Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis of ZnV and CdV/ZnV
2.3. Characterization Techniques
2.4. Photocatalytic Hydrogen Production Experiments
3. Results and Discussion
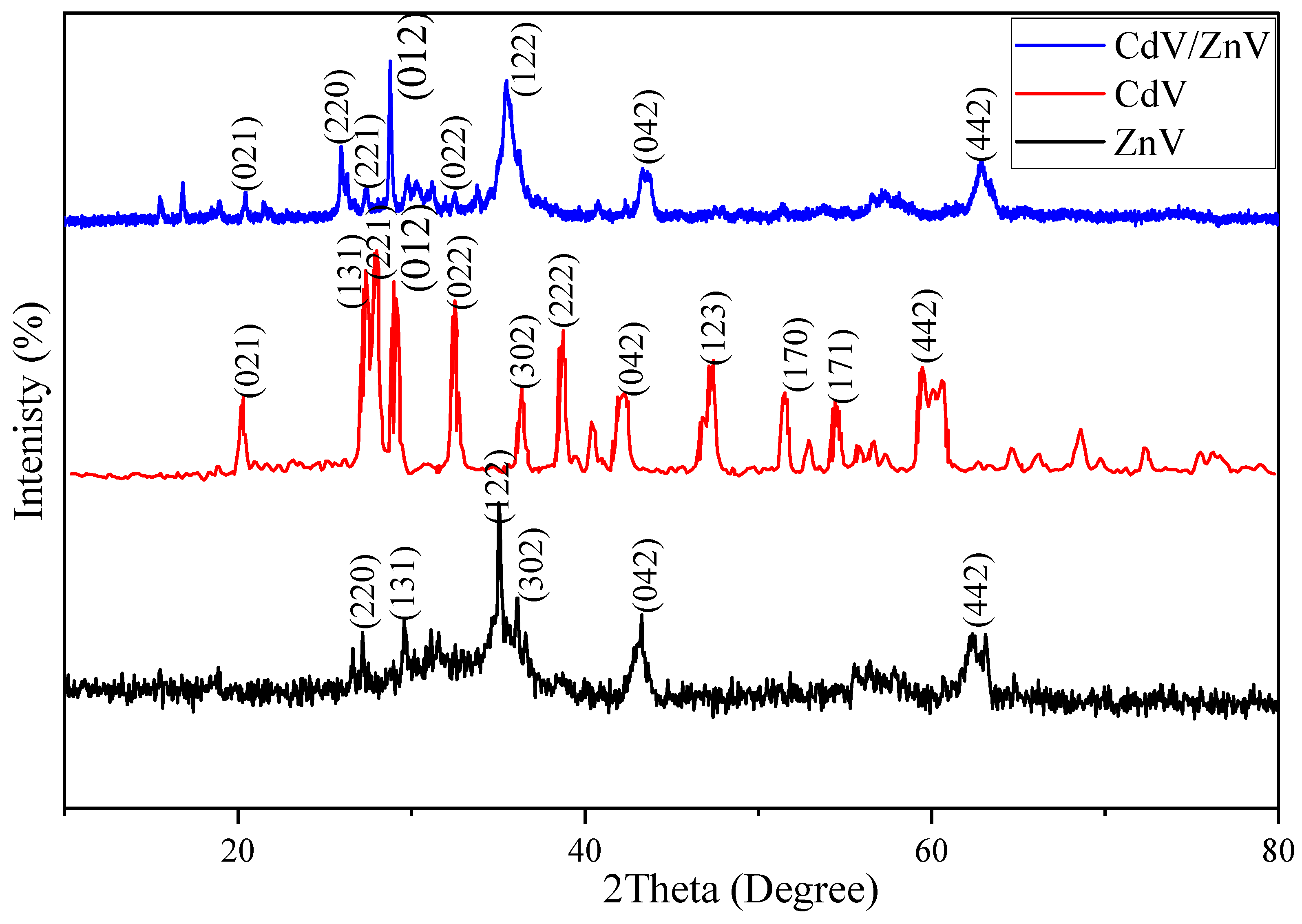
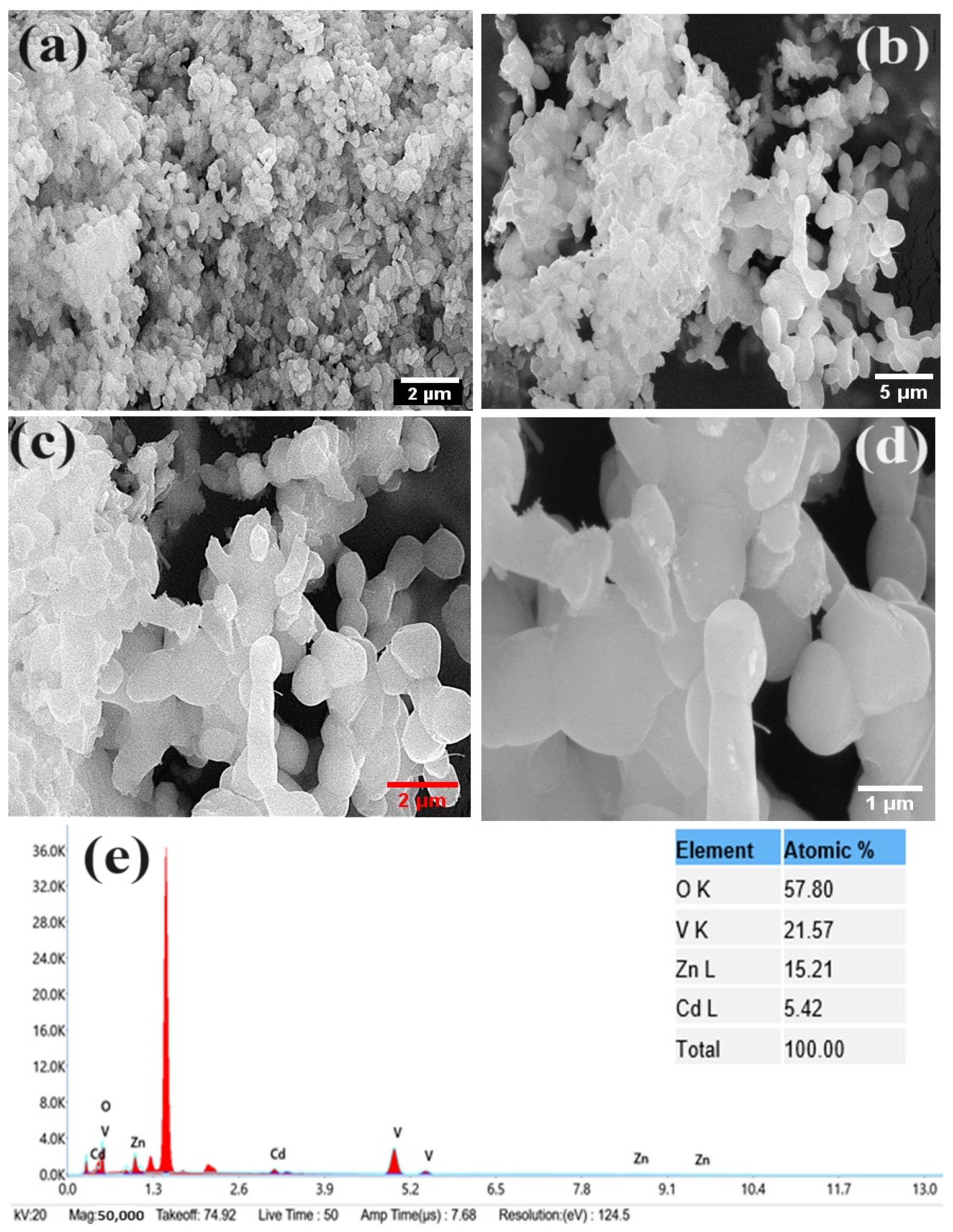
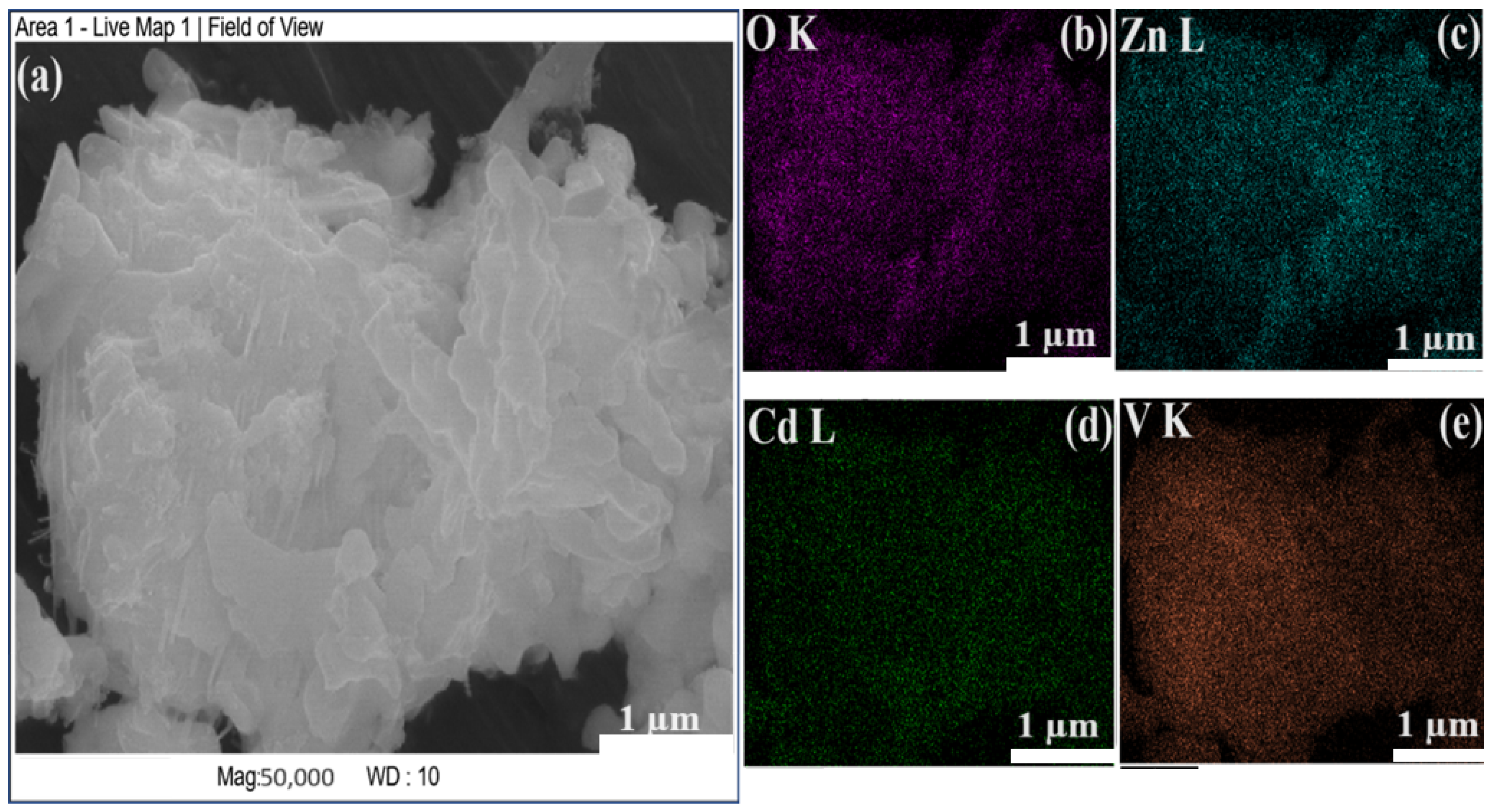
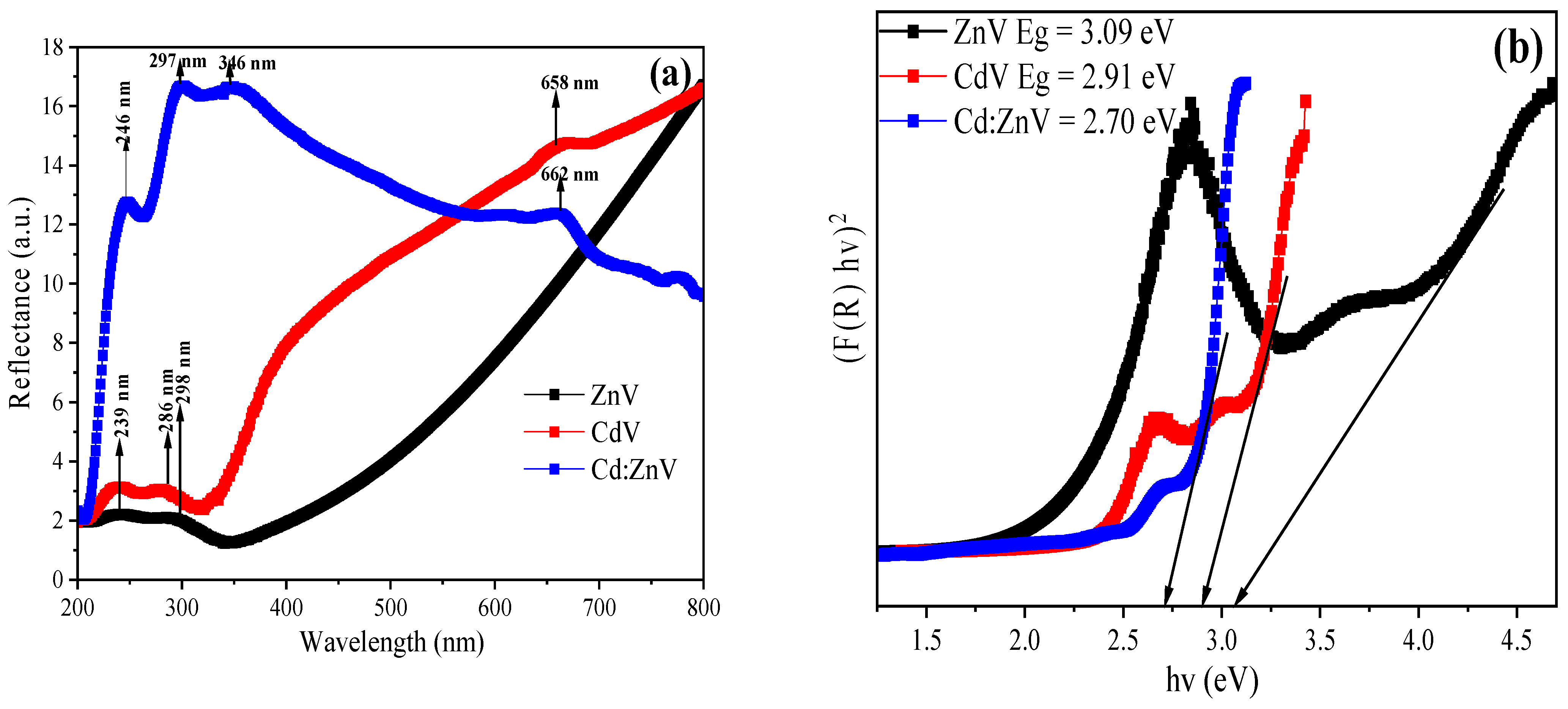
3.1. Material Characterization Studies
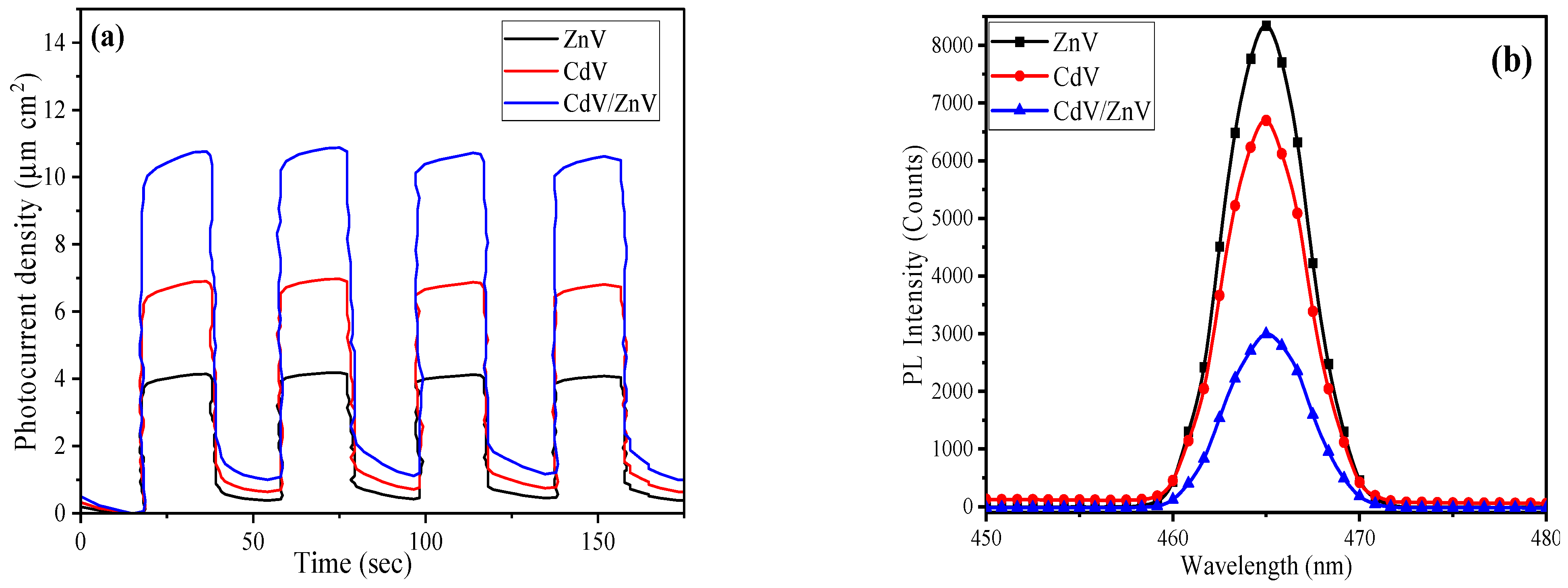
3.2. Hydrogen Production Experiments
3.2.1. Selection of Sacrificial Reagent
3.2.2. Effect of Various Reaction Parameters
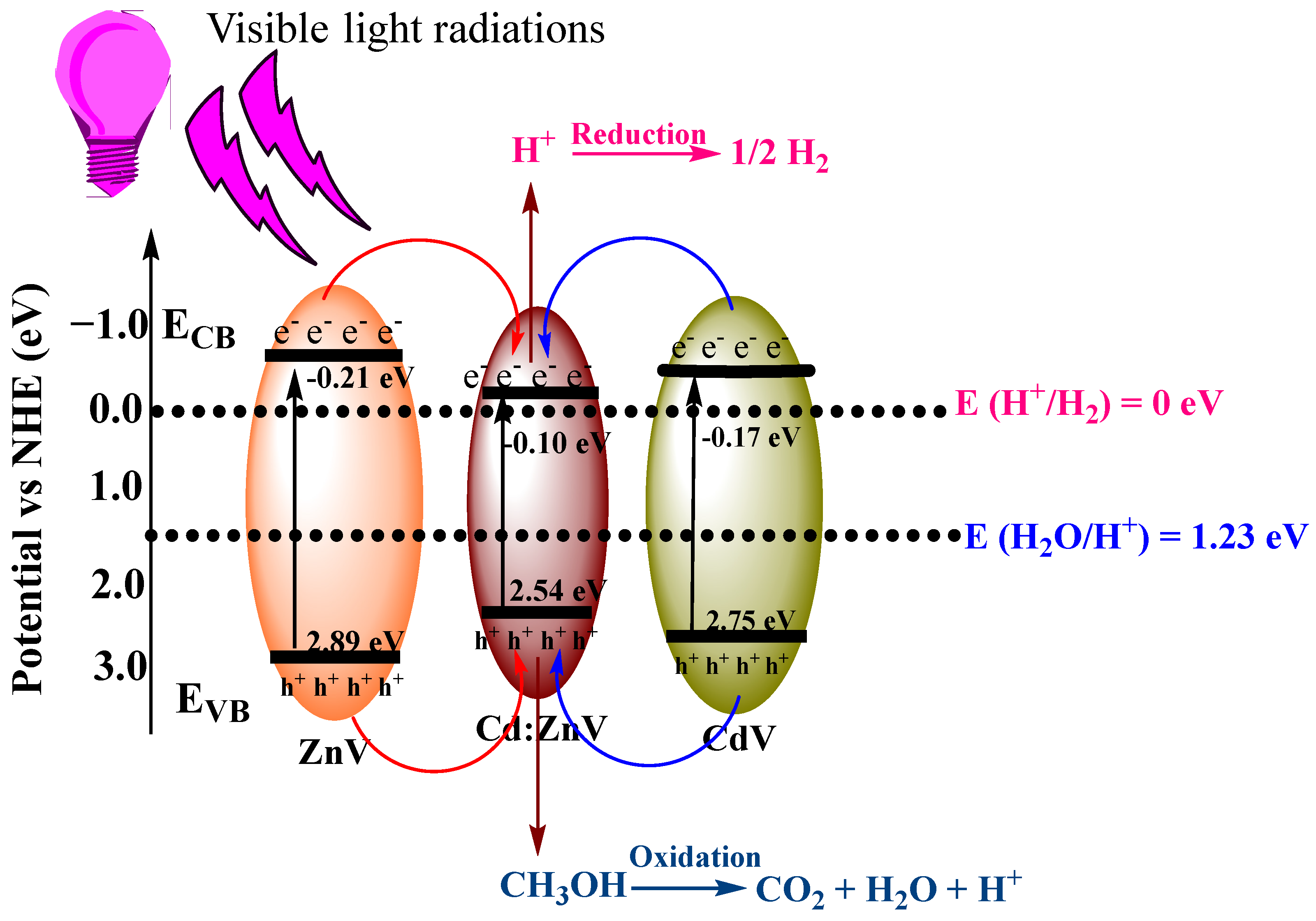
3.3. Mechanism of Reaction
3.4. Comparison with Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goveas, L.C.; Nayak, S.; Vinayagam, R.; Selvaraj, A. Pugazhendhi, Recent advances of Nano-photocatalysts involved in hydrogen production by water splitting. Fuel 2023, 348, 128460. [Google Scholar] [CrossRef]
- Zheng, D.; Xue, Y.; Wang, J.; Varbanov, P.S.; Klemeš, J.J.; Yin, C. Nanocatalysts in photocatalytic water splitting for green hydrogen generation: Challenges and opportunities. J. Clean. Prod. 2023, 414, 137700. [Google Scholar] [CrossRef]
- Goodarzi, N.; Peyman, Z.A.; Khani, E.; Moshfegh, A.Z. Recent progress on semiconductor heterogeneous photocatalysts in clean energy production and environmental remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Su, S.; Zhang, Z.; Jiang, Y.; Xu, J. NiFe-Layered Double Hydroxides/Lead-free Cs2AgBiBr6 Perovskite 2D/2D Heterojunction for Photocatalytic CO2 Conversion. Inorg. Chem. 2023, 62, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Dong, Z.; Jiang, Y.; Li, X.; Chu, Y.; Xu, J. Lead-Free Cs2AgBiBr6 Nanocrystals Confined in MCM-48 Mesoporous Molecular Sieve for Efficient Photocatalytic CO2 Reduction. Sol. RRL 2023, 7, 2300038. [Google Scholar] [CrossRef]
