The Constructing of the Oxide Phase Diagram for Fluoride Adsorption on La-Fe-Al: A Collaborative Study of Density Functional Calculation and Experimentation
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of La-Fe-Al Oxides
2.2. Physical Characterization Methods
2.3. Preparation of Fluoride Solution
2.4. Determination of Fluoride Solution Concentration
2.5. Adsorption Model
2.6. Density Functional Theory Simulation (DFT)
2.7. Operation of the Experiment
3. Results and Discussion
3.1. Adsorption Experiment
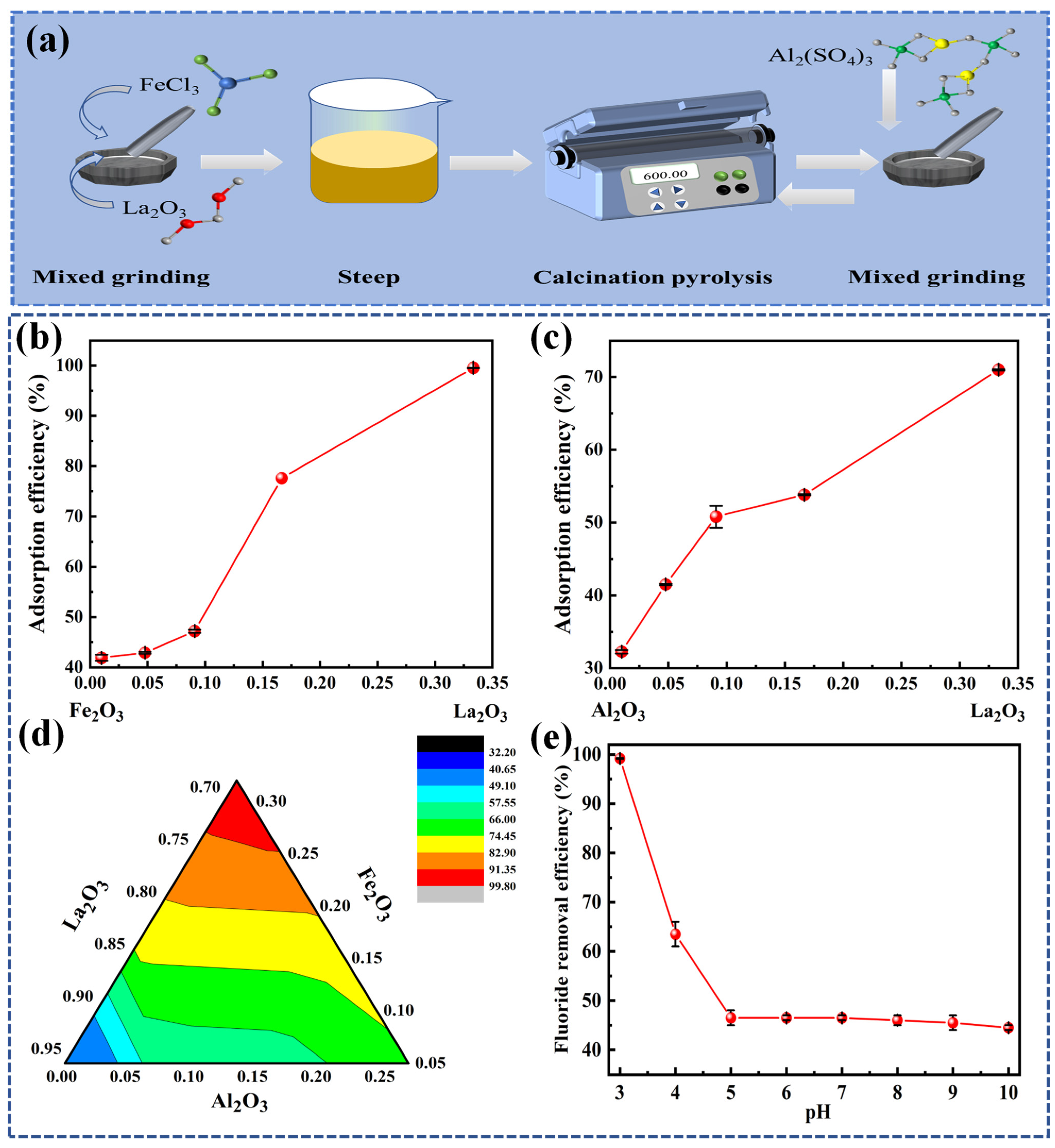
3.1.1. Adsorption Phase Diagram
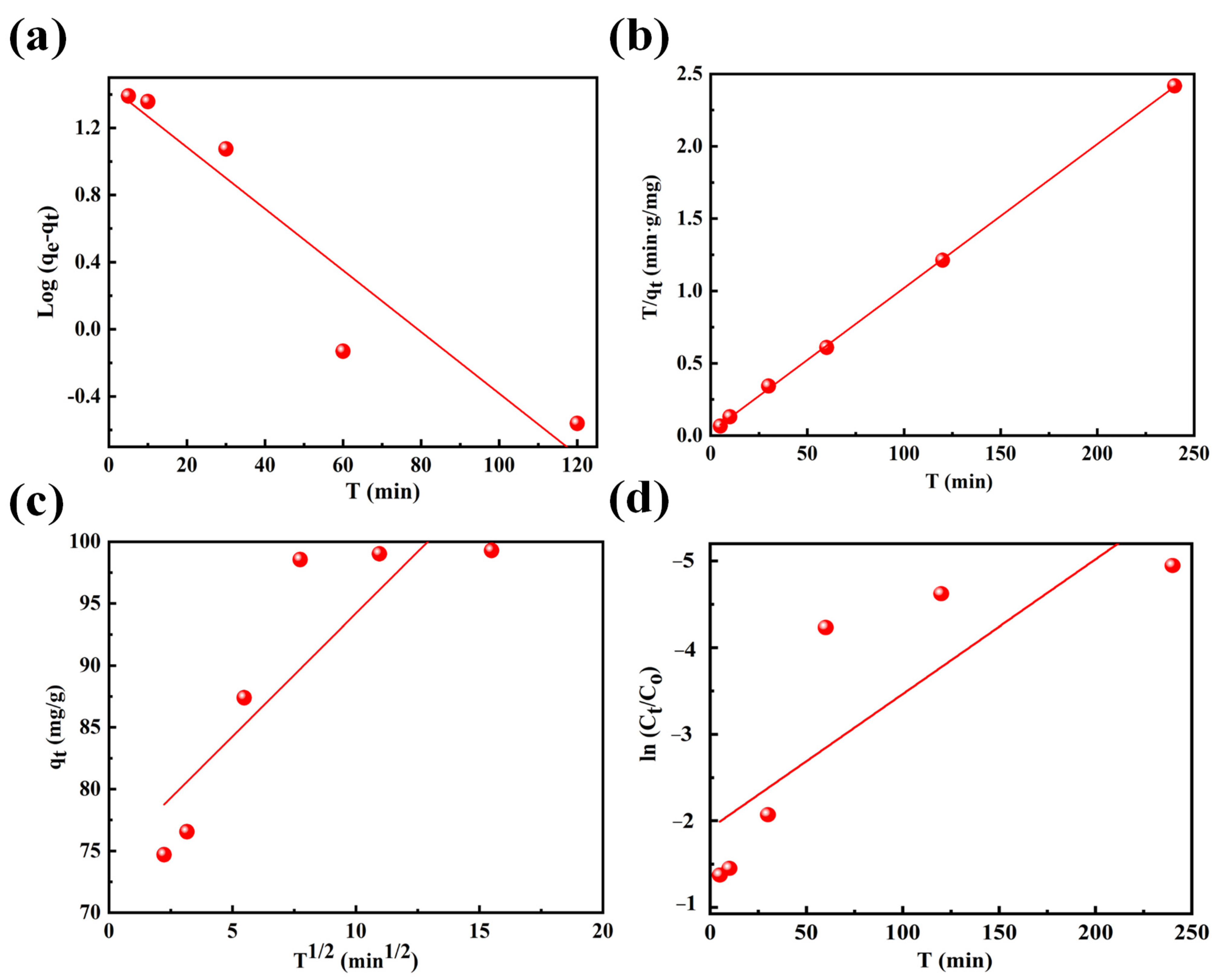
3.1.2. Adsorption Kinetics
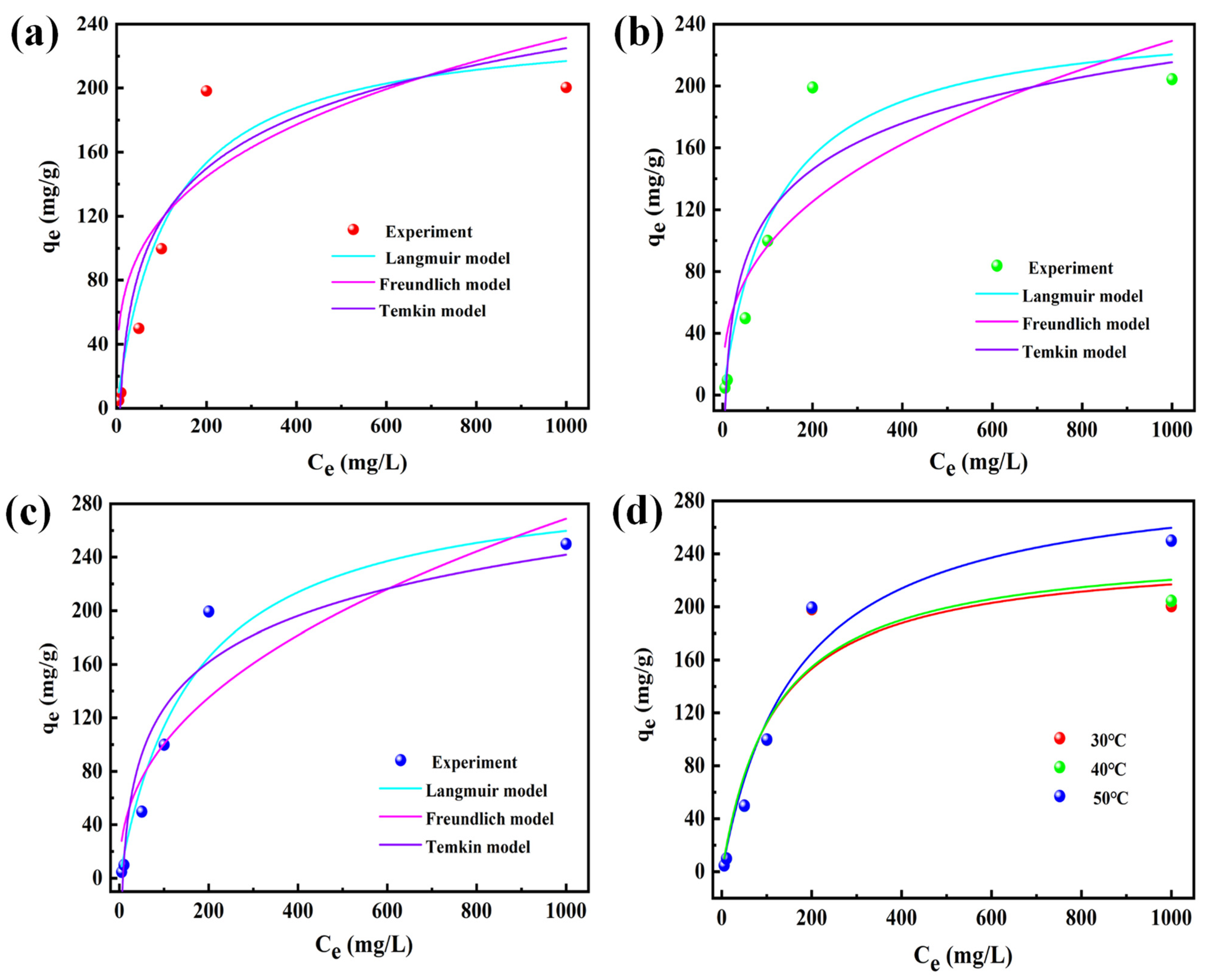
3.1.3. Adsorption Thermodynamics
3.2. Adsorption Mechanism
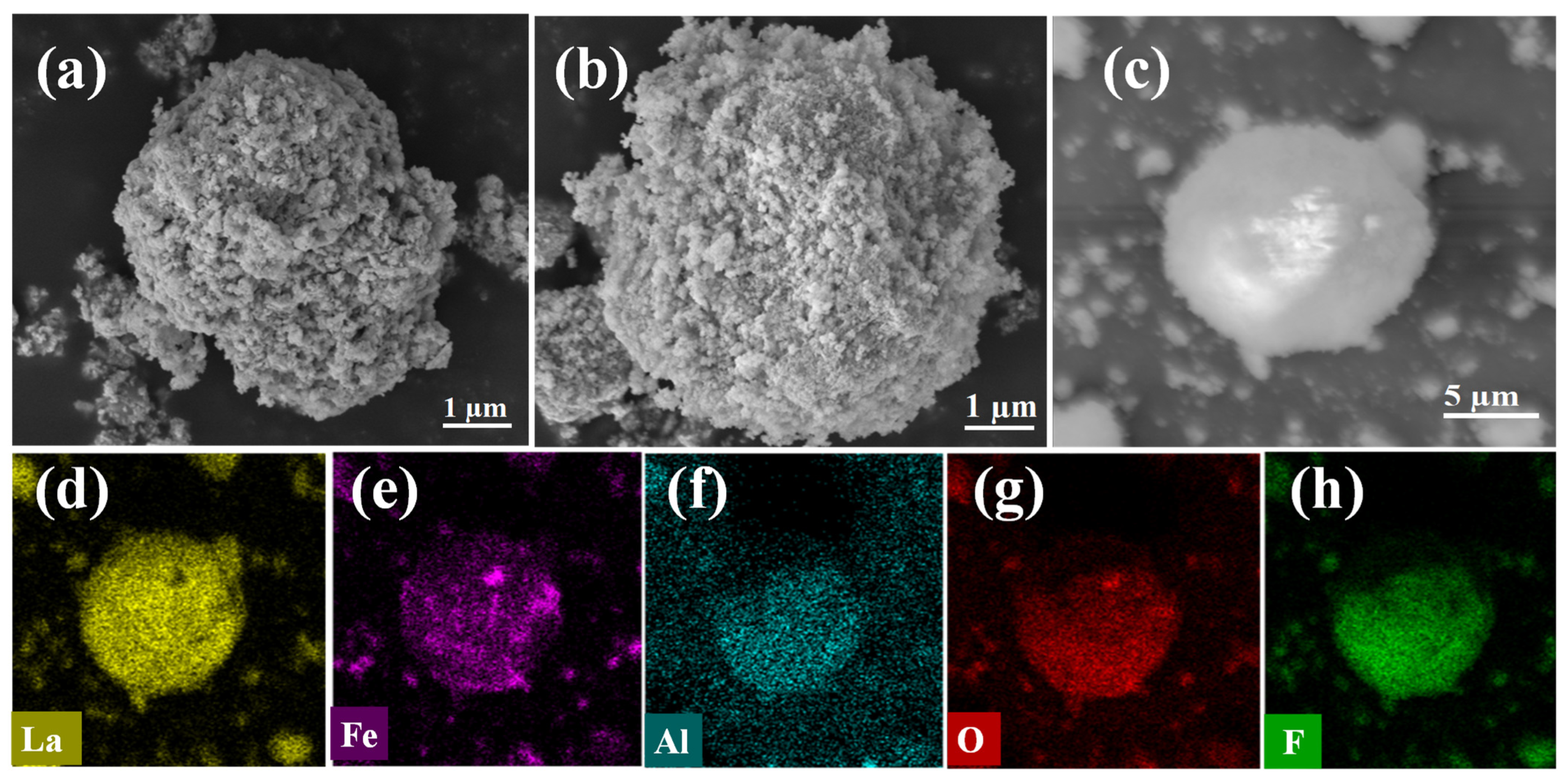
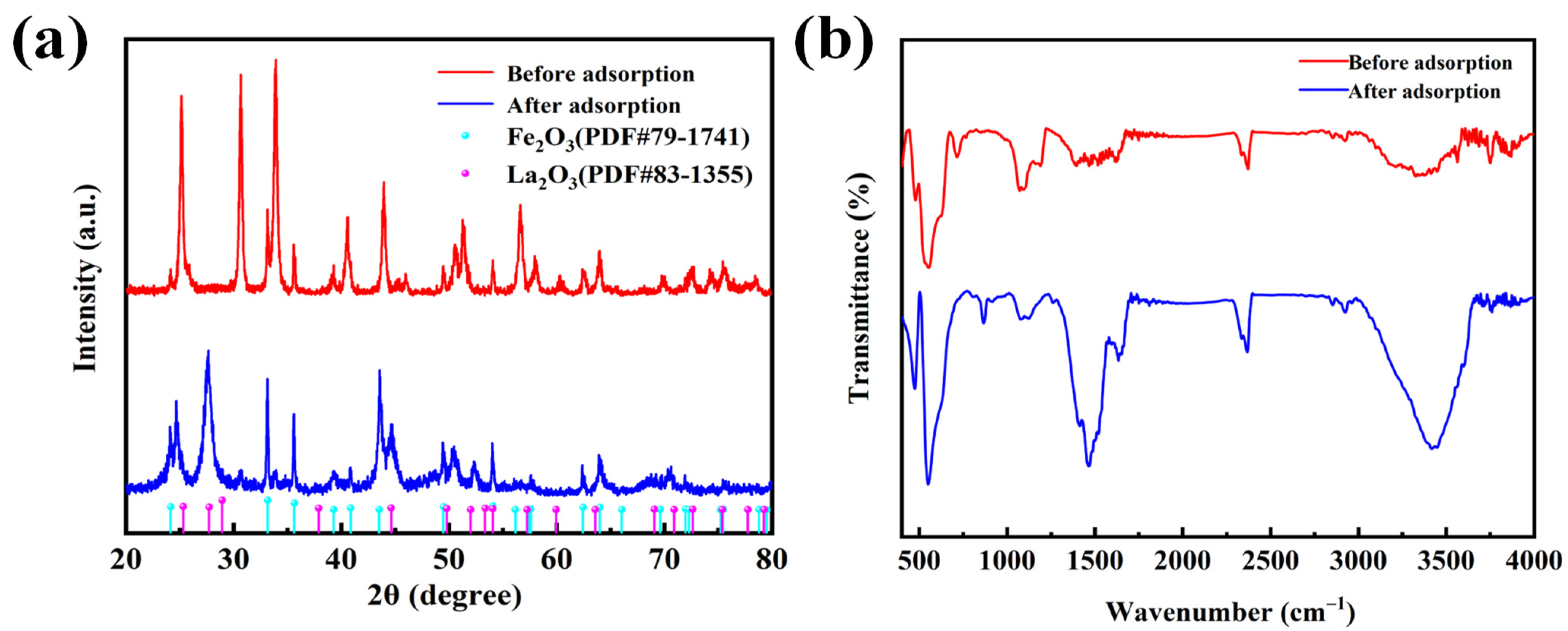
3.2.1. Adsorption Characterization
3.2.2. Changes in X-ray Photoelectron Spectroscopy
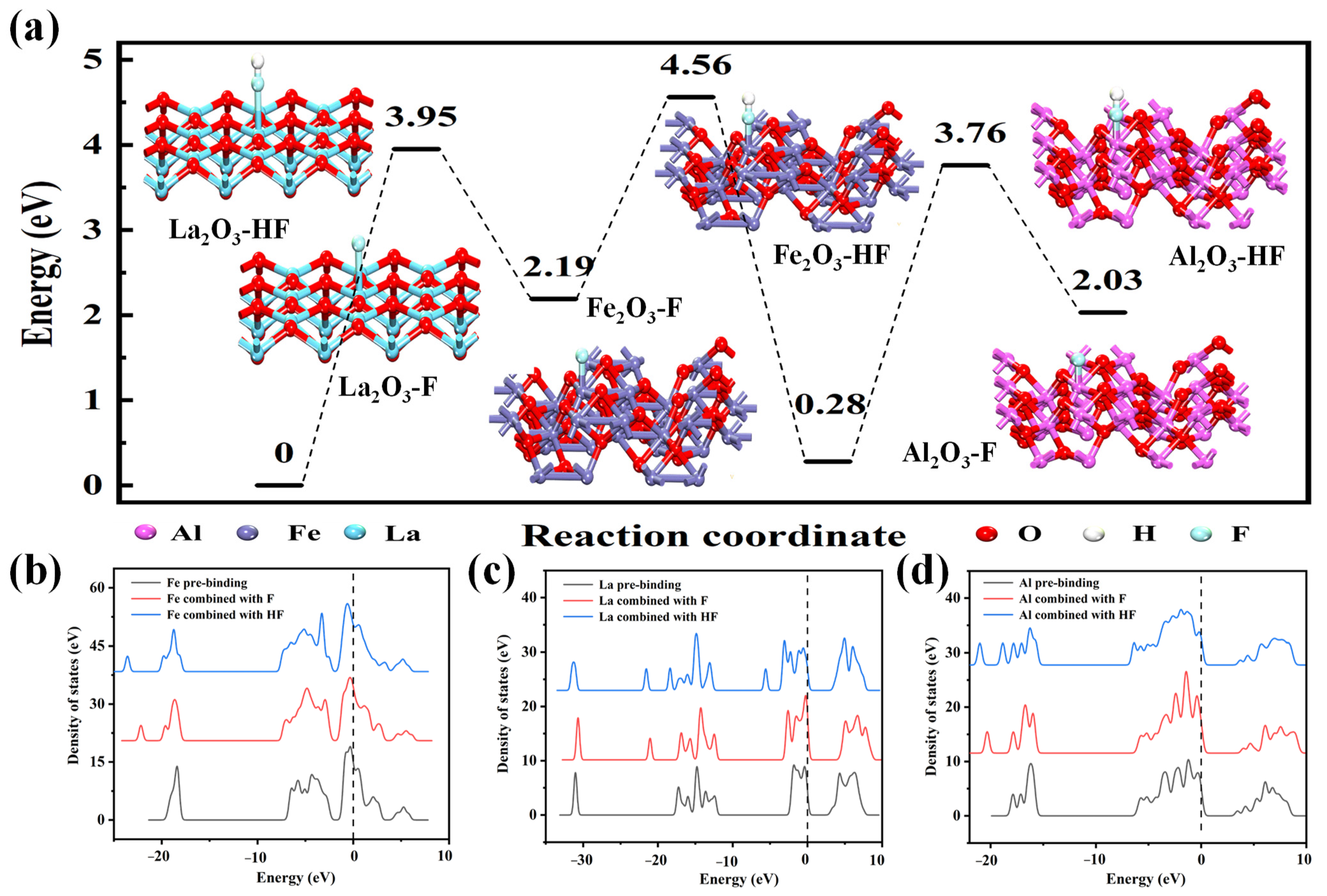
3.3. Density Functional Theory Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Zhao, H.; Roberts, D.; Van de Voorde, T.; Batelaan, O.; Fan, T.; Xu, W. Mapping center pivot irrigation systems in global arid regions using instance segmentation and analyzing their spatial relationship with freshwater resources. Remote Sens. Environ. 2023, 297, 113760. [Google Scholar] [CrossRef]
- Goyal, H.; Mondal, P. Life cycle assessment (LCA) of the arsenic and fluoride removal from groundwater through adsorption and electrocoagulation: A comparative study. Chemosphere 2022, 304, 135243. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.; Zhang, Z.; Wu, D. Performance and mechanism of lanthanum-modified zeolite as a highly efficient adsorbent for fluoride removal from water. Chemosphere 2022, 307, 136063. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Ali, A.; Sun, Y.; Li, Y.; Yang, W.; Zhang, R. Enhanced removal of fluoride, nitrate, and calcium using self-assembled fungus-flexible fiber composite microspheres combined with microbially induced calcium precipitation. Chemosphere 2022, 302, 134848. [Google Scholar] [CrossRef] [PubMed]
