MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review
Abstract
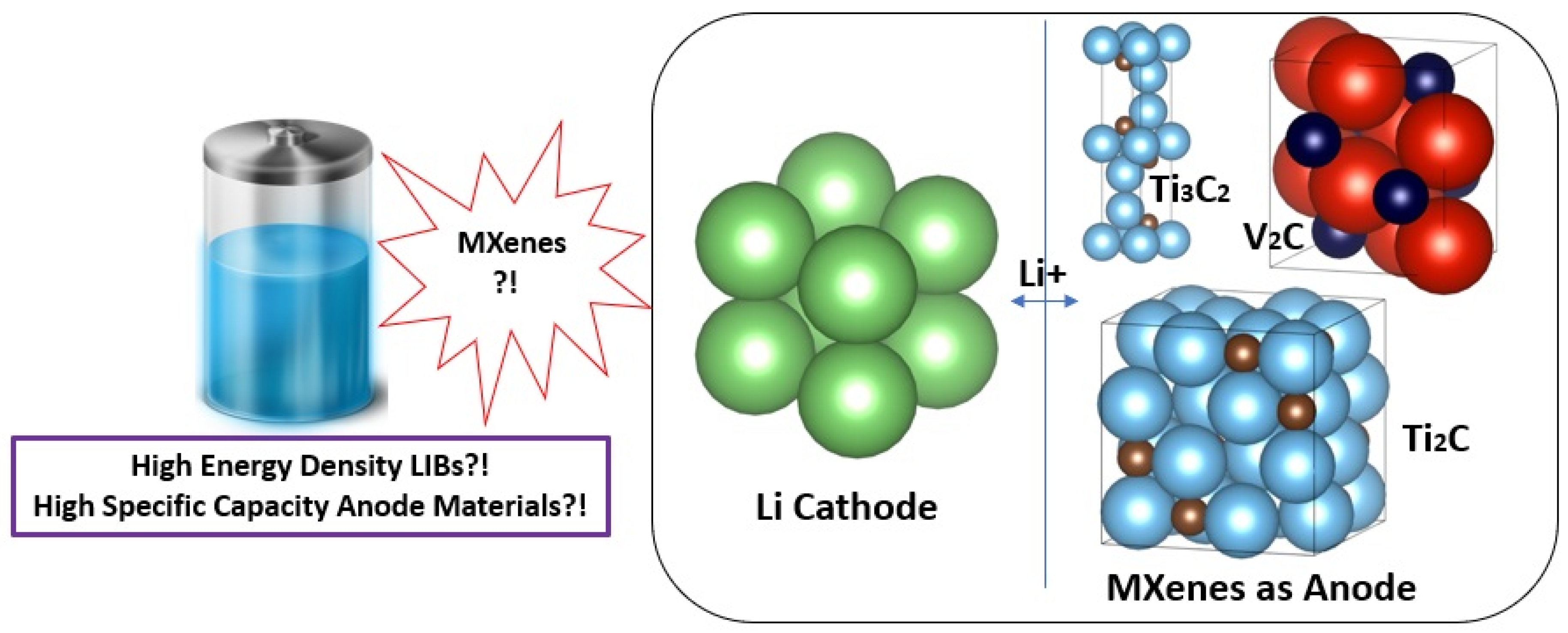
:1. Introduction
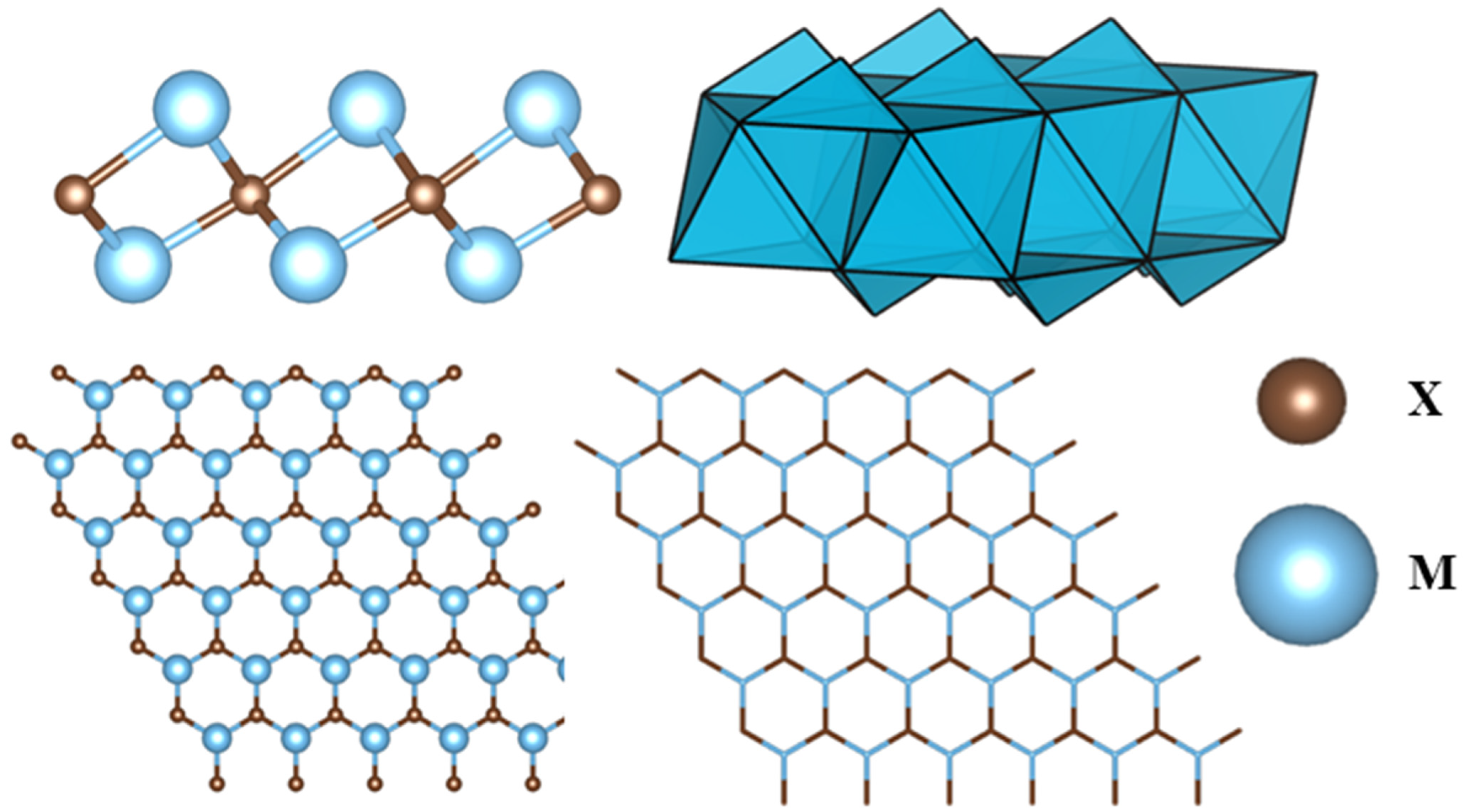
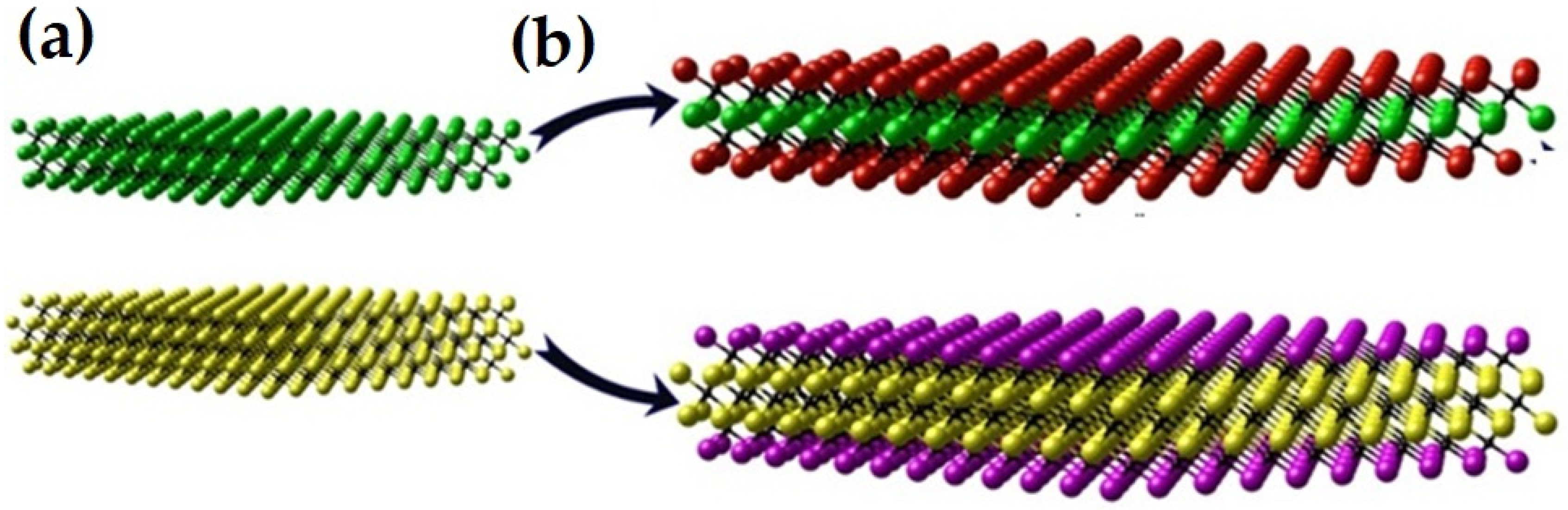
2. Structural Framework of MXene
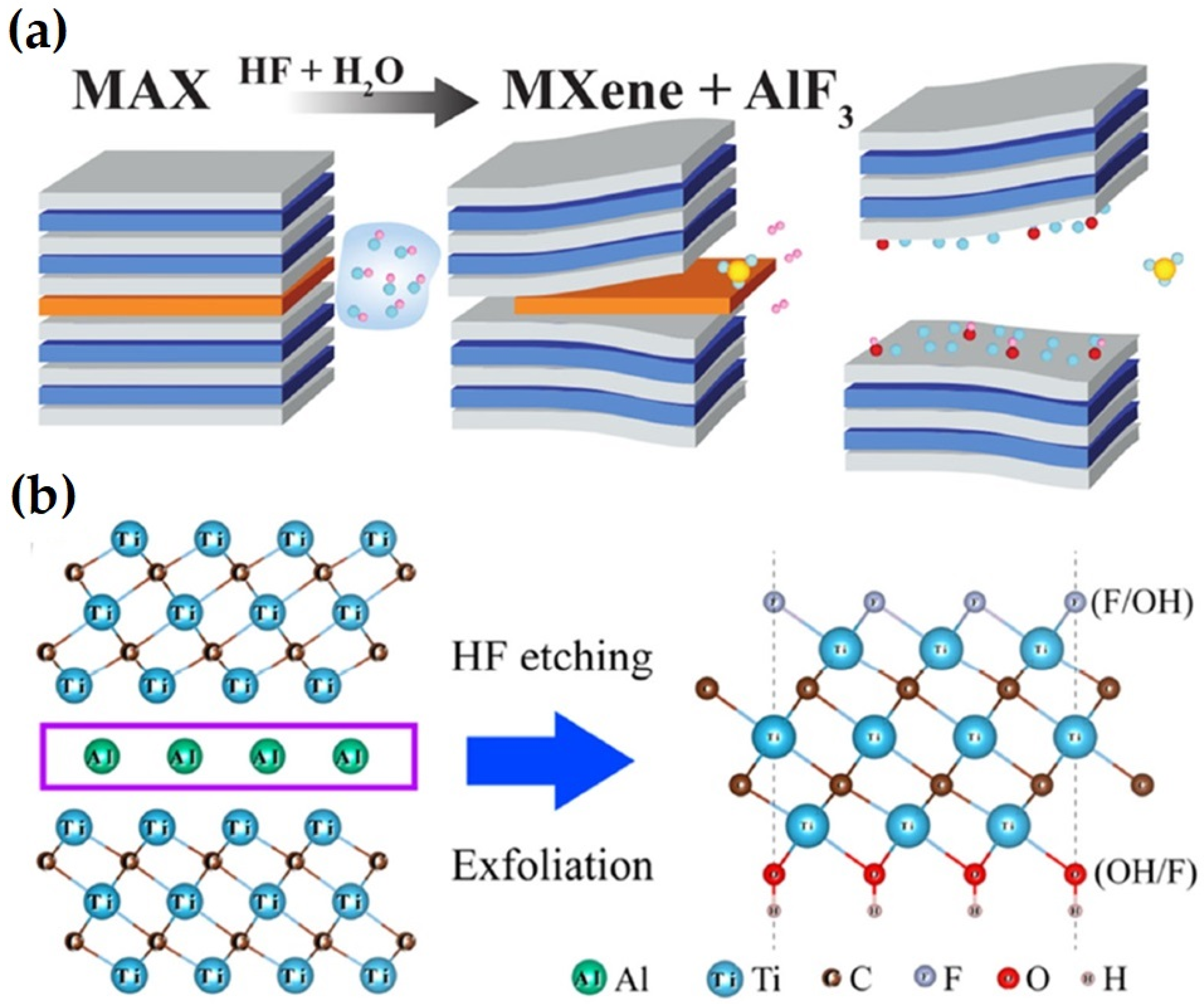
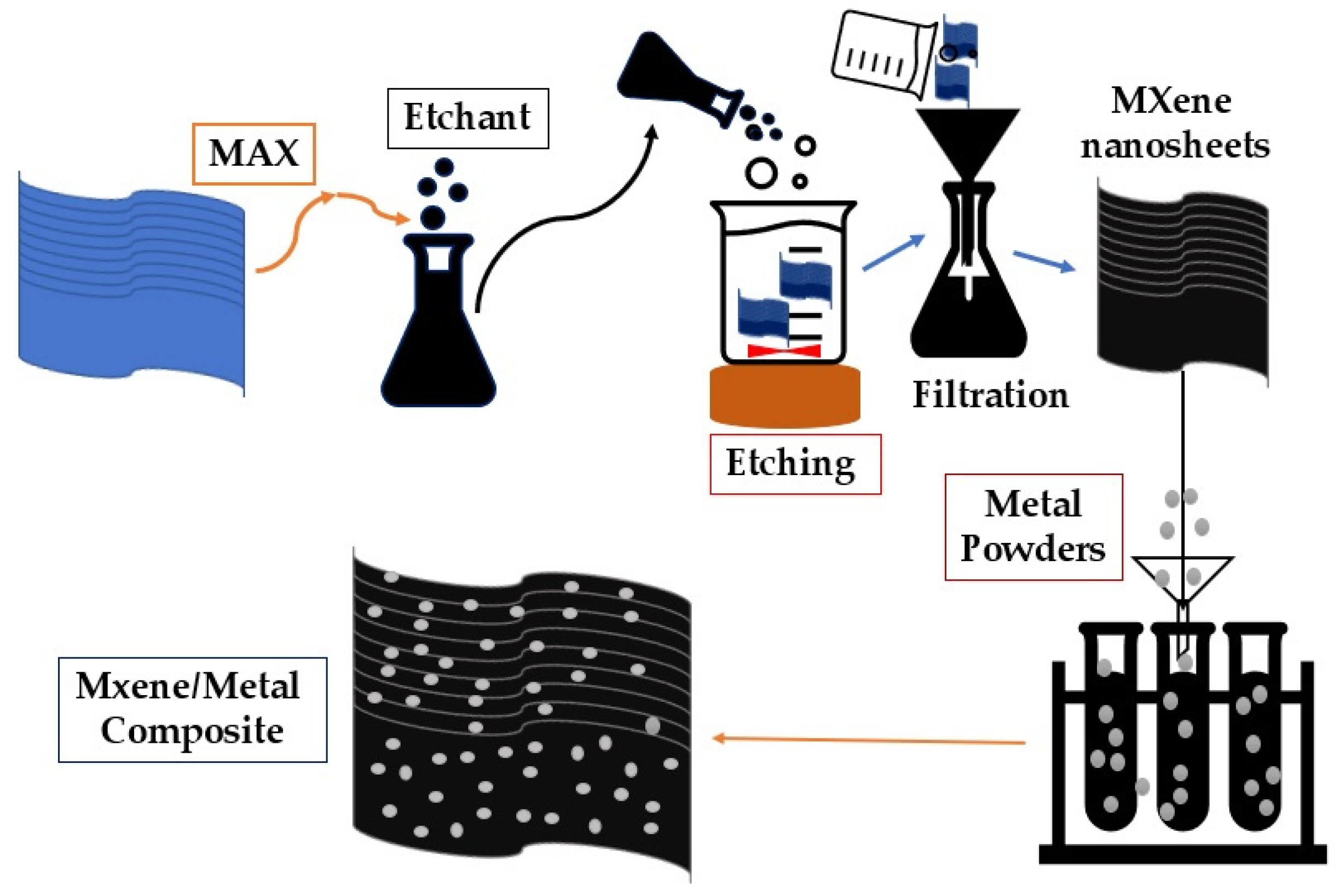
3. Synthesis of MXene
3.1. Wet Chemical Etching
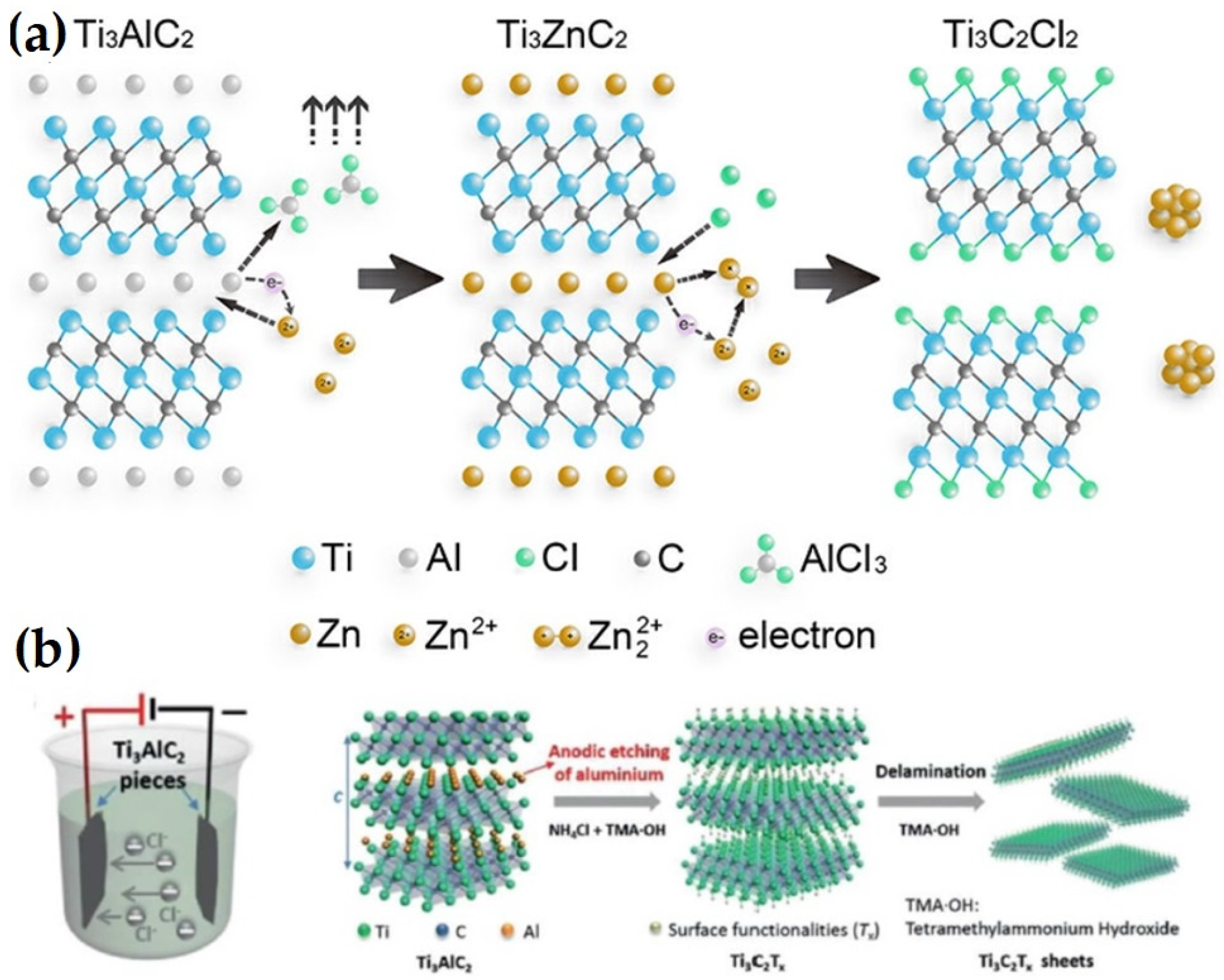
3.2. Molten Salt Etching
3.3. Electrochemical Etching
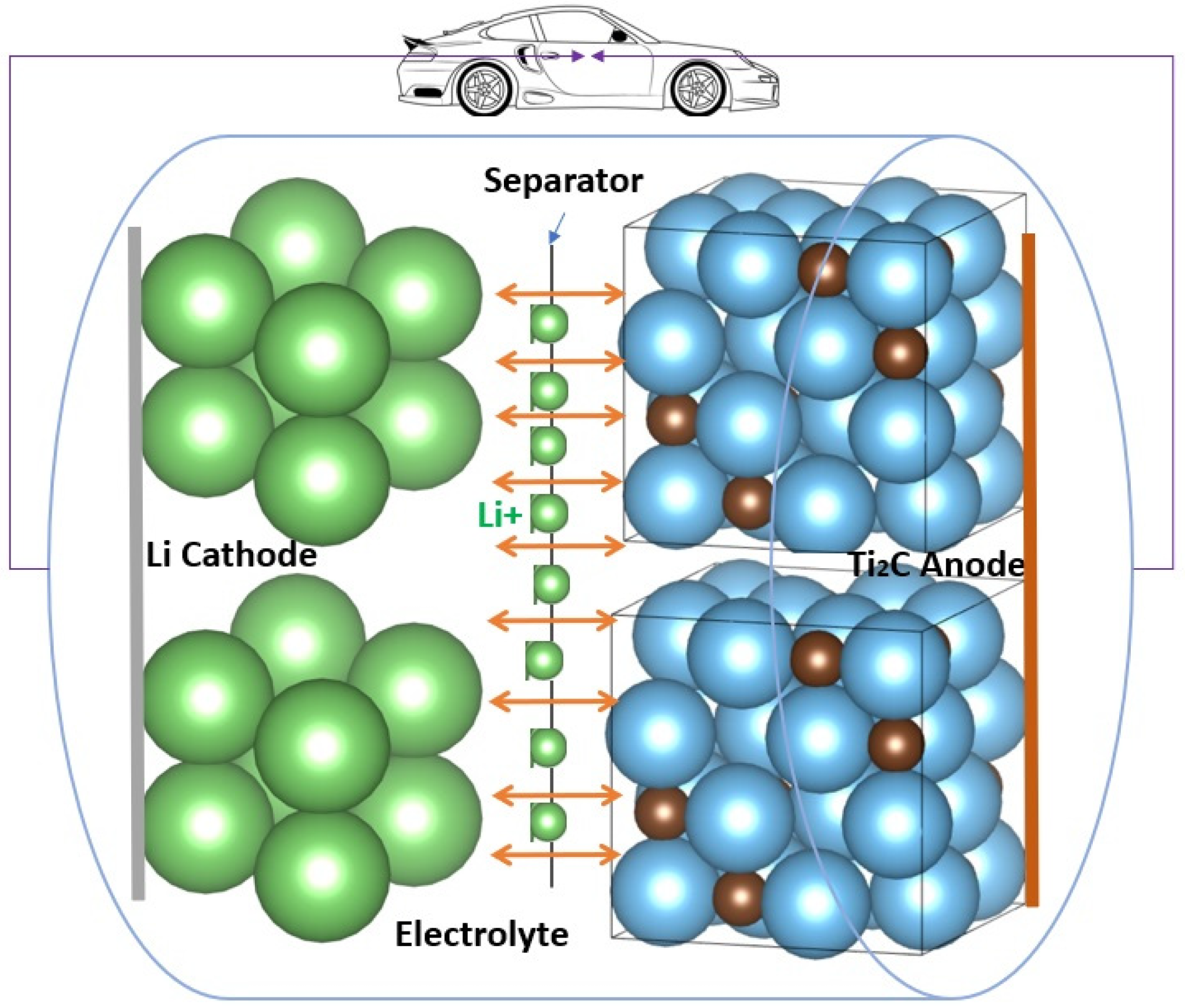
4. MXenes in LIBs
4.1. Mono-Transition Metal MXene as Anode of LIBs
4.2. Double-Transition Metal MXene (DTM) as Anode of LIBs
4.3. Composite MXenes as Anodes of LIBs
5. Outlook
- (a)
- Lithium compounds are used as cathode materials of commercial LIBs when graphite is used as the anode material. However, pure Li metal is used as a cathode material in most studies when MXene materials are used as the anode. Hence, it is necessary to identify the commercial-grade Li compound that is more suitable for the respective MXenes as anodes of LIBs.
