocol for Preparation of Binary Multi-shelled Hollow Spheres and Their Enhanced Oxidation Application. Chem. Mater. 2017, 29, 10104–10112. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Guanidine post-functionalized crystalline ZIF–90 frameworks as a promising recyclable catalyst for the production of biodiesel via soybean oil transesterification. Energy Convers. Manag. 2019, 198, 111922. [Google Scholar] [CrossRef]
- Rajamani, T.; Muthu, S.; Karabacak, M. Electronic absorption, vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N–(4–nitro–2–phenoxyphenyl) methanesulfonamide molecule by ab initio HF and density functional methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 186–196. [Google Scholar] [CrossRef]
- Khan, A.M.; Abid, O.U.R.; Mir, S. Assessment of biological activities of chitosan Schiff base tagged with medicinal plants. Biopolymers 2020, 111, e23338. [Google Scholar] [CrossRef]
- Xiao, Y.; Chu, Y.; Li, S.; Chen, F.; Gao, W.; Xu, J.; Deng, F. Host–Guest Interaction in Ethylene and Ethane Separation on Zeolitic Imidazolate Frameworks as Revealed by Solid-State NMR Spectroscopy. Chemistry 2021, 27, 11303–11308. [Google Scholar] [CrossRef]
- dos Santos, J.E.; Dockal, E.R.; Cavalheiro, T.G. Thermal behavior of Schiff bases from chitosan. J. Therm. Anal. Calorim. 2005, 79, 243–248. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kim, G.-S.; Kim, S.J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.; Galy, N.; Rouzaud, J.; Toulhoat, N.; Vaudey, C.; Simon, P.; Moncoffre, N. Characterizing various types of defects in nuclear graphite using Raman scattering: Heat treatment, ion irradiation and polishing. Carbon 2015, 95, 364–373. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-M.; Dong, H.; Zhang, X.; Sun, X.-J.; Liu, M.; Yang, D.-D.; Liu, X.; Wei, J.-Z. Postsynthetic Modification of ZIF–90 for Potential Targeted Codelivery of Two Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Z. A novel high-performance coating with hybrid nanofiller reinforcement for superior self-cleaning, anti-icing, and corrosion resistance properties. J. Build. Eng. 2023, 80, 107993. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, J.; He, Y.; Luo, J. Research progress on synthesis and structural control of graphene lubricant additives. Tribology 2021, 41, 758–772. [Google Scholar]
- Hong, S.; Chen, W.; Luo, H.Q.; Li, N.B. Inhibition effect of 4–amino–antipyrine on the corrosion of copper in 3 wt.% NaCl solution. Corros. Sci. 2012, 57, 270–278. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Ramamurthy, A.; Lorenzen, W.; Bless, S. Stone impact damage to automotive paint finishes: An introduction to impact physics and impact induced corrosion. Prog. Org. Coat. 1994, 25, 43–71. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Qiang, Y.; Zhao, H.; Wang, L. Design of smart protective coatings with autonomous self-healing and early corrosion reporting properties. Corros. Sci. 2021, 184, 109355. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Othman, N.H.; Yahya, W.Z.N.; Che Ismail, M.; Mustapha, M.; Koi, Z.K. Highly dispersed graphene oxide–zinc oxide nanohybrids in epoxy coating with improved water barrier properties and corrosion resistance. J. Coatings Technol. Res. 2020, 17, 101–114. [Google Scholar] [CrossRef]
- Li, C.; Xia, Z.; Yan, H.; Shi, Q.; Weng, J. Benzotriazole functionalized polydimethylsiloxane for reinforcement water-repellency and corrosion resistance of bio-based waterborne epoxy coatings in salt environment. Corros. Sci. 2022, 199, 110150. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Chen, Z.; Wang, Y.; Zhang, M.; Zhang, T.; Wang, J. A double-layered self-healing coating system based on the synergistic strategy of cysteine and iron polyacrylate for corrosion protection. Chem. Eng. J. 2023, 451, 138995. [Google Scholar] [CrossRef]
