Understanding Lignin Dissolution with Urea and the Formation of a Lignin Nano-Aggregate: A Multiscale Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solubility Test of Corncob Lignin in Urea Aqueous Solution
2.2.2. Equilibrium Simulation in Different Concentrations of Urea Solvents
2.2.3. Analysis
2.2.4. Density Functional Theory (DFT) Calculations
2.2.5. Preparation of Corncob Lignin Nanoparticles (CLNPs)
2.2.6. Characterization of CLNPs
3. Results and Discussion
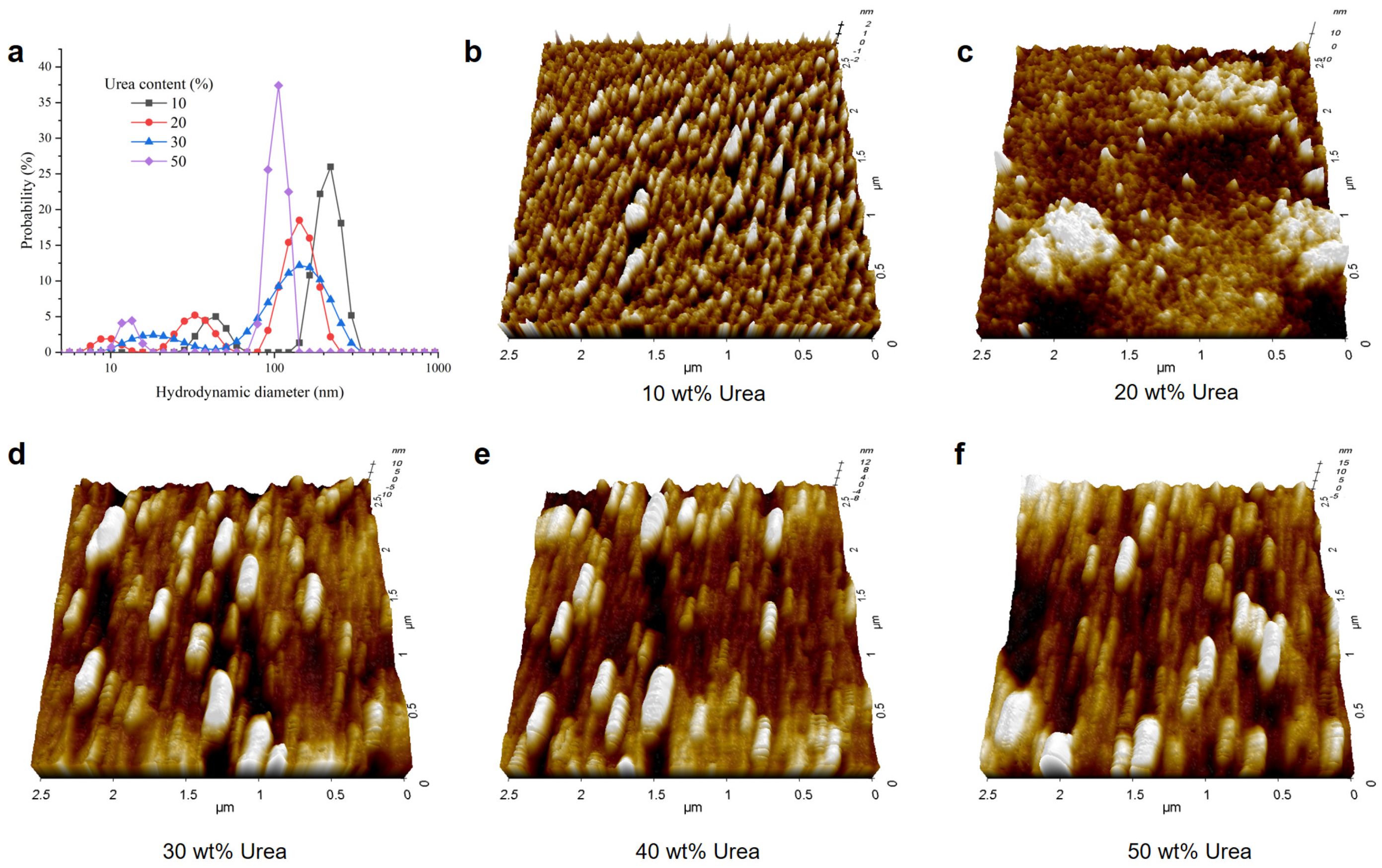
3.1. Effect of Urea Content on Lignin Dissolution Behaviors in Urea Aqueous Solution
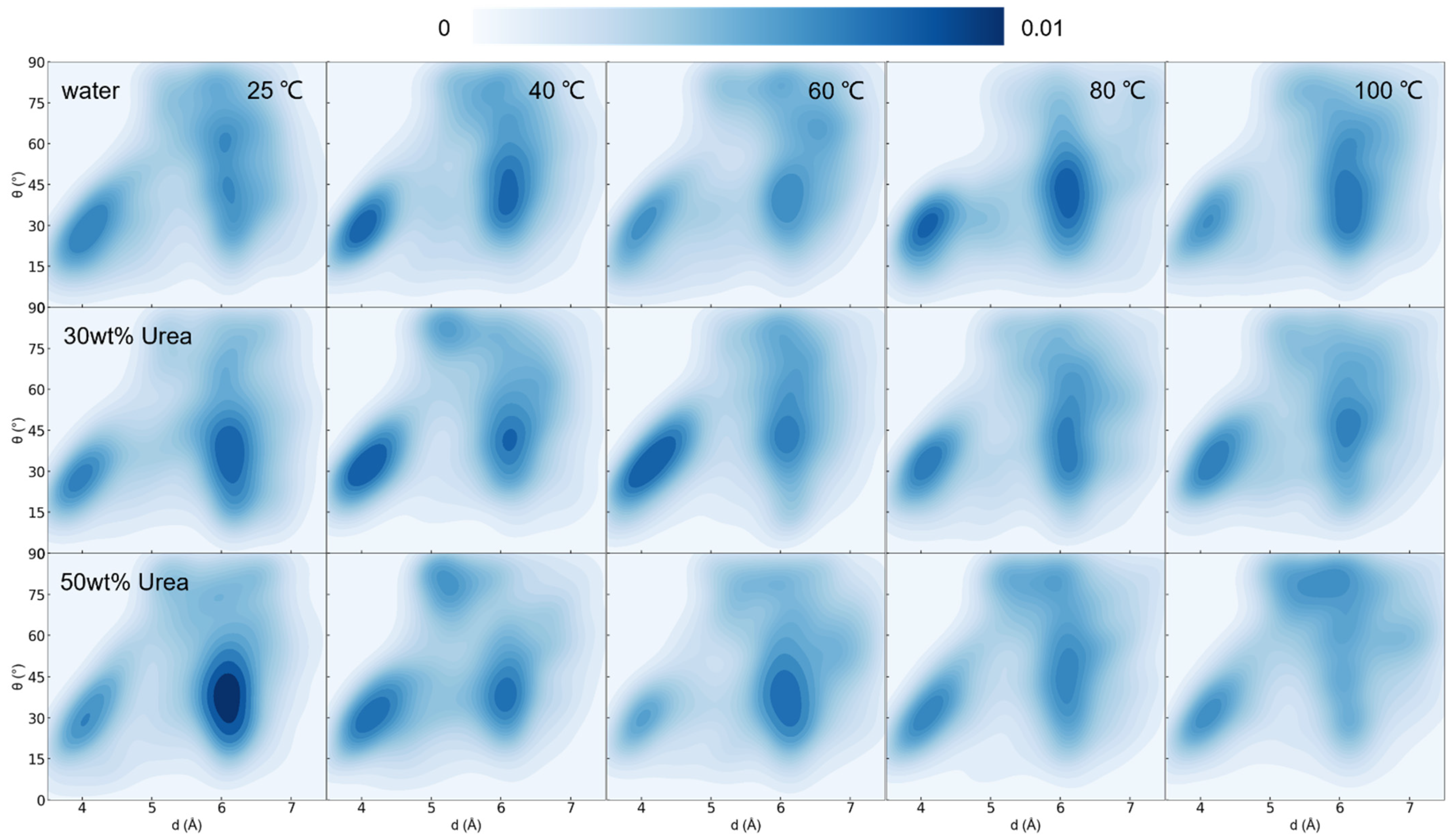
3.2. Effect of Temperature on Lignin Dissolution Behaviors in Urea Aqueous Solution
3.3. Effect of Urea on Lignin Self-Assembly by an Anti-Solvent
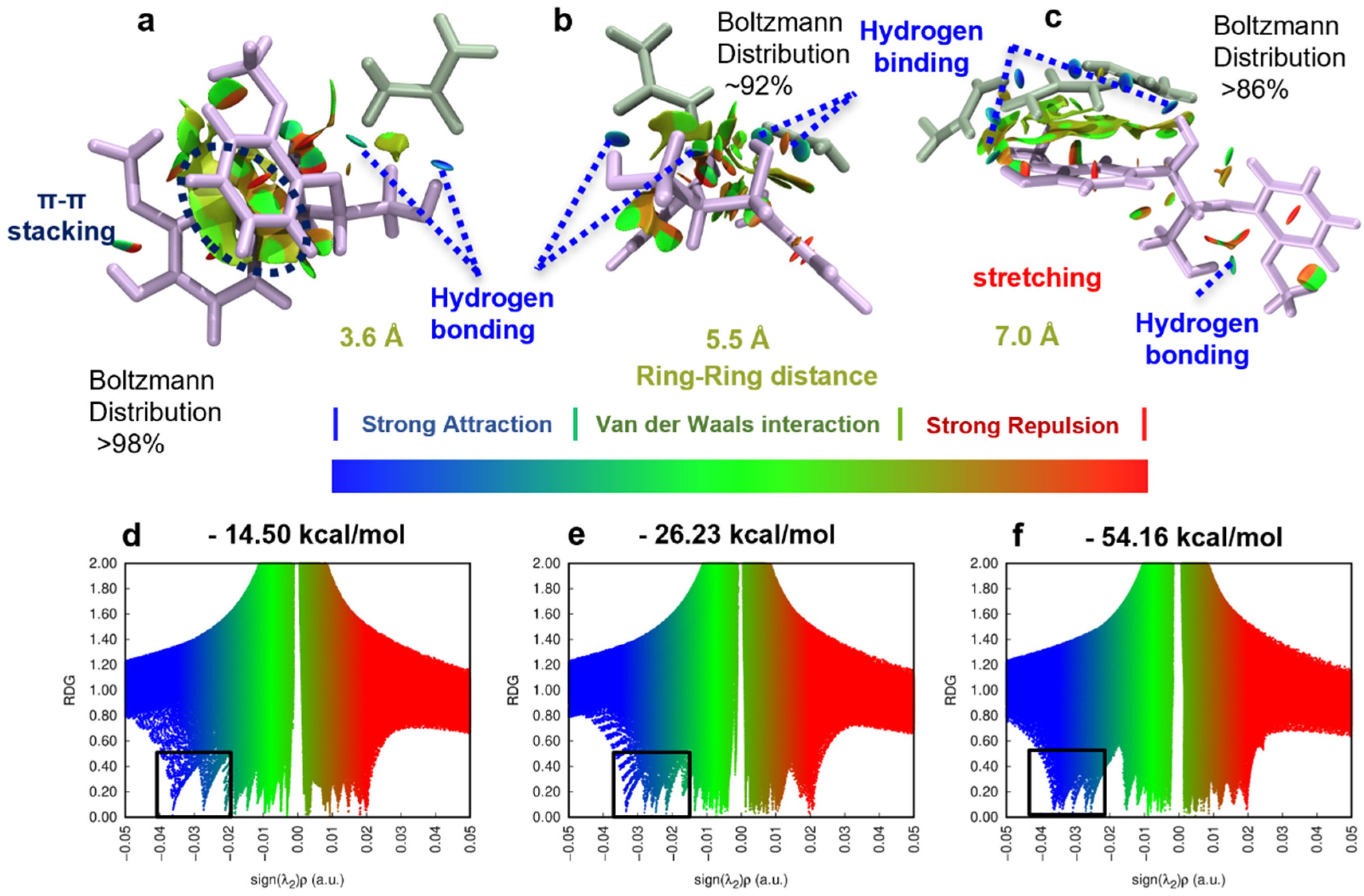
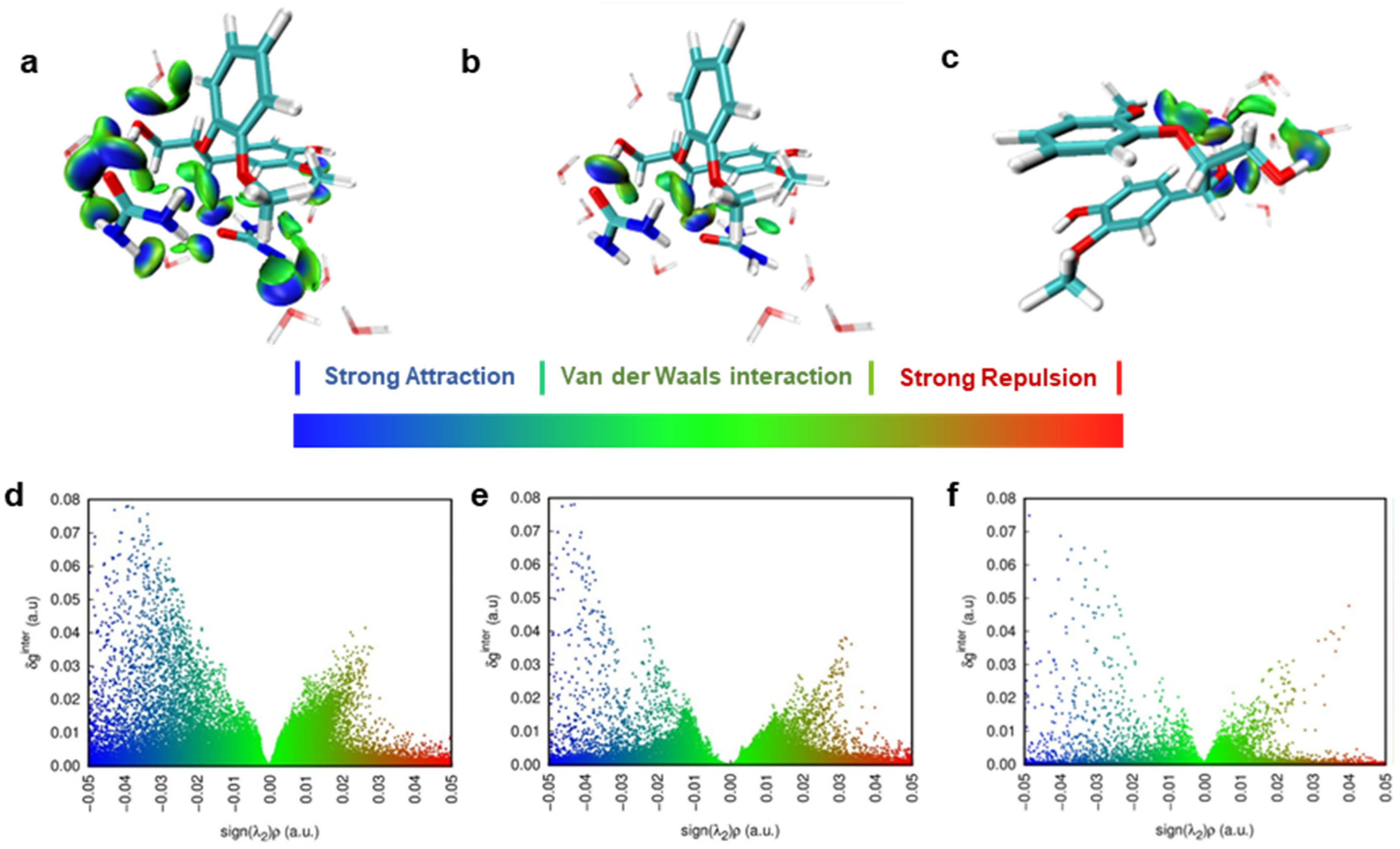
3.4. Non-Covalent Interactions (NCIs) between Urea and Lignin Unveiled by DFT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Qiu, X.; Sun, S.; Lin, X. Synergizing mitigated spatial confinement and chemical stabilization of lignin facilitates full utilization of lignocellulose. Green Chem. 2023, 25, 10415–10423. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Terrasson, V.; Guenin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Mohapatra, S.; Behera, B.C.; Thatoi, H. Recent updates on green synthesis of lignin nanoparticle and its potential applications in modern biotechnology. Crit. Rev. Biotechnol. 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, S.; Qi, Y.; Guo, H.; Guo, Y.; Li, H. State-of-the-Art: Applications and Industrialization of Lignin Micro/Nano Particles. Chemsuschem 2021, 14, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Park, J.; Kim, B.M.; Kim, J.; Kim, K.-J.; Park, J. Influence of nanoprecipitation techniques on lignin nanoparticle structure. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132803. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhuang, Y.; Chen, J.; Yang, G.; Dai, L. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254. [Google Scholar] [CrossRef]
- Osterberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Aquib, T.I. Lignin-Based Nanomaterials as Drug Delivery Vehicles: A Review. Crit. Rev. Ther. Drug Carr. Syst. 2023, 40, 1–67. [Google Scholar] [CrossRef]
- Wang, H.-M.; Yuan, T.-Q.; Song, G.-Y.; Sun, R.-C. Advanced and versatile lignin-derived biodegradable composite film materials toward a sustainable world. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, W.; Wang, J.; Qiu, X.; Huang, J.; Li, J.; Lou, H. Biomimetic high performance artificial muscle built on sacrificial coordination network and mechanical training process. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, J.; Sun, X.; Zhao, C.; Li, J.; Li, H. SiC and N, S-doped carbon nanosheets and lignin-enhanced organohydrogel for low-temperature tolerant solid-state supercapacitors. Int. J. Biol. Macromol. 2024, 258, 128759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, T.; Liu, K.; Zhang, M.; Cai, X.-M.; Si, C. Biomass-based materials for advanced supercapacitor: Principles, progress, and perspectives. Aggregate 2023, 5, e428. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Riazanova, A.; Lindstrom, M.E.; Sevastyanova, O. Structural and molecular-weight-dependency in the formation of lignin nanoparticles from fractionated soft- and hardwood lignins. Green Chem. 2021, 23, 3061–3072. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.; Wang, L.; Pranovich, A.; Hemming, J.; Dai, L.; Xu, C.; Si, C. Towards a deep understanding of the biomass fractionation in respect of lignin nanoparticle formation. Adv. Compos. Hybrid Mater. 2023, 6, 214. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, Y.; Jiang, Z.; Sun, Q.; Guo, X.; Zhang, W.; Hu, C.; Luque, R.; Shi, B.; Sels, B.F.F. Solvent effect on the production of spherical lignin nanoparticles. Green Chem. 2023, 25, 993–1003. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, H.; Xu, H.; Qing, Y.; Wu, Z.; Wu, Y. Self-assembled lignin nanospheres with solid and hollow tunable structures. Ind. Crops Prod. 2020, 144, 112063. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A simple and effective approach to fabricate lignin nanoparticles with tunable sizes based on lignin fractionation. Green Chem. 2020, 22, 2011–2017. [Google Scholar] [CrossRef]
- Zou, T.; Nonappa, N.; Khavani, M.; Vuorte, M.; Penttila, P.; Zitting, A.; Valle-Delgado, J.J.; Elert, A.M.; Silbernagl, D.; Balakshin, M.; et al. Experimental and Simulation Study of the Solvent Effects on the Intrinsic Properties of Spherical Lignin Nanoparticles. J. Phys. Chem. B 2021, 125, 12315–12328. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, S.-M.; Huo, C.-M.; Shi, Y.-F.; Feng, J.; Zhu, J.-Y.; Xue, W.; Qiu, X. New insight into lignin aggregation guiding efficient synthesis and functionalization of a lignin nanosphere with excellent performance. Green Chem. 2022, 24, 285–294. [Google Scholar] [CrossRef]
- Wang, H.; Tan, S.; Su, Z.; Li, M.; Hao, X.; Peng, F. Perforin-Mimicking Molecular Drillings Enable Macroporous Hollow Lignin Spheres for Performance-Configurable Materials. Adv. Mater. 2024, 2311073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xiao, L.-P.; Song, G.; Sun, R.-C.; He, L.; Singh, S.; Simmons, B.A.; Cheng, G. From lignin subunits to aggregates: Insights into lignin solubilization. Green Chem. 2017, 19, 3272–3281. [Google Scholar] [CrossRef]
- Hartati, I.; Sulistyo, H.; Sediawan, W.B.; Azis, M.M.; Fahrurrozi, M. Microwave-Assisted Urea-Based-Hydrotropic Pretreatment of Rice Straw: Experimental Data and Mechanistic Kinetic Models. ACS Omega 2021, 6, 13225–13239. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Delfino, I.; Crucianelli, M.; Conte, N.; Avitabile, D.; Saladino, R. Green and Scalable Preparation of Colloidal Suspension of Lignin Nanoparticles and Its Application in Eco-friendly Sunscreen Formulations. ACS Omega 2021, 6, 21444–21456. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Qiu, X.; Liu, D.; Yang, D.; Liu, W.; Qian, Y. Dissolution of lignin in green urea aqueous solution. Appl. Surf. Sci. 2017, 425, 736–741. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Petridis, L.; Ralph, J.; Crowley, M.F.; Beckham, G.T. Systematic parameterization of lignin for the CHARMM force field. Green Chem. 2019, 21, 109–122. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Huang, Z.; Qiu, X. Unraveling lignin extraction: Molecular dynamics insights into effective biomass valorization using a p-toluenesulfonic acid/solvents system. Green Chem. 2024, 26, 2783–2792. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zhao, Y.; Huang, S.; Zhao, J. Molecular docking and molecular dynamics simulation analyses of urea with ammoniated and ammoxidized lignin. J. Mol. Graph. Model. 2017, 71, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, S.; Wang, P.; Chen, H.; Zheng, J.; Lin, X.; Qin, Y.; Qiu, X. Successive organic solvent fractionation and homogenization of technical lignin for polyurethane foam with high mechanical performance. Int. J. Biol. Macromol. 