- Lacson, C.F.Z.; Lu, M.-C.; Huang, Y.-H. Fluoride-rich wastewater treatment by ballast-assisted precipitation with the selection of precipitants and discarded or recovered materials as ballast. J. Environ. Chem. Eng. 2021, 9, 105713. [Google Scholar] [CrossRef]
- Huang, L.; Sheng, L.; Wan, K.; Wang, M.; Zhang, H.; Yan, J.; Liu, Y.; Alhassan, S.I.; Chen, Y.; Arulmani, S.R.B. The diversified mechanism of adsorption and electro-adsorption technologies by using Ti3AlC2 for removing fluoride. Desalination 2024, 578, 117449. [Google Scholar] [CrossRef]
- Rathnayake, A.; Hettithanthri, O.; Sandanayake, S.; Mahatantila, K.; Rajapaksha, A.U.; Vithanage, M. Essence of hydroxyapatite in defluoridation of drinking water: A review. Environ. Pollut. 2022, 311, 119882. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, R.; Samaei, M.R.; Yousefinejad, S.; Azhdarpoor, A.; Emadi, Z.; Mohammadpour, A.; Lari, A.R.; Mousavi Khaneghah, A. Simultaneous removal of fluoride and nitrate from synthetic aqueous solution and groundwater by the electrochemical process using non-coated and coated anode electrodes: A human health risk study. Environ. Res. 2022, 214, 113938. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.; Sharma, S.; Singh, N.; Kumar, D. Use of core-shell nanomaterials as potential adsorbents for fluoride remediation: Toward a sustainable ecosystem. Groundw. Sustain. Dev. 2022, 18, 100785. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Danish, M.; Shah, M. Optimization of fluoride adsorption from aqueous solution over mesoporous titania-alumina composites using Taguchi method. Water Environ. Res. 2021, 94, e1663. