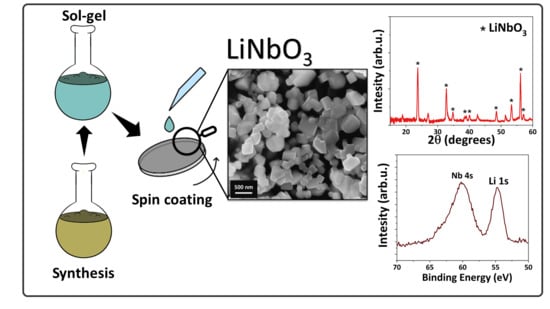
LiNbO3 Thin Films through a Sol–Gel/Spin-Coating Approach Using a Novel Heterobimetallic Lithium–Niobium Precursor
Abstract
1. Introduction
2. Materials and Methods
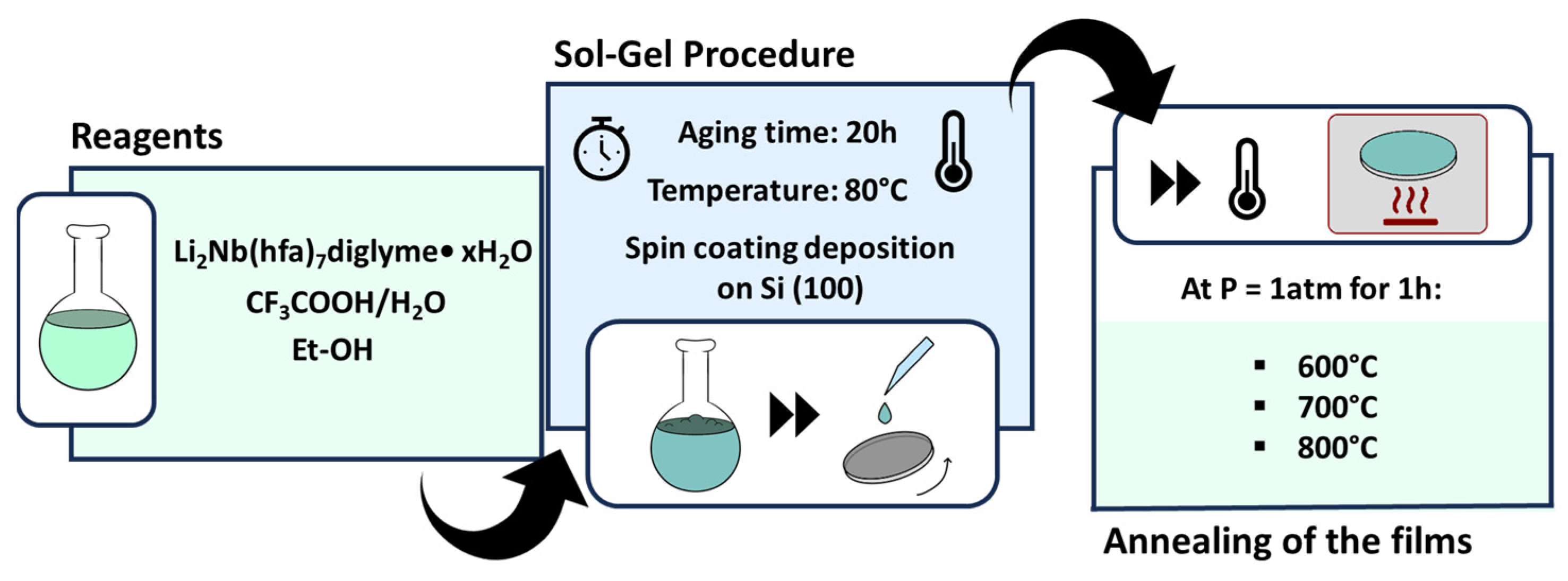
2.1. Starting Materials
2.2. Synthesis of “Li2Nb(hfa)7•diglyme•xH2O”
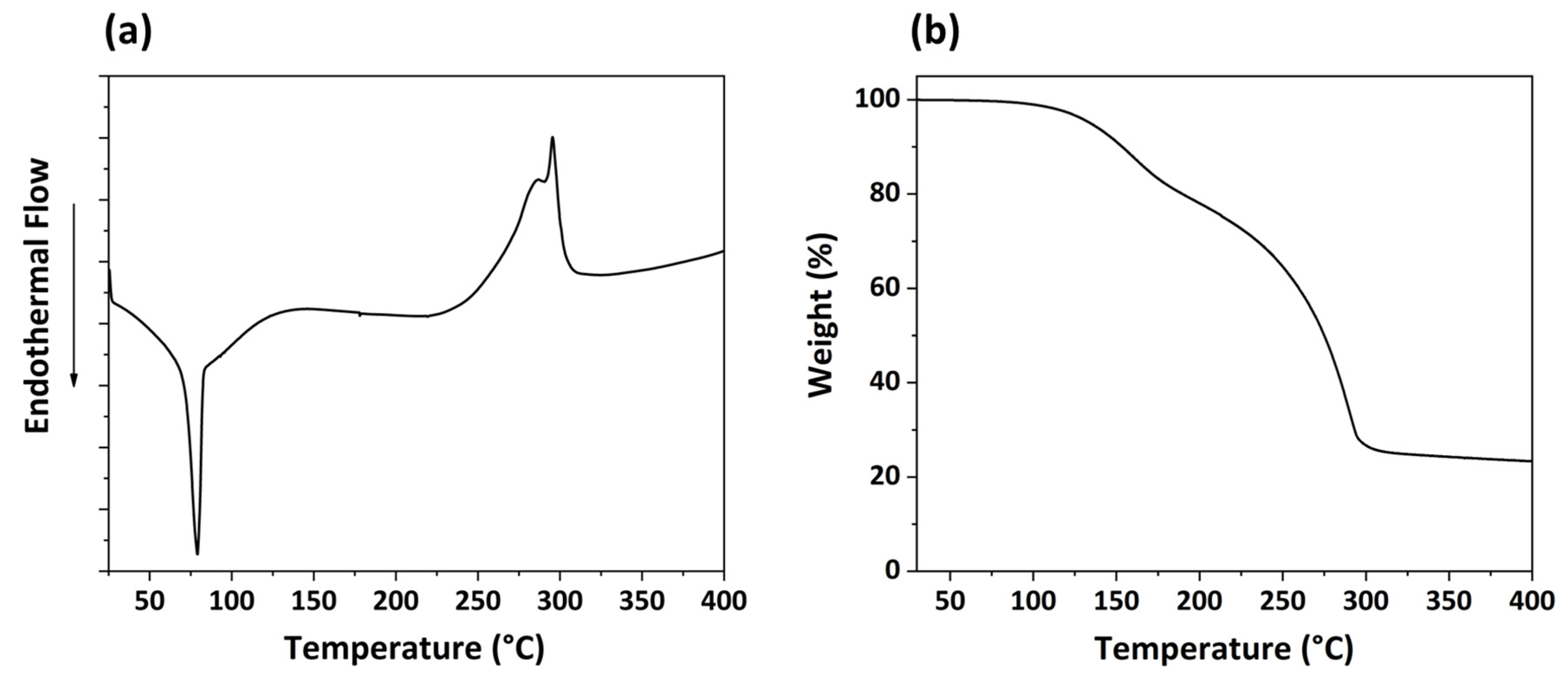
2.3. Characterization of the Precursor
2.4. Sol–Gel Deposition
2.5. Film Characterization
3. Results and Discussion
3.1. Heterobimetallic Li-Nb Single-Source
3.2. Sol–Gel Synthesis of LiNbO3 Films from the Single Li-Nb Precursor
3.3. Sol–Gel Synthesis of LiNbO3 Films from Individual Precursors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, K.; Zhu, Y.; Liu, Z.; Xue, D. State of the Art in Crystallization of LiNbO3 and Their Applications. Molecules 2021, 26, 7044. [Google Scholar] [CrossRef] [PubMed]
- Zivasatienraj, B.; Tellekamp, M.B.; Doolittle, W.A. Epitaxy of LiNbO3: Historical Challenges and Recent Success. Crystals 2021, 11, 397. [Google Scholar] [CrossRef]
- Weis, R.S.; Gaylord, T.K. Lithium niobate: Summary of physical properties and crystal structure. Appl. Phys. A 1985, 37, 191–203. [Google Scholar] [CrossRef]
- Sanna, S.; Schmidt, W.G. LiNbO3 surfaces from a microscopic perspective. J. Phys. Condens. Matter 2017, 29, 413001. [Google Scholar] [CrossRef]
- Fedotova, A.; Carletti, L.; Zilli, A.; Setzpfandt, F.; Staude, I.; Toma, A.; Finazzi, M.; De Angelis, C.; Pertsch, T.; Neshev, D.N.; et al. Zero-Order Second Harmonic Generation from AlGaAs-on-Insulator Metasurfaces. ACS Photonics 2022, 9, 3745–3763. [Google Scholar] [CrossRef]
- Bartasyte, A.; Margueron, S.; Baron, T.; Oliveri, S.; Boulet, P. Toward High-Quality Epitaxial LiNbO3 and LiTaO3 Thin Films for Acoustic and Optical Applications. Adv. Mater. Interfaces 2017, 4, 1600998. [Google Scholar] [CrossRef]
- Sánchez-Dena, O.; Fierro-Ruiz, C.D.; Villalobos-Mendoza, S.D.; Flores, D.M.C.; Elizalde-Galindo, J.T.; Farías, R. Lithium niobate single crystals and powders reviewed—Part I. Crystals 2020, 10, 973. [Google Scholar] [CrossRef]
- Lerner, P.; Legras, C.; Dumas, J.P. Stoechiométrie des monocristaux de métaniobate de lithium. J. Cryst. Growth 1968, 3–4, 231–235. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamamoto, J.K.; Iyi, N.; Kirnura, S.; Hayashi, T. Stoichiometric LiNbO3 single crystal growth by double crucible Czochralski method using automatic powder supply system. J. Cryst. Growth 1992, 116, 327–332. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G.; Yurchenko, L.P.; Proshko, V.Y.; Kokanyan, E.P.; Gabrielyan, V.T. Improvement of LiNbO3 Microstructure by Crystal Growth with Potassium. Phys. Stat. Sol. A 1992, 133, K29–K32. [Google Scholar] [CrossRef]
