Autonomous Nanorobots as Miniaturized Surgeons for Intracellular Applications
Abstract
1. Introduction
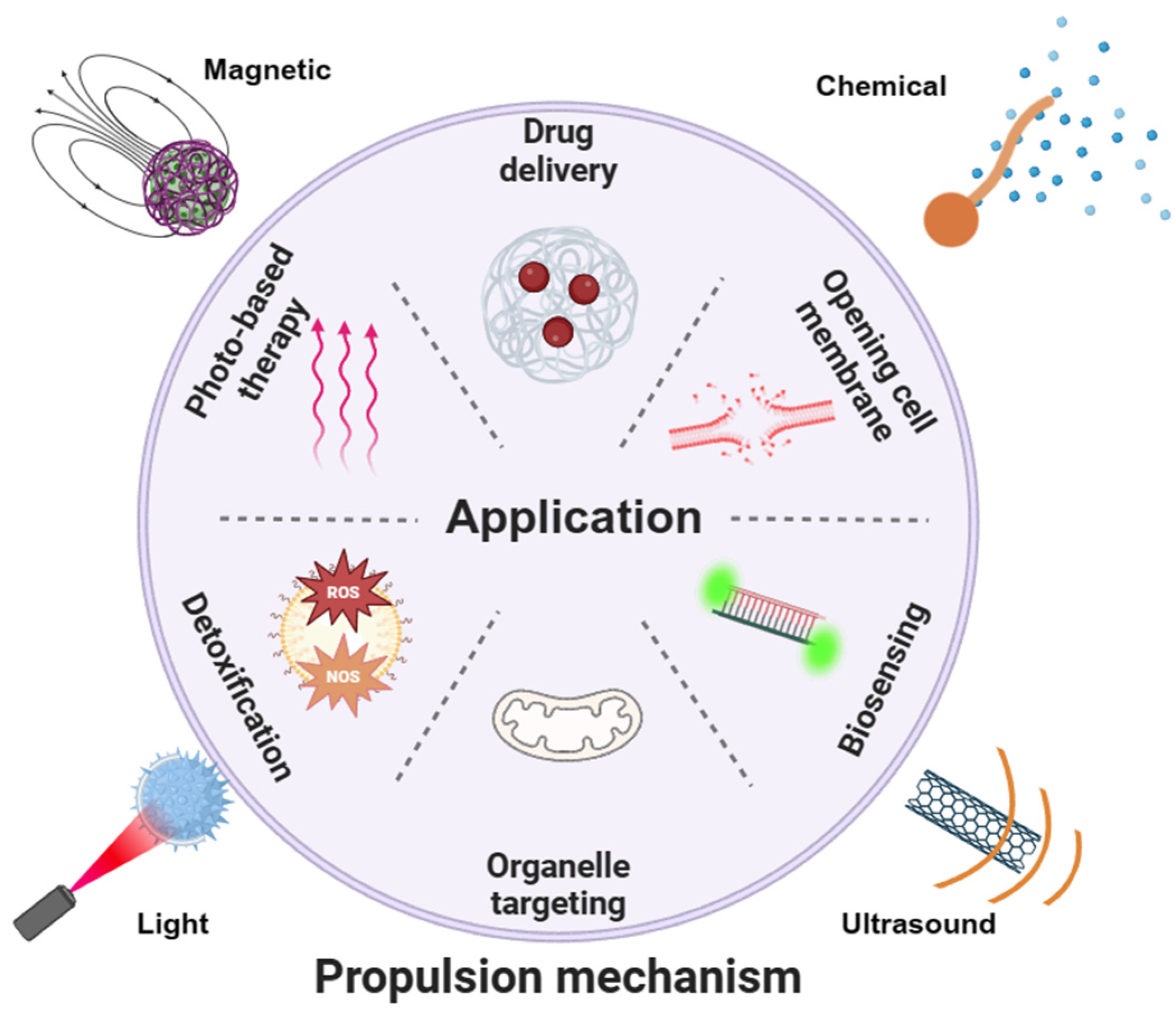
2. Propulsion Mechanisms
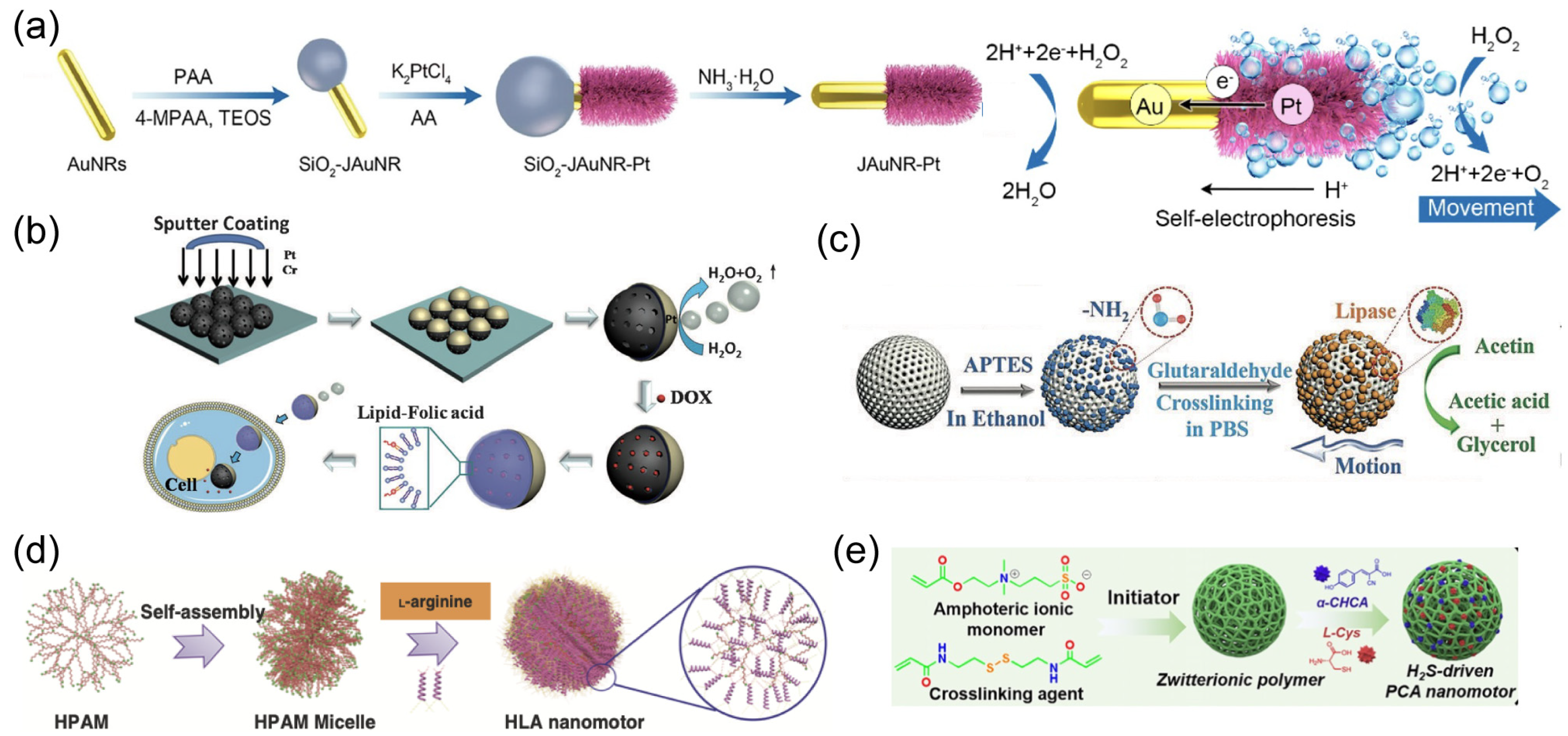
2.1. Chemical Propulsion
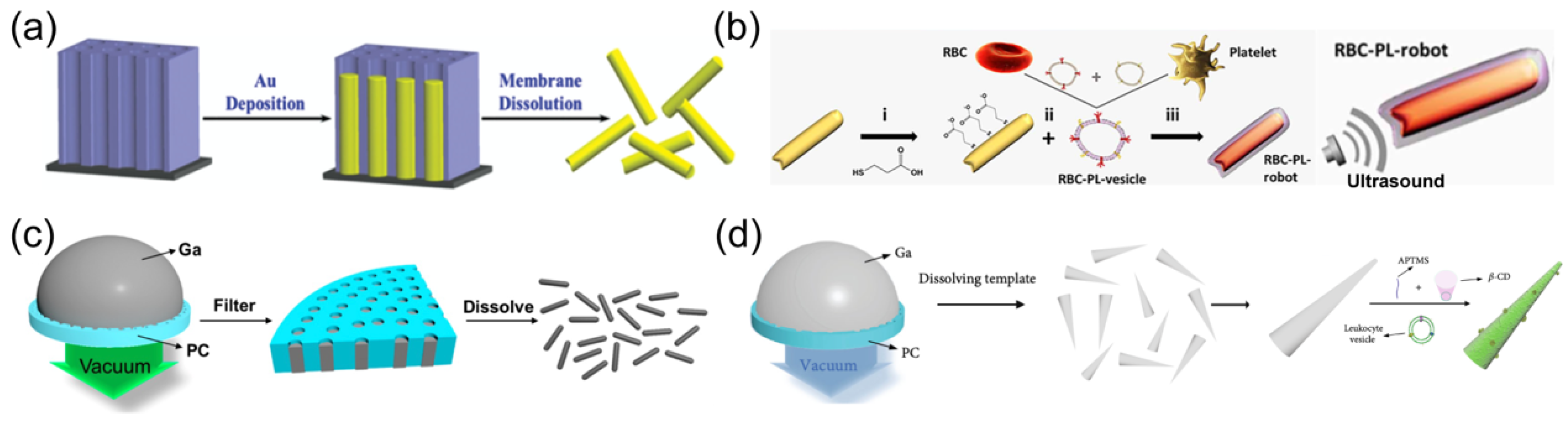
2.2. Ultrasound Propulsion
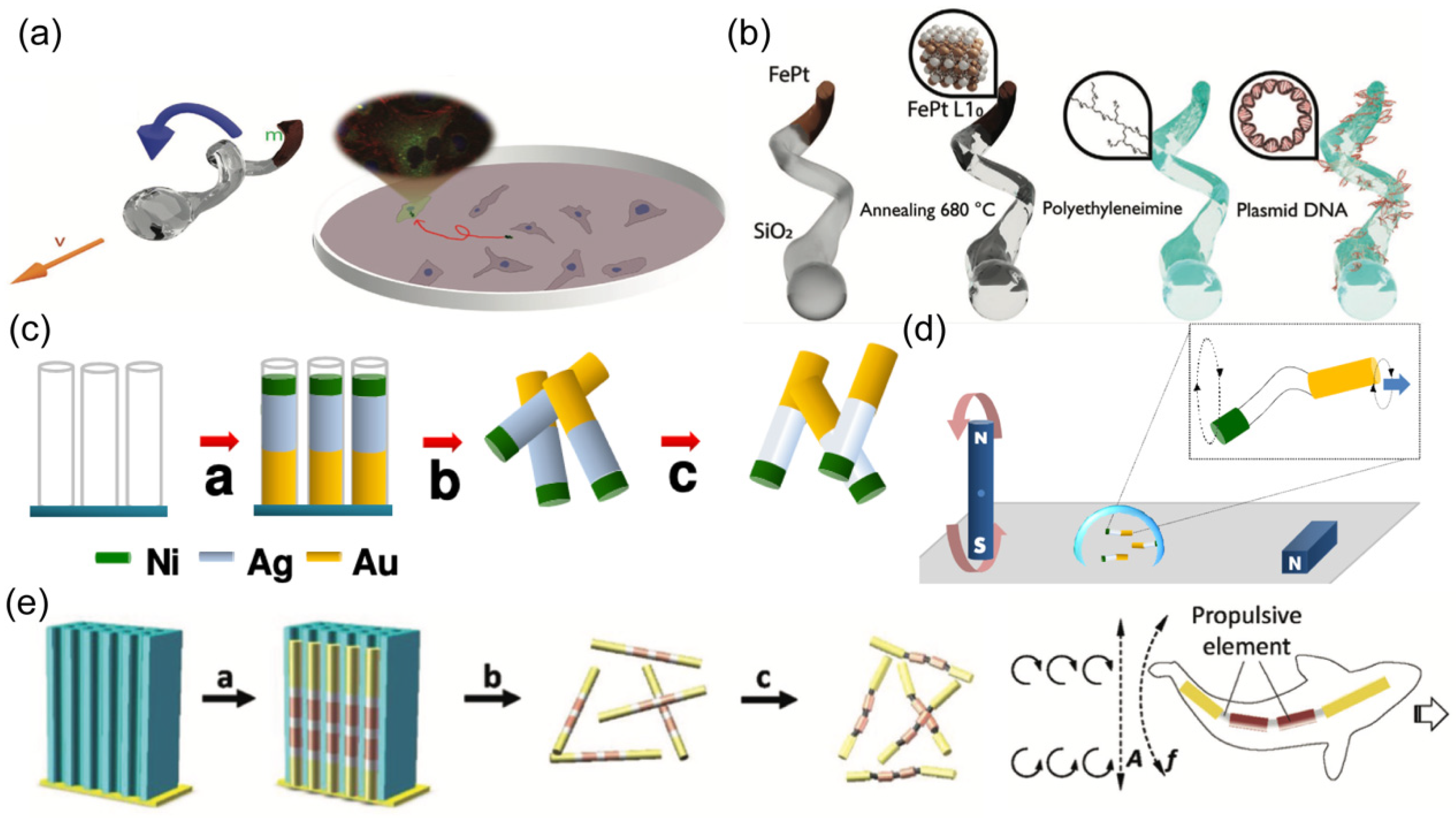
2.3. Magnetic Propulsion
2.4. Light Propulsion
3. Intracellular Applications
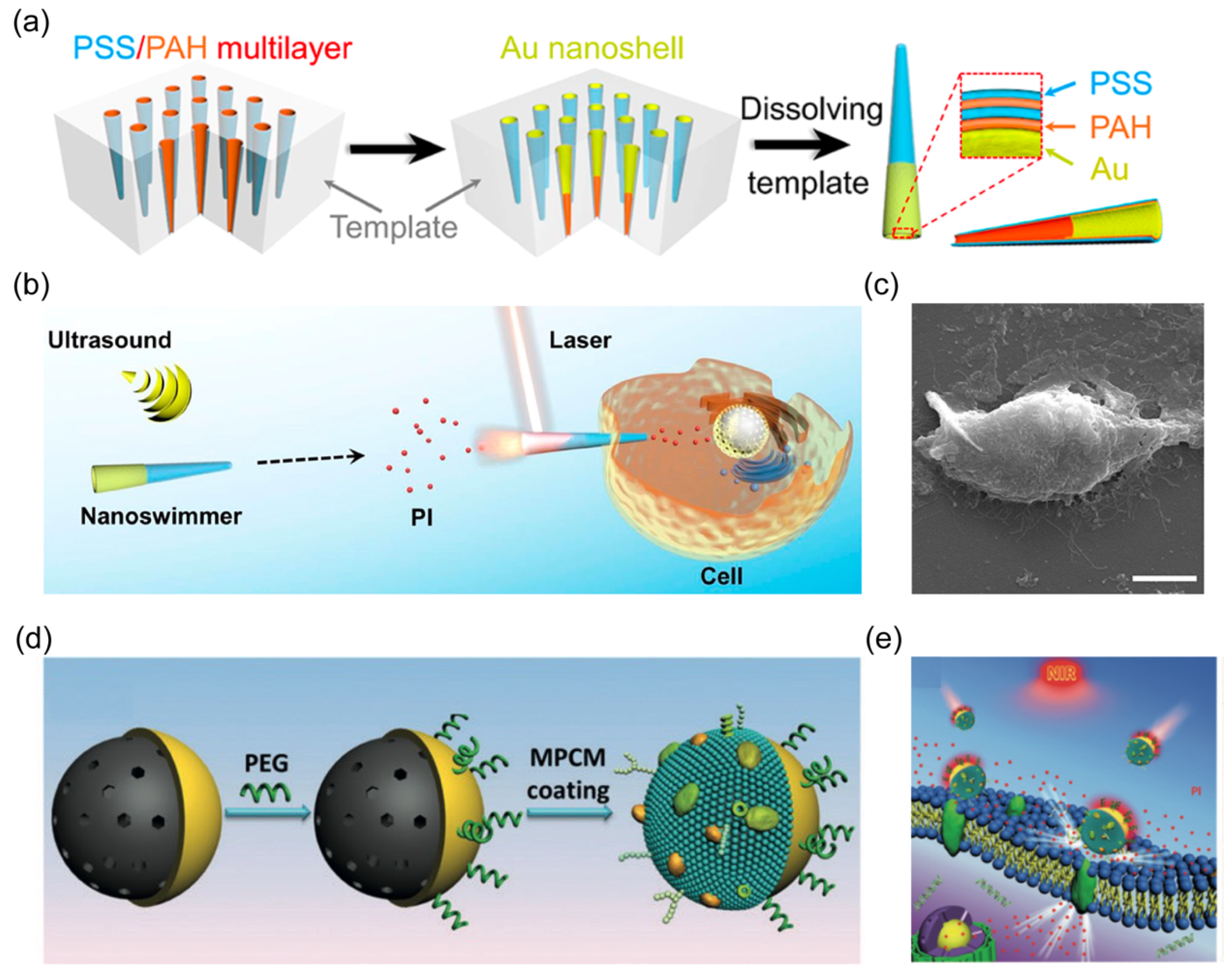
3.1. Opening Cell Membrane
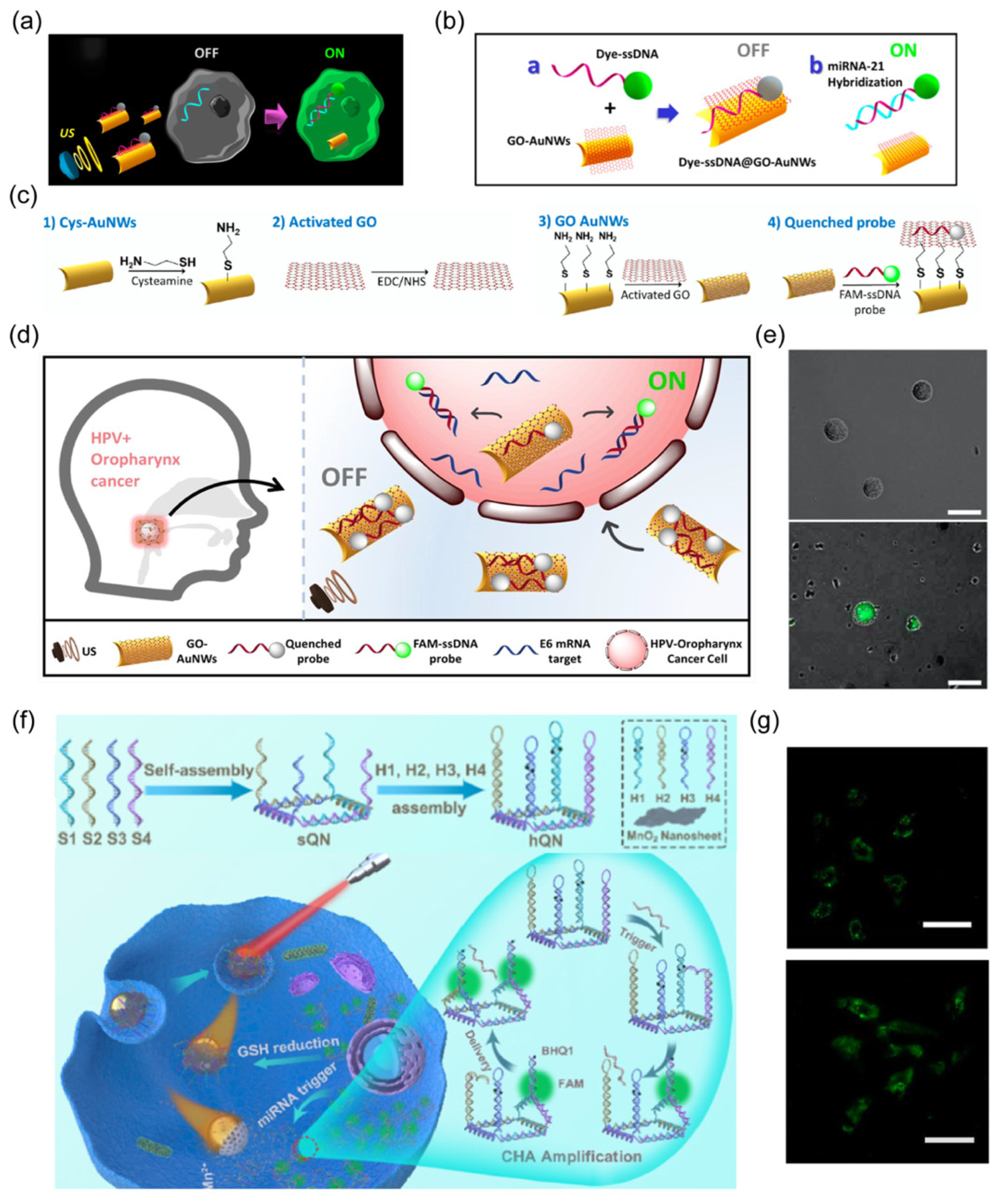
3.2. Biosensing
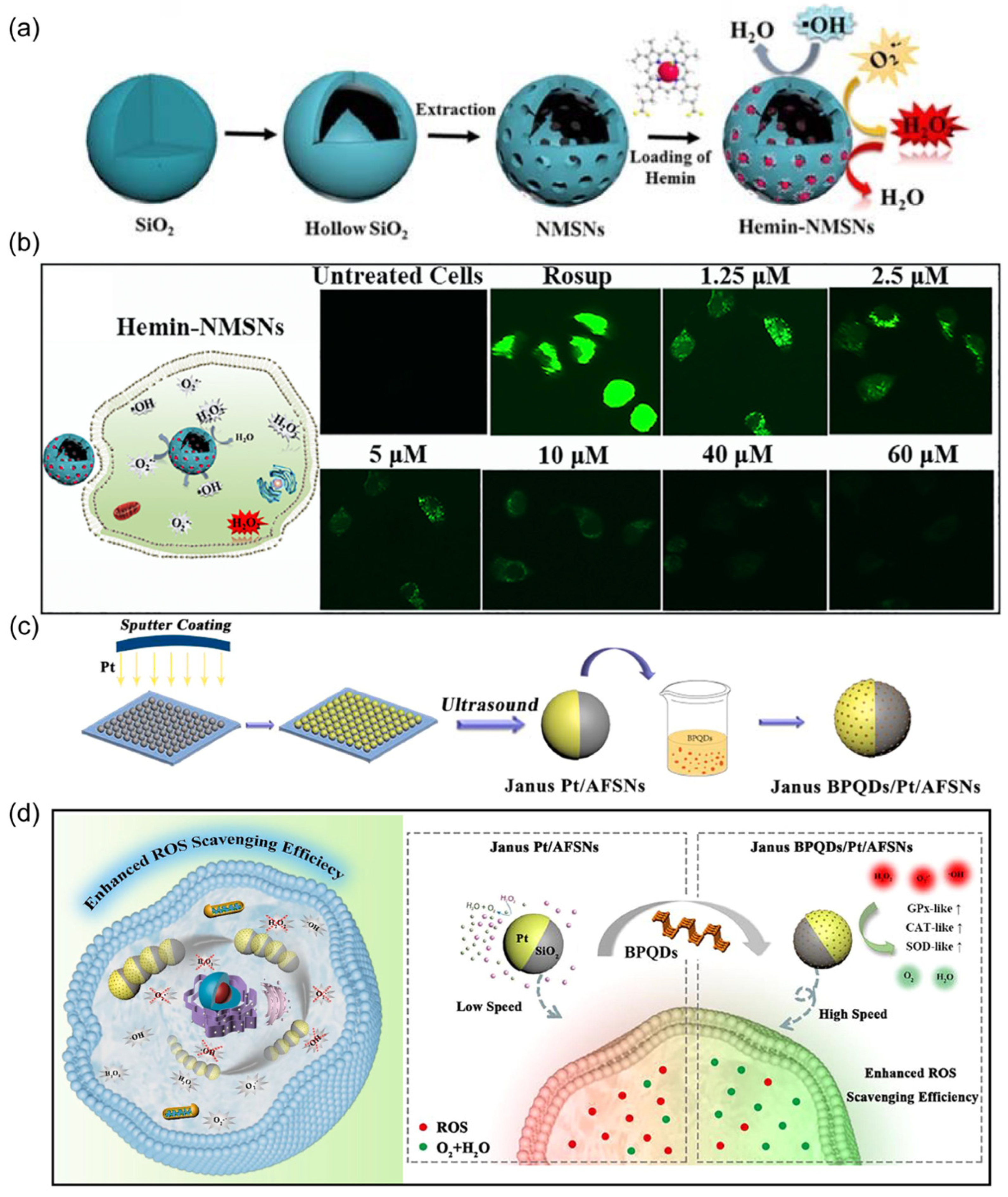
3.3. Detoxification
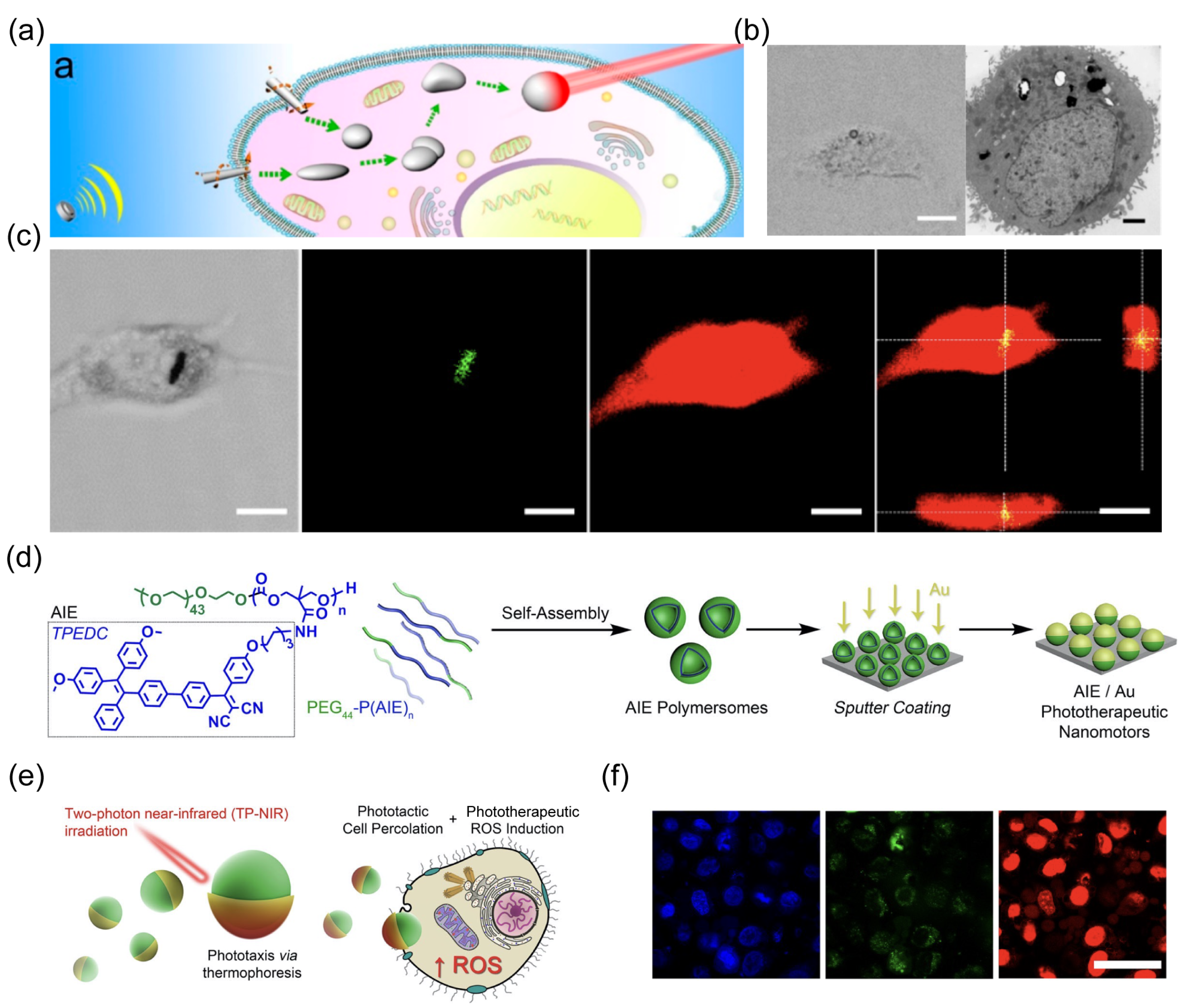
3.4. Photo-Based Therapy
3.5. Drug Delivery
3.6. Organelle Targeting
4. Conclusions and Outlook
- (1). Material Selection
- (2). Propulsion Mode
- (3). Real-Time Imaging
Funding
Conflicts of Interest
References
- Sim, S.; Aida, T. Swallowing a surgeon: Toward clinical nanorobots. Acc. Chem. Res. 2017, 50, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, H.; Raman, J.; Leveson, N.; Kalbarczyk, Z.; Iyer, R.K. Adverse events in robotic surgery: A retrospective study of 14 years of fda data. PLoS ONE 2016, 11, e0151470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Nanomachines: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, W. Nano/microscale motors: Biomedical opportunities and challenges. ACS Nano 2012, 6, 5745–5751. [Google Scholar] [CrossRef]
- Brenner, H. Dynamics of neutrally buoyant particles in low reynolds number flows. Prog. Heat Mass Transf. 1972, 6, 509–574. [Google Scholar]
- Purcell, E.M. Life at low reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef]
- Li, J.; Rozen, I.; Wang, J. Rocket science at the nanoscale. ACS Nano 2016, 10, 5619–5634. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Soler, L.; Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, L.K.E.A.; Peng, F.; Tu, Y.; Wilson, D.A. Micro- and nano-motors for biomedical applications. J. Mater. Chem. B 2014, 2, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Guo, J.; Xu, X.; Fan, D.L. Recent progress on man-made inorganic nanomachines. Small 2015, 11, 4037–4057. [Google Scholar] [CrossRef]
- Novotný, F.; Wang, H.; Pumera, M. Nanorobots: Machines squeezed between molecular motors and micromotors. Chem 2020, 6, 867–884. [Google Scholar] [CrossRef]
- Ricotti, L.; Trimmer, B.; Feinberg, A.W.; Raman, R.; Parker, K.K.; Bashir, R.; Sitti, M.; Martel, S.; Dario, P.; Menciassi, A. Biohybrid actuators for robotics: A review of devices actuated by living cells. Sci. Robot. 2017, 2, eaaq0495. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.L.; Posner, J.D. Phoretic self-propulsion. Annu. Rev. Fluid Mech. 2017, 49, 511–540. [Google Scholar] [CrossRef]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Paxton, W.F.; Sen, A.; Mallouk, T.E. Motility of catalytic nanoparticles through self-generated forces. Chemistry 2005, 11, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; An, M.; Liu, Y.; Sarwar, M.T.; Yang, H. Advances in chemically powered micro/nanorobots for biological applications: A review. Adv. Funct. Mater. 2023, 33, 2209883. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Zhang, Z.; Ji, F.; Chan, T.K.; Yang, H.; Chan, C.P.; Yang, Z.; Chen, Z.; Chang, W.T. Endoscope-assisted magnetic helical micromachine delivery for biofilm eradication in tympanostomy tube. Sci. Adv. 2022, 8, eabq8573. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Fischer, P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically driven micro and nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pané, S. Recent developments in magnetically driven micro- and nanorobots. Appl. Mate. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Xu, T.; Soto, F.; Gao, W.; Garcia-Gradilla, V.; Li, J.; Zhang, X.; Wang, J. Ultrasound-modulated bubble propulsion of chemically powered microengines. J. Am. Chem. Soc. 2014, 136, 8552–8555. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, L.-P.; Zhang, X. Ultrasound propulsion of micro-/nanomotors. Appl. Mate. Today 2017, 9, 493–503. [Google Scholar] [CrossRef]