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Aswin Kumar, I.; Viswanathan, N.; Naushad, M. Development and characterization of hydroxyapatite layered lanthanum organic frameworks by template method for defluoridation of water. J. Colloid. Interface Sci 2022, 622, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Balouch, A.; Abdullah. Remediation of toxic fluoride from aqueous media by various techniques. Int. J. Environ. Anal. Chem. 2019, 101, 482–505. [Google Scholar] [CrossRef]
- Wong, E.Y.; Stenstrom, M.K. The usage of calcium phosphate systems for onsite defluoridation treatment. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2021, 56, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, P.; Li, F.; He, M.; Gong, A.; Zhang, W.; Mo, X.; Li, K. From MOF to Al/N-doped porous carbon: Creating multiple capture sites for efficient capacitive deionization defluorination. Desalination 2022, 543, 116090. [Google Scholar] [CrossRef]
- Chaudhary, M.; Rawat, S.; Maiti, A. Defluoridation by Bare Nanoadsorbents, Nanocomposites, and Nanoadsorbent Loaded Mixed Matrix Membranes. Sep. Purif. Rev. 2022, 52, 135–153. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hua, L.-C.; Tsai, M.-H.; Chien, T.-Y.; Huang, C. Chemisorption of fluoride onto manganese-oxide-coated activated alumina in aqueous solution. J. Hazard. Mater. Adv. 2022, 6, 100095. [Google Scholar] [CrossRef]
- Ojok, W.; Ntambi, E.; Bolender, J.; Wasswa, J.; Wanasolo, W.; Moodley, B. Synthesis and characterization of hematite biocomposite using cassava starch template for aqueous phase removal of fluoride. Carbohydr. Polym. Technol. Appl. 2022, 4, 100241. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, G.; Wang, Z.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Wang, X.; Zhang, J. Effective fluorine removal using mixed matrix membrane based on polysulfone: Adsorption performance and diffusion behavior. Water Sci. Technol. 2022, 85, 3196–3207. [Google Scholar] [CrossRef]