- Hou, S.; Chen, P.; Shah, M.; Briggs, I.; Xing, W.; Liu, Z.; Fan, L. Programmable Optical Filter in Thin-Film Lithium Niobate with Simultaneous Tunability of Extinction Ratio and Wavelength. ACS Photonics 2023, 10, 3896–3900. [Google Scholar] [CrossRef]
- Zhu, D.; Shao, L.; Yu, M.; Cheng, R.; Desiatov, B.; Xin, C.J.; Hu, Y.; Holzgrafe, J.; Ghosh, S.; Shams-Ansari, A.; et al. Integrated photonics on thin-film lithium niobate. Adv. Opt. Photon. 2021, 13, 242–352. [Google Scholar] [CrossRef]
- Ke, W.; Lin, Y.; He, M.; Xu, M.; Zhang, J.; Lin, Z.; Yu, S.; Cai, X. Digitally tunable optical delay line based on thin-film lithium niobate featuring high switching speed and low optical loss. Photon. Res. 2022, 10, 2575–2583. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Salim, E.T.; Wahid, M.H.A.; Hashim, U.; Salim, Z.T. Optical investigations and optical constant of nano lithium niobate deposited by spray pyrolysis technique with injection of Li2CO3 and Nb2O5 as raw materials. J. Mater. Sci. Mater. Electron. 2018, 29, 9200–9208. [Google Scholar] [CrossRef]
- Wolff, D.; Weber, S.; Graumann, T.; Zebrowski, S.; Mainusch, N.; Dilger, N.; Cerdas, F.; Zellmer, S. An Environmental and Technical Evaluation of Vacuum-Based Thin Film Technologies: Lithium Niobate Coated Cathode Active Material for Use in All-Solid-State Battery Cells. Energies 2023, 16, 1278. [Google Scholar] [CrossRef]
- Almirall, A.; Oliveri, S.; Daniau, W.; Margueron, S.; Baron, T.; Boulet, P.; Ballandras, S.; Chamaly, S.; Bartasyte, A. High-frequency surface acoustic wave devices based on epitaxial Z-LiNbO3 layers on sapphire. Appl. Phys. Lett. 2019, 114, 162905. [Google Scholar] [CrossRef]
- Xingli, H.; Chen, K.; Kong, L.; Li, P. Single-crystalline LiNbO3 film based wideband SAW devices with spurious-free responses for future RF front-ends. Appl. Phys. Lett. 2022, 120, 113507. [Google Scholar] [CrossRef]
- Takahashi, M.; Iyoda, K.; Maeda, Y.; Miyauchi, T.; Ohkido, S.; Sato, S.; Wakita, K.; Kajitani, N.; Hotta, K.; Kurachi, M. Preparation and characterization of Eu-doped LiNbO3 films prepared by the sol-gel method. J. Appl. Phys. 2008, 103, 034101. [Google Scholar] [CrossRef]
- Dutta, S.; Zhao, Y.; Saha, U.; Farfurnik, D.; Goldschmidt, E.A.; Waks, E. An Atomic Frequency Comb Memory in Rare-Earth-Doped Thin-Film Lithium Niobate. ACS Photonics 2023, 10, 1104–1109. [Google Scholar] [CrossRef]
