Amphiphilic Graft Copolymers as Templates for the Generation of Binary Metal Oxide Mesoporous Interfacial Layers for Solid-State Photovoltaic Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Amphiphilic Graft Copolymers, PECH-g-PS
2.3. Preparation of Binary Metal Oxide Mesoporous Interfacial Layer (bi-MO Meso IF Layer)
2.4. Preparation of Photoanode
2.5. Fabrication of DSSCs
2.6. Characterization
2.7. Measurement of Dye Adsorption Value
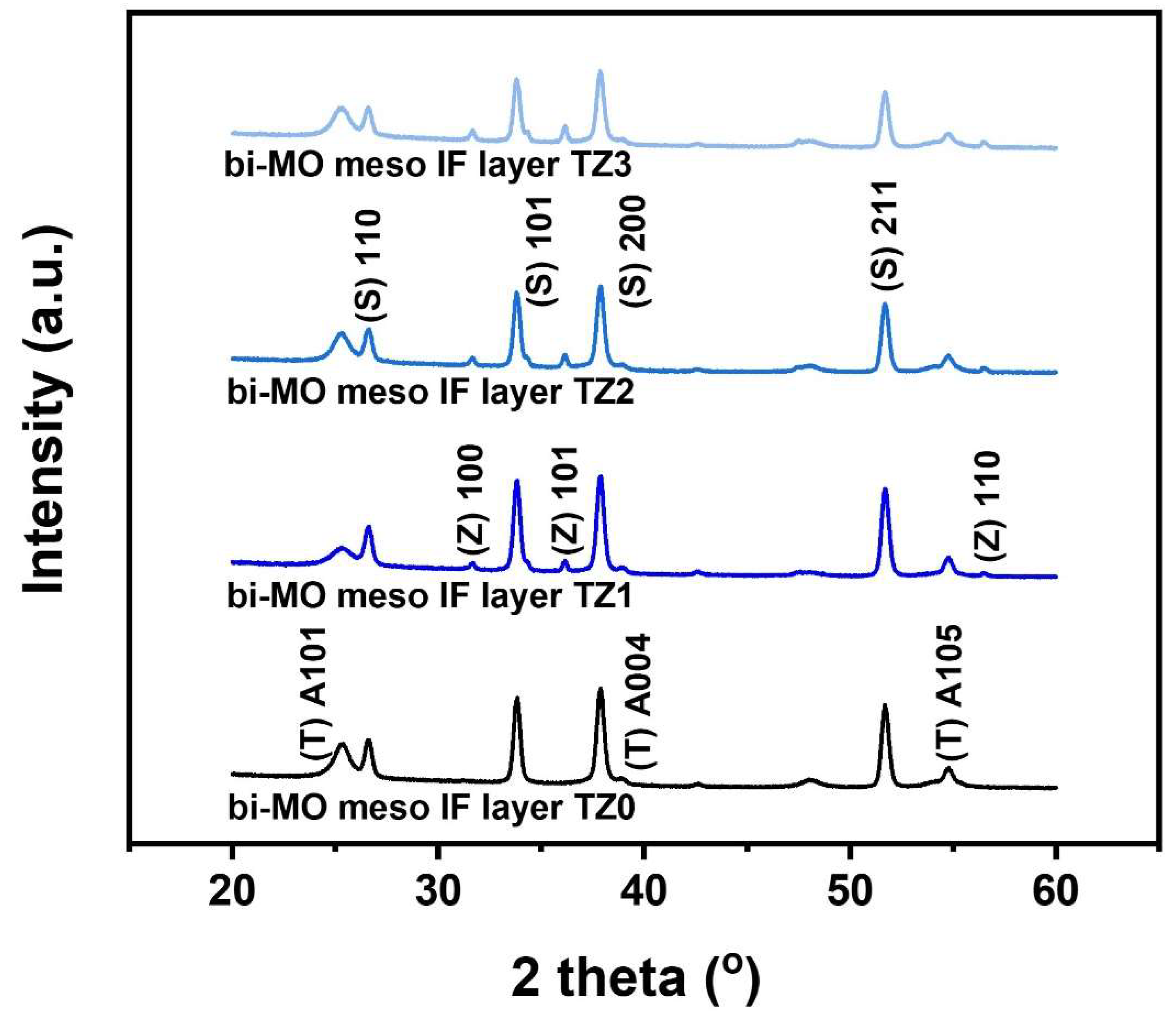
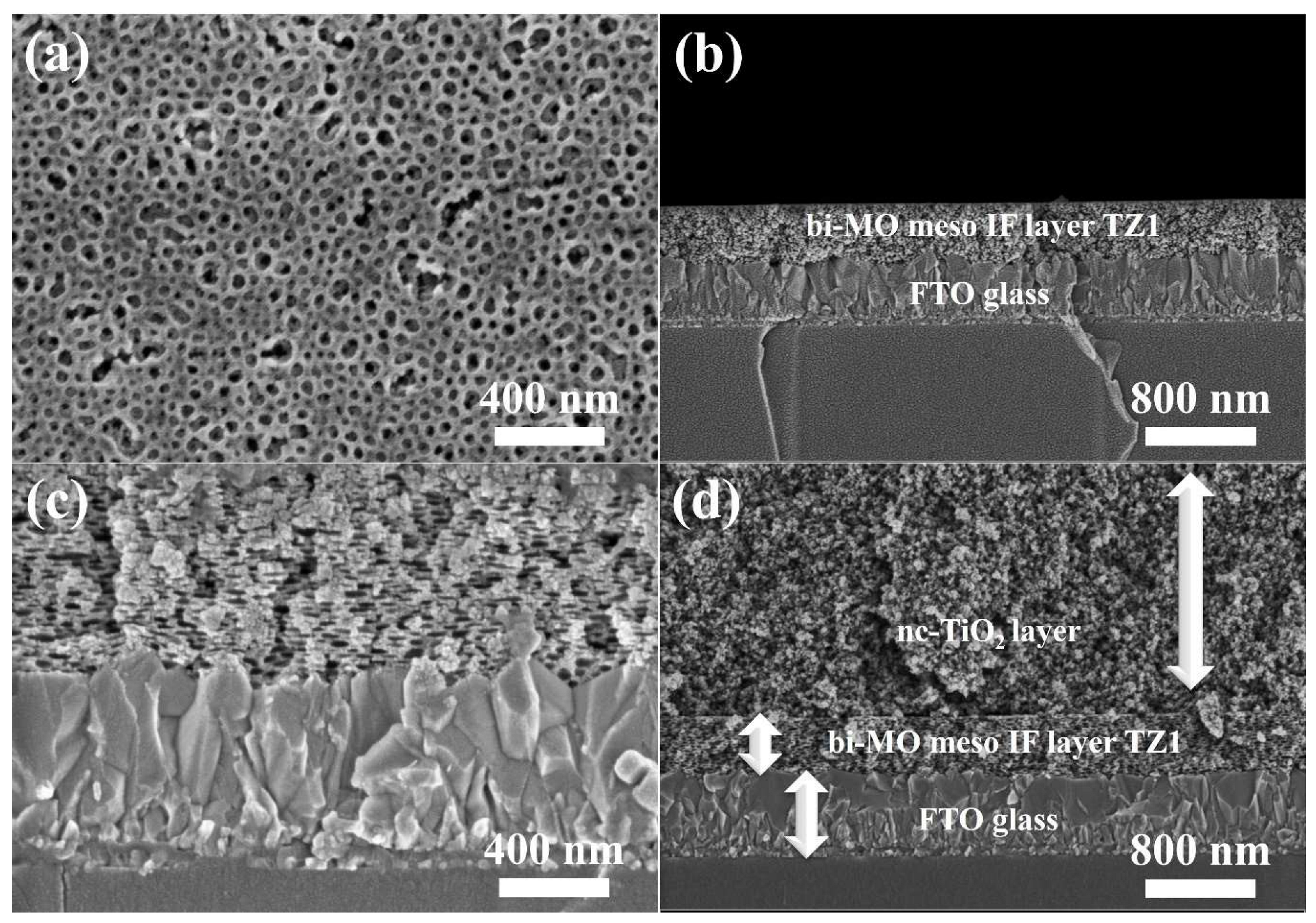
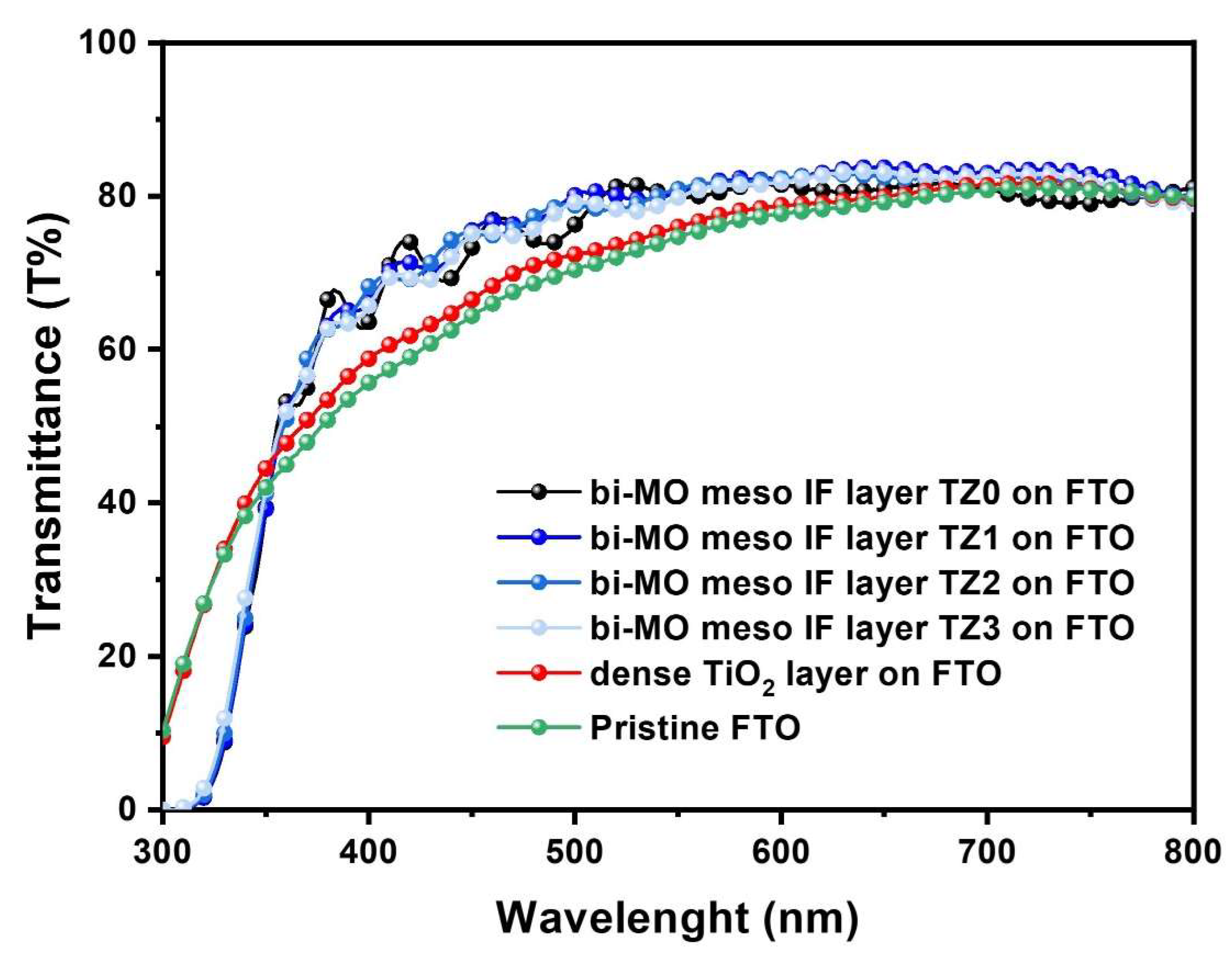
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155. [Google Scholar] [CrossRef]
- Swami, S.K.; Kumar, N.; Radu, D.R.; Cho, S.W.; Lee, J. Lithium Incorporation into TiO2 Photoanode for Performance Enhancement of Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2023, 6, 8599. [Google Scholar] [CrossRef]
- Yavuz, C.; Ela, S.E. Fabrication of g-C3N4-reinforced CdS nanosphere-decorated TiO2 nanotablet composite material for photocatalytic hydrogen production and dye-sensitized solar cell application. J. Alloy. Compd. 2023, 936, 168209. [Google Scholar] [CrossRef]
- Alizadeh, A.; Shariatinia, Z. Auspicious energy conversion performance of dye-sensitized solar cells based on Gd2O3-impregnated SmTiO3 perovskite/TiO2 nanocomposite photoelectrodes. Electrochim. Acta 2023, 450, 142280. [Google Scholar] [CrossRef]
- Koo, H.J.; Kim, Y.J.; Lee, Y.H.; Lee, W.I.; Kim, K.; Park, N.G. Nano-embossed Hollow Spherical TiO2 as Bifunctional Material for High-Efficiency Dye-Sensitized Solar Cells. Adv. Mater. 2008, 20, 195. [Google Scholar] [CrossRef]
- Yang, S.C.; Yang, D.J.; Kim, J.; Hong, J.M.; Kim, H.G.; Kim, I.D.; Lee, H. Hollow TiO2 Hemispheres Obtained by Colloidal Templating for Application in Dye-Sensitized Solar Cells. Adv. Mater. 2008, 20, 1059. [Google Scholar] [CrossRef]