- Li, J.; Mayorga-Martinez, C.C.; Ohl, C.D.; Pumera, M. Ultrasonically propelled micro-and nanorobots. Adv. Funct. Mater. 2022, 32, 2102265. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Zheng, J.; Zhan, X.; Tang, J. Light-driven micro/nanomotor for promising biomedical tools: Principle, challenge, and prospect. Acc. Chem. Res. 2018, 51, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Z.; Xiu, J.; Dai, W.; Zhao, L.; Xu, T.; Du, X.; Wen, Y.; Zhang, X. Nir powered janus nanocarrier for deep tumor penetration. Appl. Mate. Today 2020, 18, 100504. [Google Scholar] [CrossRef]
- Lin, F.; Shao, Y.; Wu, Y.; Zhang, Y. Nir light-propelled janus-based nanoplatform for cytosolic-fueled microrna imaging. ACS. Appl. Mater. Interfaces 2021, 13, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, F.; Lai, Y.; Xu, Z.; Yu, H. Engineering nanorobots for tumor-targeting drug delivery: From dynamic control to stimuli-responsive strategy. ChemBioChem 2021, 22, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; de Ávila, B.E.-F.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in micro-/nanorobotics: Materials development, actuation, localization, and system integration for biomedical applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Han, Y.; Gong, X. Micro/nanorobots for medical diagnosis and disease treatment. Micromachines 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug. Discov. 2005, 4, 255. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Lin, X.; Dai, L.; He, Q. Self-propelled janus mesoporous silica nanomotors with sub-100 nm diameters for drug encapsulation and delivery. ChemPhysChem 2014, 15, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tu, Y.; van Hest, J.C.; Wilson, D.A. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew. Chem. Int. Ed. 2015, 54, 11662–11665. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, S.; Zhang, Z.; Shi, D. Nanomaterials for cancer precision medicine. Adv. Mater. 2018, 30, 1705660. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug. Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Chou, M.-L.; Chew, C.H.; Noll, J.; Burnouf, T. Intelligent micro-/nanorobots as drug and cell carrier devices for biomedical therapeutic advancement: Promising development opportunities and translational challenges. Biomaterials 2020, 260, 120163. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xi, N.; Wang, Y.; Liu, L. Progress in nanorobotics for advancing biomedicine. IEEE. Trans. Biomed. Eng. 2020, 68, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, H.; Zhang, B.; Cao, Q.; Wang, B.; Ma, X. Recent progress of micro/nanorobots for cell delivery and manipulation. Adv. Funct. Mater. 2022, 32, 2110625. [Google Scholar] [CrossRef]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/nanorobots at work in active drug delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Prokop, A.; Davidson, J.M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008, 97, 3518–3590. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.L.; de Ávila, B.E.-F.; Pal, M.; Ghosh, A.; Wang, J. Fantastic voyage of nanomotors into the cell. ACS Nano 2020, 14, 9423–9439. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.