- (b)
- At present, acid etching remains the primary way of obtaining MXenes; nevertheless, the approach has high risk and results in a low yield. As a result, there is a pressing need for safe, effective, high-quality, and environmentally friendly methods to synthesize MXenes that have a controlled number of layers, modifiable surface groups, enhanced layer spacing, and superior quality. To support the feasible large-scale commercial production of MXenes and MXene-based materials, it is also necessary to conduct extensive research on the generation mechanism and optimize the currently available synthesis techniques. In addition, the surface chemistry of these materials is mostly unknown, and thus must be studied more, before they can be used as anodes of LIBs.
- (c)
- At present, most synthesized MXenes are in powdered form, and thus need to be glued using binder materials and pasted on the current collector. The active materials of MXenes might lose their connection during the electrochemical cycling of LIBs, and mix with the electrolyte, which would lead to low practical energy density.
- (d)
- Suitable electrolytes that will not react with MXene materials during electrochemical cycling of LIBs need to be found.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, S.; Rahman, M.A.; Islam, M.S.; Islam, M.R.; Siddiqui, S.E.T. Nanostructured Anatase TiO2 as Anode of High-Performance Lithium-Ion Batteries. Batter. Energy 2022, 1, 20220018. [Google Scholar] [CrossRef]
- Siddiqui, S.-E.-T.; Rahman, M.A.; Islam, M.S.; Kim, J.-H.; Barua, N. Microstructured Pebble Stone like Ni-NiO Composite as Anode of High-Performance Lithium-Ion Batteries. Futur. Sustain. 2024, 2, 1–13. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.M.; Song, G. A Review on Binder-Free NiO-Ni Foam as Anode of High Performance Lithium-Ion Batteries. Energy Storage 2022, 4, e278. [Google Scholar] [CrossRef]
- Paul, A.; Rahman, M.A.; Barua, N. Study of Temperature Cycling of Commercial Rechargeable Lithium-Ion Batteries. Futur. Sustain. 2023, 1, 23–31. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Zhan, C.; Sun, W.; Kent, P.R.C.; Naguib, M.; Gogotsi, Y.; Jiang, D.E. Computational Screening of MXene Electrodes for Pseudocapacitive Energy Storage. J. Phys. Chem. C 2019, 123, 315–321. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Lu, J.; Meshkian, R.; Tao, Q.; Hultman, L.; Rosen, J. Prediction and Synthesis of a Family of Atomic Laminate Phases with Kagomé-like and in-Plane Chemical Ordering. Sci. Adv. 2017, 3, e1700642. [Google Scholar] [CrossRef]
- Frey, N.C.; Wang, J.; Vega Bellido, G.I.; Anasori, B.; Gogotsi, Y.; Shenoy, V.B. Prediction of Synthesis of 2D Metal Carbides and Nitrides (MXenes) and Their Precursors with Positive and Unlabeled Machine Learning. ACS Nano 2019, 13, 3031–3041. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical Etching of Ti2AlC to Ti2CT:X (MXene) in Low-Concentration Hydrochloric Acid Solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous Heterostructured MXene/Carbon Nanotube Composite Paper with High Volumetric Capacity for Sodium-Based Energy Storage Devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Ren, C.E.; Zhao, M.Q.; Makaryan, T.; Halim, J.; Boota, M.; Kota, S.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage. ChemElectroChem 2016, 3, 689–693. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN + 1AXN Phases: A New Class of Solids. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Cheng, R.; Hu, T.; Zhang, H.; Wang, C.; Hu, M.; Yang, J.; Cui, C.; Guang, T.; Li, C.; Shi, C.; et al. Understanding the Lithium Storage Mechanism of Ti3C2Tx MXene. J. Phys. Chem. C 2019, 123, 1099–1109. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Gao, Y.; Wang, Z.; Yu, R.; Chen, L. Atomic-Scale Recognition of Surface Structure and Intercalation Mechanism of Ti3C2TX. J. Am. Chem. Soc. 2015, 137, 2715–2721. [Google Scholar] [CrossRef]
- Wang, H.W.; Naguib, M.; Page, K.; Wesolowski, D.J.; Gogotsi, Y. Resolving the Structure of Ti3C2Tx MXenes through Multilevel Structural Modeling of the Atomic Pair Distribution Function. Chem. Mater. 2016, 28, 349–359. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Bashir, T.; Ismail, S.A.; Wang, J.; Zhu, W.; Zhao, J.; Gao, L. MXene Terminating Groups O, –F or –OH, –F or O, –OH, –F, or O, –OH, –Cl? J. Energy Chem. 2023, 76, 90–104. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray Photoelectron Spectroscopy of Select Multi-Layered Transition Metal Carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.W.; Yang, X.Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Ivanovskii, A.L. Atomic Structure, Comparative Stability and Electronic Properties of Hydroxylated Ti2C and Ti3C2 Nanotubes. Comput. Theor. Chem. 2012, 989, 27–32. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.; Zhuang, Z.; Li, S.; Xia, L.; Zhao, Y.; Zhao, Y.; Han, C.; Zhou, L. MXene Surface Terminations Enable Strong Metal-Support Interactions for Efficient Methanol Oxidation on Palladium. ACS Appl. Mater. Interfaces 2020, 12, 2400–2406. [Google Scholar] [CrossRef]
- Ling, C.; Shi, L.; Ouyang, Y.; Wang, J. Searching for Highly Active Catalysts for Hydrogen Evolution Reaction Based on O-Terminated MXenes through a Simple Descriptor. Chem. Mater. 2016, 28, 9026–9032. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Ivanovskii, A.L. Structural and Electronic Properties and Stability of MX Enes Ti2C and Ti3C2 Functionalized by Methoxy Groups. J. Phys. Chem. C 2013, 117, 13637–13643. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, K.; Srikanth, N.; Pang, J.H.L.; He, X.; Wang, R. Dependence of Elastic and Optical Properties on Surface Terminated Groups in Two-Dimensional MXene Monolayers: A First-Principles Study. RSC Adv. 2016, 6, 35731–35739. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Ladha, D.G. A Review on Density Functional Theory–Based Study on Two-Dimensional Materials Used in Batteries. Mater. Today Chem. 2019, 11, 94–111. [Google Scholar] [CrossRef]
- Kumar, U.; Gaikwad, V.; Mayyas, M.; Sahajwalla, V.; Joshi, R.K. Extraordinary Supercapacitance in Activated Carbon Produced via a Sustainable Approach. J. Power Sources 2018, 394, 140–147. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Hu, C.; Chen, D.; Shen, G. Intercalation of Small Organic Molecules into Ti3C2Tx MXene Cathodes for Flexible High-Volume-Capacitance Zn-Ion Microsupercapacitor. Adv. Mater. Technol. 2022, 7, 2200158. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Du, K.; Wang, S.; Huang, Z.; Yuan, L.; Li, Z.; Wang, H.; Zheng, L.; Chai, Z.; et al. Effective Removal of U(VI) and Eu(III) by Carboxyl Functionalized MXene Nanosheets. J. Hazard. Mater. 2020, 396, 122731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, W.; Chen, Y. Chemistry of Two-Dimensional MXene Nanosheets in Theranostic Nanomedicine. Chin. Chem. Lett. 2020, 31, 937–946. [Google Scholar] [CrossRef]