- Hüger, E.; Riedel, L.; Zhu, J.; Stahn, J.; Heitjans, P.; Schmidt, H. Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results. Batteries 2023, 9, 244. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Li, J.; Li, Y.; Lai, K.; Zhang, S.; Zhao, G.; Liu, D.; Xi, Z.; Liu, C.; et al. Sulfide solid electrolyte thin film with high ionic conductive from slurry-casting strategy for all-solid-state lithium batteries. J. Electroanal. Chem. 2023, 928, 117032. [Google Scholar] [CrossRef]
- Masoud, M.; Heitjans, P. Impedance spectroscopy study of Li ion dynamics in single crystal, microcrystalline, nanocrystalline, and amorphous LiNbO3. Defect Diffus. Forum 2005, 237–240, 1016–1021. [Google Scholar] [CrossRef]
- Fan, Q.; Lei, L.; Sun, Y. Facile synthesis of a 3D-porous LiNbO3 nanocomposite as a novel electrode material for lithium ion batteries. Nanoscale 2014, 6, 7188–7192. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.; Sham, T.-K.; Sun, X. Atomic layer deposition of lithium niobium oxides as potential solid-state electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.M.; Nassau, K.; Negran, T.J. Ionic conductivity of quenched alkali niobate and tantalate glasses. J. Appl. Phys. 1978, 49, 4808–4811. [Google Scholar] [CrossRef]
- Waqar, M.; Chai, J.; Wong, L.M.; Lim, P.C.; Chen, S.; Liew, W.-H.; Wang, S.; Chen, J.; He, Q.; Yao, K.; et al. Large Electromechanical Response in a Polycrystalline Alkali-Deficient (K,Na)NbO3 Thin Film on Silicon. Nano Lett. 2023, 23, 11026–11033. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Negi, D.; Gupta, M.; Vashishtha, P.; Gupta, G.; Das, A.; Dobbidi, P.; Awasthi, K.; Nelamarri, S.R. Rapid thermal annealing induced engineering of surface and photoluminescence properties of (K,Na)NbO3 thin films for optoelectronic applications. Appl. Surf. Sci. 2022, 575, 151794. [Google Scholar] [CrossRef]
- Tudisco, C.; Pellegrino, A.L.; Malandrino, G.; Condorelli, G.G. Surface anchoring of bi-functional organic linkers on piezoelectric BiFeO3 films and particles: Comparison between carboxylic and phosphonic tethering groups. Surf. Coat. Technol. 2018, 343, 75–82. [Google Scholar] [CrossRef]
- Ren, J.; Tang, S.; Guo, C.; Wang, J.; Huang, H. Surface Effect of Thickness-Dependent Polarization and Domain Evolution in BiFeO3 Epitaxial Ultrathin Films. ACS Appl. Mater. Interfaces 2024, 16, 1074–1081. [Google Scholar] [CrossRef]
- Bartasyte, A.; Clementi, G.; Micard, Q.; Labbaveettil, I.; Moreira, A.S.L.; Boujnah, S.; Ouhabaz, M.; Verma, A.; Ichangi, A.; Malandrino, G.; et al. Material strategies to enhance the performance of piezoelectric energy harvesters based on lead-free materials. J. Micromech. Microeng. 2023, 33, 053001. [Google Scholar] [CrossRef]