- Arthi, G.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Muthamizhchelvan, C.; Ramaraj, S.G. Solvothermal synthesis of 3D hierarchical rutile TiO2 nanostructures for efficient dye-sensitized solar cells. Mater. Lett. 2023, 337, 133961. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Ding, Y.; Jia, X.; Zhong, D.; Zhang, X.; Zhao, J.; Fang, Y. Facile Synthesis of a Multifunctional SnO2 Nanoparticles/Nanosheets Composite for Dye-Sensitized Solar Cells. ACS Omega 2023, 8, 44578. [Google Scholar] [CrossRef]
- Vasanthapriya, R.; Neelakandeswari, N.; Uthayarani, K.; Chitra, M. Parthenium hysterophorous flower template assisted SnO2 nanostructure as photoanode for dye sensitized solar cell. Chem. Phys. Impact 2023, 7, 100308. [Google Scholar]
- DiMarco, B.N.; Sampaio, R.N.; James, E.M.; Barr, T.J.; Bennett, M.T.; Meyer, G.J. Efficiency Considerations for SnO2-Based Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 23923. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Soni, K.; Brajpuriya, R.; Lakshmi, N. Investigation of the structural and electrochemical properties of a ZnO-SnO2 composite and its electrical properties for application in dye-sensitized solar cells. N. J. Chem. 2023, 47, 7346. [Google Scholar] [CrossRef]
- Chen, W.; Qiu, Y.; Zhong, Y.; Wong, K.S.; Yang, S. High-Efficiency Dye-Sensitized Solar Cells Based on the Composite Photoanodes of SnO2 Nanoparticles/ZnO Nanotetrapods. J. Phys. Chem. A 2009, 114, 3127. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Wary, R.R.; Nair, R.G. Cu modified ZnO nanoflakes: An efficient visible light-driven photocatalyst and a promising photoanode for dye sensitized solar cell (DSSC). Solid State Sci. 2020, 104, 106290. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, L.; Gao, S.; Zhu, D.; Yu, X.; Cheng, S.; Zheng, D.; Xiong, Y. An investigation of the effects of ZnO inverse opal pore size in the composite of ZnO nanorods/ZnO inverse opal on the performance of quantum dot-sensitized solar cells. Dalton Trans. 2023, 52, 81. [Google Scholar] [CrossRef] [PubMed]