- Qian, J.; Hu, H.; Liang, Y.; Zhang, Z. Mesoporous TiO2 matrix embeded with Cs2CuBr4 perovskite quantum dots as a step-scheme-based photocatalyst for boosting charge separation and CO2 photoconversion. Appl. Surf. Sci. 2024, 648, 159084. [Google Scholar] [CrossRef]
- Song, M.; Wu, Y.; Zhao, Y.; Du, C.; Su, Y. Structural insight on defect-rich tin oxide for smart band alignment engineering and tunable visible-light-driven hydrogen evolution. Inorg. Chem. 2020, 59, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Mohana, P.; Isacfranklin, M.; Yuvakkumar, R.; Ravi, G.; Kungumadevi, L.; Arunmetha, S.; Han, J.H.; Hong, S.I. Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 280. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Li, J.; Cabrero-Antonino, M.; Silva, I.F.; Peng, L.; Tarakina, N.V.; Navalón, S.; García, H.; Antonietti, M. Rational design of a carbon/potassium poly(heptazine imide) heterojunction for enhanced photocatalytic H2 and H2O2 evolution. J. Mater. Chem. A Mater. 2023, 12, 475–482. [Google Scholar] [CrossRef]
- Bhuiyan, T.H.; Rahman, A.; Rahman, A.; Sultana, R.; Mostafa, R.; Tania, A.H.; Sarker, A.R. Synthesis and characterization of high-quality cobalt vanadate crystals and their applications in lithium-ion batteries. Cogent Phys. 2016, 3, 1265778. [Google Scholar] [CrossRef]
- Yavuz, C.; Erten-Ela, S. Solar Light-Responsive Fe2O3/CdS/g-C3N4 Ternary photocatalyst for photocatalytic hydrogen production and photodegradation of methylene blue. J. Alloy Compd. 2022, 908, 164584. [Google Scholar] [CrossRef]
- Huang, G.; Ye, W.; Lv, C.; Butenko, D.S.; Yang, C.; Zhang, G.; Lu, P.; Xu, Y.; Zhang, S.; Wang, H. Hierarchical red phosphorus incorporated TiO2 hollow sphere heterojunctions toward superior photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 108, 18–25. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Saruyama, M.; Takahata, R.; Sato, R.; Kitahama, Y.; Matsuzaki, H.; Yamada, T.; Hisatomi, T.; Domen, K.; Teranishi, T. Bimetallic Synergy in Ultrafine Cocatalyst Alloy Nanoparticles for Efficient Photocatalytic Water Splitting. Adv. Funct. Mater. 