- Habib, S.; Fayyed, E.; Shakoor, R.A.; Kahraman, R.; Abdullah, A. Improved self-healing performance of polymeric nanocomposites reinforced with talc nanoparticles (TNPs) and urea–formaldehyde microcapsules (UFMCs). Arab. J. Chem. 2021, 14, 102926. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Tan, B.; Li, W.; Liu, H.; Wu, S. Locust Bean Gum as a green and novel corrosion inhibitor for Q235 steel in 0.5 M H2SO4 medium. J. Mol. Liq. 2020, 310, 113239. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Génin, J.-M. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- Vengatesh, G.; Sundaravadivelu, M. Non-toxic bisacodyl as an effective corrosion inhibitor for mild steel in 1 M HCl: Thermodynamic, electrochemical, SEM, EDX, AFM, FT–IR, DFT and molecular dynamics simulation studies. J. Mol. Liq. 2019, 287, 110906. [Google Scholar] [CrossRef]
- Verma, A.; Bandyopadhyay, R.; Tiwary, C.S.; Das, B.K.; Bhattacharya, J. Engineering defect concentration and morphology of green graphene for the development of hard and anticorrosive coating on carbon steel. Corros. Sci. 2023, 224, 111523. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Wu, Y. Novel pH-responsive self-healing anti-corrosion coating with high barrier and corrosion inhibitor loading based on reduced graphene oxide loaded zeolite imidazole framework. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128641. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Li, J.; Yuan, T.; Chen, B.; Ma, X.; Jiang, D.; Luo, X.; Chen, D.; Liu, Y. Epoxy composite coating with excellent anticorrosion and self-healing performances based on multifunctional zeolitic imidazolate framework derived nanocontainers. Chem. Eng. J. 2020, 385, 123835. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, H.; Zou, Y.; Ye, Y.; Zhao, H. Corrosion protective mechanism of smart graphene-based self-healing coating on carbon steel. Corros. Sci. 2020, 174, 108825. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wu, J.; Yang, J.; Wang, B.; Wang, S.; Li, Q.; Feng, J.; You, S.; Zhu, J. High-performance, command-degradable, antibacterial Schiff base epoxy thermosets: Synthesis and properties. J. Mater. Chem. A 2019, 7, 15420–15431. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Mao, X.; Tang, S. Preparation and biological properties of Schiff-base hydrogels crosslinked by benzaldehyde substituted agarose oligosaccharides. React. Funct. Polym. 2023, 193, 105745. [Google Scholar] [CrossRef]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with pH-Regulated Recovery of Self-Healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar] [CrossRef]
- Cao, X.-X.; Liu, S.-L.; Lu, J.-S.; Zhang, Z.-W.; Wang, G.; Chen, Q.; Lin, N. Chitosan coated biocompatible zeolitic imidazolate framework ZIF–90 for targeted delivery of anticancer drug methotrexate. J. Solid State Chem. 2021, 300, 122259. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S. Facile engineering of chitosan-coated aminopterin loaded zeolitic imidazolate framework: Promising drug delivery system for breast cancer. J. Exp. Nanosci. 2023, 18, 2238129. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic Imidazolate framework–8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, Z.; Yuan, H.; Wang, C.; Qi, J.; Liu, K.; Cao, R.; Zheng, H. A protein@metal-organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018, 47, 10223–10228. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Synthesis of graphene oxide nanosheets decorated by nanoporous zeolite–imidazole (ZIF–67) based metal-organic framework with controlled-release corrosion inhibitor performance: Experimental and detailed DFT–D theoretical explorations. J. Hazard. Mater. 2021, 404, 124068. [Google Scholar] [CrossRef]

 ) and ZIF–90 (
) and ZIF–90 ( ) are indicated.
) are indicated.
 ) and ZIF–90 (
) and ZIF–90 ( ) are indicated.