- Athithya, S.; Harish, S.; Ikeda, H.; Navaneethan, M.; Archana, J. Controlled synthesis of monodispersed ZnO nanospindle decorated TiO2 mesospheres for enhanced charge transport in dye-sensitized solar cells. CrystEngComm 2023, 25, 3198. [Google Scholar] [CrossRef]
- Han, J.; Fan, F.; Xu, C.; Lin, S.; Wei, M.; Duan, X.; Wang, Z.L. ZnO nanotube-based dye-sensitized solar cell and its application in self-powered devices. Nanotechnology 2010, 21, 405203. [Google Scholar] [CrossRef] [PubMed]
- Mylsamy, S.; Govindasamy, T.; Subramanian, B. Systematic exploration of defect-rich 2D nanopetal assembled 3D ZnO nanoflowers for improved photocurrent generation and photocatalytic performance. J. Environ. Chem. Eng. 2024, 12, 111700. [Google Scholar] [CrossRef]
- Kilic, B.; Günes, T.; Besirli, I.; Sezginer, M.; Tuzemen, S. Construction of 3-dimensional ZnO-nanoflower structures for high quantum and photocurrent efficiency in dye sensitized solar cell. Appl. Surf. Sci. 2014, 318, 32. [Google Scholar] [CrossRef]
- Mir, N.; Salavati-Niasari, M.; Davar, F. Preparation of ZnO nanoflowers and Zn glycerolate nanoplates using inorganic precursors via a convenient rout and application in dye sensitized solar cells. Chem. Eng. J. 2012, 181, 779. [Google Scholar] [CrossRef]
- Aseena, S.; Nelsa, A.; Babu, V.S.; Beena, S. Effect of Carbon Nanotube Content in ZnO/Carbon Nanotube Based Photoanode for Dye Sensitized Solar Cells. ECS J. Solid State Sci. Technol. 2022, 11, 061011. [Google Scholar]
- Vijayanath, S.; Janaki, K.; Gopal, R.; Ragupathi, C.; Rangasamy, B.; Alam. M. M. Fabrication of highly efficient and cost-effective dye-sensitized solar cells using ZnO/MWCNT nanocomposite as photoanode. J. Solid State Electrochem. 2023, 27, 183. [Google Scholar] [CrossRef]
