Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sorbents
2.3. Treatment of Sauvignon Blanc Wine Samples
2.4. Protein Stability Test
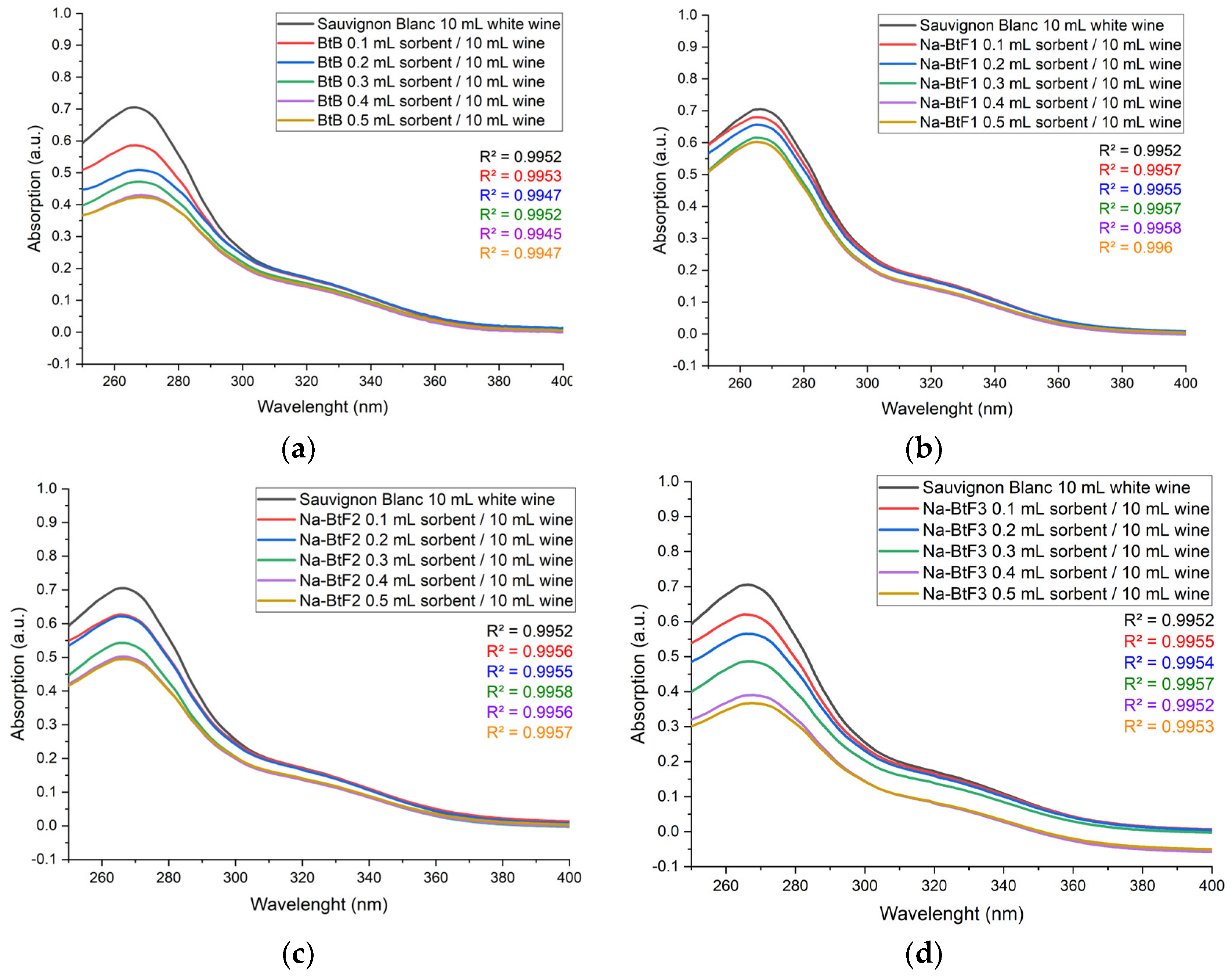
2.5. Spectrophotometric Analysis for Protein and Polyphenolic Compounds
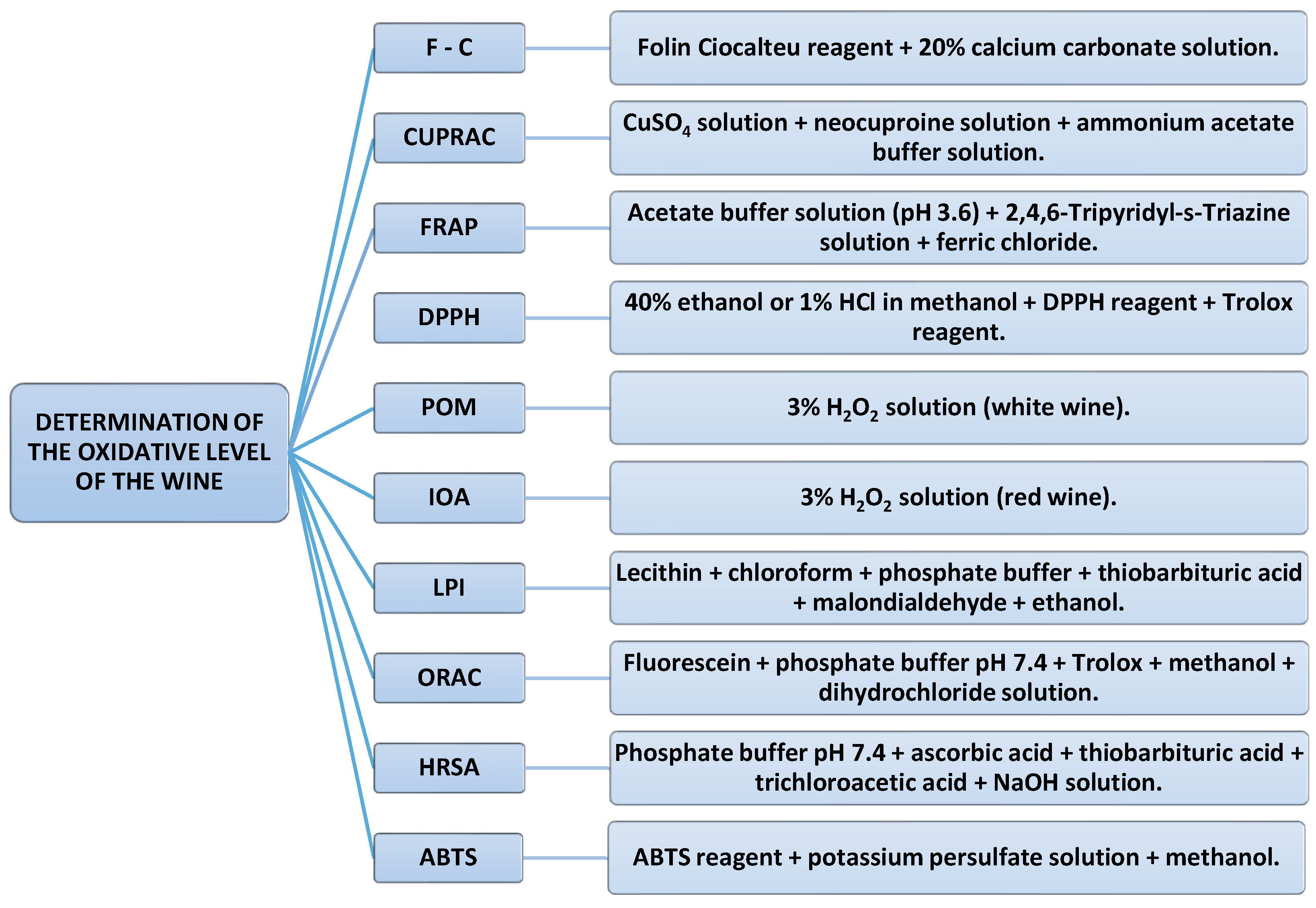
2.6. Determination of the Degree of Oxidizability
2.7. Determination of Assimilable Nitrogen Compounds in Wine
2.8. Characterization of the Prepared Sorbents
3. Results
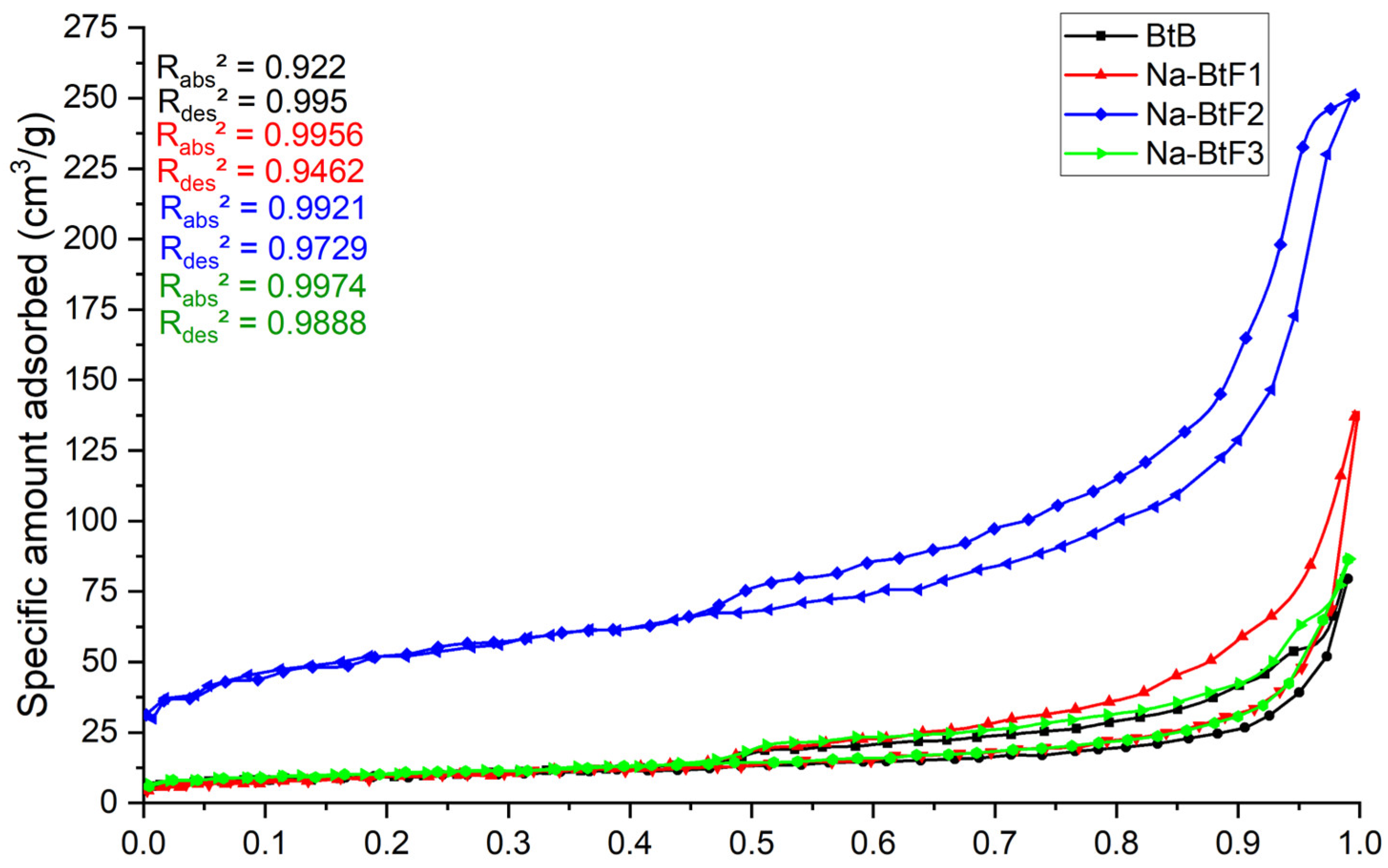
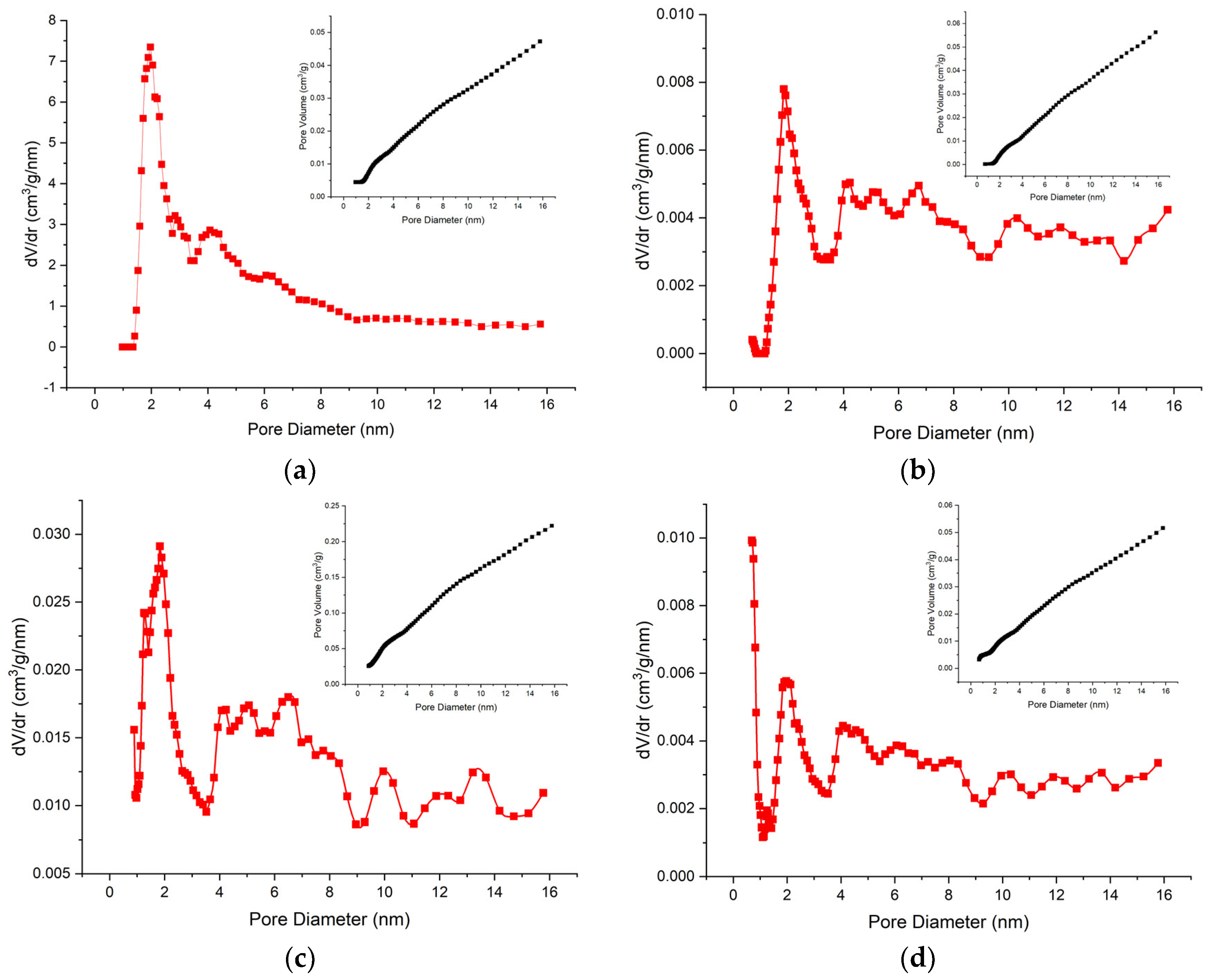
3.1. BET-BJH Analysis
3.2. XRD Analysis
3.3. FTIR-ATR Analysis
3.4. Physicochemical Parameters for Sauvignon Blanc White Wine
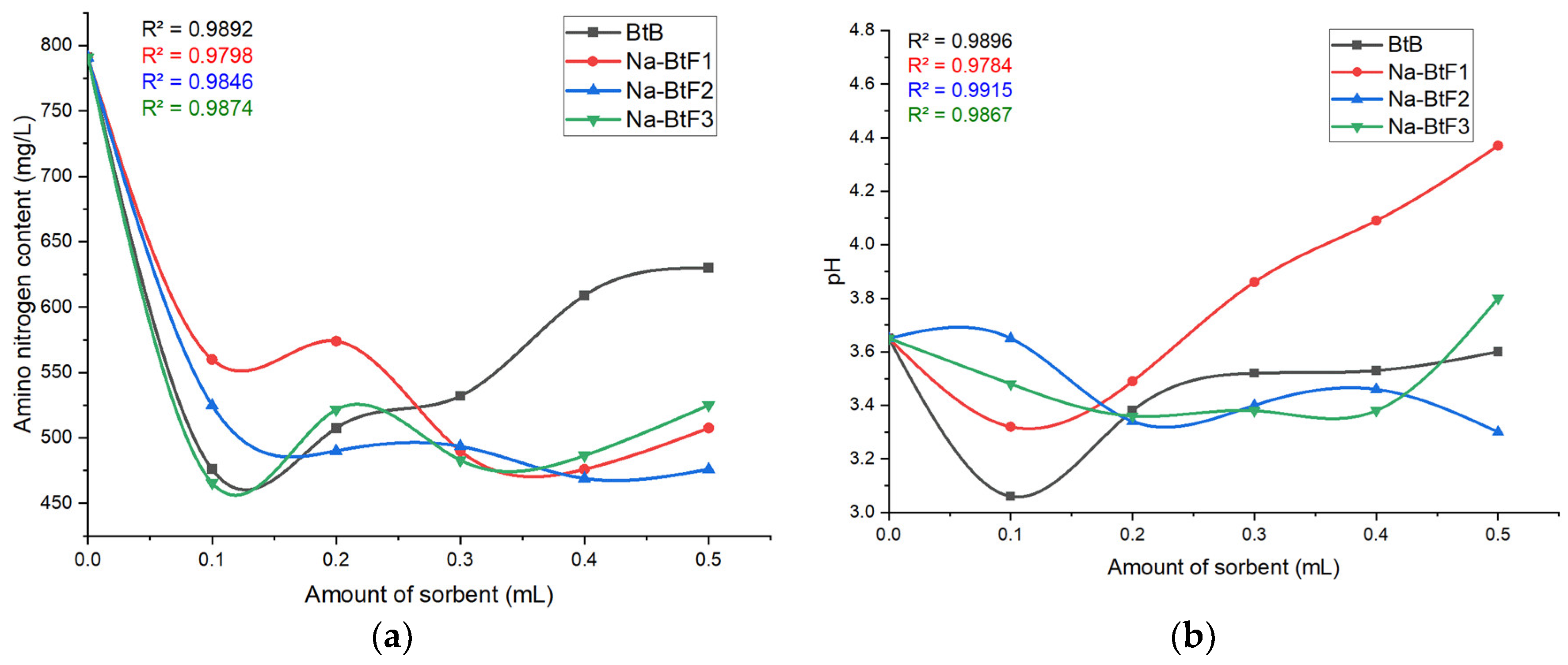
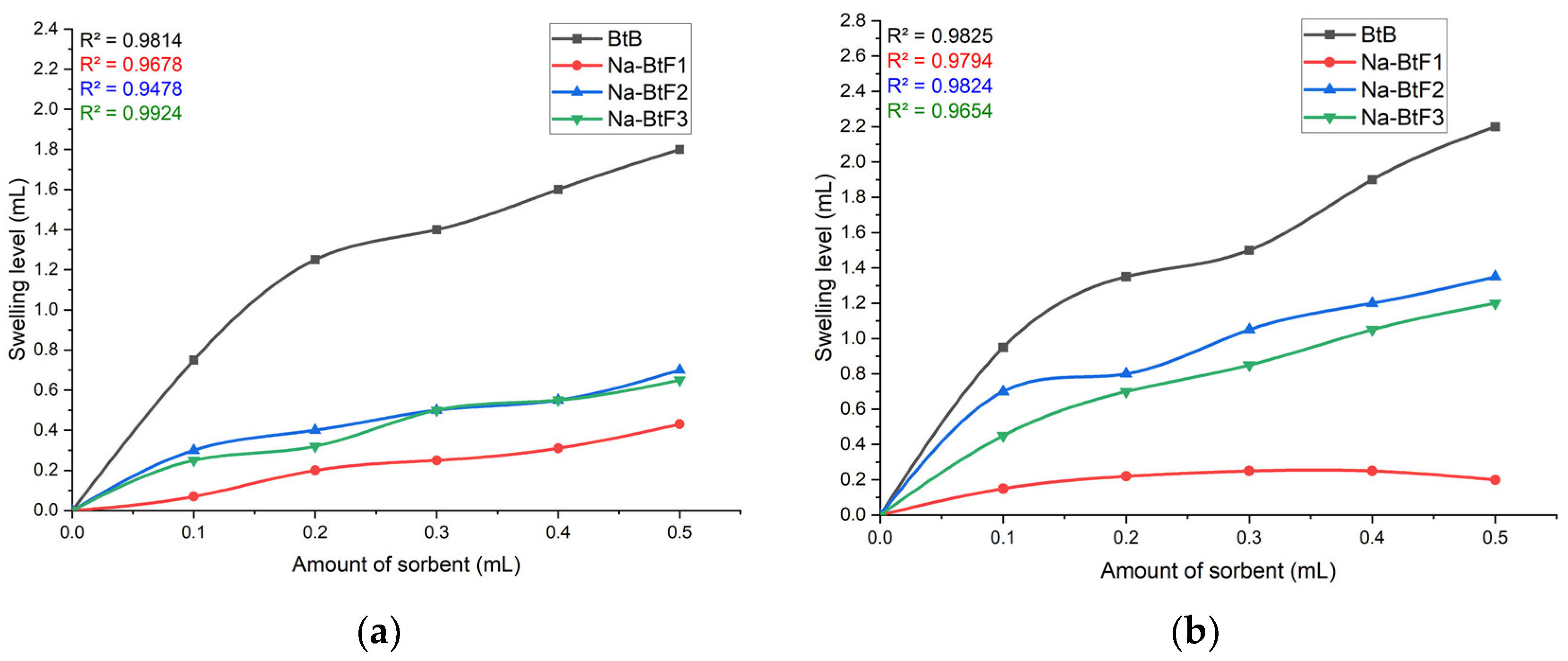
3.5. Effect of Amount of Adsorbent
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tabilo-Munizaga, G.; Gordon, T.A.; Villalobos-Carvajal, R.; Moreno-Osorio, L.; Salazar, F.N.; Pérez-Won, M.; Acuña, S. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine. Food Chem. 2014, 155, 214–220. [Google Scholar] [CrossRef]
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef]
- Tian, B.; Harrison, R.; Morton, J.; Jaspers, M.; Hodge, S.; Grose, C.; Trought, M. Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions. Molecules 2017, 22, 1164. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Perez-Wom, M.; Habib, G.; Giovagnoli-Vicuña, C.; Cañas-Sarazua, R.; Tabilo-Munizaga, G.; Salazar, F.N. Oenological and Quality Characteristic on Young White Wines (Sauvignon Blanc): Effects of High Hydrostatic Pressure Processing. J. Food Qual. 2017, 2017, 8524073. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Characterization of natural haze protein in sauvignon white wine. Food Chem. 2009, 113, 28–35. [Google Scholar] [CrossRef]
- Albuquerque, W.; Seidel, L.; Zorn, H.; Will, F.; Gand, M. Haze Formation and the Challenges for Peptidases in Wine Protein Fining. J. Agric. Food Chem. 2021, 69, 14402–14414. [Google Scholar] [CrossRef]
- Ratnayake, S.; Stockdale, V.; Grafton, S.; Munro, P.; Robinson, A.L.; Pearson, W.; McRae, J.M.; Bacic, A. Carrageenans as heat stabilisers of white wine. Aust. J. Grape Wine Res. 2019, 25, 439–450. [Google Scholar] [CrossRef]
