Synthesis and Characterization of New Nanohybrids Based on Carboxymethyl Scleroglucan and Silica Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
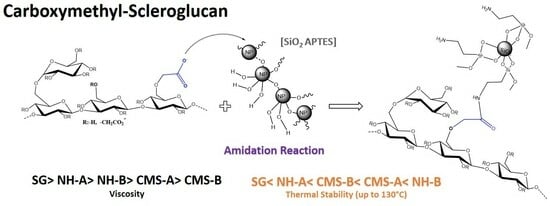
2.2. Amidation Reaction
2.3. NH-A and NH-B Characterization
2.4. SG and Nanohybrid Solution Preparation
3. Results and Discussion
3.1. NH-A and NH-B Characterization
3.1.1. ATR-FTIR Results
3.1.2. STA/TG-DSC Results
3.1.3. SEM-EDS Analysis
3.1.4. TEM Analysis
3.1.5. X-ray Diffraction (XRD) Analysis
3.1.6. ICP-OES Analysis
3.1.7. XPS Analysis
3.2. NH-A and NH-B Solution Analysis
3.2.1. DLS Analysis
3.2.2. Viscosity Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Yalpani, M. Chemistry of polysaccharide modification and degradation. In Carbohydrates: Structures, Syntheses and Dynamics; Springer: Cham, Switzerland, 1999; pp. 294–318. [Google Scholar]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Philos. Trans. R. Soc. A 2014, 372, 320. [Google Scholar] [CrossRef]
- Zia, J.; Aazam, E.S.; Riaz, U. Synthesis of nanohybrids of polycarbazole with α-MnO2 derived from Brassica oleracea: A comparison of photocatalytic degradation of an antibiotic drug under microwave and UV irradiation. Environ. Sci. Pollut. Res. 2020, 27, 24173–24189. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Chemical enhanced oil recovery and the role of chemical product design. Appl. Energy 2019, 252, 113480. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, J.; Shan, X.; Yu, S.; Zhou, J. Efficient production of scleroglucan by Sclerotium rolfsii and insights into molecular weight modification by high-pressure homogenization. Front. Bioeng. Biotechnol. 2021, 9, 748213. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Rodríguez-Mateus, Z.-P.; Angarita, R.-C.; Niño-Gómez, J.-A.; Corredor, L.-M.; Llanos Gallo, S.; Quintero, H.; Castro-García, R.-H. Biodegradation and toxicity of scleroglucan for enhanced oil recovery. Cienc. Technol. Futuro 2022, 12, 5–12. [Google Scholar] [CrossRef]
- Ruiz-Cañas, M.-C.; Cañas, M.C.R.; Garcia, R.H.C.; Bohorquez, A.R.R. Use of nanoparticles to improve thermochemical resistance of synthetic polymer to enhanced oil recovery applications: A review. Cienc. Technol. Futuro 2020, 10, 85–97. [Google Scholar]
- Franco, C.A.; Franco, C.A.; Zabala, R.D.; Bahamón, I.; Forero, A.; Cortés, F.B. Field Applications of nanotechnology in the oil and gas industry: Recent advances and perspectives. Energy Fuels 2021, 35, 19266–19287. [Google Scholar] [CrossRef]
- Alves, B.F.; Nunes, R.C.; Bertolino, L.C.; Lucas, E.F. Polymer nanocomposites to modify crude oil waxy crystallization: Influence of content and type of clay (mineral). Appl. Clay Sci. 2024, 247, 107215. [Google Scholar] [CrossRef]
- Alves, B.F.; Silva, B.K.; Silva, C.A.; Celestino, G.G.; Nunes, R.C.; Lucas, E.F. Preparation and evaluation of polymeric nanocomposites based on EVA/montmorillonite, EVA/palygorskite and EVA/halloysite as pour point depressants and flow improvers of waxy systems. Fuel 2023, 333, 126540. [Google Scholar] [CrossRef]
- Bila, A.; Torsæter, O. Experimental investigation of polymer-coated silica nanoparticles for EOR under harsh reservoir conditions of high temperature and salinity. Nanomaterials 2021, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Experimental and field applications of nanotechnology for enhanced oil recovery purposes: A review. Fuel 2022, 324, 124669. [Google Scholar] [CrossRef]