- Thate, A.G.; Pakhare, K.S.; Patil, S.S.; Bhuse, V.M. Fabrication of TiO2-ZnO nanocomposite photoanodes to enhance the dye-sensitized solar cell efficiency. Res. Chem. Intermed. 2023, 49, 147. [Google Scholar] [CrossRef]
- Yang, P.; Xiao, X.; Li, Y.; Ding, Y.; Qiang, P.; Tan, X.; Jin, H. Hydrogenated ZnO Core–Shell Nanocables for Flexible Supercapacitors and Self-Powered Systems. ACS Nano 2013, 7, 2617. [Google Scholar] [CrossRef]
- Kumar, A.; Nayak, D.; Sahoo, P.; Nandi, B.K.; Saxena, V.K.; Thangavel, R. Fabrication of porous and visible light active ZnO nanorods and ZnO@TiO2 core-shell photocatalysts for self-cleaning applications. Phys. Chem. Chem. Phys. 2023, 25, 16423. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Datt, R.; Gupta, V.; Arya, S. Synthesis and characterization of ZnO@WO3 core/shell nanoparticles as counter electrode for dye-sensitized solar cell. Surf. Interfaces 2022, 30, 101920. [Google Scholar] [CrossRef]
- Kanmani, S.S.; Ramachandran, K. Synthesis and characterization of TiO2/ZnO core/shell nanomaterials for solar cell applications. Renew. Energy 2012, 43, 149. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Gratzel, M. Analysis of Electron Transfer Properties of ZnO and TiO2 Photoanodes for Dye-Sensitized Solar Cells. ACS Nano 2014, 8, 2261. [Google Scholar] [CrossRef]
- Anta, J.A.; Guillén, E.; Tena-Zaera, R. Electron Transport and Recombination in ZnO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 11413. [Google Scholar] [CrossRef]
- Cao, D.; Wang, A.; Yu, X.; Yin, H.; Zhang, J.; Mi, B.; Gao, Z. Room-temperature preparation of TiO2/graphene composite photoanodes for efficient dye-sensitized solar cells. J. Colloid Interface Sci. 2021, 586, 326. [Google Scholar] [CrossRef]
- Yyagi, J.; Gupta, H.; Purohit, L.P. Mesoporous ZnO/TiO2 photoanodes for quantum dot sensitized solar cell. Opt. Mater. 2021, 115, 111014. [Google Scholar]
- Buapuean, T.; Jarudilokkul, S. Synthesis of mesoporous TiO2 with colloidal gas aphrons, colloidal liquid aphrons, and colloidal emulsion aphrons for dye-sensitized solar cells. Mater. Today Chem. 2020, 16, 100235. [Google Scholar] [CrossRef]