-F.; Martín, A.; Soto, F.; Lopez-Ramirez, M.A.; Campuzano, S.; Vasquez-Machado, G.M.; Gao, W.; Zhang, L.; Wang, J. Single cell real-time mirnas sensing based on nanomotors. ACS Nano 2015, 9, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gastélum, M.; de Ávila, B.E.-F.; Gong, H.; Venugopalan, P.L.; Hianik, T.; Wang, J.; Subjakova, V. Rapid detection of aib1 in breast cancer cells based on aptamer-functionalized nanomotors. ChemPhysChem 2019, 20, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Bruhn, M.; de Avila, B.E.; Beltran-Gastelum, M.; Zhao, J.; Ramirez-Herrera, D.E.; Angsantikul, P.; Vesterager Gothelf, K.; Zhang, L.; Wang, J. Active intracellular delivery of a cas9/sgrna complex using ultrasound-propelled nanomotors. Angew. Chem. Int. Ed. 2018, 57, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.-F.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Baez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically propelled nanomotors for intracellular sirna delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.-F.; Ramirez-Herrera, D.E.; Campuzano, S.; Angsantikul, P.; Zhang, L.; Wang, J. Nanomotor-enabled ph-responsive intracellular delivery of caspase-3: Toward rapid cell apoptosis. ACS Nano 2017, 11, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.; Wang, W.; Sun, M.; Guo, B.; Xie, H.; He, Q. Shape-transformable, fusible rodlike swimming liquid metal nanomachine. ACS Nano 2018, 12, 10212–10220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhuang, J.; de Ávila, B.E.-F.; Tang, S.; Zhang, Q.; Fang, R.H.; Zhang, L.; Wang, J. A nanomotor-based active delivery system for intracellular oxygen transport. ACS Nano 2019, 13, 11996–12005. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Lin, X.; Si, T.; He, Q. Gold-nanoshell-functionalized polymer nanoswimmer for photomechanical poration of single-cell membrane. J. Am. Chem. Soc. 2019, 141, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hou, S.; Wattanatorn, N.; Wang, F.; Yang, Q.; Zhao, C.; Yu, X.; Tseng, H.R.; Jonas, S.J.; Weiss, P.S. Precision-guided nanospears for targeted and high-throughput intracellular gene delivery. ACS Nano 2018, 12, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shao, J.; Wu, H.; Song, S.; De Martino, M.T.; Pijpers, I.A.B.; Friedrich, H.; Abdelmohsen, L.; Williams, D.S.; van Hest, J.C.M. Photoactivated nanomotors via aggregation induced emission for enhanced phototherapy. Nat. Commun. 2021, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Dasgupta, D.; Somalwar, N.; Reshma, V.; Tiwari, M.; Teja, D.; Narayana, S.M.; Katke, A.; Jayshree, R.; Bhat, R. Helical nanobots as mechanical probes of intra-and extracellular environments. J. Phys. Condens. Matter. 2020, 32, 224001. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Mataraza, J.M.; Qin, Z.-H.; Huang, Z.; Huang, J.; Chiles, T.C.; Carnahan, D.; Kempa, K.; Ren, Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2005, 2, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Celedon, A.; Hale, C.M.; Wirtz, D. Magnetic manipulation of nanorods in the nucleus of living cells. Biophys. J. 2011, 101, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Peng, F.; Andre, A.A.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; García-Fernández, A.; Murillo-Cremaes, N.; Hortelão, A.C.; Patiño, T.; Villalonga, R.; Sancenón, F.; Martínez-Máñez, R.; Sánchez, S. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. ACS Nano 2019, 13, 12171–12183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xia, T.; Zhang, Z.; Xie, S.; Wang, T.; Li, X. Enzyme-powered janus nanomotors launched from intratumoral depots to address drug delivery barriers. Chem. Eng. J. 2019, 375, 122109. [Google Scholar] [CrossRef]