- Kanno, I.; Ichida, T.; Adachi, K.; Kotera, H.; Shibata, K.; Mishima, T. Power-generation performance of lead-free (K, Na)NbO3 piezoelectric thin-film energy harvesters. Sens. Actuators A Phys. 2012, 179, 132–136. [Google Scholar] [CrossRef]
- Tian, A.; Ren, W.; Wang, L.; Shi, P.; Chen, X.; Wu, X.; Yao, X.I. Effect of deposition temperature on orientation and electrical properties of (K0.5Na0.5)NbO3 thin films by pulsed laser deposition. Appl. Surf. Sci. 2012, 258, 2674–2678. [Google Scholar] [CrossRef]
- Mahesh, P.; Pamu, D. Effect of deposition temperature on structural, mechanical, optical and dielectric properties of radio frequency sputtered nanocrystalline (KxNa1-x)NbO3 thin films. Thin Solid Film. 2014, 562, 471–477. [Google Scholar] [CrossRef]
- Zhao, T.; Scholl, A.; Zavaliche, F.; Lee, K.; Barry, M.; Doran, A.; Cruz, M.P.; Chu, Y.H.; Ederer, C.; Spaldin, N.A.; et al. Electrical control of antiferromagnetic domains in multiferroic BiFeO3 films at room temperature. Nat. Mater. 2006, 5, 823–829. [Google Scholar] [CrossRef]
- Alikhanov, N.M.-R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-dependent structural parameters, optical, and magnetic properties of facile synthesized pure phase BiFeO3. J. Mater. Sci. Mater. Electron. 2021, 32, 13323–13335. [Google Scholar] [CrossRef]
- Lam, S.M.; Jaffari, Z.H.; Sin, J.C.; Zeng, H.; Lin, H.; Li, H.; Mohamed, A.R. Insight into the influence of noble metal decorated on BiFeO3 for 2,4-dichlorophenol and real herbicide wastewater treatment under visible light. Colloid Surf. A 2021, 614, 126138. [Google Scholar] [CrossRef]
- Kharbanda, S.; Dhanda, N.; Sun, A.-C.A.; Thakur, A.; Thakur, P. Multiferroic perovskite bismuth ferrite nanostructures: A review on synthesis and applications. J. Magn. Magn. Mater. 2023, 572, 170569. [Google Scholar] [CrossRef]
- Orudzhev, F.; Sobola, D.; Ramazanov, S.; Cástková, K.; Papež, N.; Selimov, D.A.; Abdurakhmanov, M.; Shuaibov, A.; Rabadanova, A.; Gulakhmedov, R.; et al. Piezo-Enhanced Photocatalytic Activity of the Electrospun Fibrous Magnetic PVDF/BiFeO3 Membrane. Polymers 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dekker, P.; Dawes, J.M. Growth and characterization of lithium niobate planar waveguides by liquid phase epitaxy. J. Cryst. Growth 2009, 311, 1441–1445. [Google Scholar] [CrossRef]
- Pellegrino, A.L.; Wagner, E.; Presti, F.L.; Maudez, W.; Kolb, S.; Rani, R.; Bernard, A.; Guy, S.; Gassenq, A.; Raevskaia, M.; et al. Efficient Optimization of High-Quality Epitaxial Lithium Niobate Thin Films by Chemical Beam Vapor Deposition: Impact of Cationic Stoichiometry. Adv. Mater. Interfaces 2023, 10, 2300535. [Google Scholar] [CrossRef]