- Yan, H.; Chen, M.; Liu, W.; Wang, P.; Liu, M.; Liu, Y.; Ye, L.; Gu, M. Ti3C2 MXene quantum dots decorated mesoporous TiO2/Nb2O5 functional photoanode for dye-sensitized solar cells. Opt. Mater. 2023, 140, 113902. [Google Scholar] [CrossRef]
- Graddage, N.; Ouyang, J.; Lu, J.; Chu, T.-Y.; Zhang, Y.; Li, Z.; Wu, X.; Malenfant, P.R.L.; Tao, Y. Near-Infrared-II Photodetectors Based on Silver Selenide QuantumDots on Mesoporous TiO2 Scaffolds. ACS Appl. Nano Mater. 2020, 3, 12209. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Tang, X.; Chen, Y.; Wang, X.; Deng, Z.; Wang, C.; Yang, X.; Sun, B. Densely Packed D−π–A Photosensitizers on TiO2 Enable Efficient Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2023, 6, 4229. [Google Scholar] [CrossRef]
- Wu, T.; Deng, G.; Zhen, C. Metal oxide mesocrystals and mesoporous single crystals: Synthesis, properties and applications in solar energy conversion. J. Mater. Sci. Technol. 2021, 73, 9. [Google Scholar] [CrossRef]
- Cao, D.; Yin, H.; Yu, X.; Zhang, J.; Jiao, Y.; Zheng, W.; Mi, B.; Gao, Z. Role of Modifying Photoanodes by Organic Titanium on Charge Collection Efficiency Enhancement in Dye-Sensitized Solar Cells. Adv. Eng. Mater. 2020, 22, 1901071. [Google Scholar] [CrossRef]
- Park, J.T.; Koh, J.H.; Seo, J.A.; Roh, D.K.; Kim, J.H. Templated Formation of Silver Nanoparticles Using Amphiphilic Poly(epichlorohydrine-g-styrene) Film. Macromol. Res. 2009, 17, 301. [Google Scholar] [CrossRef]
- Park, J.T.; Moon, J.; Choi, G.H.; Lim, S.M.; Kim, J.H. Facile graft copolymer template synthesis of mesoporous polymeric metal-organic frameworks to produce mesoporous TiO2: Promising platforms for photovoltaic and photocatalytic applications. J. Ind. Eng. Chem. 2020, 84, 384. [Google Scholar] [CrossRef]