2022, 32, 2202987. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, W.; Zhang, H.; Wang, W.; Xu, S.; Song, H. Inhibited long-scale energy transfer in dysprosium doped yttrium vanadate Inverse Opal. J. Phys. Chem. C 2012, 116, 2297–2302. [Google Scholar] [CrossRef]
- Guo, H.; Guo, D.; Zheng, Z.; Wen, W.; Chen, J. Hydrothermal synthesis and visible light photocatalytic activities of Zn3(VO4)2 Nanorods. J. Mater. Res. 2014, 29, 2934–2941. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Y.; Jiang, G. 3D Hierarchical Zn3(OH)2V2O7 2H2O and Zn3(VO4)2 Microspheres: Synthesis, Characterization and Photoluminescence. Mater. Res. Bull. 2012, 47, 18–23. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nawaz, T.; Sagir, M.; Alzaid, M.; Alrobei, H.; Shahzad, K.; Ali, A.M. Muhammad, Functionalized Role of Highly Porous Activated Carbon in Bismuth Vanadate Nanomaterials for Boosted Photocatalytic Hydrogen Evolution and Synchronous Activity in Water. Int. J. Hydrogen Energy 2021, 46, 39778–39785. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Ma, X.; Zhang, Y.; Wang, H. Properties of iron vanadate over CdS nanorods for efficient photocatalytic hydrogen production. New J. Chem. 2019, 43, 3609–3618. [Google Scholar] [CrossRef]
- Iqbal, T.; Hassan, A.; Ijaz, M.; Salim, M.; Farooq, M.; Zafar, M.; Tahir, M.B. Chromium incorporated copper vanadate nano-materials for hydrogen evolution by water splitting. Appl. Nanosci. 2021, 11, 1661–1671. [Google Scholar] [CrossRef]
- Seabold, J.A.; Neale, N.R. All First Row Transition Metal Oxide Photoanode for Water Splitting Based on Cu3V2O8. Chem. Mater. 2015, 27, 1005–1013. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, Y.; Wu, D.; Yang, Y.; Cao, Y. TiO2/Vanadate (Sr10V6O25, Ni3V2O8, Zn2V2O7) Heterostructured Photocatalysts with Enhanced Photocatalytic Activity for Photoreduction of CO2 into CH4. Nanoscale 2015, 8, 949–958. [Google Scholar] [CrossRef]