- Kadota, M.; Suzuki, Y.; Ito, Y. Properties of LiNbO3 thin film deposited by chemical vapor deposition and frequency characteristics of film bulk acoustic wave resonator. Jpn. J. Appl. Phys. 2011, 50, 07HD10/1–07HD10/5. [Google Scholar] [CrossRef]
- Lee, S.; Feigelson, R. C-axis lithium niobate thin film growth on silicon using solid-source metalorganic chemical vapor deposition. J. Mater. Res. 1999, 14, 2662–2667. [Google Scholar] [CrossRef]
- Akiyama, Y.; Shitanaka, K.; Murakami, H.; Shin, Y.-S.; Yoshida, M.; Imaishi, N. Epitaxial growth of lithium niobate film using metalorganic chemical vapor deposition. Thin Solid Film. 2007, 515, 4975–4979. [Google Scholar] [CrossRef]
- Ocón, J.A.; Murillo, J.G.; Miki-Yoshida, M.; Cardoza, M.N.; Contreras-López, O.E. Synthesis and characterization of LiNbO3 nanocrystals prepared by the aerosol assisted chemical vapor deposition method. J. Cryst. Growth 2014, 408, 64–70. [Google Scholar] [CrossRef]
- Sauze, L.C.; Vaxelaire, N.; Templier, R.; Rouchon, D.; Pierre, F.; Guedj, C.; Remiens, D.; Rodriguez, G.; Bousquet, M.; Dupont, F. Homo-epitaxial growth of LiNbO3 thin films by Pulsed Laser deposition. J. Cryst. Growth 2023, 601, 126950. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.; Man, W.; Jia, J.; Shi, X. Effect of laser fluence on c-axis orientation of LiNbO3 piezoelectric films on nanocrystalline diamond by pulsed laser deposition. Cryst. Res. Technol. 2012, 47, 719–722. [Google Scholar] [CrossRef]
- Tellekamp, M.B.; Shank, J.C.; Goorsky, M.S.; Doolittle, W.A. Molecular Beam Epitaxy Growth of High Crystalline Quality LiNbO3. J. Electron. Mater. 2016, 45, 6292–6299. [Google Scholar] [CrossRef]
- Dabirian, A.; Kuzminy, Y.; Sandu, S.C.; Wagner, E.; Benvenuti, G.; Parsons, C.; Rushworth, S.; Hoffmann, P. Combinatorial Chemical Vapor Deposition of Lithium Niobate Thin Films. ECS Trans. 2009, 25, 1221. [Google Scholar] [CrossRef]
- Zeng, X.; Pelenovich, V.; Xu, C.; Neena, D.; Jiang, Y.; Zhang, X.; Pogrebnjak, A.; Rakhimov, R.; Zhang, J.; Yang, B.; et al. Morphology of lithium niobium oxide thin film ultrasonic transducers deposited by RF magnetron sputtering. Ceram. Int. 2023, 49, 16297–16304. [Google Scholar] [CrossRef]
- Akazaw, H. Crystallization characteristics of Li2O–Nb2O5 compound films on sapphire substrates and formation of crack-free and thick LiNbO3 epitaxial films. Ceram. Int. 2023, 49, 271–281. [Google Scholar] [CrossRef]