- Andhari, S.S.; Wavhale, R.D.; Dhobale, K.D.; Tawade, B.V.; Chate, G.P.; Patil, Y.N.; Khandare, J.J.; Banerjee, S.S. Self-propelling targeted magneto-nanobots for deep tumor penetration and ph-responsive intracellular drug delivery. Sci. Rep. 2020, 10, 4703. [Google Scholar] [CrossRef] [PubMed]
- Hortelão, A.C.; Patiño, T.; Perez-Jiménez, A.; Blanco, À.; Sánchez, S. Enzyme-powered nanobots enhance anticancer drug delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Yu, W.; Lin, R.; He, X.; Yang, X.; Zhang, H.; Hu, C.; Liu, R.; Huang, Y.; Qin, Y.; Gao, H. Self-propelled nanomotor reconstructs tumor microenvironment through synergistic hypoxia alleviation and glycolysis inhibition for promoted anti-metastasis. Acta Pharm. Sin. B 2021, 11, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; García-Fernández, A.; Lucena-Sánchez, E.; Díez, P.; Sancenón, F.; Villalonga, R.; Wilson, D.A.; Martínez-Máñez, R. Stimulus-responsive nanomotors based on gated enzyme-powered janus au–mesoporous silica nanoparticles for enhanced cargo delivery. Chem. Commun. 2019, 55, 13164–13167. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Xu, D.; Pan, X.; Ma, X. Self-propelled enzymatic nanomotors for enhancing synergetic photodynamic and starvation therapy by self-accelerated cascade reactions. Appl. Mate. Today 2019, 16, 508–517. [Google Scholar] [CrossRef]
- Lian, M.; Xue, Z.; Qiao, X.; Liu, C.; Zhang, S.; Li, X.; Huang, C.; Song, Q.; Yang, W.; Chen, X.; et al. Movable hollow nanoparticles as reactive oxygen scavengers. Chem 2019, 5, 2378–2387. [Google Scholar] [CrossRef]
- Yan, M.; Chen, Q.; Liu, T.; Li, X.; Pei, P.; Zhou, L.; Zhou, S.; Zhang, R.; Liang, K.; Dong, J.; et al. Site-selective superassembly of biomimetic nanorobots enabling deep penetration into tumor with stiff stroma. Nat. Commun. 2023, 14, 4628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Lian, M.-L.; Yang, W.-S.; Chen, X. An engineered, self-propelled nanozyme as reactive oxygen species scavenger. Chem. Eng. J. 2022, 446, 136794. [Google Scholar] [CrossRef]
- Ren, J.; Hu, P.; Ma, E.; Zhou, X.; Wang, W.; Zheng, S.; Wang, H. Enzyme-powered nanomotors with enhanced cell uptake and lysosomal escape for combined therapy of cancer. Appl. Mate. Today 2022, 27, 101445. [Google Scholar] [CrossRef]
- Peng, X.; Tang, S.; Tang, D.; Zhou, D.; Li, Y.; Chen, Q.; Wan, F.; Lukas, H.; Han, H.; Zhang, X. Autonomous metal-organic framework nanorobots for active mitochondria-targeted cancer therapy. Sci. Adv. 2023, 9, eadh1736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, L.; Yang, C.; Guo, W.; Huang, Y.; Wang, W.; Wan, M.; Mao, C.; Shen, J. Nanomotor-based H2S donor with mitochondrial targeting function for treatment of parkinson’s disease. Bioact. Mater. 2024, 31, 578–589. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Gao, C.; Wang, W.; Dai, L.; He, Q. Self-propelled nanomotors for thermomechanically percolating cell membranes. Angew. Chem. Int. Ed. 2018, 57, 12463–12467. [Google Scholar] [CrossRef] [PubMed]
- Qualliotine, J.R.; Bolat, G.; Beltrán-Gastélum, M.; de Ávila, B.E.-F.; Wang, J.; Califano, J.A. Acoustic nanomotors for detection of human papillomavirus–associated head and neck cancer. Otolaryngol.–Head Neck Surg. 2019, 161, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Pumera, M. Geometric asymmetry driven janus micromotors. Nanoscale 2014, 6, 11177–11180. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xu, D.; Sun, L.; Henzie, J.; Lopes, A.; Gu, Q.; Yamauchi, Y.; Liu, B. Asymmetric multimetallic mesoporous nanospheres. Nano. Lett. 2019, 19, 3379–3385. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; Angelo, S.K.S.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic nanomotors: Autonomous movement of striped nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.R.; Paxton, W.F.; Mallouk, T.E.; Sen, A. Catalytic nanomotors: Remote-controlled autonomous movement of striped metallic nanorods. Angew. Chem. Int. Ed. 2005, 117, 754–756. [Google Scholar] [CrossRef]