- Falconer, R.J.; Marangon, M.; Van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Waters, E.J. Thermal Stability of Thaumatin-Like Protein, Chitinase, and Invertase Isolated from Sauvignon blanc and Semillon Juice and Their Role in Haze Formation in Wine. J. Agric. Food Chem. 2010, 58, 975–980. [Google Scholar] [CrossRef]
- He, S.; Hider, R.; Zhao, J.; Tian, B. Effect of Bentonite Fining on Proteins and Phenolic Composition of Chardonnay and Sauvignon Blanc Wines. J. S. Afr. J. Enol. Viticult. 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Bamogo, H.; Ouedraogo, M.; Sanou, I.; Aubert, J.-E.; Millogo, Y. Physical, Hydric, Thermal and Mechanical Properties of Earth Renders Amended with Dolomitic Lime. Materials 2022, 15, 4014. [Google Scholar] [CrossRef]
- Jaeckels, N.; Meier, M.; Dietrich, H.; Will, F.; Decker, H.; Fronk, P. Influence of polysaccharides on wine protein aggregation. Food Chem. 2016, 200, 38–45. [Google Scholar] [CrossRef]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The complexity of protein haze formation in wines. Food Chem. 2009, 112, 169–177. [Google Scholar] [CrossRef]
- Vicente, M.A.; Gil, A.; Bergaya, F. Chapter 10.5—Pillared Clays and Clay Minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 523–557. [Google Scholar]
- Bai, B.; He, F.; Yang, L.; Chen, F.; Reeves, M.J.; Li, J. Comparative study of phenolic compounds in Cabernet Sauvignon wines made in traditional and Ganimede fermenters. Food Chem. 2013, 141, 3984–3992. [Google Scholar] [CrossRef]
- Andrea-Silva, J.; Cosme, F.; Ribeiro, L.F.; Moreira, A.S.P.; Malheiro, A.C.; Coimbra, M.A.; Domingues, M.R.M.; Nunes, F.M. Origin of the Pinking Phenomenon of White Wines. J. Agric. Food Chem. 2014, 62, 5651–5659. [Google Scholar] [CrossRef]
- Revi, M.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Effect of packaging material on enological parameters and volatile compounds of dry white wine. Food Chem. 2014, 152, 331–339. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissedre, P.L.; López-Tamames, E. Kinetics of Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial Sparkling Wines. J. Agric. Food Chem. 2014, 62, 1159–1166. [Google Scholar] [CrossRef]