- Paldi, R.L.; Qi, Z.; Misra, S.; Lu, J.; Sun, X.; Phuah, X.L.; Kalaswad, M.; Bischoff, J.; Branch, D.W.; Siddiqui, A.; et al. Nanocomposite-Seeded Epitaxial Growth of Single-Domain Lithium Niobate Thin Films for Surface Acoustic Wave Devices. Adv. Photonics Res. 2021, 2, 2000149. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Q.H.; Chai, X.J.; Jiang, J.; Yang, J.G.; Jiang, A.Q. Improved Ferroelectric Performance of Mg-Doped LiNbO3 Films by an Ideal Atomic Layer Deposited Al2O3 Tunnel Switch Layer. Nanoscale Res. Lett. 2019, 14, 131. [Google Scholar] [CrossRef]
- Østreng, E.; Sønsteby, H.H.; Sajavaara, T.; Nilsena, O.; Fjellvåga, H. Atomic layer deposition of ferroelectric LiNbO3. J. Mater. Chem. C 2013, 1, 4283–4290. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Salim, E.T.; Abdulwahhab, A.W.; Hashim, U.; Munshid, M.A.; Salim, Z.T. The effect of annealing temperature on optical and photoluminescence properties of LiNbO3. Surf. Rev. Lett. 2018, 26, 1950068. [Google Scholar] [CrossRef]
- Satapathy, S.; Mukherjee, C.; Shaktawat, T.; Gupta, P.K.; Sathe, V.G. Blue shift of optical band-gap in LiNbO3 thin films deposited by sol–gel technique. Thin Solid Film. 2012, 520, 6510–6514. [Google Scholar] [CrossRef]
- Nashimoto, K.; Cima, M.J. Epitaxial LiNbO3 thin films prepared by a sol-gel process. Mater. Lett. 1991, 10, 348–354. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Salim, E.T.; Wahid, M.H.A.; Hashim, U.; Salim, Z.T.; Ismail, R.A. Synthesis and characterization of nanostructured LiNbO3 films with variation of stirring duration. J. Mater. Sci. Mater. Electron. 2017, 28, 11813–11822. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, S.W. Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing; Brinker, C.J., Scherer, G.W., Eds.; Academic Press: Boston, MA, USA, 1990. [Google Scholar] [CrossRef]
- Torma, V.; Peterlik, H.; Bauer, U.; Rupp, W.; Hu, N.; Bernstorff, S.; Steinhart, M.; Goerigk, G.; Schubert, U. Mixed Silica Titania Materials Prepared from a Single-Source Sol−Gel Precursor: A Time-Resolved SAXS Study of the Gelation, Aging, Supercritical Drying, and Calcination Processes. Chem. Mater. 2005, 17, 3146–3153. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamauchi, K.; Yagi, T.; Nishiwaki, A.; Wakita, K.; Ohnishi, N.; Hotta, K.; Sahashi, I. Preparation and characterization of high-quality stoichiometric LiNbO3 thick films prepared by the sol–gel method. Thin Solid Film. 2004, 458, 108–113. [Google Scholar] [CrossRef]
- Yoon, J.-G.; Kim, K. Growth of highly textured LiNbO3 thin film on Si with MgO buffer layer through the sol-gel process. Appl. Phys. Lett. 1996, 68, 2523–2525. [Google Scholar] [CrossRef]