- Lim, J.M.; Park, J.; Park, J.T.; Bae, S. Preparation of quasi-solid-state electrolytes using a coal fly ash derived zeolite-X and -A for dye-sensitized solar cells. J. Ind. Eng. Chem. 2019, 71, 378. [Google Scholar] [CrossRef]
- Lim, S.M.; Moon, J.; Baek, U.C.; Lee, J.Y.; Chae, Y.; Park, J.T. Shape-Controlled TiO2 Nanomaterials-Based Hybrid Solid-State Electrolytes for Solar Energy Conversion with a Mesoporous Carbon Electrocatalyst. Nanomaterials 2021, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381. [Google Scholar] [CrossRef]
- Toro, R.G.; Diab, M.; Caro, T.d.; Al-shemy, M.; Adel, A.; Caschera, D. Study of the effect of Titanium Dioxide Hydrosol on the Photocatalytic and Mechanical Properties of Paper Sheets. Materials 2020, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas Sensing Properties and Mechanism of Nano-SnO2-Based Sensor for Hydrogen and Carbon Monoxide. J. Nanomater. 2012, 20, 612420. [Google Scholar] [CrossRef]
- Zheng, S.; Li, X.; Zhang, J.; Wang, J.; Zhao, C.; Hu, X.; Wu, Y.; He, Y. One-step preparation of MoOx/ZnS/ZnO composite and its excellent performance in piezocatalytic degradation of Rhodamine B under ultrasonic vibration. J. Environ. Sci. 2023, 125, 1. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, R.J.; Caseri, W.R.; Smith, P.; Tervoort, T. Polymer-TiO2 Nanocomposites: A Route Towards Visually Transparent Broadband UV Filters and High Refractive Index Materials. Macromol. Mater. Eng. 2003, 288, 44. [Google Scholar] [CrossRef]
- Gumus, C.; Ozkendir, O.M.; Kavak, H.; Ufuktepe, Y. Structural and optical properties of zinc oxide thin films prepared by spray pyrolysis method. J. Optoelectron. Adv. Mater. 2006, 8, 299. [Google Scholar]
- Mohsenzadegan, N.; Nouri, E.; Mohammadi, M.R. Efficient quasi-solid-state dye-sensitized solar cells aided by mesoporous TiO2 beads and a non-volatile gel polymer electrolyte. CrystEngComm 2023, 25, 3210. [Google Scholar] [CrossRef]