- Ma, L.; Waterhouse, A.L. Flavanols react preferentially with quinones through an electron transfer reaction, stimulating rather than preventing wine browning. Anal. Chim. Acta 2018, 1039, 162–171. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Romanini, E.; Colangelo, D.; Lucini, L.; Lambri, M. Identifying chemical parameters and discriminant phenolic compounds from metabolomics to gain insight into the oxidation status of bottled white wines. Food Chem. 2019, 288, 78–85. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Mattioli, M.; Giardini, L.; Roselli, C.; Desideri, D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl. Clay Sci. 2016, 119, 449–454. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, Z.; Zhang, S.; Yang, Y.; Wu, Y.; Liu, L.; Liu, Q. Purification of montmorillonite and the influence of the purification method on textural properties. Appl. Clay Sci. 2020, 187, 105491. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Giribaldi, M.; Violetta, M.R.; Giuffrida, M.G. Heat-unstable protein removal by different bentonite labels in white wines. LWT-Food Sci. Technol. 2012, 46, 460–467. [Google Scholar] [CrossRef]
- Ellen Cristine, G.; Maria Chacón, O.; Nuria Barrajón, S.; Ana, I.B.P.; Robert, F.H.D.; Aneli, M.B. Evaluation of the Components Released by Wine Yeast Strains on Protein Haze Formation in White Wine. Orbital Electron. J. Chem. 2022, 8, 307–313. [Google Scholar] [CrossRef]
- Sarmento, M.R.; Oliveira, J.C.; Boulton, R.B. Selection of low swelling materials for protein adsorption from white wines. Int. J. Food Sci. Technol. 2000, 35, 41–47. [Google Scholar] [CrossRef]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and Wine Proteins: Their Fractionation by Hydrophobic Interaction Chromatography and Identification by Chromatographic and Proteomic Analysis. J. Agric. Food Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Williams, P.J. Proteins in white wine, II: Their resistance to proteolysis is not due to either phenolic association or glycosylation. Aust. J. Grape Wine Res. 1995, 1, 94–99. [Google Scholar] [CrossRef]