- Gong, Y.; Li, L.; Huang, W.; Zou, J.; Zhong, X.; Wang, L.; Kang, D.; Zhang, Z.; Zhang, Z. A study of alkali-silica nanoparticle-polymer (ANP) flooding for enhancing heavy oil recovery. J. Pet. Sci. Eng. 2022, 213, 110465. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. The effect of the coupling agents KH550 and KH570 on the nanostructure and interfacial interaction of zinc oxide/chiral poly (amide–imide) nanocomposites containing l-leucine amino acid moieties. J. Mater. Sci. 2014, 49, 5112–5118. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Allam, N.K. Impact of nanotechnology on enhanced oil recovery: A mini-review. Ind. Eng. Chem. Res. 2019, 58, 16287–16295. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Effect of hydrophobic and hydrophilic metal oxide nanoparticles on the performance of xanthan gum solutions for heavy oil recovery. Nanomaterials 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Corredor, L.M.; Aliabadian, E.; Husein, M.; Chen, Z.; Maini, B.; Sundararaj, U. Heavy oil recovery by surface modified silica nanoparticle/HPAM nanofluids. Fuel 2019, 252, 622–634. [Google Scholar] [CrossRef]
- Corredor, L.M.; Espinosa, C.; Delgadillo, C.L.; Llanos, S.; Castro, R.H.; Quintero, H.I.; Cañas, M.C.R.; Bohorquez, A.R.R.; Manrique, E. Flow Behavior through Porous Media and Displacement Performance of a SILICA/PAM Nanohybrid: Experimental and Numerical Simulation Study. ACS Omega 2024, 9, 7923–7936. [Google Scholar] [CrossRef]
- El-Hoshoudy, A.; Desouky, S.; Betiha, M.; Alsabagh, A. Use of 1-vinyl imidazole based surfmers for preparation of polyacrylamide–SiO2 nanocomposite through aza-Michael addition copolymerization reaction for rock wettability alteration. Fuel 2016, 170, 161–175. [Google Scholar] [CrossRef]
- Mobaraki, S.; Tabatabaee, H.; Torkmani, R.S.; Khalilinezhad, S.S.; Ghorashi, S. The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach. E-Polymers 2022, 22, 454–467. [Google Scholar] [CrossRef]
- Azli, N.B.; Junin, R.; Agi, A.A.; Risal, A.R. Partially hydrolyzed polyacrylamide apparent viscosity in porous media enhancement by silica dioxide nanoparticles under high temperature and high salinity. J. Appl. Sci. Eng. 2021, 25, 481–493. [Google Scholar]
- Chancellor, A.J.; Seymour, B.T.; Zhao, B. Characterizing polymer-grafted nanoparticles: From basic defining parameters to behavior in solvents and self-assembled structures. Anal. Chem. 2019, 91, 6391–6402. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yoo, B.R. Advanced silica/polymer composites: Materials and applications. J. Ind. Eng. Chem. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Castro, R.H.; Corredor, L.M.; Llanos, S.; Causil, M.A.; Arias, A.; Pérez, E.; Quintero, H.I.; Bohórquez, A.R.R.; Franco, C.A.; Cortés, F.B. Experimental Investigation of the Viscosity and Stability of Scleroglucan-Based Nanofluids for Enhanced Oil Recovery. Nanomaterials 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.H.; Burgos, I.; Corredor, L.M.; Llanos, S.; Franco, C.A.; Cortés, F.B.; Bohórquez, A.R.R. Carboxymethyl Scleroglucan Synthesized via O-Alkylation Reaction with Different Degrees of Substitution: Rheology and Thermal Stability. Polymers 2024, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.C.; Hess, G.P. A new method of forming peptide bonds. J. Am. Chem. Soc. 1955, 77, 1067–1068. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Goodman, M.; Hess, G.P. Peptide derivatives containing hydroxyamino acids. J. Am. Chem. Soc. 1956, 78, 1367–1369. [Google Scholar] [CrossRef]
- Williams, A.; Ibrahim, T.I. Carbodiimide chemistry: Recent advances. Chem. Rev. 1981, 81, 589–636. [Google Scholar] [CrossRef]