- Kim, M.; Joshi, B.; Yoon, H. Electrosprayed copper hexaoxodivanadate (CuV2O6) and pyrovanadate(Cu2V2O7) photoanodes for efficient solar water splitting. J. Alloys Compd. 2017, 708, 444–450. [Google Scholar] [CrossRef]
- Ronde, H.; Blasse, G. The nature of the electronic transitions of the vanadate group. J. Inorg. Nucl. Chem. 1978, 40, 215–219. [Google Scholar] [CrossRef]
- Min, X.; Huang, Z.; Fang, M.; Tang, Y.C.; Wu, X. Luminescence Properties of Self-Activated M3(VO4)2 (M = Mg, Ca, Sr, and Ba) Phosphors Synthesized by Solid-State Reaction Method. J. Nanosci. Nanotechnol. 2016, 16, 3684–3689. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ababtain, A.S.; Alanazi, H.S.; Alshayiqi, A.A.; Hasan, I. Zinc Vanadate (Zn3V2O8) immobilized multiwall carbon nanotube (MWCNT) heterojunction as an efficient photocatalyst for visible light driven hydrogen production. Molecules 2023, 28, 1362. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, F.A.; Marghany, A.E.; Abduh, N.A.Y.; Hasan, I. Hydrothermal Synthesis of a Magnesium Vanadate-Functionalized Reduced Graphene Oxide Nanocomposite for an Efficient Photocatalytic Hydrogen Production. ACS Omega 2023, 8, 31493–31499. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Marghany, A.E.; Abduh, N.A.Y.; Hasan, I. Efficient light-driven hydrogen evolution and azo dye degradation over the GdVO4@g-C3N4 heterostructure. RSC Adv. 2023, 13, 20417–20429. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ababtain, A.S.; Alanazi, H.S.; Al-Nafaei, W.S.; Hasan, I. Synthesis of Zn3V2O8/rGO nanocomposite for photocatalytic hydrogen production. Inorganics 2023, 11, 93. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Lee, S.H.; Ryu, K.S. Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications. RSC Adv. 2015, 5, 91822–91828. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y.; Liu, Y.; Wang, P.; Li, Y.; Huang, B. Doping strategy to promote the charge separation in BiVO4 photoanodes. Appl. Catal. B 2017, 211, 258–265. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Ravindranadh, K.; Reddy, K.R.; Shim, J.; Cheolho, B. Au-doped BiVO4 nanostructure-based photoanode with enhanced photoelectrochemical solar water splitting and electrochemical energy storage ability. Appl. Surf. Sci. 2021, 545, 149030. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A Mater. 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Quang, N.D.; Majumder, S.; Van, P.C.; Jeong, J.R.; Kim, C.; Kim, D. Co3O4/reduced graphene oxide/BiVO4 nanorod as high performance photoanode for water oxidation. Electrochim. Acta 2020, 364, 137283. [Google Scholar] [CrossRef]
- Pan, Y.; Qiao, K.; Ning, C.; Wang, X.; Liu, Z.; Chen, Z. Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation. Nanomaterials 2023, 13, 2964. [Google Scholar] [CrossRef] [PubMed]
- Saif, O.M.; Elogail, Y.; Abdolkader, T.M.; Shaker, A.; Zekry, A.; Abouelatta, M.; Salem, M.S.; Fedawy, M. Comprehensive Review on Thin Film Homojunction Solar Cells: Technologies, Progress and Challenges. Energies 2023, 16, 4402. [Google Scholar] [CrossRef]
- Wang, X.; Xia, R.; Muhire, E.; Jiang, S.; Huo, X.; Gao, M. Highly enhanced photocatalytic performance of TiO2 nanosheets through constructing TiO2/TiO2 quantum dots homojunction. Appl. Surf. Sci. 2018, 459, 9–15. [Google Scholar] [CrossRef]
- Tay, Q.; Liu, X.; Tang, Y.; Jiang, Z.; Sum, T.C.; Chen, Z. Enhanced photocatalytic hydrogen production with synergistic two-phase anatase/brookite TiO2 nanostructures. J. Phys. Chem. C 2013, 117, 14973–14982. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Chen, R.; Hu, F.; Zou, H.; Wei, M. A 3-D chiral organic–inorganic hybrid zinc vanadate assembled from helical units. Dalton Trans. 