- Celotti, E.; Osorio Barahona, M.S.; Bellantuono, E.; Cardona, J.; Roman, T.; Nicolini, G.; Natolino, A. High-power ultrasound on the protein stability of white wines: Preliminary study of amplitude and sonication time. LWT 2021, 147, 111602. [Google Scholar] [CrossRef]
- Hortolomeu, A.; Mirila, D.-C.; Georgescu, A.-M.; Rosu, A.-M.; Scutaru, Y.; Nedeff, F.-M.; Sturza, R.; Nistor, I.D. Retention of Phthalates in Wine Using Nanomaterials as Chemically Modified Clays with H20, H30, H40 Boltron Dendrimers. Nanomaterials 2023, 13, 2301. [Google Scholar] [CrossRef] [PubMed]
- Rastuti, U.; Diastuti, H.; Chasani, M.; Purwati; Hidayatullah, R. Chemical composition and antioxidant activities of citronella essential oil Cymbopogon nardus (L.) rendle fractions. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2020; Volume 2237. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Zhang, J.W.; Hayakawa, S.; Mine, Y. Immunochemical and Structural Analysis of Pepsin-Digested Egg White Ovomucoid. J. Agric. Food Chem. 2000, 48, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Lacorn, M.; Ristow, R.; Weiss, T.; Immer, U.J.F.A.M. Collaborative Tests of ELISA Methods for the Determination of Egg White Protein and Caseins Used as Fining Agents in Red and White Wines. Food Anal. Methods 2014, 7, 417–429. [Google Scholar] [CrossRef]
- Oberholster, A.; Carstens, L.M.; du Toit, W.J. Investigation of the effect of gelatine, egg albumin and cross-flow microfiltration on the phenolic composition of Pinotage wine. Food Chem. 2013, 138, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Marangon, M.; Stockdale, V.J.; Munro, P.; Trethewey, T.; Schulkin, A.; Holt, H.E.; Smith, P.A. Addition of Carrageenan at Different Stages of Winemaking for White Wine Protein Stabilization. J. Agric. Food Chem. 2013, 61, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Vasile Arhip, I.S. Vine culture in the Republic of Moldova at the beginning of the third millennium. J. Soc. Sci. 2019, 2, 87–94. [Google Scholar] [CrossRef]