- Tang, M.; Li, J.; Wang, Y.; Han, W.; Xu, S.; Lu, M.; Zhang, W.; Li, H. Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry 2022, 14, 2232. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for Photocatalytic Degradation of Antiepileptic Drug Carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Zhi, C. Environmental Stability of MXenes as Energy Storage Materials. Front. Mater. 2019, 6, 2–10. [Google Scholar] [CrossRef]
- Mehta, V.; Saini, H.S.; Srivastava, S.; Kashyap, M.K.; Tankeshwar, K. S-Functionalized Mo2C Monolayer as a Novel Electrode Material in Li-Ion Batteries. J. Phys. Chem. C 2019, 123, 25052–25060. [Google Scholar] [CrossRef]
- Urbankowski, P.S. Synthesis of Two-Dimensional Transition Metal Nitrides. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2019. [Google Scholar]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kim, J.H.; Hossain, S. Recent Advances of Energy Storage Technologies for Grid: A Comprehensive Review. Energy Storage 2022, 4, e322. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. Enhanced Electrochemical Performance of Li-Ion Batteries with Nanoporous Titania as Negative Electrodes. J. Energy Chem. 2015, 24, 157–170. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; San Baek, D.; Joo, S.H.; Lee, K. MXene: An Emerging Two-Dimensional Material for Future Energy Conversion and Storage Applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wong, Y.C.; Song, G.; Zhu, D.M.; Wen, C. Improvement on Electrochemical Performances of Nanoporous Titania as Anode of Lithium-Ion Batteries through Annealing of Pure Titanium Foils. J. Energy Chem. 2018, 27, 250–263. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured Silicon Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Ghidiu, M.; Kota, S.; Halim, J.; Sherwood, A.W.; Nedfors, N.; Rosen, J.; Mochalin, V.N.; Barsoum, M.W. Alkylammonium Cation Intercalation into Ti3C2 (MXene): Effects on Properties and Ion-Exchange Capacity Estimation. Chem. Mater. 2017, 29, 1099–1106. [Google Scholar] [CrossRef]
- Magnuson, M.; Greczynski, G.; Eriksson, F.; Hultman, L.; Högberg, H. Electronic Structure of β-Ta Films from X-Ray Photoelectron Spectroscopy and First-Principles Calculations. Appl. Surf. Sci. 2019, 470, 607–612. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A Promising Transition Metal Carbide Anode for Lithium-Ion Batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.Z.; Zhou, A. Two-Dimensional Ti3C2 as Anode Material for Li-Ion Batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl + LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2016, 695, 818–826. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Liu, Q.; Gu, J. Fluorine-Free Ti3C2Tx as Anode Materials for Li-Ion Batteries. Electrochem. Commun. 2019, 104, 106472. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Zhu, X.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Partially Etched Ti3AlC2 as a Promising High-Capacity Lithium-Ion Battery Anode. ChemSusChem 2018, 11, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Wan, P.; He, L.; Xu, B. Flexible 3D Porous MXene Foam for High-Performance Lithium-Ion Batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Zheng, Y.; Wang, R.; Bai, Y. Improving the Electrochemical Properties of MXene Ti3C2 Multilayer for Li-Ion Batteries by Vacuum Calcination. Electrochim. Acta 2018, 265, 140–150. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, F.; Zhang, L.; Liu, L.; Chen, J.; Yang, B.; Yan, X. Rolling up MXene Sheets into Scrolls to Promote Their Anode Performance in Lithium-Ion Batteries. J. Energy Chem. 2020, 46, 256–263. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, H.; Huang, P.; Wang, J.; Zhang, Z.; Yang, T.; Han, W.Q. Rational Design of Pillared SnS/Ti3C2Tx MXene for Superior Lithium-Ion Storage. ACS Nano 2020, 14, 17665–17674. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, X.; Xu, H.; Yang, J.; Zhou, J.; Chen, Q.; Sun, G. Design of Vertically Aligned Two-Dimensional Heterostructures of Rigid Ti3C2Tx MXene and Pliable Vanadium Pentoxide for Efficient Lithium Ion Storage. ACS Nano 2022, 16, 5556–5565. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene Hybrids with Ultrahigh Volumetric Capacity as an Anode Material for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Li, T.; Ding, B.; Wang, J.; Qin, Z.; Fernando, J.F.S.; Bando, Y.; Nanjundan, A.K.; Kaneti, Y.V.; Golberg, D.; Yamauchi, Y. Sandwich-Structured Ordered Mesoporous Polydopamine/MXene Hybrids as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 14993–15001. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhu, Y.; Cao, B.; Zhu, Q.; Zhang, P.; Xu, B.; Wu, F.; Chen, R. Electrostatic Self-Assembly of 0D–2D SnO2 Quantum Dots/Ti3C2Tx MXene Hybrids as Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2019, 11, 65. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Wang, S.; Wang, B.; Shen, C.; Wang, L.; Hu, Q.; Huang, Q.; Zhou, A. Preparation of High-Purity V2C MXene and Electrochemical Properties as Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A709–A713. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.; Gogotsi, Y.; Gao, Y. Li-Ion Uptake and Increase in Interlayer Spacing of Nb4C3 MXene. Energy Storage Mater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Huang, Y.; Tong, P.; Zhao, B.; Zhu, X.; Sun, Y. Synthesis and Lithium Ion Storage Performance of Two-Dimensional V4C3 MXene. Chem. Eng. J. 2019, 373, 203–212. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, P.; Jin, N.; Wang, B.; Liu, Y.; Lin, Z. Molten Salt Derived Nb2CTx MXene Anode for Li-Ion Batteries. ChemElectroChem 2021, 8, 957–962. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, J.; Bai, L.; Xiao, J.; Zheng, R.; Shan, X.; Li, L.; Gao, H.; Zhang, X. One-Step Synthesis of Few-Layer Niobium Carbide MXene as a Promising Anode Material for High-Rate Lithium Ion Batteries. Dalt. Trans. 2019, 48, 14433–14439. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Y.; Li, F.; Huo, J.; Zhao, D.; Zhu, J.; Guo, S. H2O2 Assisted Hydrothermal Oxidation of Partially Etched Vanadium Carbides (MXene) and Their Electrochemical Properties as Anode for Li-Ion Batteries. Appl. Surf. Sci. 2020, 523, 146387. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, D.; Huang, B.; Wang, L.; Xia, Q.; Zhou, A. Effects of Surface Compositions and Interlayer Distance on Electrochemical Performance of Mo2CTx MXene as Anode of Li-Ion Batteries. J. Phys. Chem. Solids 2023, 176, 111238. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Tang, H.; Pan, L.; Zhang, T.; Lu, H.; Xiong, J.; Yang, J.; Zhang, C.J. Environmental Friendly Scalable Production of Colloidal 2D Titanium Carbonitride MXene with Minimized Nanosheets Restacking for Excellent Cycle Life Lithium-Ion Batteries. Electrochim. Acta 2017, 235, 690–699. [Google Scholar] [CrossRef]