- Mandal, S.; Tripathy, S.; Padhi, T.; Sahu, M.K.; Patel, R.K. Removal efficiency of fluoride by novel Mg-Cr-Cl layered double hydroxide by batch process from water. J. Environ. Sci. 2013, 25, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, N.; Wei, K.; Li, S.; Shang, H.; Sun, H.; Zhang, L. Zero-valent iron coupled calcium hydroxide: A highly efficient strategy for removal and magnetic separation of concentrated fluoride from acidic wastewater. Sci. Total Environ. 2022, 838, 156336. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, M.; Iqbal, S.; Bolan, N.; Zubair, S.; Shafique, M.A.; Shah, A. Effectiveness of the engineered pinecone-derived biochar for the removal of fluoride from water. Environ. Res. 2022, 212, 113540. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Gu, Y.; Lin, H.; Jie, B.; Zhang, Q.; Zhang, X. Adsorption property of fluoride in water by metal organic framework: Optimization of the process by response surface methodology technique. Surf. Interfaces 2022, 28, 101649. [Google Scholar] [CrossRef]
- Borgohain, X.; Rashid, H. Rapid and enhanced adsorptive mitigation of groundwater fluoride by Mg(OH)2 nanoflakes. Environ. Sci. Pollut. Res. Int. 2022, 29, 70056–70069. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zhang, D.; Chen, X.; Zhao, H.; Yang, D.; Li, Y.; Bao, M.; Wang, Z. Preparation of MOF/polypyrrole and flower-like MnO2 electrodes by electrodeposition: High-performance materials for hybrid capacitive deionization defluorination. Water Res. 2023, 229, 119441. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wan, L.; Wang, H.; Zhong, C.; Min, X.; Zhang, L. Unexpected F- removal by Co2Al-LDHs: Performance and new insight. Chem. Eng. J. 2023, 452, 139400. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, X.; Ling, C.; Deng, Z.; Zhang, X. Revisiting regeneration performance and mechanism of anion exchanger-supported nano-hydrated zirconium oxides for cyclic water defluoridation. Sep. Purif. Technol. 2022, 301, 121906. [Google Scholar] [CrossRef]