- Demirok, U.K.; Laocharoensuk, R.; Manesh, K.M.; Wang, J. Ultrafast catalytic alloy nanomotors. Angew. Chem. Int. Ed. 2008, 47, 9349–9351. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, L.; Huo, H.; Su, L.; Wu, Y.; Lin, H.; Ge, X.; Mu, J.; Zhang, X.; Zheng, L. Nanosized janus aunr-pt motor for enhancing nir-ii photoacoustic imaging of deep tumor and Pt2+ ion-based chemotherapy. ACS Nano 2022, 16, 7947–7960. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hortelao, A.C.; Huang, X.; Sanchez, S. Lipase-powered mesoporous silica nanomotors for triglyceride degradation. Angew. Chem. Int. Ed. 2019, 58, 7992–7996. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Chen, H.; Wang, Q.; Niu, Q.; Xu, P.; Yu, Y.; Zhu, T.; Mao, C.; Shen, J. Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 2019, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Liu, Z.; Li, T.; Chen, H.; Wang, Q.; Chen, T.; Tao, Y.; Mao, C. Zwitterion-based hydrogen sulfide nanomotors induce multiple acidosis in tumor cells by destroying tumor metabolic symbiosis. Angew. Chem. Int. Ed. 2021, 60, 16139–16148. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.; Cham, J.T.; Reithofer, M.R.; Hor, T.S.; Chin, J.M. Motorized janus metal organic framework crystals. Chem. Commun. 2014, 50, 15175–15178. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, R.A.; Sengupta, S.; McFadden, T.; Zhang, H.; Sen, A. A polymerization-powered motor. Angew. Chem. Int. Ed. 2011, 50, 9374–9377. [Google Scholar] [CrossRef] [PubMed]
- Simmchen, J.; Baeza, A.; Ruiz, D.; Esplandiu, M.J.; Vallet-Regi, M. Asymmetric hybrid silica nanomotors for capture and cargo transport: Towards a novel motion-based DNA sensor. Small 2012, 8, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Hortelao, A.C.; Carrascosa, R.; Murillo-Cremaes, N.; Patino, T.; Sanchez, S. Targeting 3d bladder cancer spheroids with urease-powered nanomotors. ACS Nano 2018, 13, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Mathesh, M.; Sun, J.; Wilson, D.A. Enzyme catalysis powered micro/nanomotors for biomedical applications. J. Mater. Chem. B 2020, 8, 7319–7334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gentile, K.; Mohajerani, F.; Sen, A. Powering motion with enzymes. Acc. Chem. Res. 2018, 51, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ibele, M.E.; Sen, A. Fantastic voyage: Designing self-powered nanorobots. Angew. Chem. Int. Ed. 2012, 51, 8434–8445. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug. Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound activated sensitizers and applications. Angew. Chem. Int. Ed. 2019, 59, 14212–14233. [Google Scholar] [CrossRef]
- Rao, K.J.; Li, F.; Meng, L.; Zheng, H.; Cai, F.; Wang, W. A force to be reckoned with: A review of synthetic microswimmers powered by ultrasound. Small 2015, 11, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Gao, W.; Xu, T.; Jurado-Sánchez, B.; Li, J.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv. Funct. Mater. 2015, 25, 3881–3887. [Google Scholar] [CrossRef]