2022, 221, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Qiu, X.; Hao, S.; Ravichandran, S.; Song, J.; Zhang, W. Electrochemical conversion of lignin to short-chain carboxylic acids. Green Chem. 2023, 25, 3127–3136. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Dellon, L.D.; Broadbelt, L.J.; Beckham, G.T.; Crowley, M.F. Automated Transformation of Lignin Topologies into Atomic Structures with LigninBuilder. Acs Sustain. Chem. Eng. 2019, 7, 3443–3453. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, J.V.; Hardy, D.J.; Stone, J.E.; Tajkhorshid, E.; Kohlmeyer, A. TopoGromacs: Automated Topology Conversion from CHARMM to GROMACS within VMD. J. Chem. Inf. Model. 2016, 56, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Molclus Program, Version 1.9.9.9. Available online: http://www.keinsci.com/research/molclus.html (accessed on 23 January 2022).
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-Garcia, J.; Henon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
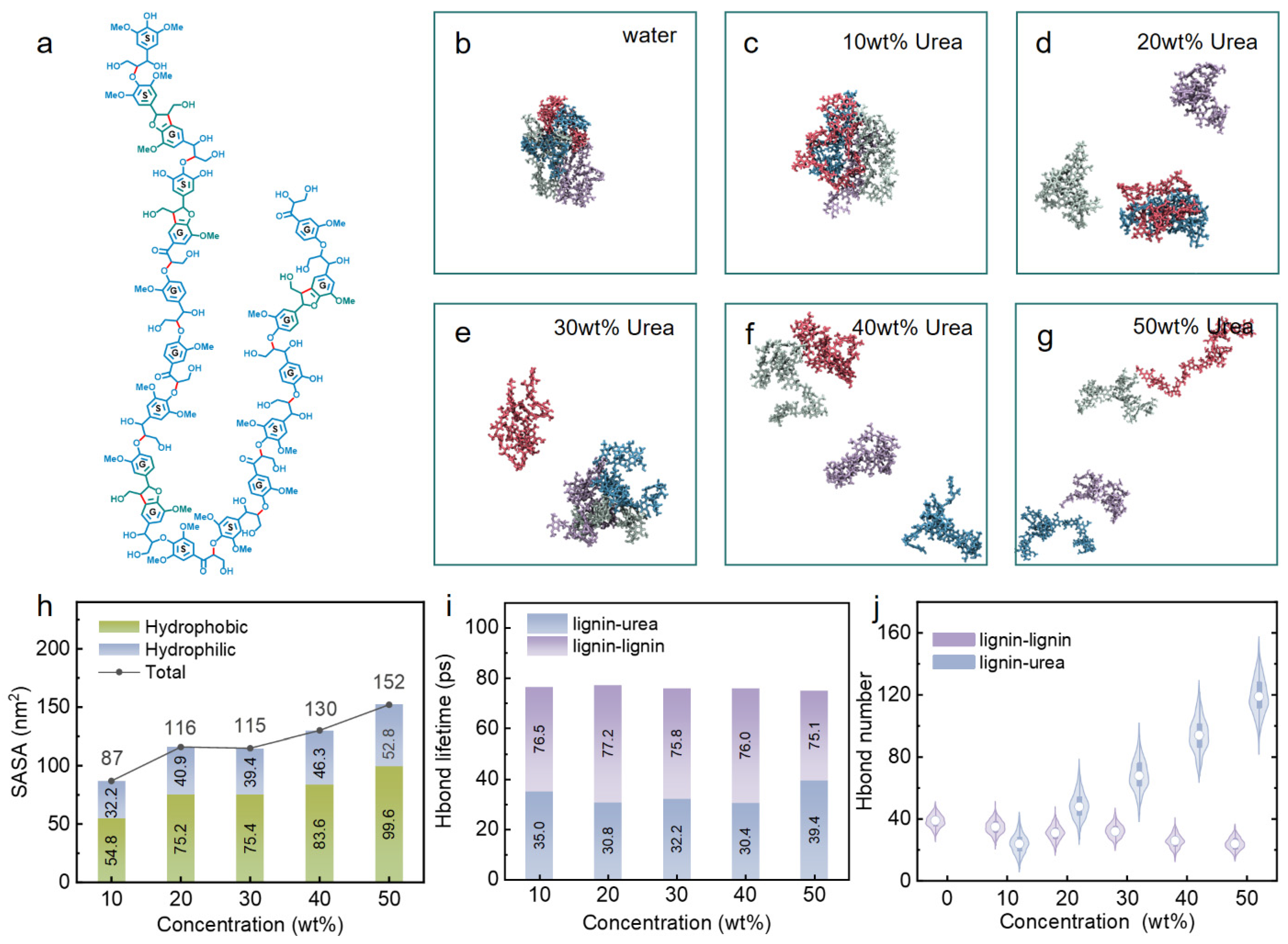