- Ledoux, V.; Dulau, L.; Dubourdieu, D. Interprétation de l’amélioration de la stabilité protéique des vins au cours de l’élevage sur lies. OENO One 1992, 26, 239–251. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Serrano, M.; Vannier, A.-C.; Ribéreau-Gayon, P. Etude comparée des tests de stabilité protéique. OENO One 1998, 22, 261–273. [Google Scholar] [CrossRef]
- Pocock, K.F.; Alexander, G.M.; Hayasaka, Y.; Jones, P.R.; Waters, E.J. Sulfatea Candidate for the Missing Essential Factor That Is Required for the Formation of Protein Haze in White Wine. J. Agric. Food Chem. 2007, 55, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Bergaya, F.; Lagaly, G.; Vayer, M. Chapter 12.10 Cation and Anion Exchange. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 979–1001. [Google Scholar]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Mesquita, P.R.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Effect of Wine Composition on Protein Stability. J. Am. J. Enol. Vitic. 2001, 52, 324–330. [Google Scholar] [CrossRef]
- Ruth, H.-O.; María Reyes, G.-C.; Kleopatra, C.; Michaël, J.; Pierre-Louis, T. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Fernanda, C., Fernando, M.N., Luís, F.-R., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 1. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 716–734. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Somers, T.C.; Vérette, E. Phenolic Composition of Natural Wine Types. In Wine Analysis; Linskens, H.-F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 219–257. [Google Scholar] [CrossRef]
- Du, W.; Yang, Y.; Hu, L.; Chang, B.; Cao, G.; Nasir, M.; Lv, J. Combined determination analysis of surface properties evolution towards bentonite by pH treatments. Coll. Surf. A Physicochem. Eng. Asp. 2021, 626, 127067. [Google Scholar] [CrossRef]
- Shattar, S.F.A.; Foo, K.Y. Sodium salt-assisted low temperature activation of bentonite for the adsorptive removal of methylene blue. Sci. Rep. 2022, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Hernández-Artiga, M.P.; Palacios-Ponce de León, L.; Bellido-Milla, D. Selective methods for polyphenols and sulphur dioxide determination in wines. Food Chem. 2015, 182, 47–54. [Google Scholar] [CrossRef]