- Tang, X.; Xia, W.; Qu, X.; Wang, C.; Wang, W.; Liang, Y.; Zeng, Y.; Xiong, W.; Cheng, M.; Song, B.; et al. Structure-performance correlation guided cerium-based metal-organic frameworks: Superior adsorbents for fluoride removal in water. Chemosphere 2023, 312, 137335. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lv, F.; Li, H.; Xu, L.; Wei, J.; He, Y.; Qian, J.; Gao, P. Evaluation of fluoride adsorption in solution by synthetic Al2O3 /CeO2: A fixed-bed column study. Water Environ. Res. 2021, 93, 2559–2575. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseelan, A.; Kumar, I.A.; Viswanathan, N.; Naushad, M. Rationally designed and hierarchically structured functionalized aluminium organic frameworks incorporated chitosan hybrid beads for defluoridation of water. Int. J. Biol. Macromol. 2022, 207, 941–951. [Google Scholar] [CrossRef]
- Wang, X.; Pfeiffer, H.; Wei, J.; Wang, J.; Zhang, J. Fluoride ions adsorption from water by CaCO3 enhanced Mn-Fe mixed metal oxides. Front. Chem. Sci. Eng. 2022, 17, 236–248. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Dai, Z.; Lei, Y.; Liu, X.; Liu, G. Simple preparation and efficient fluoride removal of La anchored Zr-based metal–organic framework adsorbent. J. Environ. Chem. Eng. 2022, 10, 108807. [Google Scholar] [CrossRef]
- Su, J.; Yuan, M.; Han, L.; Deng, H.; Chang, J.; Zhuang, Y.; Wang, J.; Zhang, Y. Ultrathin metal organic framework nanosheets with rich defects for enhanced fluoride removal. Chem. Eng. J. 2023, 451, 138989. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Peng, W.; Zhang, J.; Liu, J.; Wang, Y.; Wang, X. Removal of F− from water by magnetic floriform magnesium zirconium hydrotalcite-like material doped with Fe2O3 and ZrO2. Desalination 2022, 544, 116142. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Yu, T. Effectively arsenic(V) and fluoride removal in geothermal water using magnetic Fe3O4@MgO nanoparticles. Chin. Chem. Lett. 2023, 34, 107748. [Google Scholar] [CrossRef]
- Dong, C.; Wu, X.; Gao, Z.; Yang, P.; Khan, M.Y.A. A Novel and Efficient Metal Oxide Fluoride Absorbent for Drinking Water Safety and Sustainable Development. Sustainability 2021, 13, 883. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Zhou, J.; Peng, Z.; Shen, L.; Li, W. Insights of the adsorption mechanism of methylene blue on biochar from phytoextraction residues of Citrus aurantium L.: Adsorption model and DFT calculations. J. Environ. Chem. Eng. 2023, 11, 110496. [Google Scholar] [CrossRef]
- Hussain, Z.; Chang, N.; Sun, J.; Xiang, S.; Ayaz, T.; Zhang, H.; Wang, H. Modification of coal fly ash and its use as low-cost adsorbent for the removal of directive, acid and reactive dyes. J. Hazard. Mater. 2022, 422, 126778. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, F.; Zheng, X.; Liu, J. Bismuth tungstate nanosheets sensors based on Temkin adsorption model for triethylamine detection. Solid-State Electron. 2023, 213, 108850. [Google Scholar] [CrossRef]
- Ngobeni, W.A.; Mulaba-Bafubiandi, A.F. Investigating the thermodynamics and adsorption mechanisms of alkaline gelatinised novel depressants on pyrite surface. J. Mol. Liq. 2023, 384, 122173. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Panther, J.G.; Wang, Q.; Chakir, S.; Ding, Y.; Huang, Y.; Wang, H. Micro/nanostructured MgO hollow spheres with selective adsorption performance and their application for fluoride monitoring in water. Sep. Purif. Technol. 2022, 299, 121703. [Google Scholar] [CrossRef]