- de Ávila, B.E.-F.; Angsantikul, P.; Ramírez-Herrera, D.E.; Soto, F.; Teymourian, H.; Dehaini, D.; Chen, Y.; Zhang, L.; Wang, J. Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci. Robot. 2018, 3, eaat0485. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.; Zhou, C.; Lin, Z.; He, Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research 2020, 2020, 3676954. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.R.; Carlson, J.D.; Munoz, B.C. A model of the behaviour of magnetorheological materials. Smart Mater. Struct. 1996, 5, 607. [Google Scholar] [CrossRef]
- Kuhn, S.J.; Hallahan, D.E.; Giorgio, T.D. Characterization of superparamagnetic nanoparticle interactions with extracellular matrix in an in vitro system. Ann. Biomed. Eng. 2006, 34, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, F.; Prins, M.; Van Ijzendoorn, L. Magnetization and actuation of polymeric microstructures with magnetic nanoparticles for application in microfluidics. J. Magn. Magn. Mater. 2009, 321, 1843. [Google Scholar] [CrossRef]
- Di Leonardo, R.; Angelani, L.; Dell’arciprete, D.; Ruocco, G.; Iebba, V.; Schippa, S.; Conte, M.P.; Mecarini, F.; De Angelis, F.; Di Fabrizio, E. Bacterial ratchet motors. Proc. Natl. Acad. Sci. USA 2010, 107, 9541–9545. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Tang, S.; Xie, L.; Yang, M.; Li, Y.; Lu, D.; Li, J.; Yang, Q.; Chen, Q.; Zhang, Z. Magnetic-powered janus cell robots loaded with oncolytic adenovirus for active and targeted virotherapy of bladder cancer. Adv. Mater. 2022, 34, 2201042. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Somalwar, N.; Singh, A.; Bhat, R.; Eswarappa, S.M.; Saini, D.K.; Ghosh, A. Maneuverability of magnetic nanomotors inside living cells. Adv. Mater. 2018, 30, e1800429. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dasgupta, D.; Pal, M.; Morozov, K.I.; Leshansky, A.M.; Ghosh, A. Helical nanomachines as mobile viscometers. Adv. Funct. Mater. 2018, 28, 1705687. [Google Scholar] [CrossRef]
- Kadiri, V.M.; Bussi, C.; Holle, A.W.; Son, K.; Kwon, H.; Schutz, G.; Gutierrez, M.G.; Fischer, P. Biocompatible magnetic micro- and nanodevices: Fabrication of fept nanopropellers and cell transfection. Adv. Mater. 2020, 32, e2001114. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K.M.; Weihs, D.; Wang, J. Magnetically powered flexible metal nanowire motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Zhang, H.; Chang, X.; Song, W.; Hu, Y.; Shao, G.; Sandraz, E.; Zhang, G.; Li, L.; et al. Magnetically propelled fish-like nanoswimmers. Small 2016, 12, 6098–6105. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zentgraf, T.; Liu, Y.; Bartal, G.; Zhang, X. Light-driven nanoscale plasmonic motors. Nat. Nanotechnol. 2010, 5, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Wang, W.; Xu, D.; Zeng, F.; Zhan, C.; Gu, J.; Li, M.; Zhao, W.; Zhang, J. Photocatalytically powered matchlike nanomotor for light-guided active sers sensing. Angew. Chem. Int. Ed. 2018, 130, 13294–13297. [Google Scholar] [CrossRef]
- Song, Y.; Zhan, G.; Zhou, S.F. Design of near infrared light-powered copper phyllosilicate nanomotors for cuproptosis-based synergistic cancer therapy. Adv. Funct. Mater. 2024, 2314568. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Li, J.; Ning, P.; Shi, Z.; Zhang, W.; Li, Z.; Zhou, R.; Tong, Y.; Li, Y. Photomagnetically powered spiky nanomachines with thermal control of viscosity for enhanced cancer mechanotherapy. Adv. Mater. 2023, 35, 2204996. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, Z.; Zhu, Y.; Zhao, H.; Wu, K.; Wu, J.; Zhang, W.; Zhang, Q.; Guo, H. Nir-ii light powered asymmetric hydrogel nanomotors for enhanced immunochemotherapy. Angew. Chem. Int. Ed. 2023, 62, e202212866. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xiu, J.; Zhou, M.; Xu, T.; Zhang, M.; Li, H.; Li, X.; Du, X.; Ma, T.; Zhang, X. Copper single-atom jellyfish-like nanomotors for enhanced tumor penetration and nanocatalytic therapy. ACS Nano 2023, 17, 6789–6799. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Feng, A.; Cheng, X.; Xiong, X.; Wang, Z.; He, Z.; Guo, J.; Wang, S.; Yan, X. Photoactivated organic nanomachines for programmable enhancement of antitumor efficacy. Small 2022, 18, 2201525. [Google Scholar] [CrossRef] [PubMed]