- Mulero, J.; Martínez, G.; Oliva, J.; Cermeño, S.; Cayuela, J.M.; Zafrilla, P.; Martínez-Cachá, A.; Barba, A. Phenolic compounds and antioxidant activity of red wine made from grapes treated with different fungicides. Food Chem. 2015, 180, 25–31. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, Q.; Zhang, S.; Wu, Y. The mineralogical characteristics between opaline silica in bentonite and α-cristobalite. Solid State Sci. 2019, 96, 105948. [Google Scholar] [CrossRef]
- Hurduc, M. Physical Chemistry. Part II. Chemical Kinetics and Dispersed Systems; Polytechnic Institute: Iasi, Romania, 1985. [Google Scholar]
- Atkins, P.; Paula, J. Physical Chemistry; Agir: Bucharest, Romania, 2003; p. 1175. [Google Scholar]
- Marchal, R.; Waters, E.J. 7—New directions in stabilization, clarification and fining of white wines. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 188–225. [Google Scholar] [CrossRef]
- Jackson, R.S. Post-Fermentation Treatments and Related Topics. In Wine Science; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- Farha, S. The Effect of Chelating Agent on the Separation of Some Metal Ions from Binary Mixture Solution by Cation-Exchange Resin. Nat. Sci. 2010, 8, 16–25. [Google Scholar]
- Cotea, V.D. Treatise on Oenochemistry; Romanian Academy: Bucharest, Romania, 2009; Volume II. [Google Scholar]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]






| Test Type SP | Used Materials | Dosage in Wine | Purpose/Working Principle | References | |
|---|---|---|---|---|---|
| Thermal stability | Cold | Sodium bentonite changed with Boltron dendrimers | 10–50 mL sorbent/L white wine |
| [25,26,27,28,29] |
| Hot | - | - |
| [30,31] | |
| Thermal stability (hot/cold) with inorganic organic materials | Egg albumin, blood albumin | 50–250 mg/L red wine; |
| [32,33,34,35] | |
| Milk casein | 150–300 mg/L white wine, 7–10.5 mg/L |
| [36] | ||
| Carrageenan | 1.5–2% solution, 2 g in 200 mL wine |
| [7,37] | ||
| Sodium bentonite (BS) | 200–400 mg/L red + white wine |