- Panda, B.; Mondal, D.; Mandal, S.; Khatun, J.; Mukherjee, A.; Dhak, D. One-pot solution combustion synthesis of porous spherical-shaped magnesium zinc binary oxide for efficient fluoride removal and photocatalytic degradation of methylene blue and Congo red dye. Environ. Sci. Pollut. Res. Int. 2023, 30, 81386–81402. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, K.; Yan, J.; Li, Q.; Guo, Y.; Huang, L.; Arulmani, S.R.B.; Luo, J. The function of doping nitrogen on removing fluoride with decomposing La-MOF-NH2: Density functional theory calculation and experiments. J. Environ. Sci. 2024, 135, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shi, F.; Jiang, W.; Chen, Y.; Zhang, K.; Jian, S.; Jiang, S.; Zhang, C.; Hu, J. Outstanding fluoride removal from aqueous solution by a La-based adsorbent. RSC Adv. 2022, 12, 30522–30528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ou, H.; Liu, H.; Ke, Y.; Zhang, W.; Liao, G.; Wang, D. Polyimide-based carbon nanofibers: A versatile adsorbent for highly efficient removals of chlorophenols, dyes and antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 92–101. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panigrahi, G.K.; Dhal, J.P.; Sahoo, J.K.; Behera, A.K.; Panda, P.C.; Patel, P.; Mund, S.K.; Muduli, S.M.; Panda, L. Co-axial electrospun hollow MgO nanofibers for efficient removal of fluoride ions from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129877. [Google Scholar] [CrossRef]
- Yi, M.; Wang, K.; Wei, H.; Wei, D.; Wei, X.; Wei, B.; Shao, L.; Fujita, T.; Cui, X. Efficient preparation of red mud-based geopolymer microspheres (RM@GMs) and adsorption of fluoride ions in wastewater. J. Hazard. Mater. 2023, 442, 130027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Y.; Yao, Q.; Zhang, F.; Chen, W.; Liu, Y. Comparison of fluorine removal performance and mechanism of spheroidal magnesium oxide before and after lanthanum modification. Environ. Sci. Pollut. Res. Int. 2022, 29, 80477–80490. [Google Scholar] [CrossRef]