2013, 42, 5603–5606. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, J.; Hojamberdiev, M.; Lu, Z.; Ren, B.; Xu, Y. Effect of SDS on morphology tailoring of GdVO4:Eu3+ powders under hydrothermal conditions in a wide pH range. J. Alloys Compd. 2014, 597, 282–290. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Zhang, L.; Zhang, Z. Design and controllable synthesis of α-/γ-Bi2O3 homojunction with synergetic effect on photocatalytic activity. Chem. Eng. J. 2012, 211–212, 161–167. [Google Scholar] [CrossRef]
- Martha, S.; Mansingh, S.; Parida, K.M.; Thirumurugan, A. Exfoliated metal free homojunction photocatalyst prepared by a biomediated route for enhanced hydrogen evolution and Rhodamine B degradation. Mater. Chem. Front. 2017, 1, 1641–1653. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; Du, X.; Zhang, Y. Vertically Aligned Nanosheets-Array-like BiOI Homojunction: Three-in-One Promoting Photocatalytic Oxidation and Reduction Abilities. ACS Sustain. Chem. Eng. 2017, 5, 5253–5264. [Google Scholar] [CrossRef]
- Mazloom, F.; Masjedi-Arani, M.; Salavati-Niasari, M. Controllable synthesis, characterization and photocatalytic studies on cadmium vanadate nanostructures. J. Mol. Liq. 2016, 220, 566–572. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Chen, X.; Ning, X.; Zhan, L.; Zhou, X. Construction of cerium oxide nanoparticles immobilized on the surface of zinc vanadate nanoflowers for accelerated photocatalytic degradation of tetracycline under visible light irradiation. J. Colloid Interface Sci. 2021, 587, 831–844. [Google Scholar] [CrossRef]
- Kubelka, P. Ein Beitrag Zur Optik Der Farbanstriche (Contribution to the Optic of Paint). Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Hussain, E.; Majeed, I.; Nadaem, M.A.; Badshah, A.; Chen, Y.; Nadeem, M.A.; Jin, R. Titania-Supported Palladium/Strontium Nanoparticles (Pd/Sr-NPs@P25) for Photocatalytic H2 Production from Water Splitting. J. Phys. Chem. C 2016, 120, 17205–17213. [Google Scholar] [CrossRef]