- Yèagle, P.L. Lipid regulation of cell membrane structure and function. FASEB J. 1989, 3, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, S.; Gupta, G.K.; Avci, P.; Chandran, R.; Sadasivam, M.; Jorge, A.E.S.; Hamblin, M.R. Physical energy for drug delivery; poration, concentration and activation. Adv. Drug. Deliv. Rev. 2014, 71, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; He, Q. Swimming nanorobots for opening a cell membrane mechanically. View 2020, 1, 20200005. [Google Scholar] [CrossRef]
- Kong, L.; Guan, J.; Pumera, M. Micro-and nanorobots based sensing and biosensing. Curr. Opin. Electrochem. 2018, 10, 174–182. [Google Scholar] [CrossRef]
- Campuzano, S.; Esteban-Fernandez de Avila, B.; Yanez-Sedeno, P.; Pingarron, J.M.; Wang, J. Nano/microvehicles for efficient delivery and (bio)sensing at the cellular level. Chem. Sci. 2017, 8, 6750–6763. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.; Mair, L.; Ahmed, S.; Huang, T.J.; Mallouk, T.E. Acoustic propulsion of nanorod motors inside living cells. Angew. Chem. Int. Ed. 2014, 53, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Ros. Curr. Biol. 2013, 23, R100–R102. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K. Transport of toxins across intracellular membranes. Bact. Protein Toxins 2003, 157–172. [Google Scholar]
- Menon, J.U.; Jadeja, P.; Tambe, P.; Vu, K.; Yuan, B.; Nguyen, K.T. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 2013, 3, 152. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762. [Google Scholar] [CrossRef] [PubMed]
- Wieder, M.E.; Hone, D.C.; Cook, M.J.; Handsley, M.M.; Gavrilovic, J.; Russell, D.A. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: Cancer therapy using a ‘trojan horse’. Photochem. Photobiol. Sci. 2006, 5, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, M.; Liu, H.; Zhang, Z.; Hu, Y.; Shi, J.; Wang, Z.-H. Light-driven micro/nanomotors in biomedical applications. Nanoscale 2023, 15, 18550–18570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, Y.; Ma, P.; Wang, Y.; Zhang, F.; Cai, B.; Chen, P.; Liu, B.F. Intelligent micro-/nanorobots for cancer theragnostic. Adv. Mater. 2022, 34, 2201051. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer. Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Sun, J.; Mathesh, M.; Li, W.; Wilson, D.A. Enzyme-powered nanomotors with controlled size for biomedical applications. ACS Nano 2019, 13, 10191–10200. [Google Scholar] [CrossRef] [PubMed]
- Mallouk, T.E.; Sen, A. Powering nanorobots. Sci. Am. 2009, 300, 72–77. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gong, N.; Zhong, L.; Sun, J.; Liang, X.-J. Future of nanotherapeutics: Targeting the cellular sub-organelles. Biomaterials 2016, 97, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Schliwa, M.; Woehlke, G. Molecular motors. Nature 2003, 422, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Soto, F.; Karshalev, E.; Li, J.; Wang, J. Hybrid nanovehicles: One machine, two engines. Adv. Funct. Mater. 2019, 29, 1806290. [Google Scholar] [CrossRef]

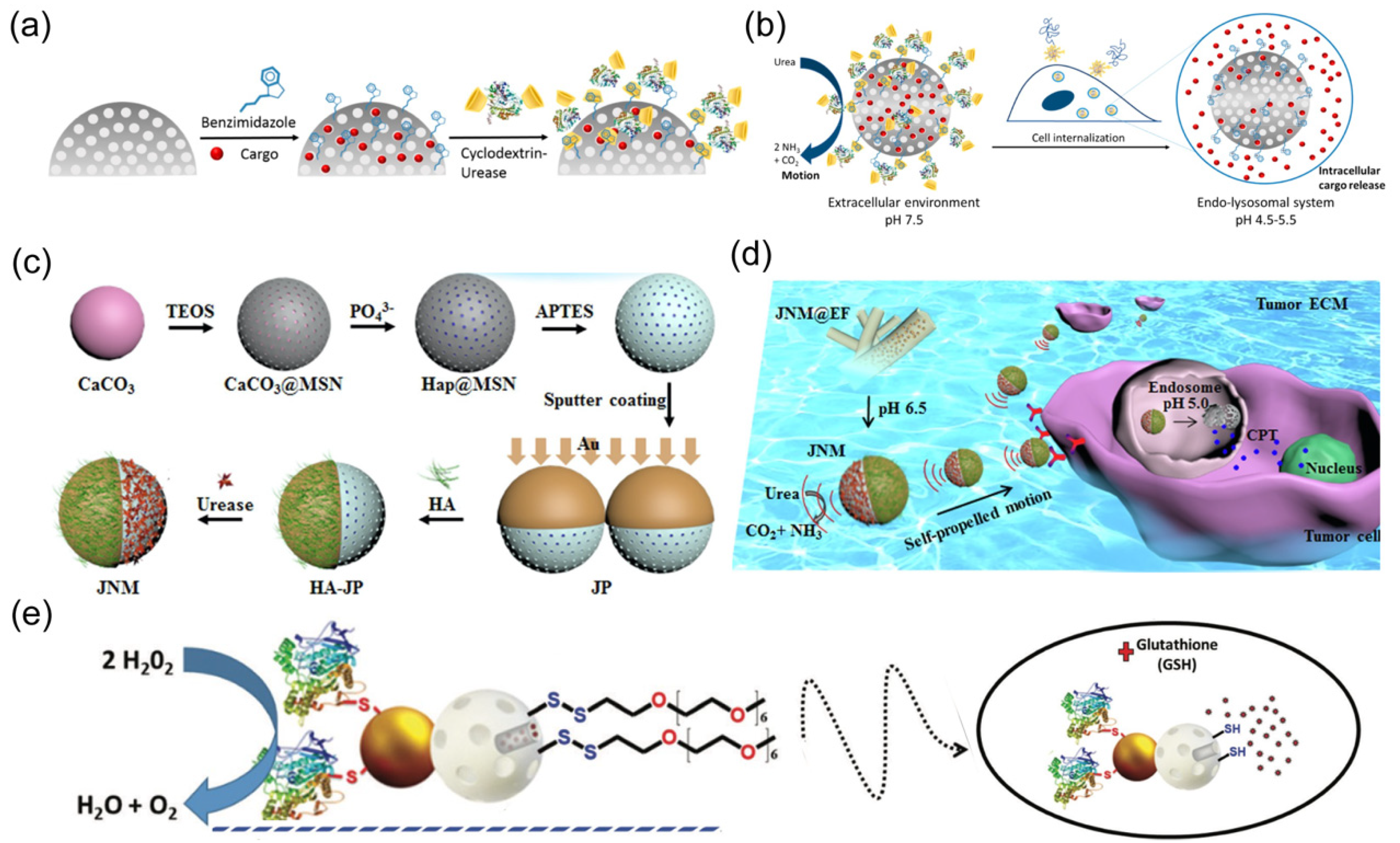
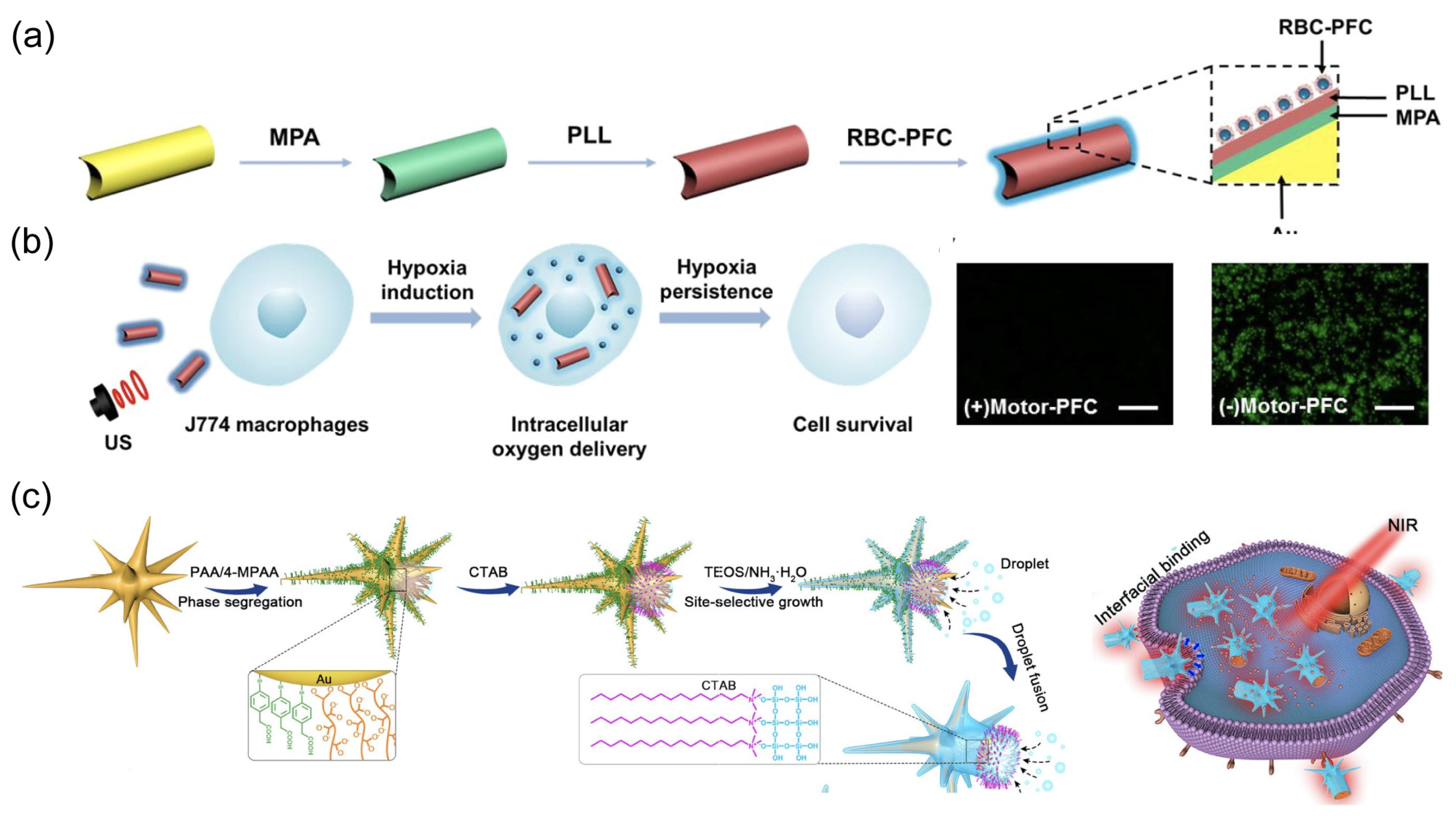
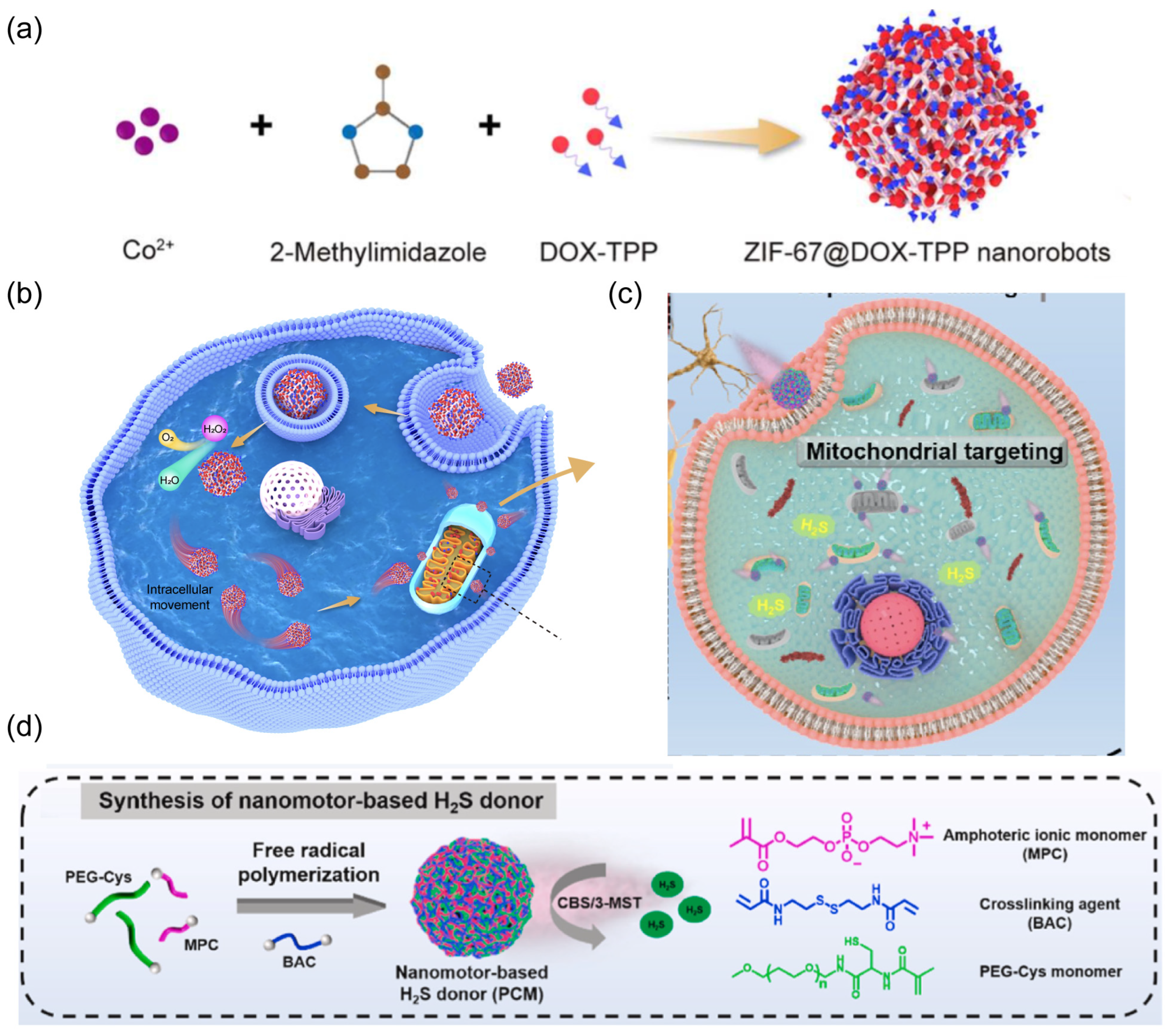

| Materials | Size | Propulsion Mechanism | Application | Ref. |