- Kim, S.J.; Naguib, M.; Zhao, M.; Zhang, C.; Jung, H.T.; Barsoum, M.W.; Gogotsi, Y. High Mass Loading, Binder-Free MXene Anodes for High Areal Capacity Li-Ion Batteries. Electrochim. Acta 2015, 163, 246–251. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ayoko, G.A.; Hu, C.; Bell, J.M.; Sun, Z. Two-Dimensional Fluorine-Free Mesoporous Mo2C MXene via UV-Induced Selective Etching of Mo2Ga2C for Energy Storage. Sustain. Mater. Technol. 2020, 25, e00156. [Google Scholar] [CrossRef]
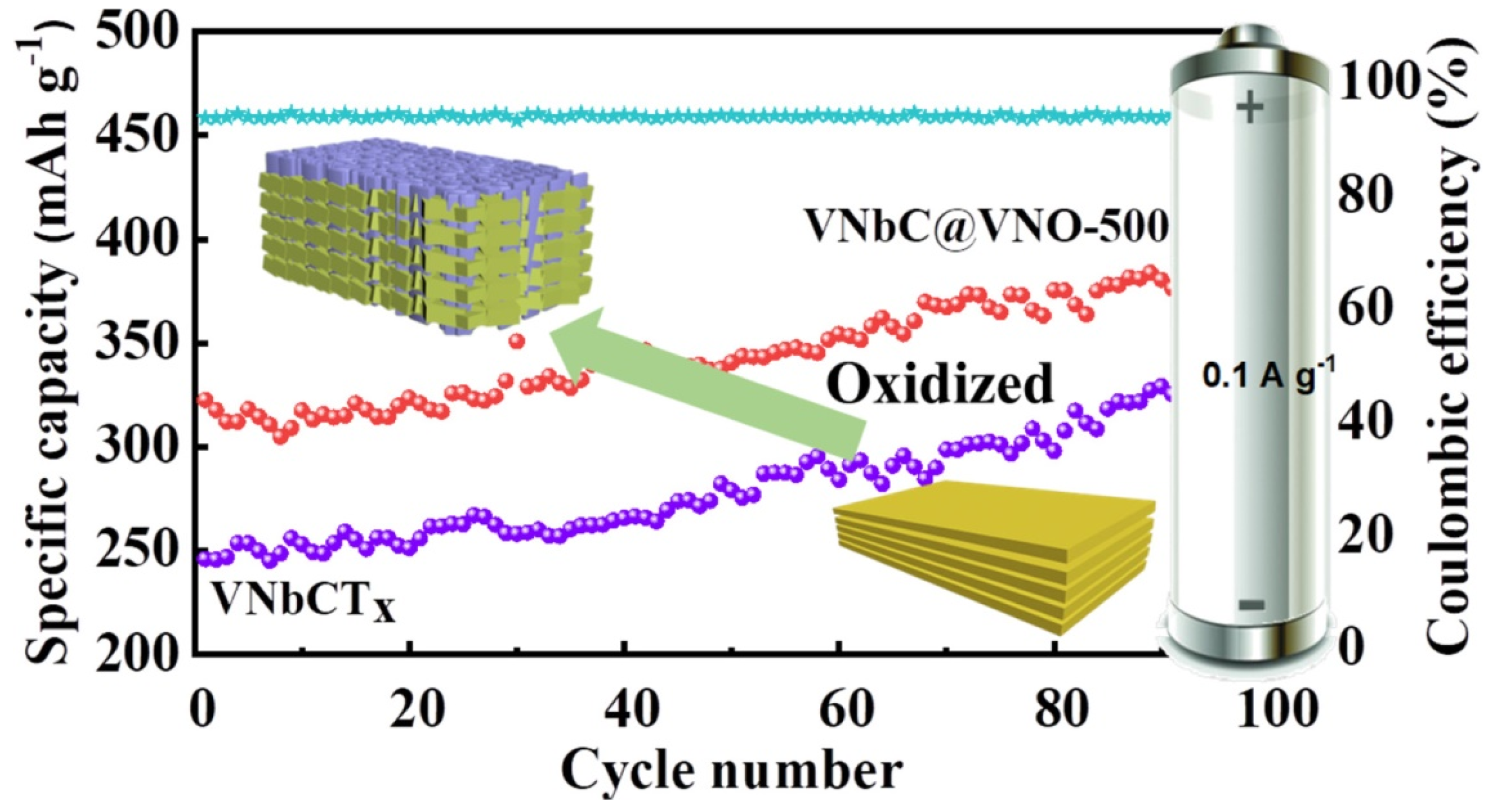
- Cheng, Y.; Yang, L.; Yin, S. Synthesis and Lithium Ion Storage Performance of Novel Two Dimensional Vanadium Niobium Carbide (VNbCTx) MXene. Compos. Commun. 2023, 40, 101588. [Google Scholar] [CrossRef]
- Liu, W.; Cao, J.; Song, F.; Zhang, D.D.; Okhawilai, M.; Yi, J.; Qin, J.Q.; Zhang, X.Y. A Double Transition Metal Ti2NbC2Tx MXene for Enhanced Lithium-Ion Storage. Rare Met. 2023, 42, 100–110. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Zhang, P.; Tian, W.; Chen, J.; Sun, Z.M. Preparation of (Vx, Ti1−x)2C MXenes and Their Performance as Anode Materials for LIBs. J. Mater. Sci. 2019, 54, 11991–11999. [Google Scholar] [CrossRef]
- Syamsai, R.; Rodriguez, J.R.; Pol, V.G.; Van Le, Q.; Batoo, K.M.; Farooq, S.A.; Pandiaraj, S.; Muthumareeswaran, M.R.; Raslan, E.H.; Grace, A.N. Double Transition Metal MXene (TixTa4−xC3) 2D Materials as Anodes for Li-Ion Batteries. Sci. Rep. 2021, 11, 688. [Google Scholar] [CrossRef]
- Yang, J.; Naguib, M.; Ghidiu, M.; Pan, L.M.; Gu, J.; Nanda, J.; Halim, J.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nb-Based M4C3 Solid Solutions (MXenes). J. Am. Ceram. Soc. 2016, 99, 660–666. [Google Scholar] [CrossRef]
- Li, C.; Xue, Z.; Qin, J.; Sawangphruk, M.; Yu, P.; Zhang, X.; Liu, R. Synthesis of Nickel Hydroxide/Delaminated-Ti3C2 MXene Nanosheets as Promising Anode Material for High Performance Lithium Ion Battery. J. Alloys Compd. 2020, 842, 155812. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, Z.; Qin, J.; Sawangphruk, M.; Zhang, X.; Liu, R. NiCo-LDH/Ti3C2 MXene Hybrid Materials for Lithium Ion Battery with High-Rate Capability and Long Cycle Life. J. Energy Chem. 2020, 50, 143–153. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Cao, J.; Yu, P.; Okhawilai, M.; Yi, J.; Qin, J.; Zhang, X. Ti3C2MXene-Encapsulated NiFe-LDH Hybrid Anode for High-Performance Lithium-Ion Batteries and Capacitors. ACS Appl. Energy Mater. 2021, 4, 7821–7828. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-Temperature Reduction Strategy Synthesized Si/Ti3C2 MXene Composite Anodes for High-Performance Li-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Liu, J.; Feng, S.; Li, C.; Marsili, E.; Zhang, X. Silicon Nanospheres Supported on Conductive MXene Nanosheets as Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 160–169. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Zhang, Z.; Ying, H.; Huang, P.; Zhang, S.; Zhang, Z.; Yang, T.T.; Han, W.Q. Porous Si Decorated on MXene as Free-Standing Anodes for Lithium-Ion Batteries with Enhanced Diffusion Properties and Mechanical Stability. Chem. Eng. J. 2023, 451, 138785. [Google Scholar] [CrossRef]
- Bashir, T.; Li, X.; Yang, S.; Song, Y.; Zhou, S.; Wang, J.; Zhu, W.; Yang, J.; Zhao, J.; Gao, L. Enhancing Role of Structurally Integrated V2C MXene Nanosheets on Silicon Anode for Lithium Storage. J. Alloys Compd. 2022, 922, 166213. [Google Scholar] [CrossRef]
- Maughan, P.A.; Bouscarrat, L.; Seymour, V.R.; Shao, S.; Haigh, S.J.; Dawson, R.; Tapia-Ruiz, N.; Bimbo, N. Pillared Mo2TiC2 MXene for High-Power and Long-Life Lithium and Sodium-Ion Batteries. Nanoscale Adv. 2021, 3, 3145–3158. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2020, 16, 1905430. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial Nitrogen Engineering of Robust Silicon/MXene Anode toward High Energy Solid-State Lithium-Ion Batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, D.; Qian, Z.; Gu, X.; Yang, J.; Qian, Y. N-Doped Ti3C2Tx MXene Sheet-Coated SiOx to Boost Lithium Storage for Lithium-Ion Batteries. Sci. China Mater. 2023, 66, 51–60. [Google Scholar] [CrossRef]
- Choudhury, R.; Kurra, N.; Meduri, P. Doped Micro-Silicon and Vanadium Carbide MXene Composite as Anode for High Stability and High Capacity Li-Ion Batteries. Results Eng. 2023, 19, 101338. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Y.; Yi, D.; Xiao, Y.; Wang, K.; Cui, W.; Wang, X. Regulating Fe–O Bond in Ti3C2Tx MXene Anode for High-Capacity Li-Ion Batteries. Chem. Eng. J. 2021, 422, 130018. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, S.; Ying, H.; Yang, W.; Wang, J.; Guo, R.; Han, W. Fabrication of Fe Nanocomplex Pillared Few-Layered Ti3C2Tx MXene with Enhanced Rate Performance for Lithium-Ion Batteries. Nano Res. 2021, 14, 1218–1227. [Google Scholar] [CrossRef]
- Wan, L.; Chua, D.H.C.; Sun, H.; Chen, L.; Wang, K.; Lu, T.; Pan, L. Construction of Two-Dimensional Bimetal (Fe-Ti) Oxide/Carbon/MXene Architecture from Titanium Carbide MXene for Ultrahigh-Rate Lithium-Ion Storage. J. Colloid Interface Sci. 2021, 588, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Weng, L.; Rao, Q.; Yang, S.; Hu, J.; Cai, J.; Min, Y. Highly-Dispersed Iron Oxide Nanoparticles Anchored on Crumpled Nitrogen-Doped MXene Nanosheets as Anode for Li-Ion Batteries with Enhanced Cyclic and Rate Performance. J. Power Sources 2019, 439, 227107. [Google Scholar] [CrossRef]
- He, L.; Tan, C.; Sheng, C.; Chen, Y.; Yu, F.; Chen, Y. A β-FeOOH/MXene Sandwich for High-Performance Anodes in Lithium-Ion Batteries. Dalt. Trans. 2020, 49, 9268–9273. [Google Scholar] [CrossRef]
- Nam, S.; Umrao, S.; Oh, S.; Shin, K.H.; Park, H.S.; Oh, I.K. Sonochemical Self-Growth of Functionalized Titanium Carbide Nanorods on Ti3C2 Nanosheets for High Capacity Anode for Lithium-Ion Batteries. Compos. Part B Eng. 2020, 181, 107583. [Google Scholar] [CrossRef]