| [30,38] | ||
| Calcium bentonite (BC) | 700–2000 mg/L white/red wine | ||||
| BS + BC | 700–1500 mg/L white/red wine | ||||
| Active coal | 100–500 mg/L wine |
| [38] | ||
| Kaolin | 5000–6000 mg/L wine |
| [38] | ||
| Sodium alginate | 40–80 mg/L wine |
| [38] | ||
| Trichloroacetic (TCA) | TCA solution | 1 mL TCA solution concentration 55% in 10 mL wine |
| [2,39,40] | |
| With tannin | - | Oenological, ethereal alcoholic | 5 mg/10 mL wine |
| [5,26,40] |
| Hot | Condensed tannin (TC) | 0.5 mL tannin solution (TC/TH) in water with 10% ethanol | [30] | ||
| Hydrolyzed tannin (TH) | |||||
| Cold | Condensed tannin (TC) | 5% TC/TH solution in water with 10% ethanol | |||
| Hydrolyzed tannin (TH) | |||||
| Bentotest | Phosphomolybdic acid (FA) | 100 mL FA reagent in 10 mL wine |
| [2,40] | |
| Protochek (PC) | Reagent PC | It is added to wine in a ratio of 1:2 |
| [30] | |
| Prostab (PS) | Reagent PS | 0.05–0.1 mL PS/L white wine |
| [30] | |
| With inorganic solvents | Ethanol | 3% solution in white wine |
| [2,26] | |
| With mannoproteins | - | - |
| [30,39] | |
| With inorganic salts | Ammonium sulfate | 0.5–2 g/L white wine |
| [41] | |
| BtB % | ||||
|---|---|---|---|---|
| Chemical Composition | Mineralogical Composition | |||
| Component | % | Component | Before Purification% | After Purification % |
| SiO2 | 67.98 | Montmorillonite | 67.98 | 99.00 |
| Al2O3 | 14.5 | Quartz | 9.50 | ~0.50 |
| MgO | 2.15 | Cristobalite | 24.00 | ~0.50 |
| Fe2O3 | 1.13 | Illite | 0.50 | Traces |
| Na2O | 2.15 | Beidelite | - | - |
| K2O | 0.60 | Alkaline and alkaline terous mineral compounds | 5.80 | Traces |
| CaO | 0.90 | Amorphous substance | Traces | - |
| MnO | - | |||
| PC * calcination losses | 5.67 | |||
| Bentonite Samples | Specific Surface Area (SBET, m2g−1) | Total Pore Volume (Vt, cm3g−1) | Mesopore Volume (Vme, cm3g−1) | Micropore Volume (Vmi, cm3g−1) |
|---|---|---|---|---|
| BtB | 34 | 0.131 | 0.307 | 0.003 |
| Na-BtF1 | 32 | 0.213 | 0.213 | 0.0 |
| Na-BtF2 | 189 | 0.392 | 0.362 | 0.030 |
| Na-BtF3 | 37 | 0.134 | 0.130 | 0.004 |