- Ruiz-Cañas, M.C.; Quintero, H.I.; Corredor, L.M.; Manrique, E.; Bohórquez, A.R.R. New nanohybrid based on hydrolyzed polyacrylamide and silica nanoparticles: Morphological, structural and thermal properties. Polymers 2020, 12, 1152. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Moraillon, A.; Gouget-Laemmel, A.C.; Ozanam, F.; Chazalviel, J.-N. Amidation of monolayers on silicon in physiological buffers: A quantitative IR study. J. Phys. Chem. C 2008, 112, 7158–7167. [Google Scholar] [CrossRef]
- Meiser, F.; Cortez, C.; Caruso, F. Biofunctionalization of fluorescent rare-earth-doped lanthanum phosphate colloidal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 43, 5954–5957. [Google Scholar] [CrossRef]
- Abraham, T.W.; Sumner, E.S. Method for Solubilizing Biopolymer Solids for Enhanced Oil Recovery Applications. U.S. Patent 20190112518, 18 April 2019. [Google Scholar]
- Castro-García, R.-H.; Gallo, S.L.; Ardila, J.L.R.; Pérez, H.I.Q.; Ventura, E.J.M.; Arango, J.F.Z. Heavy oil and high-temperature polymer EOR applications. Cienc. Technol. Futuro 2020, 10, 73–83. [Google Scholar] [CrossRef]
- Casadei, M.A.; Matricardi, P.; Fabrizi, G.; Feeney, M.; Paolicelli, P. Physical gels of a carboxymethyl derivative of scleroglucan: Synthesis and characterization. Eur. J. Pharm. Biopharm. 2007, 67, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Corrente, F.; Paolicelli, P.; Matricardi, P.; Tita, B.; Vitali, F.; Casadei, M.A. Novel pH-sensitive physical hydrogels of carboxymethyl scleroglucan. J. Pharm. Sci. 2012, 101, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Cerreto, A.; Corrente, F.; Botta, B.; Pacelli, S.; Paolicelli, P.; Mannina, L.; Casadei, M.A. NMR characterization of carboxymethyl scleroglucan. Int. J. Polym. Anal. Charact. 2013, 18, 587–595. [Google Scholar] [CrossRef]
- Arora, S.; Lal, S.; Kumar, S.; Kumar, M.; Kumar, M. Comparative degradation kinetic studies of three biopolymers: Chitin, chitosan and cellulose. ASEAN J. Psychiatry 2011, 3, 188–201. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Mansa, R.; Dzene, L.; Quintela, A.; Rocha, F.; Detellier, C. Preparation and characterization of novel clay/scleroglucan nanocomposites. Appl. Clay Sci. 2016, 126, 235–244. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.; Fatah, A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Doh, S.J.; Lee, J.Y.; Lim, D.Y.; Im, J.N. Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers. Fibers Polym. 2013, 14, 2176–2184. [Google Scholar] [CrossRef]
- Ianăşi, C.; Pascu, B.; Nemeş, N.; Popa, A. Synthesis and Characterization of Amorphous SiO2−FexOy Materials Starting from Iron Sulfate for Preliminary Studies of CO2 Adsorption. Separations 2023, 10, 352. [Google Scholar] [CrossRef]
- Ciolacu, D.; Chiriac, A.I.; Pastor, F.J.; Kokol, V. The influence of supramolecular structure of cellulose allomorphs on the interactions with cellulose-binding domain, CBD3b from Paenibacillus barcinonensis. Bioresour. Technol. 2014, 157, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kambara, O.; Tamura, A.; Uchino, T.; Yamamoto, K.; Tominaga, K. Terahertz time-domain spectroscopy of poly-l-lysine. Biopolymers 2010, 93, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Werner, W.S.; Powell, C. Applications of the National Institute of Standards and Technology (NIST) database for the simulation of electron spectra for surface analysis for quantitative x-ray photoelectron spectroscopy of nanostructures. J. Vac. Sci. Technol. A 2021, 39, 63205. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Kozakevych, R.B. Silica nanocomposites based on silver nanoparticles-functionalization and pH effect. Appl. Nanosci. 2018, 8, 1649–1668. [Google Scholar] [CrossRef]