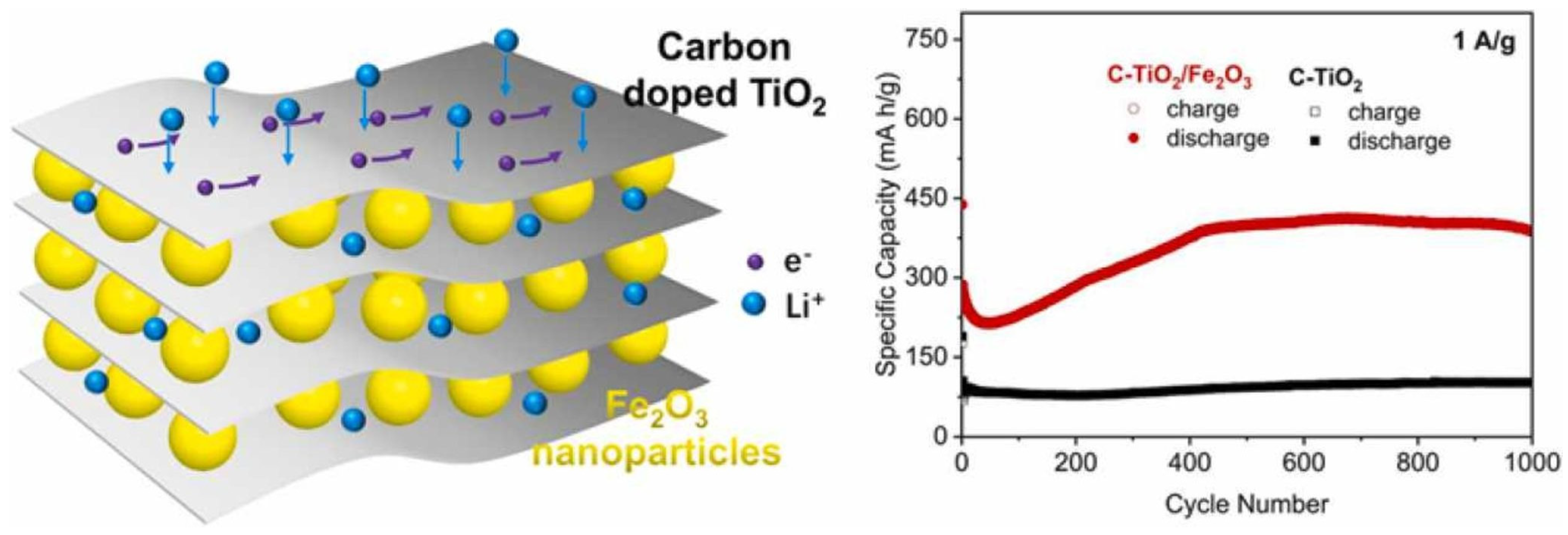
- Lv, X.; Deng, Z.; Wang, M.; Deng, J. Ti3C2 MXene Derived Carbon-Doped TiO2 Multilayers Anchored with Fe2O3 Nanoparticles as Anode for Enhanced Lithium-Ion Storage. J. Alloys Compd. 2022, 918, 165697. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, S.; Bai, X.; Liang, M.; Zhang, X.; Zhao, N. One-Step in-Situ Synthesis of Sn-Nanoconfined Ti3C2Tx MXene Composites for Li-Ion Battery Anode. Electrochim. Acta 2022, 407, 139916. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic Layer Deposition of SnO2 on MXene for Li-Ion Battery Anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; He, Y.; Liu, L.; Yang, Z.; Wang, B.; Xia, Q.; Hu, Q.; Zhou, A. Enhanced Reversible Capacity and Cyclic Performance of Lithium-Ion Batteries Using SnO2 Interpenetrated MXene V2C Architecture as Anode Materials. Energy Technol. 2021, 9, 2000753. [Google Scholar] [CrossRef]
- Fan, W.; Xue, J.; Wang, D.; Chen, Y.; Liu, H.; Xia, X. Sandwich-Structured Sn4P3@MXene Hybrid Anodes with High Initial Coulombic Efficiency for High-Rate Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 61055–61066. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, L.; Li, Y.; Li, J.; Zhu, G.; Zhang, X.; Lu, T.; Pan, L. MXene-Decorated SnS2/Sn3S4 Hybrid as Anode Material for High-Rate Lithium-Ion Batteries. Chem. Eng. J. 2020, 380, 122590. [Google Scholar] [CrossRef]
- Zhu, M.; Deng, X.; Ke, J.; Feng, Z.; He, M.; Feng, Y.; Xiong, D. Graphite Nano-Modified SnO2-Ti2C MXene as Anode Material for High-Performance Lithium-Ion Batteries. J. Alloys Compd. 2021, 886, 161139. [Google Scholar] [CrossRef]
- Zuo, D.C.; Song, S.C.; An, C.S.; Tang, L.B.; He, Z.J.; Zheng, J.C. Synthesis of Sandwich-like Structured Sn/SnOx@MXene Composite through in-Situ Growth for Highly Reversible Lithium Storage. Nano Energy 2019, 62, 401–409. [Google Scholar] [CrossRef]
- Abdurehman Tariq, H.; Nisar, U.; James Abraham, J.; Ahmad, Z.; AlQaradawi, S.; Kahraman, R.; Shakoor, R.A. TiO2 Encrusted MXene as a High-Performance Anode Material for Li-Ion Batteries. Appl. Surf. Sci. 2022, 583, 152441. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Shang, L. Layered Structure 2D MXene/TiO2 Composites as High-Performance Anodes for Lithium-Ion Batteries. Ionics 2023, 29, 531–537. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Gogotsi, Y.; Alshareef, H.N. H2O2 Assisted Room Temperature Oxidation of Ti2C MXene for Li-Ion Battery Anodes. Nanoscale 2016, 8, 7580–7587. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Han, L.; Li, H.; Wang, J.; Lu, T.; Pan, L. In-Situ Fabrication of Few-Layered MoS2 Wrapped on TiO2-Decorated MXene as Anode Material for Durable Lithium-Ion Storage. J. Colloid Interface Sci. 2021, 604, 30–38. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Wang, X.; Shen, C.; Hu, Q.; Zhou, A.; Liu, X. Surface Reformation of 2D MXene by in Situ LaF3-Decorated and Enhancement of Energy Storage in Lithium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 6735–6743. [Google Scholar] [CrossRef]
- Yuan, K.; Hao, P.; Hu, X.; Zhang, J.; Zhou, Y. Experimental and Computational Studies on S-Decorated Ti3C2 MXene as Anode Material in Li-Ion Batteries. J. Mater. Sci. 2022, 57, 7001–7011. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, M.; Luo, G.; Feng, X.; Wu, C.; Qin, W. Improved Lithium Ion Storage Performance of Ti3C2Tx MXene @ S Composite with Carboxymethyl Cellulose Binder. J. Colloid Interface Sci. 2023, 641, 15–25. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, Z.; Guo, J.; Liu, B.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of MXene/Ag Composites for Extraordinary Long Cycle Lifetime Lithium Storage at High Rates. ACS Appl. Mater. Interfaces 2016, 8, 22280–22286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, C.; Zhou, Y.; Wen, F.; Lu, Q.; Liu, D.; Wang, F. Ag Decorated 3D Honeycomb-like MXene Architecture as an Advanced Lithium-Ion Anode Material towards High Capacity and Long-Term Cycle Capability. Appl. Surf. Sci. 2023, 615, 156406. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Zhang, Y.; Zhang, Y.; Wu, Y.; Tian, M.; Chen, P.; Trout, R.; Ma, Y.; Wu, T.H.; et al. A Safe and Fast-Charging Lithium-Ion Battery Anode Using MXene Supported Li3VO4. J. Mater. Chem. A 2019, 7, 11250–11256. [Google Scholar] [CrossRef]
- Liu, R.; Cao, W.; Han, D.; Mo, Y.; Zeng, H.; Yang, H.; Li, W. Nitrogen-Doped Nb2CTx MXene as Anode Materials for Lithium Ion Batteries. J. Alloys Compd. 2019, 793, 505–511. [Google Scholar] [CrossRef]
- Zhong, S.; Ju, S.; Shao, Y.; Chen, W.; Zhang, T.; Huang, Y.; Zhang, H.; Xia, G.; Yu, X. Magnesium Hydride Nanoparticles Anchored on MXene Sheets as High Capacity Anode for Lithium-Ion Batteries. J. Energy Chem. 2021, 62, 431–439. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Yi, R.; Wu, H.; Yang, W.; Li, Y.; Mitrovic, I.; Taylor, S.; Chalker, P.; Liu, R.; et al. Enhanced Electrochemical Performance by GeOx-Coated MXene Nanosheet Anode in Lithium-Ion Batteries. Electrochim. Acta 2020, 358, 136923. [Google Scholar] [CrossRef]
- Melchior, S.A.; Palaniyandy, N.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. Probing the Electrochemistry of MXene (Ti2CTx)/Electrolytic Manganese Dioxide (EMD) Composites as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2019, 297, 961–973. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, D.; Zhu, Q.; Sun, N.; Fu, F.; Xu, B. Plate-to-Layer-Bi2MoO6/MXene-Heterostructured Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2019, 11, 81. [Google Scholar] [CrossRef]
- Amirul, M.; Mohd, A.; Cherusseri, J.; Akmaliah, N.; Mokhtar, M.; Sukor, M.; Seng, Y.; Norhaf, M.; Khalid, M.; Numan, A.; et al. Facile Synthesis of Microwave-Etched Ti3C2 MXene/Activated Carbon Hybrid for Lithium-Ion Battery Anode. J. Electroanal. Chem. 2023, 928, 117050. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Cao, J.; Duan, T.; Kong, Q.; Yao, W. Multidimensional VO2 Nanotubes/Ti3C2 MXene Composite for Efficient Electrochemical Lithium/Sodium-Ion Storage. J. Power Sources 2022, 521, 230946. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Z.; Kou, P.; Wang, D.; Wang, Z.; Sun, H.; Zheng, R.; Liu, Y. In-Situ Synthesis of Niobium-Doped TiO2 Nanosheet Arrays on Double Transition Metal MXene (TiNbCTx) as Stable Anode Material for Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 617, 147–155. [Google Scholar] [CrossRef]