- Peddagopu, N.; Rossi, P.; Bonaccorso, C.; Bartasyte, A.; Paoli, P.; Malandrino, G. Facile synthesis of novel lithium β-diketonate glyme adducts: The effect of molecular engineering on the thermal properties. Dalton Trans. 2020, 49, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Battiato, S.; Rossi, P.; Paoli, P.; Malandrino, G. Heterobimetallic Sodium Rare-Earth Complexes: “Third-Generation” MOCVD Precursors for the Deposition of NaREF4 (RE = Y, Gd) Films. Inorg. Chem. 2018, 57, 15035–15039. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.L.; Moshier, R.W.; Sievbrs, R.E. Infrared Spectra of Metal Chelate Compounds of Hexafluoroacetylacetone. Inorg. Chem. 1963, 2, 411–412. [Google Scholar] [CrossRef]
- Gulino, A.; Castelli, F.; Dapporto, P.; Rossi, P.; Fragalà, I. Synthesis and Characterization of Novel Self-Generating Liquid MOCVD Precursors for Thin Films of Zinc Oxide. Chem. Mater. 2000, 12, 548–554. [Google Scholar] [CrossRef]
- Joshi, V.; Mecartney, M.L. The influence of water of hydrolysis on microstructural development in sol-gel derived LiNbO3 thin films. J. Mater. Res. 1993, 8, 2668–2678. [Google Scholar] [CrossRef]
- Margueron, S.; Bartasyte, A.; Glazer, A.M.; Simon, E.; Hlinka, J.; Gregora, I.; Gleize, J. Resolved E-symmetry zone-centre phonons in LiTaO3 and LiNbO3. J. Appl. Phys. 2012, 111, 104105. [Google Scholar] [CrossRef]
- Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Stanionyte, S.; Margueron, S.; Boulet, P.; Kobata, T.; Uesu, Y.; Gleize, J. Identification of LiNbO3, LiNb3O8 and Li3NbO4 phases in thin films synthesized with different deposition techniques by means of XRD and Raman spectroscopy. J. Phys. Condens. Matter 2013, 25, 205901. [Google Scholar] [CrossRef] [PubMed]
- Bartasyte, A.; Margueron, S.; Glazer, A.M.; Simon, E.; Gregora, I.; Huband, S.; Thomas, P.A. Vibrational modes and overlap matrix of LiNb1−xTaxO3 mixed crystals. Phys. Rev. B 2019, 99, 094306. [Google Scholar] [CrossRef]
- Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Stanionyte, S.; Margueron, S.; Kubilius, V.; Boulet, P.; Huband, S.; Thomas, P.A. Thickness dependent stresses and thermal expansion of epitaxial LiNbO3 thin films on C-sapphire. Mater. Chem. Phys. 2015, 149–150, 622–631. [Google Scholar] [CrossRef]
- Tao, Y.; Gitmans, F.; Sitar, Z.; Pierhofer, H.; Kundig, A.; Gamboni, I.; Gunter, P. Dielectric, pyroelectric and structural properties of LiTaO3 thin films grown on silicon by a modified molecular beam epitaxy. Ferroelectrics 1997, 201, 245. [Google Scholar] [CrossRef]