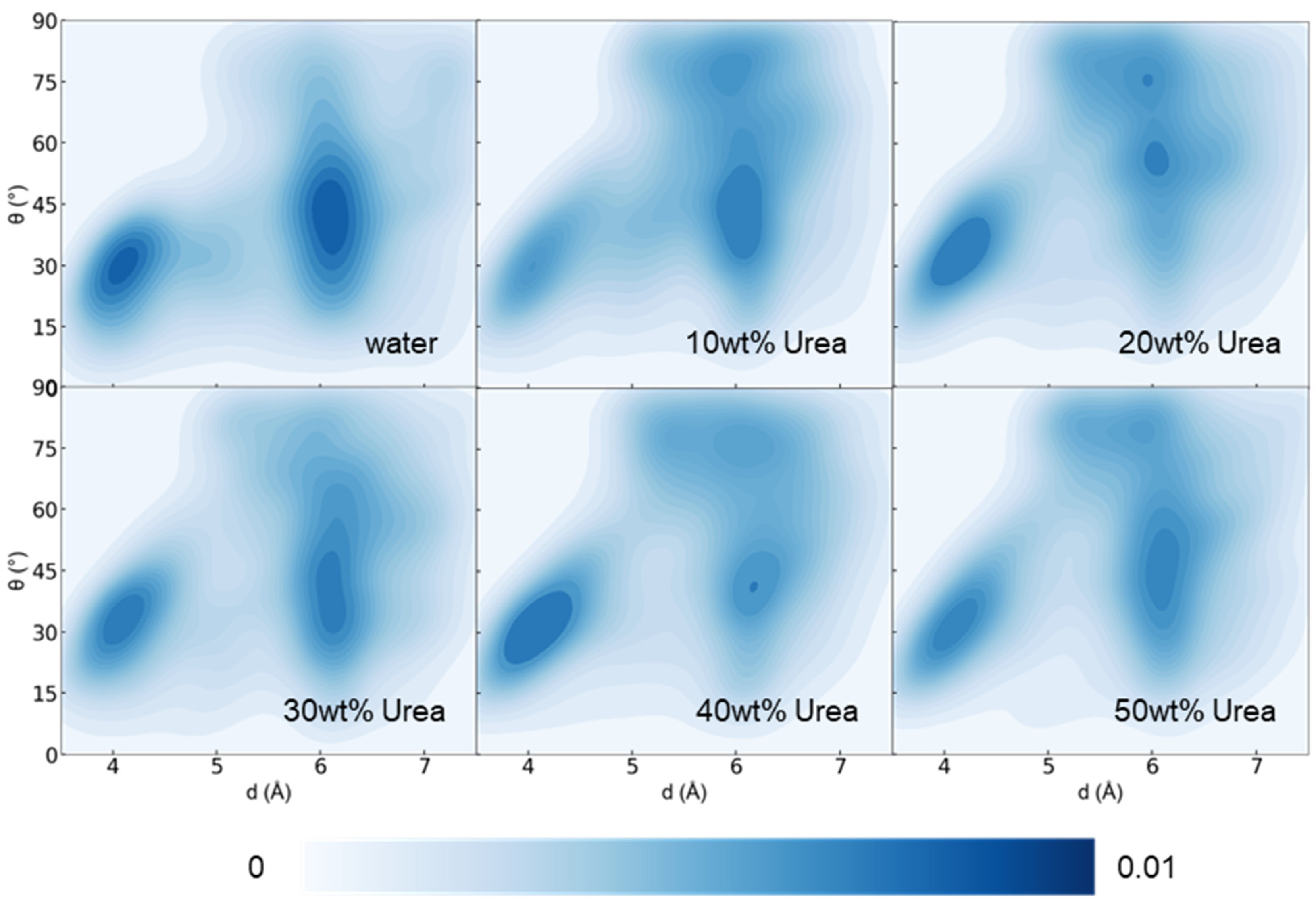
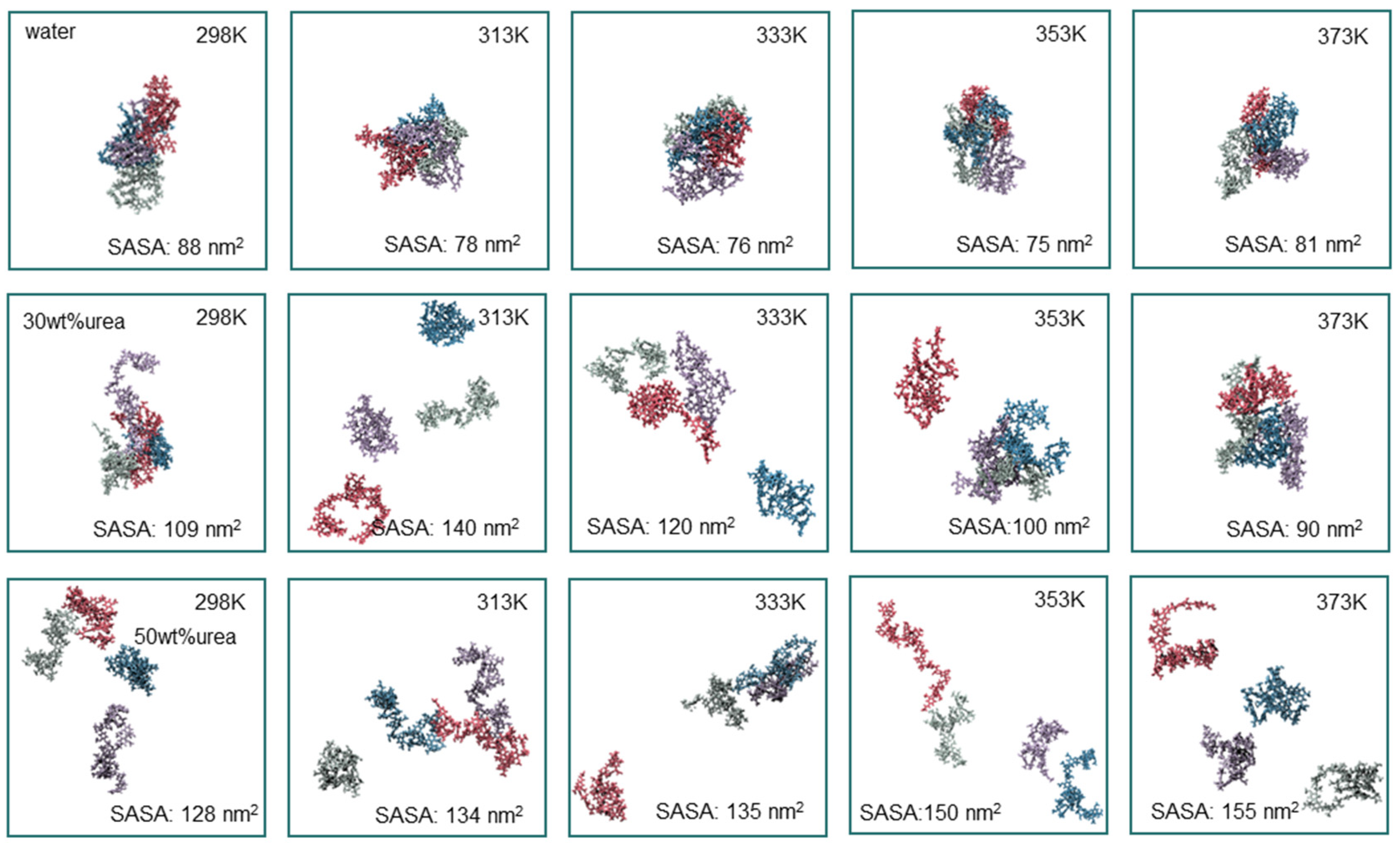
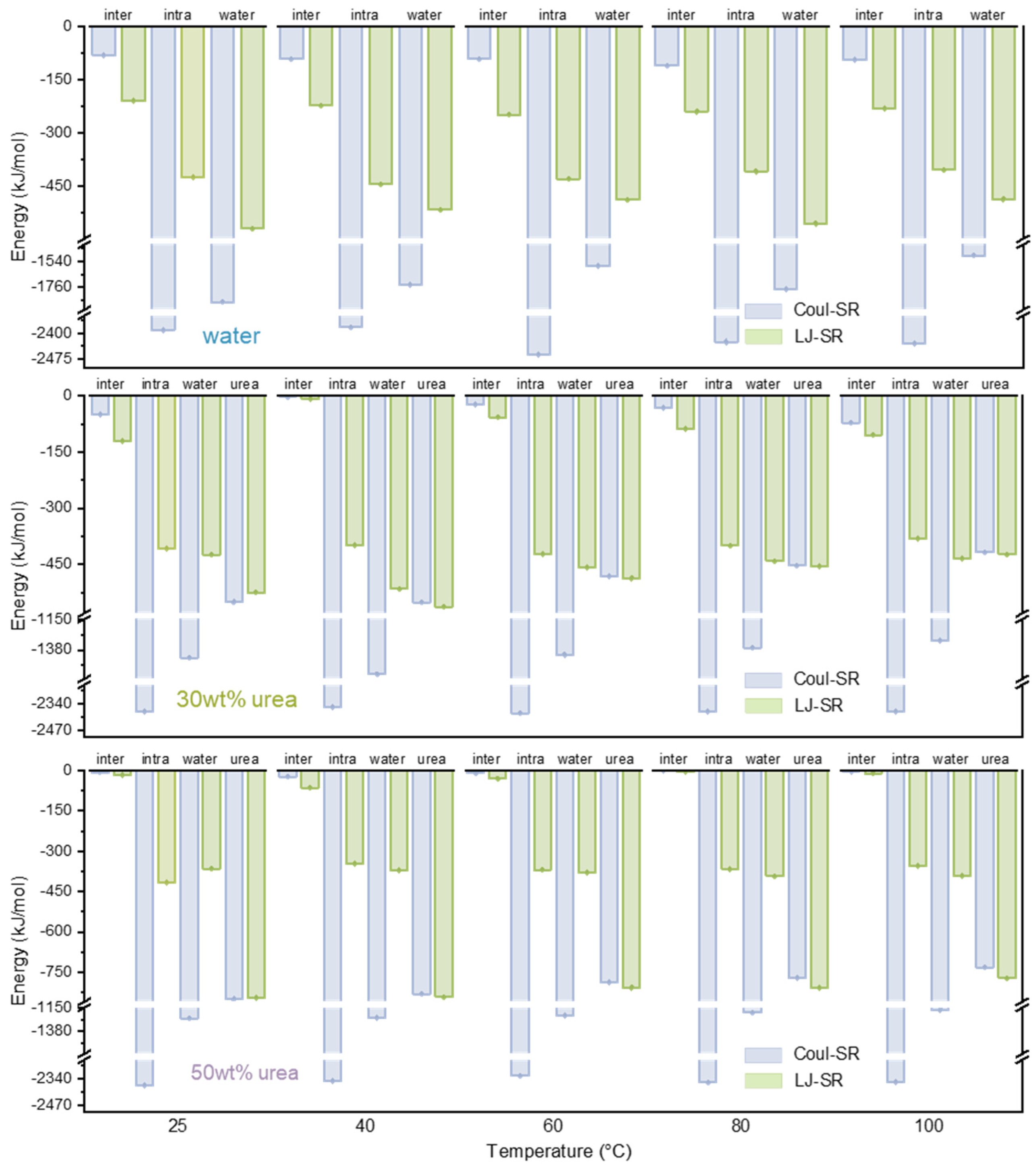
| Urea Aqueous Solution Concentration (wt%) | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|
| Lignin solubility (mg/mL) | 0.87 | 2.49 | 2.99 | 3.23 | 5.68 |
| Solution Temperature (K) | 298 | 313 | 333 | 353 |
|---|---|---|---|---|
| Lignin solubility (mg/mL) | 1.34 | 3.56 | 5.68 | 12.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Chen, L.; Qin, Y.; Qiu, X. Understanding Lignin Dissolution with Urea and the Formation of a Lignin Nano-Aggregate: A Multiscale Approach. Nanomaterials 2024, 14, 593. https://doi.org/10.3390/nano14070593
Lin J, Chen L, Qin Y, Qiu X. Understanding Lignin Dissolution with Urea and the Formation of a Lignin Nano-Aggregate: A Multiscale Approach. Nanomaterials. 2024; 14(7):593. https://doi.org/10.3390/nano14070593
Chicago/Turabian StyleLin, Jinxin, Liheng Chen, Yanlin Qin, and Xueqing Qiu. 2024. "Understanding Lignin Dissolution with Urea and the Formation of a Lignin Nano-Aggregate: A Multiscale Approach" Nanomaterials 14, no. 7: 593. https://doi.org/10.3390/nano14070593