) are indicated.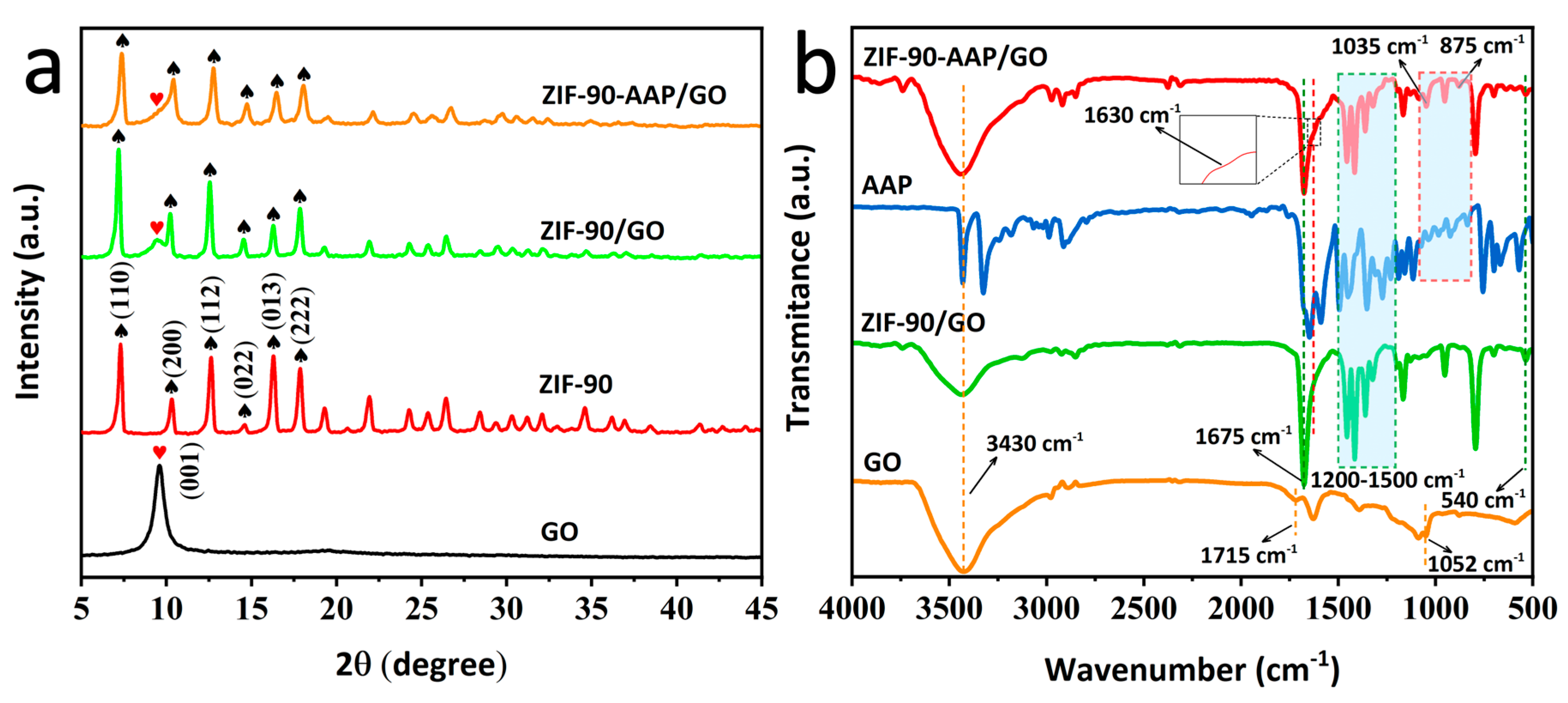





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cao, Y.; Wan, J.; Zhang, M.; Li, Y.; Wang, Y.; Song, D.; Zhang, T.; Wang, J. pH-Responsive Graphene Oxide-Based 2D/3D Composite for Enhancing Anti-Corrosion Properties of Epoxy Coating. Nanomaterials 2024, 14, 323. https://doi.org/10.3390/nano14040323
Wang J, Cao Y, Wan J, Zhang M, Li Y, Wang Y, Song D, Zhang T, Wang J. pH-Responsive Graphene Oxide-Based 2D/3D Composite for Enhancing Anti-Corrosion Properties of Epoxy Coating. Nanomaterials. 2024; 14(4):323. https://doi.org/10.3390/nano14040323
Chicago/Turabian StyleWang, Jian, Yangyang Cao, Jieru Wan, Meng Zhang, Yunqiang Li, Yanli Wang, Dalei Song, Tao Zhang, and Jun Wang. 2024. "pH-Responsive Graphene Oxide-Based 2D/3D Composite for Enhancing Anti-Corrosion Properties of Epoxy Coating" Nanomaterials 14, no. 4: 323. https://doi.org/10.3390/nano14040323