- Wen, J.; Liu, Y.; Li, T.; Liu, C.; Wang, T.; Liu, Y.; Zhou, Y.; Li, G.; Sun, Z. Low Cost and Strongly Adsorbed Melamine Formaldehyde Sponge Electrolyte for Nontraditional Quasi-Solid Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2023, 6, 4952. [Google Scholar] [CrossRef]
- Fang, D.; Tan, Y.; Ren, Y.; Zheng, S.; Xiong, F.; Wang, A.; Chang, K.; Mi, B.; Cao, D.; Gao, Z. Simple Solution Preparation of Cs2SnI6 Films and Their Applications in Solid-State DSSCs. ACS Appl. Mater. Interfaces 2023, 15, 32538. [Google Scholar] [CrossRef]
- Moon, J.; Shin, W.; Park, J.T.; Jang, H. Solid-state solar energy conversion from WO3 nano and microstructures with charge transportation and light scattering characteristics. Nanomaterials 2019, 9, 1797. [Google Scholar] [CrossRef]
- Lim, S.M.; Moon, J.; Choi, G.H.; Baek, U.C.; Lim, J.H.; Park, J.T.; Kim, J.H. Surface carbon shell-functionalized ZrO2 as nanofiller in polymer gel electrolytes-based dye-sensitized solar cells. Nanomaterials 2019, 9, 1418. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, G.H.; Moon, J.; Choi, W.S.; Park, J.T. 1D Co4S3 Nanoneedle Array with Mesoporous Carbon Derived from Double Comb Copolymer as an Efficient Solar Conversion Catalyst. Appl. Surf. Sci. 2021, 535, 147637. [Google Scholar] [CrossRef]
- Song, E.; Moon, J.; Lee, J.Y.; Lee, C.O.; Chi, W.S.; Park, J.T. High-Voltage Solar Energy Conversion Based on a ZIF-67 Derived Binary Redox-Quasi-Solid-State Electrolyte. J. Electroanal. Chem. 2021, 893, 115264. [Google Scholar] [CrossRef]

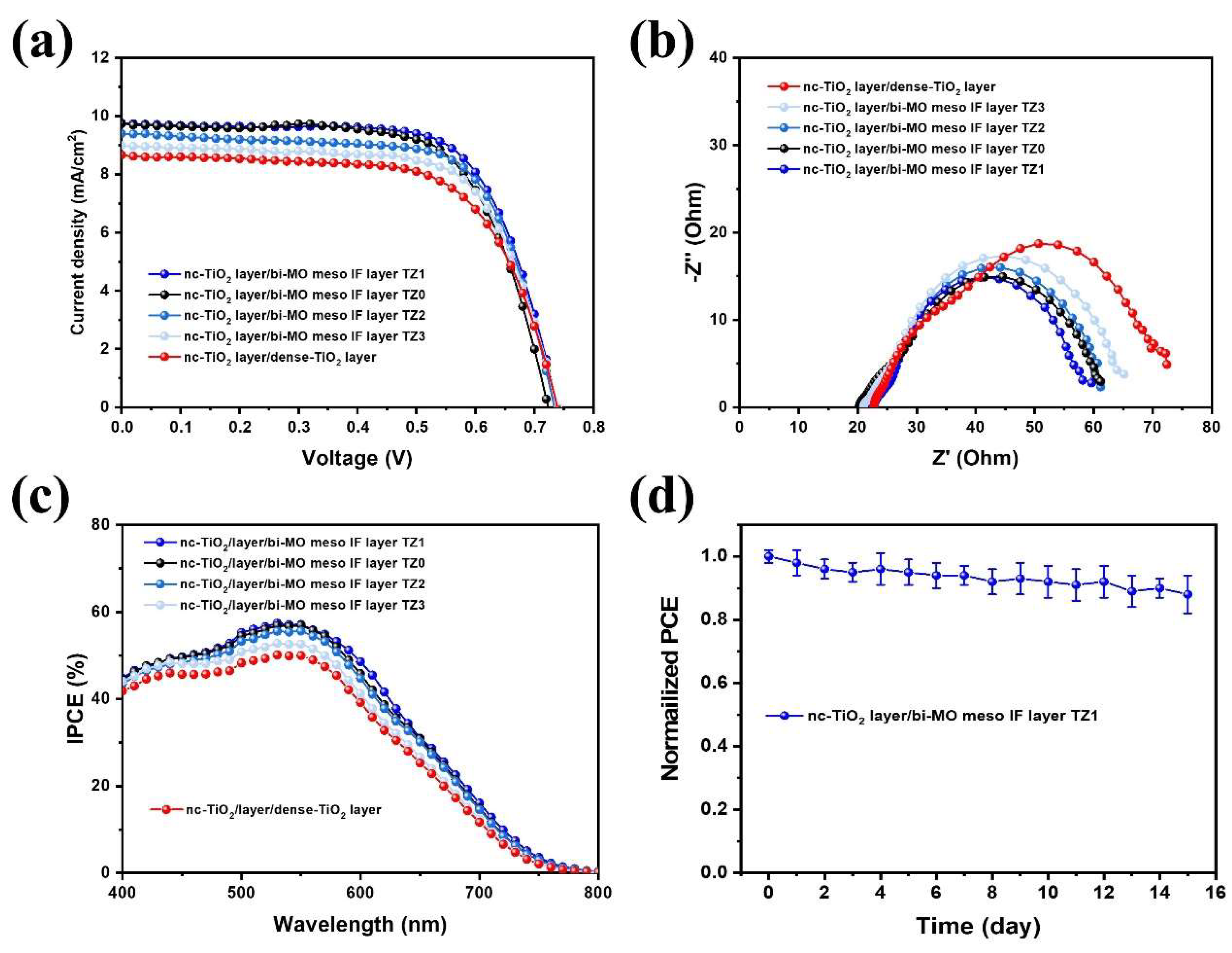
| Photoanode | Voc (V) | Jsc d (mA/cm2) | Jsc e (mA/cm2) | FF | η (%) | Dye Adsorption Value (nmol/cm2) |
|---|---|---|---|---|---|---|
| nc-TiO2 layer /bi-MO meso IF layer TZ0 | 0.72 ± 0.04 | 9.7 ± 0.5 | 9.3 | 0.68 ± 0.02 | 4.7 ± 0.15 | 68 |
| nc-TiO2 layer /bi-MO meso IF layer TZ1 | 0.74 ± 0.04 | 9.8 ± 0.5 | 9.5 | 0.69 ± 0.02 | 5.0 ± 0.15 | 70 |