- Ren, C.; Wang, H.; Ji, J.; Li, W. Molecular scale assessment of defluoridation of coal-mining wastewater by calcined Mg/Al layered double hydroxide using 19F solid-state NMR, XPS, and HRTEM. Chemosphere 2022, 303, 135072. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, B.; Ma, B.; Cao, Z.; Shao, S.; Liu, Y.; Li, X.; Wang, C.; Yang, W. Removal of fluoride from waste acid using lanthanum chloride: Defluoridation behavior and reaction kinetics of recovery process. Process Saf. Environ. Prot. 2022, 167, 322–331. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Xiao, Y.; Huang, L.; Li, J.; Yan, J.; Li, Q.; Li, M.; Zhang, H. The Constructing of the Oxide Phase Diagram for Fluoride Adsorption on La-Fe-Al: A Collaborative Study of Density Functional Calculation and Experimentation. Nanomaterials 2024, 14, 619. https://doi.org/10.3390/nano14070619
Xie S, Xiao Y, Huang L, Li J, Yan J, Li Q, Li M, Zhang H. The Constructing of the Oxide Phase Diagram for Fluoride Adsorption on La-Fe-Al: A Collaborative Study of Density Functional Calculation and Experimentation. Nanomaterials. 2024; 14(7):619. https://doi.org/10.3390/nano14070619
Chicago/Turabian StyleXie, Shaojian, Yao Xiao, Lei Huang, Jiaxin Li, Jia Yan, Qian Li, Meng Li, and Hongguo Zhang. 2024. "The Constructing of the Oxide Phase Diagram for Fluoride Adsorption on La-Fe-Al: A Collaborative Study of Density Functional Calculation and Experimentation" Nanomaterials 14, no. 7: 619. https://doi.org/10.3390/nano14070619
APA StyleXie, S., Xiao, Y., Huang, L., Li, J., Yan, J., Li, Q., Li, M., & Zhang, H. (2024). The Constructing of the Oxide Phase Diagram for Fluoride Adsorption on La-Fe-Al: A Collaborative Study of Density Functional Calculation and Experimentation. Nanomaterials, 14(7), 619. https://doi.org/10.3390/nano14070619