| Clays Samples | POM-Test 420 nm (%) | POM-Test 520 nm (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 mL | 0.2 mL | 0.3 mL | 0.4 mL | 0.5 mL | 0.1 mL | 0.2 mL | 0.3 mL | 0.4 mL | 0.5 mL | |
| Sauvignon Blanc | 91.07 | 62.35 | ||||||||
| BtB | 19.51 | 13.9 | 13.8 | 16.37 | 19.51 | 15.46 | 29.03 | 31.26 | 20.24 | 15.46 |
| Na-BtF1 | 16.45 | 13.25 | 11.83 | 12.96 | 16.45 | 12.67 | 28.88 | 12.59 | 13.69 | 12.67 |
| Na-BtF2 | 13.38 | 13.04 | 11.01 | 11.84 | 13.38 | 28.58 | 25.11 | 12.11 | 23.94 | 25.58 |
| Na-BtF3 | 12.33 | 11.98 | 9.87 | 10.35 | 12.33 | 17.81 | 19.42 | 12.01 | 17.43 | 17.81 |
| IPT | SFT (Gallic Acid Eq. mgL−1) | |||||||||
| Sauvignon Blanc | 5.57 | 46.20 | ||||||||
| BtB | 4.8 | 4.45 | 4.07 | 3.8 | 3.79 | 23.60 | 23.60 | 23.60 | 23.60 | 23.60 |
| Na-BtF1 | 5.34 | 5.34 | 5.34 | 5.34 | 5.34 | 46.93 | 46.93 | 46.93 | 46.93 | 46.93 |
| Na-BtF2 | 4.99 | 4.99 | 4.99 | 4.99 | 4.99 | 30.70 | 30.70 | 30.70 | 30.70 | 30.70 |
| Na-BtF3 | 4.95 | 4.95 | 4.95 | 4.95 | 4.95 | 29.35 | 29.35 | 29.35 | 29.35 | 29.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hortolomeu, A.; Mirila, D.C.; Roșu, A.-M.; Nedeff, F.M.; Scutaru, I.; Ureche, D.; Sturza, R.; Fînaru, A.-L.; Nistor, I.D. Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine. Nanomaterials 2024, 14, 588. https://doi.org/10.3390/nano14070588
Hortolomeu A, Mirila DC, Roșu A-M, Nedeff FM, Scutaru I, Ureche D, Sturza R, Fînaru A-L, Nistor ID. Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine. Nanomaterials. 2024; 14(7):588. https://doi.org/10.3390/nano14070588
Chicago/Turabian StyleHortolomeu, Andreea, Diana Carmen Mirila, Ana-Maria Roșu, Florin Marian Nedeff, Iuri Scutaru, Dorel Ureche, Rodica Sturza, Adriana-Luminița Fînaru, and Ileana Denisa Nistor. 2024. "Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine" Nanomaterials 14, no. 7: 588. https://doi.org/10.3390/nano14070588
APA StyleHortolomeu, A., Mirila, D. C., Roșu, A.-M., Nedeff, F. M., Scutaru, I., Ureche, D., Sturza, R., Fînaru, A.-L., & Nistor, I. D. (2024). Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine. Nanomaterials, 14(7), 588. https://doi.org/10.3390/nano14070588