| nc-TiO2 layer /bi-MO meso IF layer TZ2 | 0.73 ± 0.05 | 9.4 ± 0.6 | 9.2 | 0.69 ± 0.03 | 4.8 ± 0.20 | 69 |
| nc-TiO2 layer /bi-MO meso IF layer TZ3 | 0.73 ± 0.05 | 9.0 ± 0.7 | 8.9 | 0.68 ± 0.02 | 4.6 ± 0.20 | 64 |
| nc-TiO2 layer /dense-TiO2 layer | 0.72 ± 0.04 | 8.7 ± 0.7 | 8.6 | 0.67 ± 0.03 | 4.2 ± 0.20 | 55 |
| Photoanode | Rs (Ω) | R1 (Ω) | R2 (Ω) | Ws (Ω) |
|---|---|---|---|---|
| nc-TiO2 layer /bi-MO meso IF layer TZ0 | 19.9 ± 3.0 | 9.9 ± 1.5 | 37 ± 5.5 | 2.4 ± 0.3 |
| nc-TiO2 layer /bi-MO meso IF layer TZ1 | 22.2 ± 2.8 | 9.6 ± 1.3 | 33 ± 4.5 | 2.1 ± 0.1 |
| nc-TiO2 layer /bi-MO meso IF layer TZ2 | 21.8 ± 3.1 | 9.7 ± 1.6 | 38 ± 5.7 | 2.4 ± 0.2 |
| nc-TiO2 layer /bi-MO meso IF layer TZ3 | 21.0 ± 3.0 | 9.9 ± 1.5 | 40 ± 5.6 | 2.6 ± 0.2 |
| nc-TiO2 layer /dense-TiO2 layer | 23.2 ± 3.3 | 10.1 ± 1.8 | 43 ± 5.8 | 3.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.M.; Jeong, H.; Moon, J.; Park, J.T. Amphiphilic Graft Copolymers as Templates for the Generation of Binary Metal Oxide Mesoporous Interfacial Layers for Solid-State Photovoltaic Cells. Nanomaterials 2024, 14, 352. https://doi.org/10.3390/nano14040352
Lim SM, Jeong H, Moon J, Park JT. Amphiphilic Graft Copolymers as Templates for the Generation of Binary Metal Oxide Mesoporous Interfacial Layers for Solid-State Photovoltaic Cells. Nanomaterials. 2024; 14(4):352. https://doi.org/10.3390/nano14040352
Chicago/Turabian StyleLim, Seung Man, Hayeon Jeong, Juyoung Moon, and Jung Tae Park. 2024. "Amphiphilic Graft Copolymers as Templates for the Generation of Binary Metal Oxide Mesoporous Interfacial Layers for Solid-State Photovoltaic Cells" Nanomaterials 14, no. 4: 352. https://doi.org/10.3390/nano14040352
APA StyleLim, S. M., Jeong, H., Moon, J., & Park, J. T. (2024). Amphiphilic Graft Copolymers as Templates for the Generation of Binary Metal Oxide Mesoporous Interfacial Layers for Solid-State Photovoltaic Cells. Nanomaterials, 14(4), 352. https://doi.org/10.3390/nano14040352