- Xu, C.; Feng, K.; Yang, X.; Cheng, Y.; Zhao, X.; Yang, L.; Yin, S. In-Situ Construction of Metallic Oxide (VNbO5) on VNbCTx MXene for Enhanced Li-Ion Batteries Performance. J. Energy Storage 2023, 69, 107888. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, Y.; Ma, J.; Huang, Y.; Han, S.; Zhou, M.; Lin, H. Sandwich-like Na2Ti3O7Nanosheet/Ti3C2 MXene Composite for High-Performance Lithium/Sodium-Ion Batteries. J. Phys. Chem. C 2022, 126, 18229–18237. [Google Scholar] [CrossRef]
- Gong, S.; Wang, Y.; Zhang, P.; Li, M.; Wen, Y.; Qiu, J.; Xu, B.; Wang, H. Self-Assembly TiNb2O7@MXene Anode Material for Fast and Stable Lithium Storage. Energy Fuels 2023, 37, 3159–3165. [Google Scholar] [CrossRef]
- Qi, X.; Xu, W.; Tang, J.; Xu, Y.; Gao, Y.; Li, L.; Sasaki, S.I.; Tamiaki, H.; Wang, X. Chlorophyll Derivative Intercalation into Nb2C MXene for Lithium-Ion Energy Storage. J. Mater. Sci. 2022, 57, 9971–9979. [Google Scholar] [CrossRef]
- Tian, S.; Wang, D.; Liu, Z.; Liu, G.; Zeng, Q.; Sun, X.; Yang, H.; Han, C.; Tao, K.; Peng, S. Highly Reversible Lithium-Ion Battery with Excellent Rate Performance and Cycle Stability Based on a Ti3C2/CoS2 Composite Anode. ACS Appl. Mater. Interfaces 2023, 15, 44996–45004. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, L.; Zhou, A.; Zhang, H.; Chen, Z.; Hu, Q.; Qin, G. MoS2-Decorated Ti3C2 MXene Nanosheet as Anode Material in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2654–A2659. [Google Scholar] [CrossRef]
- Chen, C.; Xie, X.; Anasori, B.; Sarycheva, A.; Makaryan, T.; Zhao, M.; Urbankowski, P.; Miao, L.; Jiang, J.; Gogotsi, Y. MoS2-on-MXene Heterostructures as Highly Reversible Anode Materials for Lithium-Ion Batteries. Angew. Chemie—Int. Ed. 2018, 57, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Kamat, R.S.; Padwal, C.; Pham, H.D.; Wang, X.; Jadhav, L.D.; Dubal, D.P. Selenium Enriched Over-Oxidized Mo3Se4 Decorated MXene as a High-Performance Li-Ion Battery Anode Material. J. Energy Storage 2023, 73, 108916. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, B.; Lin, S.; Li, K.; Zhou, J.; Dai, J.; Zhu, X.; Sun, Y. Construction of Hierarchical V4C3-MXene/MoS2/C Nanohybrids for High Rate Lithium-Ion Batteries. Nanoscale 2020, 12, 1144–1154. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Zeng, G.; Feng, J.; Qian, Y. Room-Temperature Liquid Metal Confined in MXene Paper as a Flexible, Freestanding, and Binder-Free Anode for Next-Generation Lithium-Ion Batteries. Small 2019, 15, 1903214. [Google Scholar] [CrossRef]
- Zhang, P.; Soomro, R.A.; Guan, Z.; Sun, N.; Xu, B. 3D Carbon-Coated MXene Architectures with High and Ultrafast Lithium/Sodium-Ion Storage. Energy Storage Mater. 2020, 29, 163–171. [Google Scholar] [CrossRef]
- Wang, C.; Xie, H.; Chen, S.; Ge, B.; Liu, D.; Wu, C.; Xu, W.; Chu, W.; Babu, G.; Ajayan, P.M.; et al. Atomic Cobalt Covalently Engineered Interlayers for Superior Lithium-Ion Storage. Adv. Mater. 2018, 30, 1802525. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Fang, Y.; Lv, W.; Wang, F.; Liu, Y.; Liu, H. Hydrothermal Preparation of CoO/Ti3C2 Composite Material for Lithium-Ion Batteries with Enhanced Electrochemical Performance. J. Electroanal. Chem. 2018, 817, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Yi, R.; Li, Z.; Chen, Y.; Li, Y.; Mitrovic, I.; Taylor, S.; Chalker, P.; Yang, L.; et al. Facile Preparation of Co3O4 Nanoparticles Incorporating with Highly Conductive MXene Nanosheets as High-Performance Anodes for Lithium-Ion Batteries. Electrochim. Acta 2020, 345, 136203. [Google Scholar] [CrossRef]
- Oh, H.G.; Park, S. Two-Dimensional Composite of Nitrogen-Doped Graphitic Carbon-Coated Cobaltosic Oxide Nanocrystals on MXene Nanosheets as High-Performance Anode for Lithium-Ion Batteries. Appl. Surf. Sci. 2021, 564, 150415. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, H.; Zhang, X.; Xu, Y.; Fransaer, J. Cu2O Hybridized Titanium Carbide with Open Conductive Frameworks for Lithium-Ion Batteries. Electrochim. Acta 2016, 202, 24–31. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, D.; Huang, Q.; Yang, J.; Barsoum, M.W.; Yan, X. Carbon Nanofiber Bridged Two-Dimensional Titanium Carbide as a Superior Anode for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 14096–14100. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and Characterization of 2D Molybdenum Carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, Z.; Cui, C.; Hu, T.; Fan, B.; Wang, H.; Liang, Y.; Zhang, C.; Zhang, H.; Wang, X. One-Step Incorporation of Nitrogen and Vanadium between Ti3C2Tx MXene Interlayers Enhances Lithium Ion Storage Capability. J. Phys. Chem. C 2020, 124, 6012–6021. [Google Scholar] [CrossRef]
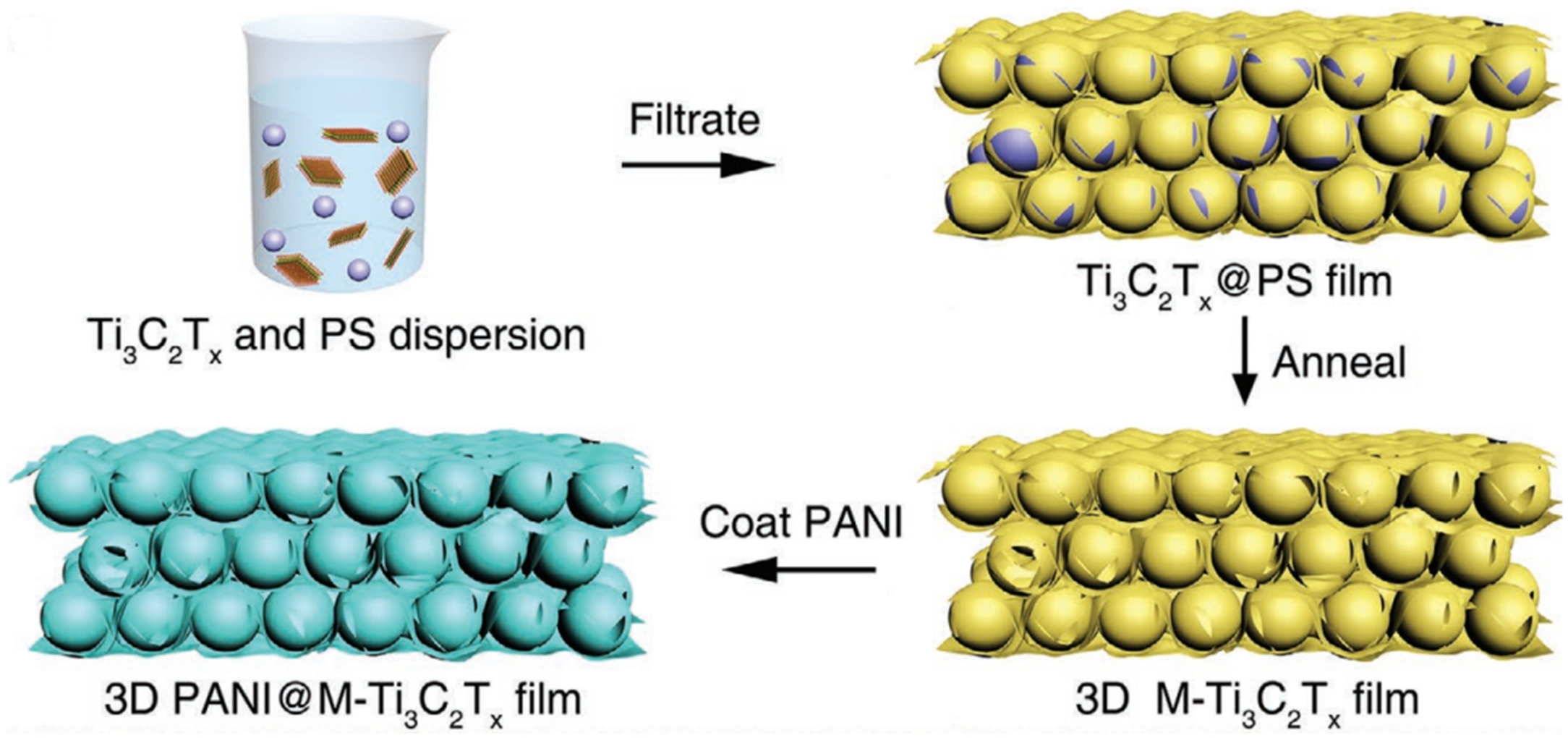
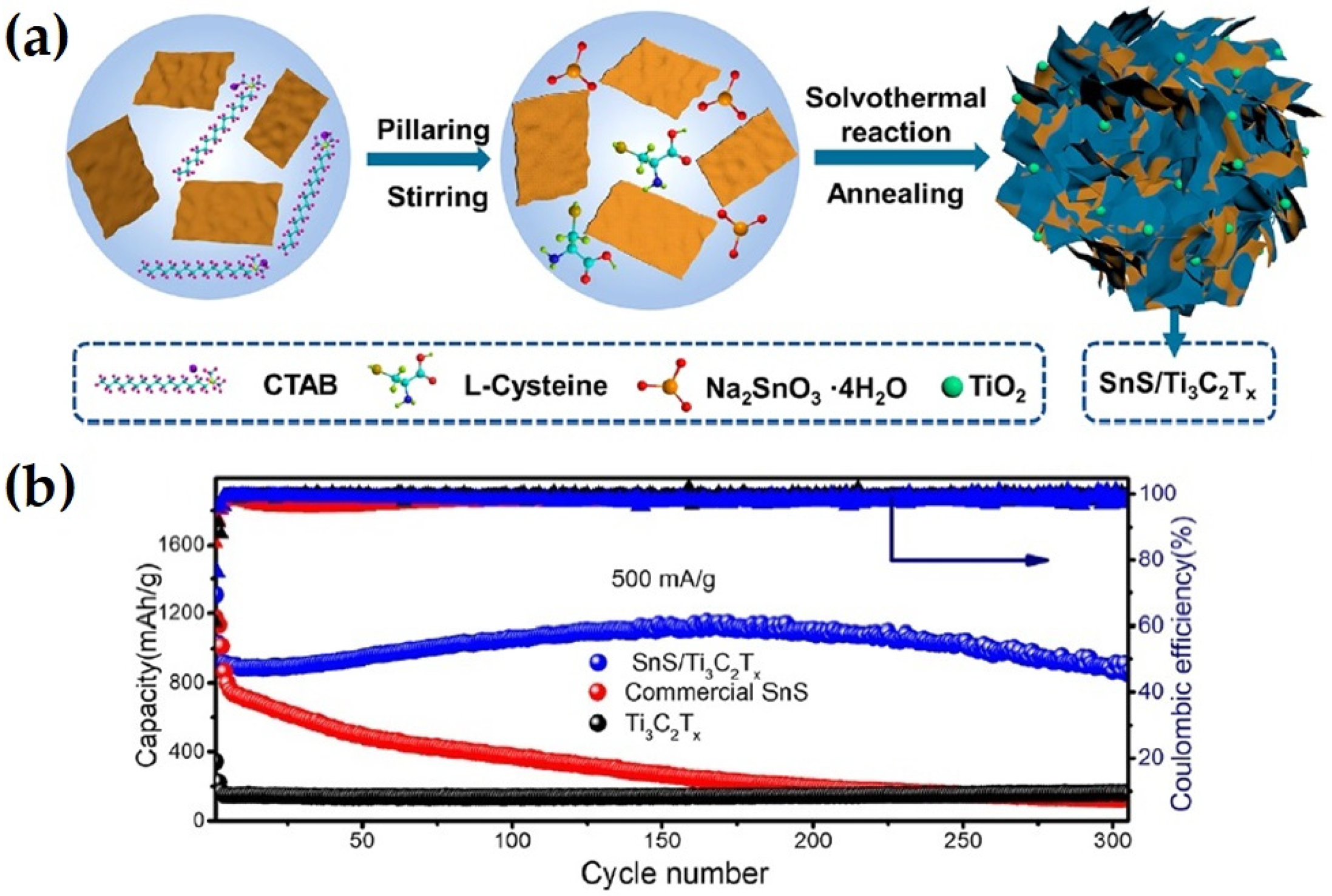
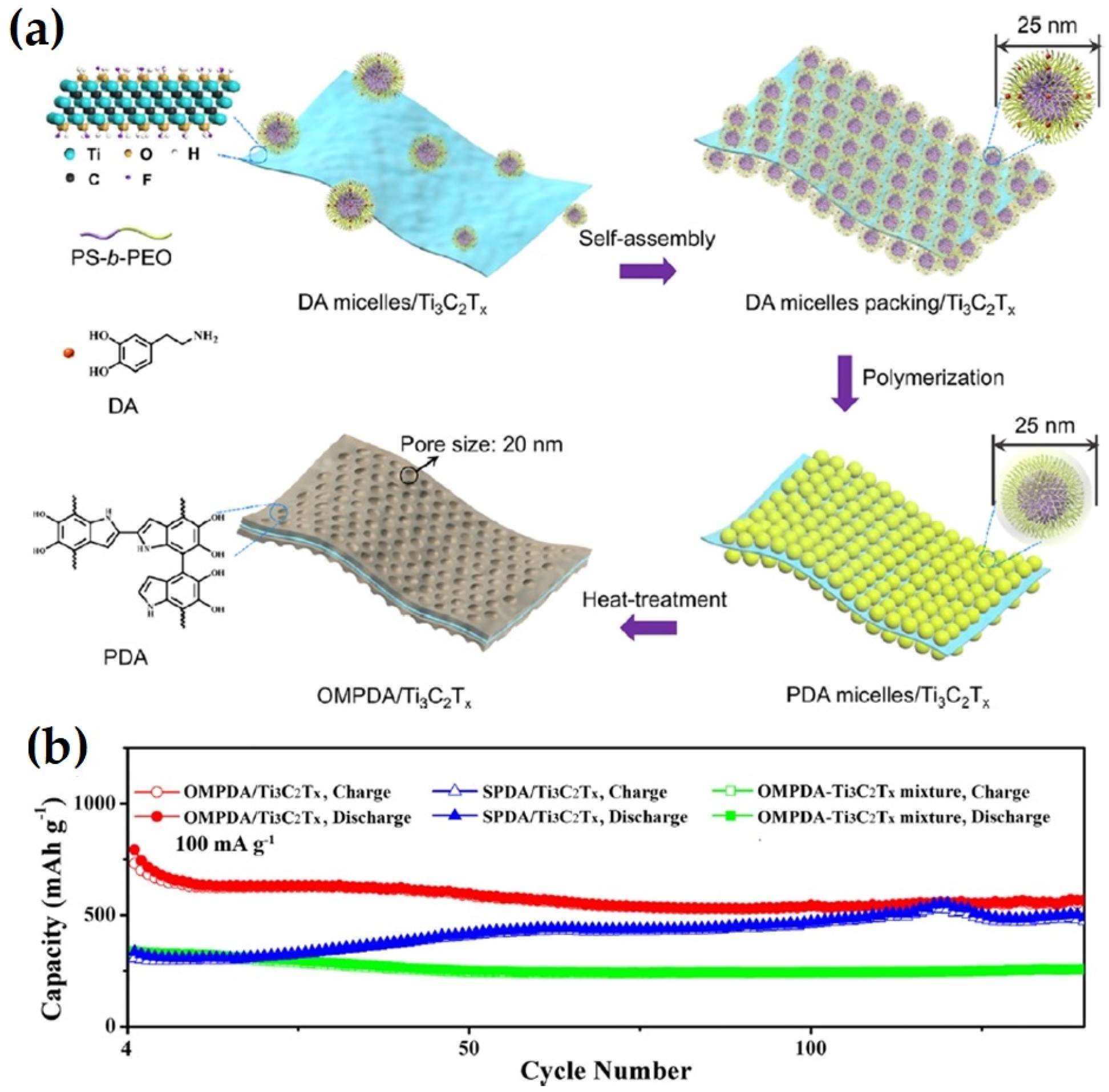
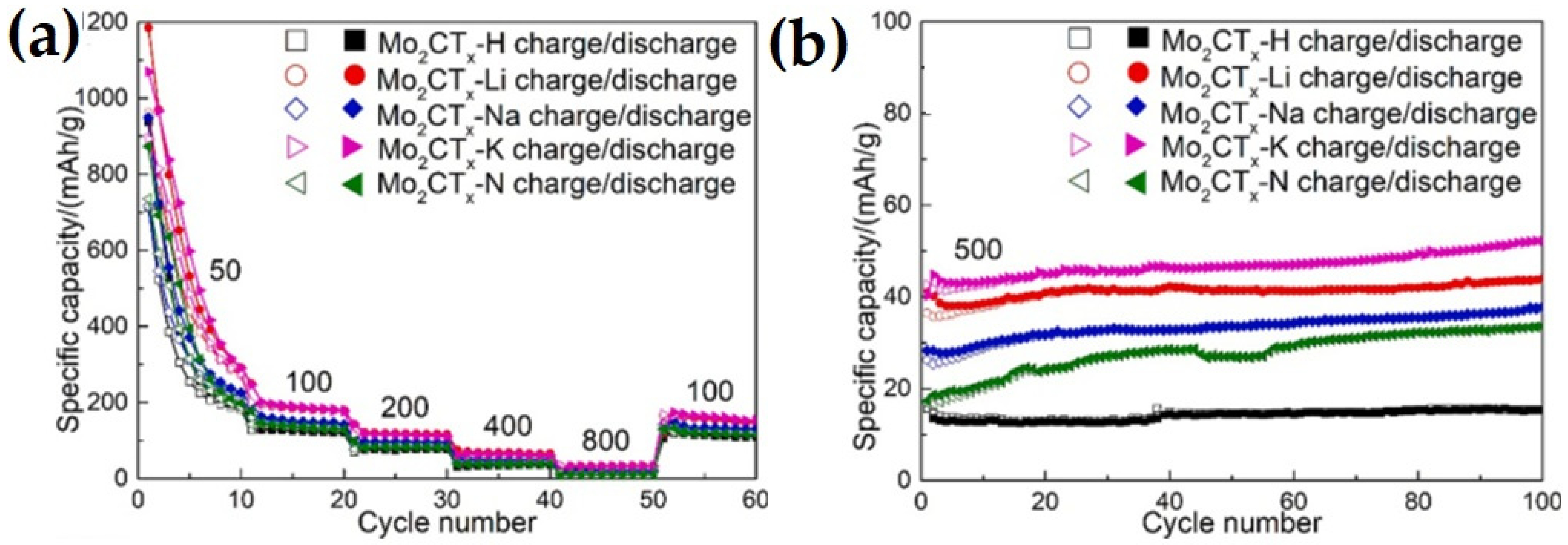


| Anode Material | Etchant | Etching Time (h) | Discharge Capacity (mAhg−1) and Current Rate (Ag−1) | Number of Cycle | Ref. |
|---|---|---|---|---|---|
| Ti2C | 10 wt% HF at RT | 10 | 70 at 10 C | 200 | [53] |
| Ti3C2 | 49 wt% HF at 60 °C | 24 | 118.7 at 1 C | 75 | [54] |
| Ti3C2 | HCl + LiF at 35 °C | 24 | 47.9 at 3 Ag−1 | 100 | [55] |
| Ti3C2 | NaOH | 104.8 at 0.5 Ag−1 | 500 | [56] | |
| Partially etched Ti3C2 | 40 wt% HF at RT | 0.5 | 160 at 1 C | 100 | [57] |
| Porous Ti3C2 | HCl + LiF at 35 °C | 24 | 220 at 1 Ag−1 | 3500 | [58] |
| Ti3C2Tx-400 | 40 wt% HF at 60 °C | 48 | 126.4 at 1 C | 100 | [60] |
| Ti3C2Tx-700 | 40 wt% HF at 60 °C | 48 | 147.4 at 1 C | 100 | [60] |
| Scroll Ti3C2 | HCl + LiF at 40 °C | 24 | ~112 at 0.4 Ag−1 | 500 | [61] |
| V2C | HCl + NaF at 90 °C | 72 | 243 at 0.5 Ag−1 | 500 | [67] |
| Nb4C3 | 49 wt% HF at RT | 140 | 320 at 1 Ag−1 | 750 | [68] |
| V4C3 | 40 wt% HF at 55 °C | 96 | 125 at 1 Ag−1 | 300 | [69] |
| Nb2CTx | Lewis acid at 750 °C | 5 | 150 at 1 Ag−1 | 500 | [70] |
| Nb2C | 40 wt% HF at 60 °C | 90 | 225 at 1 Ag−1 | 800 | [71] |
| Partially etched V2CTx | 40 wt% HF at RT | 7 days | 125 at 1 Ag−1 | 1000 | [72] |
| d-Mo2CTx | HCl + KF at 180 °C | 24 | 118.8 at 2 Ag−1 | 100 | [73] |
| d-Hf3C2Tx | 35 wt% HF at RT | 60 | 146 at 0.2 Ag−1 | 200 | [74] |
| Ti3CNTx | HCl + LiF at 30 °C | 12 | 300 at 0.5 Ag−1 | 1000 | [75] |
| Mo2C | H3PO4 | 3-5 | 90 at 0.01 Ag−1 | 140 | [78] |
| Anode Material | Etchant | Etching Time (h) | Discharge Capacity (mAhg−1) and Current Rate (Ag−1) | Number of Cycle | Ref. |
|---|---|---|---|---|---|
| VNbCTx | 50 wt% HF at 40 °C | 48 | 520.5 at 0.1 | 100 | [79] |
| Ti2NbC2Tx | 48 wt% HF at 50 °C | 24 | 95.2 at 1 | 4000 | [80] |
| (V0.7Ti0.3)2C | LiF + HF at 90 °C | 5 | 177 at 1 | 1000 | [81] |