- Hussain, E.; Majeed, I.; Nadeem, M.A.; Iqbal, A.; Chen, Y.; Choucair, M.; Jin, R. Remarkable effect of BaO on photocatalytic H2 evolution from water splitting via TiO2 (P25) supported palladium nanoparticles. J. Environ. Chem. Eng. 2019, 7, 102729. [Google Scholar] [CrossRef]
- Liang, S.; Sui, G.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Li, J.; Wang, C. g-C3N4-wrapped nickel doped zinc oxide/carbon core-double shell microspheres for high-performance photocatalytic hydrogen production. J. Colloid Interface Sci. 2023, 635, 83–93. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H. Facile synthesis of multitasking composite of Silver nanoparticle with Zinc oxide for 4-nitrophenol reduction, photocatalytic hydrogen production, and 4-chlorophenol degradation. J. Alloys Compd. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Shao, Y.B.; Wang, L.H.; Huang, J.H. ZnS/CuS nanotubes for visible light-driven photocatalytic hydrogen generation. RSC Adv. 2016, 6, 84493–84499. [Google Scholar] [CrossRef]
- Oskenbay, A.; Salikhov, D.; Rofman, O.; Rakhimbek, I.; Shalabayev, Z.; Khan, N.; Soltabayev, B.; Mentbayeva, A.; Baláž, M.; Tatykayev, B. Solid-state synthesis of ZnS/ZnO nanocomposites and their decoration with NiS cocatalyst for photocatalytic hydrogen production. Ceram. Int. 2023, 49, 32246–32260. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Sun, K.; Tan, L.; Guo, F.; Du, X.; Shi, W. Plasmonic coupling-boosted photothermal composite photocatalyst for achieving near-infrared photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 661, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, S.U.H.; Zada, A.; Raziq, F.; Ali, S.; Shah, M.I.A.; Ateeq, M.; Khan, M.; Alei, D.; Fazil, P.; Naeem, M.; et al. Zinc phthalocyanine sensitized g-C3N4 photocatalyst for exceptional photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrogen Energy 2023, 48, 16320–16329. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Guo, X.; Jin, Z. Type-II CoMoO4/Graphdiyne heterojunction promotes visible-light-driven photocatalytic hydrogen production activity. Sep. Purif. Technol. 2024, 332, 125786. [Google Scholar] [CrossRef]

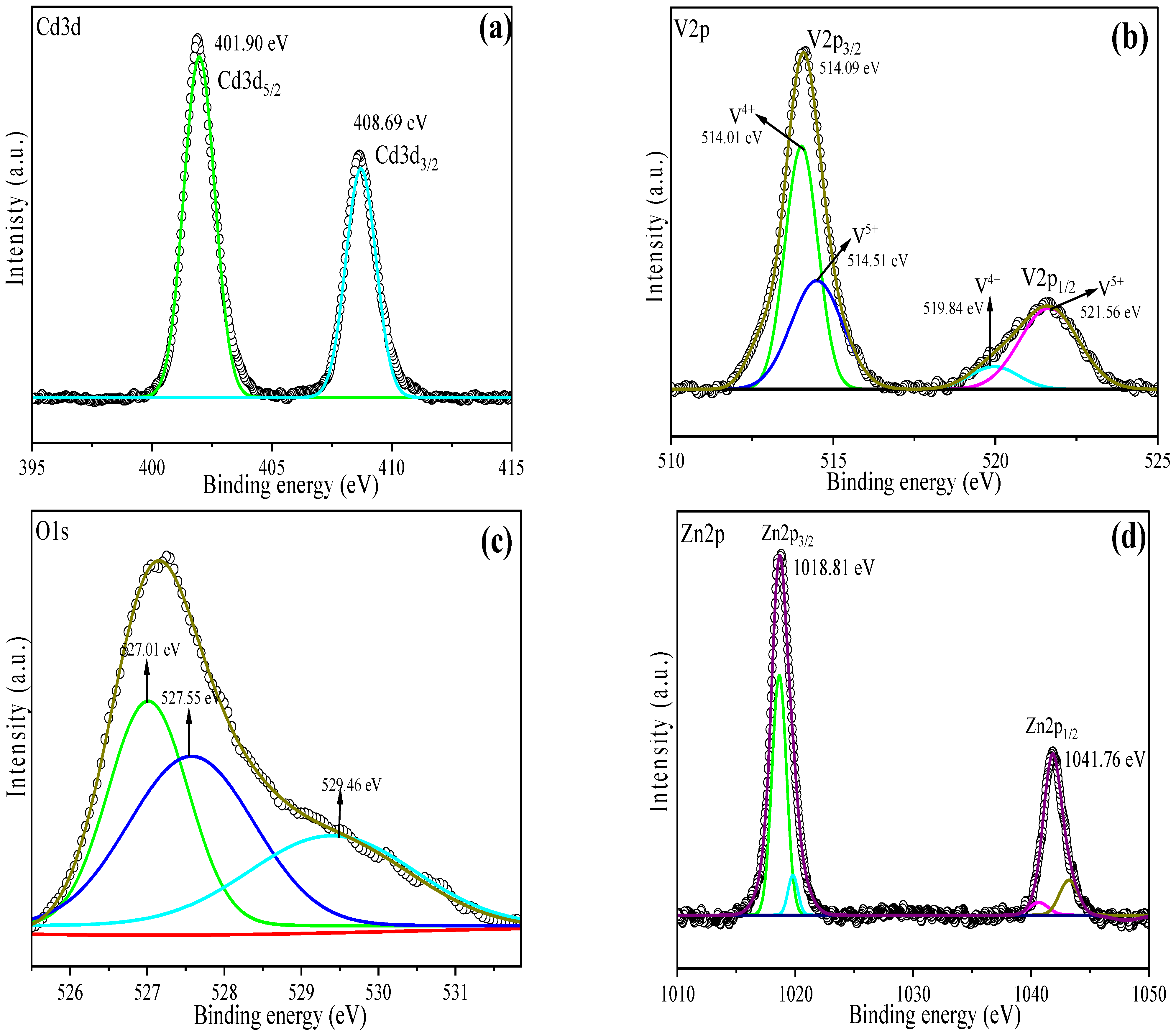


| O1s | V2p3 | Zn2p3 | Cd3d5 | RSF Corrected |
| 57.57 | 20.65 | 14.72 | 7.06 |
| Photocatalyst | Light Used | SR | H2 Production (µmol g−1 h−1) | Reference |
|---|---|---|---|---|
| ZnO@C/g-C3N4 | simulated sunlight | TEOA | 336.08 | [49] |
| Ag/ZnO | mercury xenon lamp | Ethanol | 12.00 | [50] |
| ZnS@CuS | Visible | 56.47 | [51] | |
| ZnO/ZnS-3h-NiS | Visible | Ethanol | 26.00 | [52] |
| WO3-x/ZnIn2S4 | NIR | TEOA | 14.05 | [53] |
| ZPCN | Visible | Methanol | 90.00 | [54] |
| CoMoO4/GDY | Visible | TEOA | 121.00 | [55] |
| CdV/ZnV | Visible | Methanol | 366.34 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; El Marghany, A.; Abduh, N.A.Y.; Alharthi, F.A. Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution. Nanomaterials 2024, 14, 492. https://doi.org/10.3390/nano14060492
Hasan I, El Marghany A, Abduh NAY, Alharthi FA. Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution. Nanomaterials. 2024; 14(6):492. https://doi.org/10.3390/nano14060492
Chicago/Turabian StyleHasan, Imran, Adel El Marghany, Naaser A. Y. Abduh, and Fahad A. Alharthi. 2024. "Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution" Nanomaterials 14, no. 6: 492. https://doi.org/10.3390/nano14060492
APA StyleHasan, I., El Marghany, A., Abduh, N. A. Y., & Alharthi, F. A. (2024). Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution. Nanomaterials, 14(6), 492. https://doi.org/10.3390/nano14060492