- Burg, P.; Fydrych, P.; Cagniant, D.; Nanse, G.; Bimer, J.; Jankowska, A. The characterization of nitrogen-enriched activated carbons by IR, XPS and LSER methods. Carbon 2002, 40, 1521–1531. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, X.; Lai, N.; Peng, Q.; Li, X.; Li, C. Synthesis and performance of an acrylamide copolymer containing nano-SiO2 as enhanced oil recovery chemical. Chem. EOR 2013, 2013, 437309. [Google Scholar]

















| Sample | T, °C | Weight Loss, % | Endothermic Peaks of DSC, °C | DTG Peaks, °C | MWLR, (% min−1) |
|---|---|---|---|---|---|
| CMS-A | 30–122 | 7.0 | 62.9 | 58 | 0.80 |
| 123–230 | 3.0 | 172.0 | 177 | 1.20 | |
| 231–521 | 65.0 | 240.0 | 298 | 4.31 | |
| 522–999 | 12.0 | 399.8 | |||
| Residue, 1000 °C | 13.0 | ||||
| NH-A | 30–122 | 9.0 | 57.0 | 58.2 | - |
| 122–400 | 69.0 | 294.0 | 295 | 1.40 | |
| 401–800 | 7.0 | - | - | - | |
| 801–999 | 6.0 | 821 | 879 | - | |
| Residue, 1000 °C | 13.0 | ||||
| CMS-B | 30–122 | 8.0 | 54.5 | 59 | 0.86 |
| 122–208 | 2.0 | 171.8 | 175 | 2.42 | |
| 209–524 | 60.0 | 234.7 | 285 | 3.86 | |
| 525–999 | 13.0 | 396.0 | |||
| Residue, 1000 °C | 17.0 | ||||
| NH-B | 30–123 | 7.0 | - | 56 | - |
| 124–200 | 2.0 | 165 | 167 | 2.42 | |
| 201–388 | 53.0 | 270 (exothermic) | 276 | 0.9 | |
| 389–800 | 10.0 | - | - | - | |
| 801–999 | 8.0 | - | - | - | |
| Residue, 1000 °C | 20.0 | ||||
| SiO2_APTES_120 | 30–139 | 5.0 | 61.0 | 57 | 0.1 |
| 139–999 | 5.0 | 767.0; 821 (exothermic) | - | - | |
| Residue, 1000 °C | 90.0 |
| Sample | Si (ppm) | SiO2 (ppm) |
|---|---|---|
| CMS-A | 0.07 | 0.15 |
| NH-A | 2.66 | 5.70 |
| CMS-B | 0.06 | 0.12 |
| NH-B | 2.84 | 6.07 |
| Energy Level | Functional Groups | CMS-B | NH-A | NH-B | NIST Database |
|---|---|---|---|---|---|
| C 1s | C-(CH2) | 283.19 | 283.02 | 283.30 | |
| C-(C) | 284.60 | 284.60 | 284.60 | 284.60 | |
| (C)-O | 285.33 | 285.00 | |||
| (C)-O-(C) | 285.87 | 286.01 | 286.00 | ||
| O-(CH2) | 287.00 | 287.07 | 287.00 | ||
| (C)O=O | 288.92 | 288.05 | 287.69 | 288.50 | |
| (C)=O | 289.62 | 289.60 | 288–290 | ||
| O 1s | C-(O)-C | 530.67 | 530.76 | 530.75 | |
| C=(O) | 532.03 | 531.95 | 532.38 | 531.5–532 | |
| C-(O) | 533.19 | 533.16 | 533.83 | 533.00 | |
| (O)-CH2 | 534.15 | 534.1 | |||
| Si 2p | (Si)–O–C | 103.37 | 102.82 | 102.8 | |
| Na 2s | (Na)Cl | 1071.66 | 1071.6 | ||
| (Na)Cl | 1072.21 | 1072.2 | |||
| Cl 2p | Na(Cl) | 198.45 | 198.91 | 198.06 | 200 |
| Cl organic | 200.93 | 200.61 | 199.57 | 198.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, R.H.; Corredor, L.M.; Burgos, I.; Llanos, S.; Franco, C.A.; Cortés, F.B.; Idrobo, E.A.; Bohórquez, A.R.R. Synthesis and Characterization of New Nanohybrids Based on Carboxymethyl Scleroglucan and Silica Nanoparticles. Nanomaterials 2024, 14, 499. https://doi.org/10.3390/nano14060499
Castro RH, Corredor LM, Burgos I, Llanos S, Franco CA, Cortés FB, Idrobo EA, Bohórquez ARR. Synthesis and Characterization of New Nanohybrids Based on Carboxymethyl Scleroglucan and Silica Nanoparticles. Nanomaterials. 2024; 14(6):499. https://doi.org/10.3390/nano14060499
Chicago/Turabian StyleCastro, Rubén H., Laura M. Corredor, Isidro Burgos, Sebastián Llanos, Camilo A. Franco, Farid B. Cortés, Eduardo A. Idrobo, and Arnold R. Romero Bohórquez. 2024. "Synthesis and Characterization of New Nanohybrids Based on Carboxymethyl Scleroglucan and Silica Nanoparticles" Nanomaterials 14, no. 6: 499. https://doi.org/10.3390/nano14060499
APA StyleCastro, R. H., Corredor, L. M., Burgos, I., Llanos, S., Franco, C. A., Cortés, F. B., Idrobo, E. A., & Bohórquez, A. R. R. (2024). Synthesis and Characterization of New Nanohybrids Based on Carboxymethyl Scleroglucan and Silica Nanoparticles. Nanomaterials, 14(6), 499. https://doi.org/10.3390/nano14060499