- Skryleva, E.A.; Senatulin, B.R.; Kiselev, D.A.; Ilina, T.S.; Podgorny, D.A.; Parkhomenko, Y.N. Ar gas cluster ion beam assisted XPS study of LiNbO3 Z cut surface. Surf. Interfaces 2021, 26, 101428. [Google Scholar] [CrossRef]
- Skryleva, E.A.; Kubasov, I.V.; Kiryukhantsev-Korneev, P.V.; Senatulin, B.R.; Zhukov, R.N.; Zakutailov, K.V.; Malinkovich, M.D.; Parkhomenko, Y.N. XPS study of Li/Nb ratio in LiNbO3 crystals. Effect of polarity and mechanical processing on LiNbO3 surface chemical composition. Appl. Surf. Sci. 2016, 389, 387–394. [Google Scholar] [CrossRef]

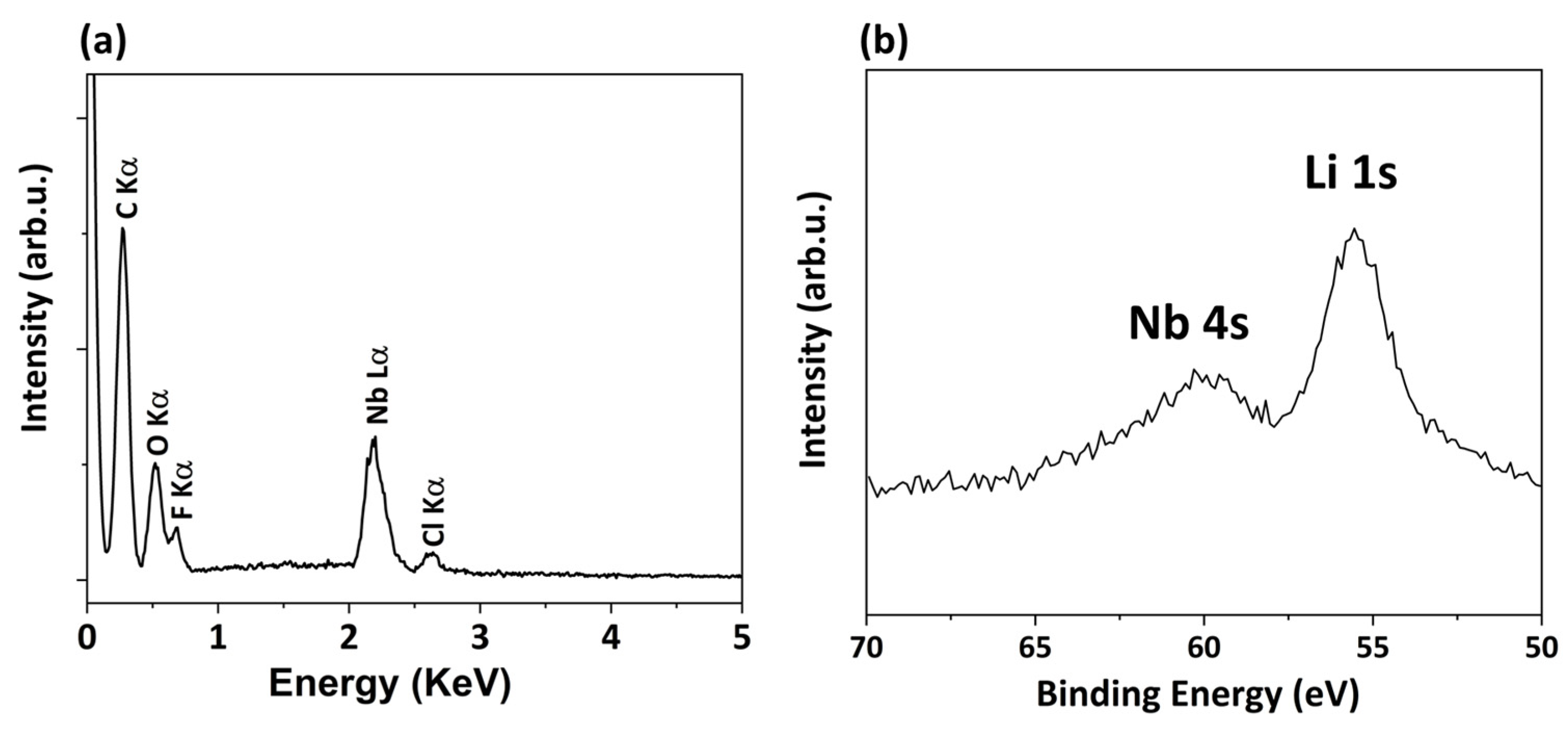


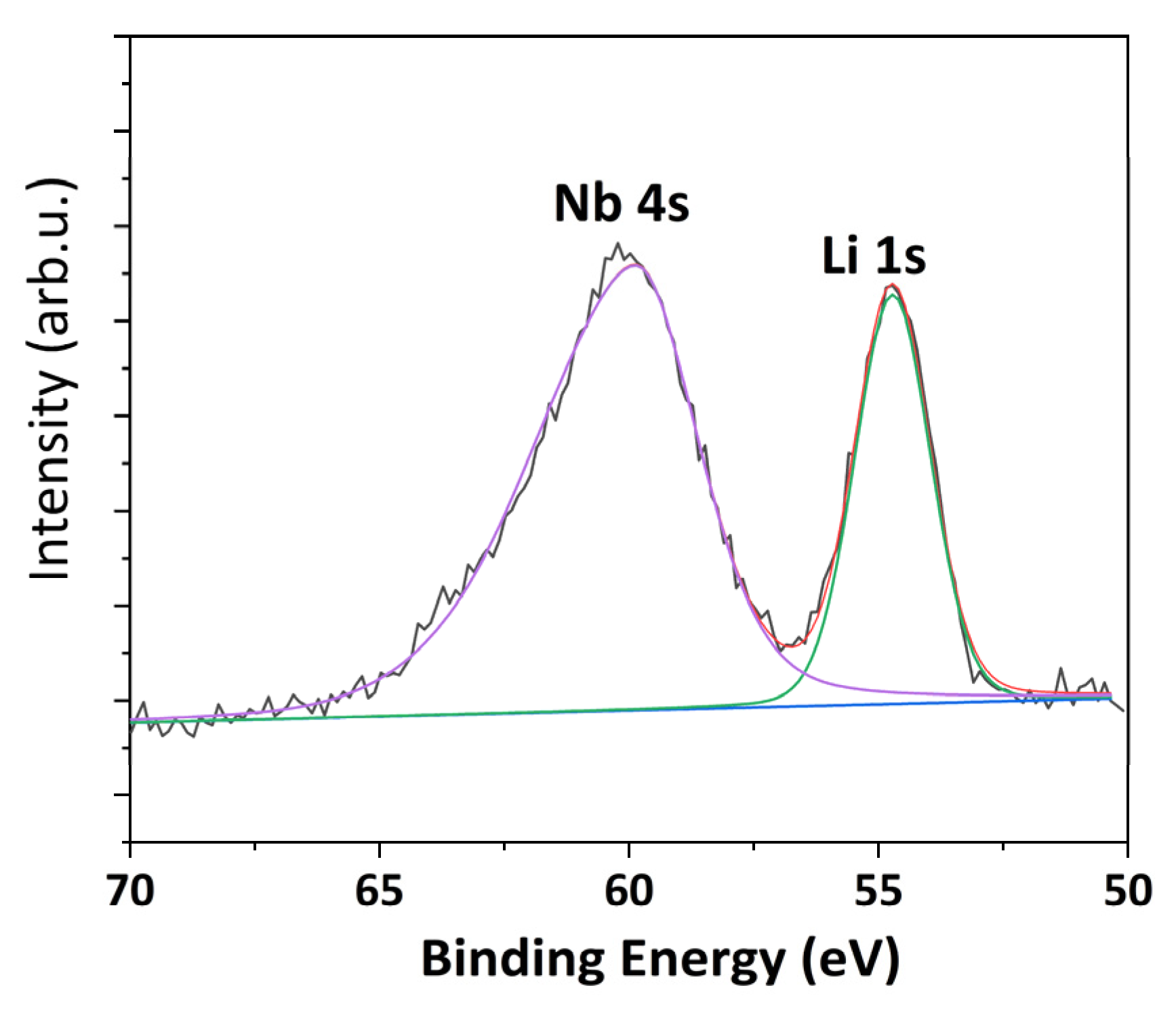
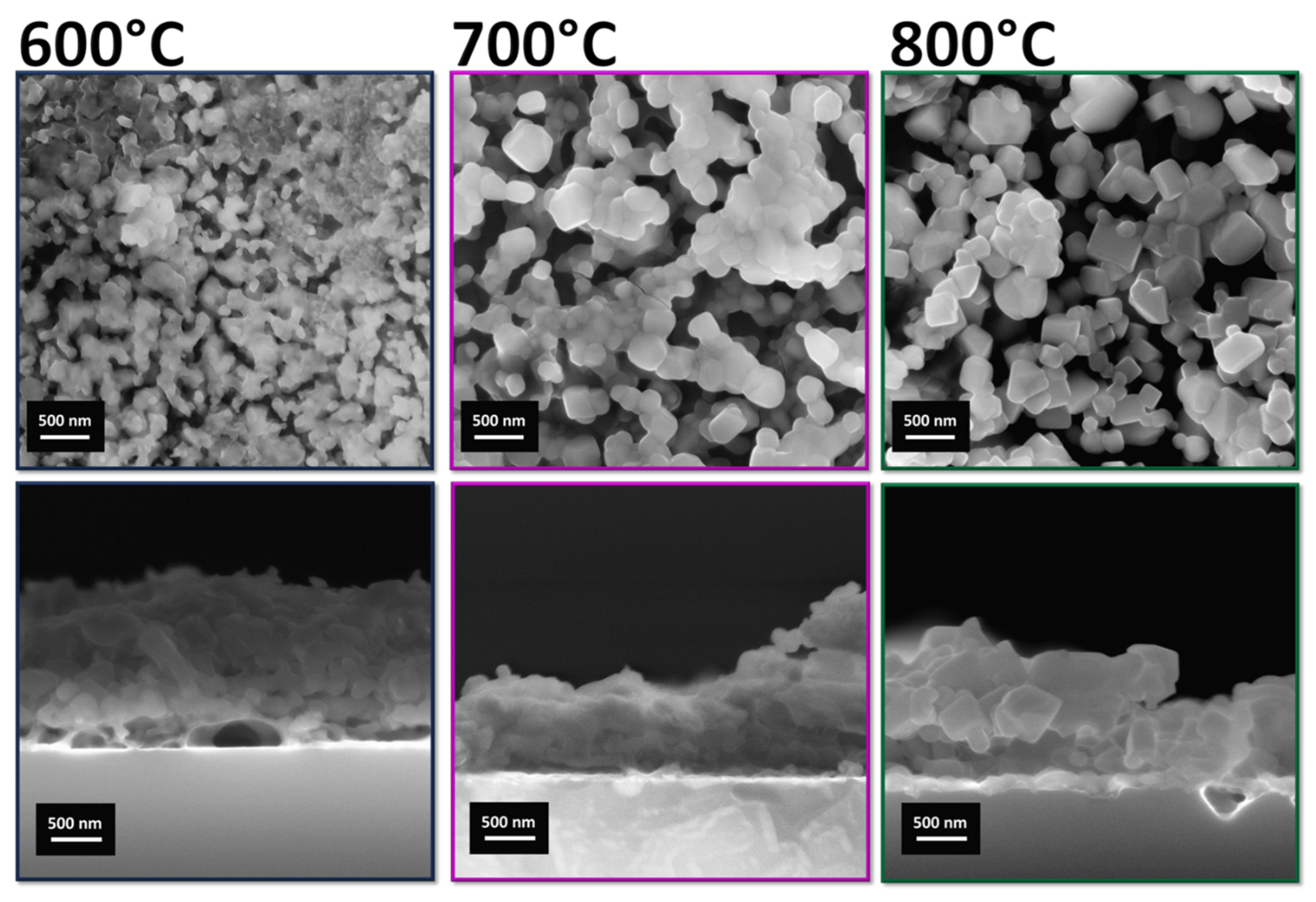
| XPS Quantitative Analysis | |||||
|---|---|---|---|---|---|
| Li 1s | Nb 3d | C 1s | F 1s | O 1s | Cl 2p |
| 4.3 | 2.1 | 32.5 | 42.0 | 18.9 | 0.1 |
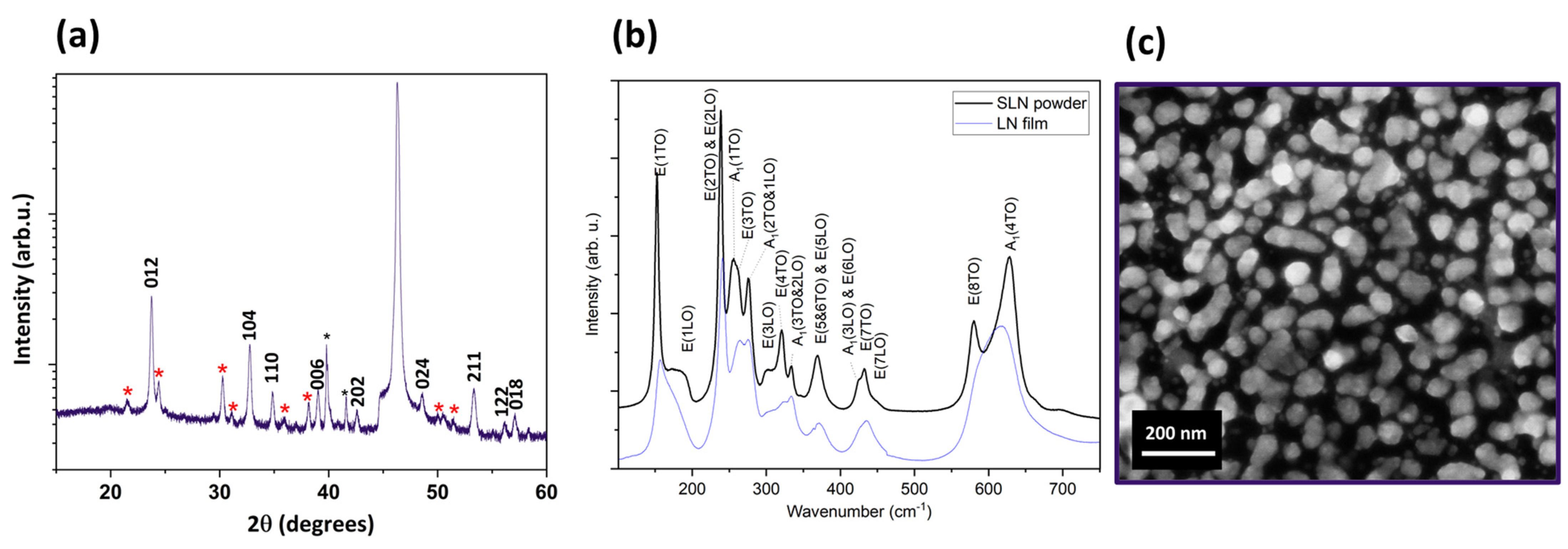
| Raman Modes | ||
|---|---|---|
| E Modes | Our Work (cm−1) | Ref. [67] (cm−1) |
| 1TO | 153 | 155 |
| 2TO-2LO | 239 | 240–241 |
| 3TO | 262 | 265 |
| 4TO | 322 | 322 |
| 6TO | 368 | 370 |
| 7TO | 434 | 433 |
| 8TO | 583 | 579 |
| A1 modes | ||
| 1LO-2TO | 277 | 275–276 |
| 2LO-3TO | 335 | 334 |
| 4TO | 624 | 632 |
| Element | Atomic Percentage |
|---|---|
| C 1s | 15.8% |
| O 1s | 49% |
| F 1s | 6.6% |
| Nb 3d | 11.2% |
| Li 1s | 13.3% |
| Si 2p | 4.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, F.; Pellegrino, A.L.; Micard, Q.; Condorelli, G.G.; Margueron, S.; Bartasyte, A.; Malandrino, G. LiNbO3 Thin Films through a Sol–Gel/Spin-Coating Approach Using a Novel Heterobimetallic Lithium–Niobium Precursor. Nanomaterials 2024, 14, 345. https://doi.org/10.3390/nano14040345
Lo Presti F, Pellegrino AL, Micard Q, Condorelli GG, Margueron S, Bartasyte A, Malandrino G. LiNbO3 Thin Films through a Sol–Gel/Spin-Coating Approach Using a Novel Heterobimetallic Lithium–Niobium Precursor. Nanomaterials. 2024; 14(4):345. https://doi.org/10.3390/nano14040345
Chicago/Turabian StyleLo Presti, Francesca, Anna Lucia Pellegrino, Quentin Micard, Guglielmo Guido Condorelli, Samuel Margueron, Ausrine Bartasyte, and Graziella Malandrino. 2024. "LiNbO3 Thin Films through a Sol–Gel/Spin-Coating Approach Using a Novel Heterobimetallic Lithium–Niobium Precursor" Nanomaterials 14, no. 4: 345. https://doi.org/10.3390/nano14040345
APA StyleLo Presti, F., Pellegrino, A. L., Micard, Q., Condorelli, G. G., Margueron, S., Bartasyte, A., & Malandrino, G. (2024). LiNbO3 Thin Films through a Sol–Gel/Spin-Coating Approach Using a Novel Heterobimetallic Lithium–Niobium Precursor. Nanomaterials, 14(4), 345. https://doi.org/10.3390/nano14040345