| (V0.5Ti0.5)2C | LiF + HF at 90 °C | 24 | 204.9 at 1 | 1000 | [81] |
| (V0.3Ti0.7)2C | LiF + HF at 90 °C | 36 | 184.9 at 1 | 1000 | [81] |
| TixTa(4−x)C3 | 40 wt% HF at RT | 72 | 459 at 0.5 C | 200 | [82] |
| Anode Material | Synthesis Process (Etchant) | Discharge Capacity (mAhg−1) and Current Rate (Ag−1) | Number of Cycles | Ref. |
|---|---|---|---|---|
| Ni(OH)2/d-Ti3C2 | Hydrothermal (HCl + LiF) | 372 at 1 Ag−1 | 1000 | [84] |
| NiCo-LDH/Ti3C2 | Hydrothermal (HCl + LiF) | 562 at 5 Ag−1 | 800 | [85] |
| NiFe-LDH/Ti3C2Tx | Hydrothermal (HCl + LiF) | 726.1 at 1 Ag−1 | 400 | [86] |
| Ti3C2/Si | Orthosilicate hydrolysis and low-temp. reduction (HF) | 973 at 1 Ag−1 | 800 | [87] |
| Si/Ti3C2 | Electrostatic self-assembly (HCl + LiF) | 1654.8 at 1 Ag−1 | 300 | [88] |
| Si/Ti3C2Tx | Vacuum filtration (HCl + LiF) | 1672 at 1 Ag−1 | 200 | [89] |
| Porous Si/Ti3C2Tx-2:1 | Vacuum filtration (HCl + LiF) | 555.5 at 5 Ag−1 | 500 | [90] |
| Si-V2C | Ultrasonic mixing (NaF + HCl) | 430 at 3 Ag−1 | 150 | [91] |
| Mo2TiC2–Si-400 | Pillaring and calcination (HCl + LiF) | 108 at 1 Ag−1 | 500 | [92] |
| SiO2/Ti3C2Tx | Stober method spray drying (40 wt% HF) | 635 at 1 Ag−1 | 200 | [93] |
| Si-N-Ti3C2Tx | Heat treatment (40 wt% HF) | 760 at 3.2 Ag−1 | 900 | [94] |
| SiOx-N-Ti3C2Tx | Ball milling and annealing (40 wt% HF) | 700 at 1 Ag−1 | 800 | [95] |
| d-Si/G/V2C | 48% HF + HCl | 2003 at 1C | 500 | [96] |
| Fe-Ti3C2Tx | HCl + LiF | 418.8 at 0.2 Ag−1 | 500 | [97] |
| Fe-Ti3C2 | HF | 310 at 5 Ag−1 | 850 | [98] |
| (Fe-Ti) oxide/carbon/Ti3C2Tx | Solvothermal, ultrasound hybridizing, and annealing | 452.5 at 10 Ag−1 | 1200 | [99] |
| N-Ti3C2/Fe2O3 | Thermal decomposition (HCl + LiF) | 549 at 2 Ag−1 | 400 | [100] |
| β-FeOOH/Ti3C2Tx | (HCl + LiF) | 671 at 1 Ag−1 | 100 | [101] |
| FTCN-Ti3C2 | Sonochemical method (HCl + LiF) | 1034 at 0.1 C | 250 | [102] |
| C-TiO2/Fe2O3-Ti3C2 | Heat treatment | 387.7 at 1 Ag−1 | 1000 | [103] |
| Sn-Ti3C2Tx | Molten salt reaction method (Lewis acid) | 226.2 at 0.2 Ag−1 | 1000. | [104] |
| Sn4+/Ti3C2 | Liquid phase immersion process (40 wt% HF) | 544 at 0.5 Ag−1 | 200 | [105] |
| V2CTx-SnO2 | Ultrasound and annealing (NH4F + HCl) | 274 at 8 Ag−1 | 200 | [107] |
| Sn4P3-Ti3C2Tx | Solvothermal phosphorization (50 wt% HF) | 847 at 1 Ag−1 | 300 | [108] |
| SnS/Ti3C2Tx | Solvothermal and annealing (HF) | 866 at 0.5 Ag−1 | 300 | [62] |
| SnS2/Sn3S4- Ti3C2Tx | Solvothermal and calcination (45 wt% HF) | 101.4 at 5 Ag−1 | 500 | [109] |
| SnO2-Ti2C-C | Hydrothermal | 763.18 at 2 Ag−1 | 500 | [110] |
| Sn/SnOx-Ti3C2Tx | Annealing (40 wt% HF) | 594.2 at 0.05 Ag−1 | 200 | [111] |
| 5 wt% Ti3C2/TiO2 | Hydrolysis (HCl + LiF) | 180 at 0.1 C | 100 | [112] |
| Ti3C2/TiO2 | Hydrothermal (46 wt% HF) | 186 at 1 Ag−1 | 300 | [113] |
| TiO2/Ti2C | Oxidation (10 wt% HF) | 280 at 1 Ag−1 | 1000 | [114] |
| Ti3C2/TiO2@f-MoS2 | Hydrothermal and annealing (48 wt% HF) | 403 at 2 Ag−1 | 1200 | [115] |
| Ti3C2/LaF3 | Heat treatment (HCl + LiF) | 89.2at 1 Ag−1 | 50 | [116] |
| Ti3C2/S | 166.3 at 0.5 Ag−1 | 400 | [117] | |
| S-Ti3C2Tx (CMC) | Calcination and annealing (HCl + LiF) | 858.2 at 5 Ag−1 | 3600 | [118] |
| S-Ti3C2Tx (PVDF) | Calcination and annealing (HCl + LiF) | 322.2 at 5 Ag−1 | 3600 | [118] |
| Ti3C2/Ag | Self-reduction (40 wt% HF) | 310 at 1 C | 800 | [119] |
| 3D Ti3C2Tx/Ag | HCl + LiF | 310 at 3 Ag−1 | 2000 | [120] |
| Li3VO4/Ti3C2 | Sol–gel method (48 wt% HF) | 146 at 5 C | 1000 | [121] |
| N-Nb2CTx | Hydrothermal (50 wt% HF) | 238 at 5 C | 100 | [122] |
| MgH2/Ti3C2-60 | HCl + LiF | 328 at 2 Ag−1 | 50 | [123] |
| GeOx/Ti3C2/PVDF(NMP) | One-pot method (HCl + LiF) | 483 at 0.2 Ag−1 | 100 | [124] |
| GeOx/Ti3C2/Li-PAA(DI-water). | One-pot method (HCl + LiF) | 950 at 0.5 Ag−1 | 100 | [124] |
| Ti2C/EMD | 490 at 0.1 Ag−1 | 100 | [125] | |
| Bi2MoO6/Ti3C2Tx-30% | Electrostatic self-assembly (HCl + LiF) | 545.1 at 1 Ag−1 | 1000 | [126] |
| Activated carbon-Ti3C2 | Slurry casting method (HCl + LiF) | 881.9 at 0.2 Ag−1 | 117 | [127] |
| VO2-NTs/Ti3C2 | Solvothermal self-assembly (HCl + LiF) | 516 at 5 Ag−1 | 2000 | [128] |
| TiNbC@NTO-500 | Hydrothermal (HF) | 261 at 1 Ag−1 | 500 | [129] |
| VNbC@VNO-500 | Partial oxidation (40 wt% HF) | 323.9 at 1 Ag−1 | 1000 | [130] |
| Na2Ti3O7/Ti3C2 | Alkalization and oxidation (HCl + LiF) | 158 at 4 Ag−1 | 1200 | [131] |
| TiNb2O7/Ti3C2 | Electrostatic self-assembly (HCl + LiF) | 192.3 at 10 C | 500 | [132] |
| Ti3C2/CoS2 | Hydrothermal (HCl + LiF) | ~165 at 1 Ag−1 | 1000 | [134] |
| MoS2/Ti3C2 | Etching and solid-state sintering (HCl + LiF) | 131.6 at 1 Ag−1 | 200 | [135] |
| MoS2/Mo2TiC2Tx | Sulfidation | 509 at 0.1 Ag−1 | 100 | [136] |
| Mo3Se4-Ti3C2Tx | Hydrothermal (40 wt% HF) | 1092.37 at 0.268 Ag−1 | 378 | [137] |
| V4C3/MoS2/C | 40 wt% HF | 622.6 at 1 Ag−1 | 450 | [138] |
| T-Ti3C2Tx@C | HCl + LiF | 337.3 at 2C | 600 | [140] |
| V2C@Co | HF | 248.8 at 8 Ag−1 | 15,000 | [141] |
| CoO/Ti3C2Tx | Hydrothermal (40 wt% HF) | 324 at 0.1 Ag−1 | 100 | [142] |
| Co3O4/Ti3C2Tx | HCl + LiF | 307 at 5 C | 1000 | [143] |
| Co3O4@NGC/Ti3C2 | 830 at 1 Ag−1 | 500 | [144] | |
| Cu2O/Ti2C | Solvothermal (HCl + LiF) | 143 at 1 Ag−1 | 250 | [145] |
| Ti3C2/CNF | CVD | 97 at 100 C | 2900 | [146] |
| Nb2C/CNT | CVD | 430 at 2.5 C | 300 | [147] |
| d-Mo2C/CNT | Vacuum filtration (HCl + LiF) | 76 at 10 Ag−1 | 1000 | [148] |
| p-Ti3C2Tx/CNT | Chemical etching method (HF) | ~500 at 0.5 C | 100 | [14] |
| V0.1-Ti3C2Tx | Microwave irradiation (HF) | 72.9 at 3 C | 1000 | [149] |
| V0.2-Ti3C2Tx | Microwave irradiation (HF) | 92.4 at 3 C | 1000 | [149] |
| V0.5-Ti3C2Tx | Microwave irradiation (HF) | 98.3 at 3 C | 1000 | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chy, M.N.U.; Rahman, M.A.; Kim, J.-H.; Barua, N.; Dujana, W.A. MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review. Nanomaterials 2024, 14, 616. https://doi.org/10.3390/nano14070616
Chy MNU, Rahman MA, Kim J-H, Barua N, Dujana WA. MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review. Nanomaterials. 2024; 14(7):616. https://doi.org/10.3390/nano14070616
Chicago/Turabian StyleChy, Mohammad Nezam Uddin, Md. Arafat Rahman, Jin-Hyuk Kim, Nirjhor Barua, and Wasif Abu Dujana. 2024. "MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review" Nanomaterials 14, no. 7: 616. https://doi.org/10.3390/nano14070616
APA StyleChy, M. N. U., Rahman, M. A., Kim, J.-H., Barua, N., & Dujana, W. A. (2024). MXene as Promising Anode Material for High-Performance Lithium-Ion Batteries: A Comprehensive Review. Nanomaterials, 14(7), 616. https://doi.org/10.3390/nano14070616