|---|---|---|---|---|
| AuNW | 4 μm in length and 200 nm in diameter | US | biosensing | [45] |
| AuNW | 1.7 μm in length and 400 nm in diameter | US | biosensing | [46] |
| AuNW | 2 μm in length and 400 nm in diameter | US | cargo delivery | [47] |
| AuNW | 4 μm in length and 200 nm in diameter | US | cargo delivery | [48] |
| AuNW | 4 μm in length and 200 nm in diameter | US | cargo delivery | [49] |
| gallium | 5.5 μm in length and 500 nm in diameter | US | photo-based therapy | [50] |
| RBC-PFC, AuNW, | 2 μm in length and 400 nm in diameter | US | cargo delivery | [51] |
| gold, polymer | 10 pm in length, diameters of the two openings are ~200 nm and ~800 nm | US | cell membrane penetration | [52] |
| Au/Ni/Si | ~5 μm in length with tip diameters < 50 nm | MF | cargo delivery | [53] |
| polymersomes, Au | around 400 nm | NIR | biosensing | [54] |
| SiO2-Co/Fe | 2.4 μm in length and 250 nm in width | MF | biosensing | [55] |
| Ni-carbon | <2 μm in length | MF | cargo delivery | [56] |
| Ni/Pt/Ni | 200 nm in diameter and 1.5 mm in length | MF | cargo delivery | [57] |
| Pt, polymer | 500 nm | H2O2 | cargo delivery | [58] |
| mesoporous silica NPs | average diameter ~420 nm | urea | cargo delivery | [59] |
| mesoporous silica NPs, gold | average diameter of sub-100 nm | urea | cargo delivery | [60] |
| carbon, Fe3O4 | outer diameter of 10–15 nm, length of 1–5 μm | MF | cargo delivery | [61] |
| cetyltrimethylammonium bromide (CTAB) and tetraethylorthosilicate (TEOS) | average diameter 344 ± 3 nm | urease | cargo delivery | [62] |
| gold | average diameter 171.53 + 1.40 nm | H2O2 | cargo delivery | [63] |
| AuNW, red blood cell membrane | 2 μm in length and 400 nm in diameter. | US | cargo delivery | [51] |
| Au-mesoporous silica | Au 20nm, SiO2 80 nm | H2O2 | cargo delivery | [64] |
| mesoporous silica | 418 ± 21 nm | urea | cargo delivery | [59] |
| Yb mof | 41 ± 2 nm | GOx-Cat | cargo delivery | [65] |
| mesoporous silica nanoparticles, hemin | diameter of about 630 nm | ROS | detoxification | [66] |
| AuNS, SiO2 | length (~13–94 nm), tail length (~0–510 nm), and large tunable hollow diameter (~100–240 nm) | NIR | cargo delivery | [67] |
| Au, MnO2 | 93.4 nm | NIR | biosensing | [28] |
| polymersomes, Au | around 400 nm | NIR | photo-based therapy | [54] |
| Pt, silica, black phosphorous | diameter (450 nm) | H2O2 | detoxification | [68] |
| calcium carbonate nanoparticle | a diameter of 60.0 ± 5.0 nm | H2O2 | cargo delivery | [69] |
| ZIF-67, DOX-TPP | average size of 140.0 nm | H2O2 | organelle targeting | [70] |
| PEG-Cys, MP, PEG | average size 200 nm | H2S | organelle targeting | [71] |
| mesoporous silica | 67.8 nm to 80.6 nm | NIR | cell membrane penetration | [72] |
| gold nanowire | 2 μm in length and 400 nm in diameter | US | biosensing | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, D.; Peng, X.; Wu, S.; Tang, S. Autonomous Nanorobots as Miniaturized Surgeons for Intracellular Applications. Nanomaterials 2024, 14, 595. https://doi.org/10.3390/nano14070595
Tang D, Peng X, Wu S, Tang S. Autonomous Nanorobots as Miniaturized Surgeons for Intracellular Applications. Nanomaterials. 2024; 14(7):595. https://doi.org/10.3390/nano14070595
Chicago/Turabian StyleTang, Daitian, Xiqi Peng, Song Wu, and Songsong Tang. 2024. "Autonomous Nanorobots as Miniaturized Surgeons for Intracellular Applications" Nanomaterials 14, no. 7: 595. https://doi.org/10.3390/nano14070595
APA StyleTang, D., Peng, X., Wu, S., & Tang, S. (2024). Autonomous Nanorobots as Miniaturized Surgeons for Intracellular Applications. Nanomaterials, 14(7), 595. https://doi.org/10.3390/nano14070595







