Photocatalytic Activity of Ag Nanoparticles Deposited on Thermoexfoliated g-C3N4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Photocatalytic Test
2.4. Scavenger Testing
3. Results and Discussion
3.1. Characterization Results
3.2. Photocatalytic Activity Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganesan, S.; Kokulnathan, T.; Sumathi, S.; Palaniappan, A. Efficient Photocatalytic Degradation of Textile Dye Pollutants Using Thermally Exfoliated Graphitic Carbon Nitride (TE– g-C3N4). Sci. Rep. 2024, 14, 2284. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qiao, S.; Li, X.; Li, Q. Synthesis and Photocatalytic Performance of g-C3N4/MeTMC-COP Composite Photocatalyst. Front. Chem. 2023, 11, 1138789. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Khan, A.; Marwat, M.A.; Bououdina, M.; Ali, S.; Rahman, A.U.; Ali, F.; Wang, C. Au Decorated Chemically Exfoliated g-C3N4 as Highly Efficient Visible Light Catalyst for Hydrogen Production. J. Photochem. Photobiol. A 2024, 450, 115472. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Luque, R.; Fernández-García, M.; Kubacka, A. g-C3N4/TiO2 Composite Catalysts for the Photo-Oxidation of Toluene: Chemical and Charge Handling Effects. Chem. Eng. J. 2019, 378, 122228. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, T.; Zhang, L.; Zhu, J.; Wang, X. Ag/gC3N4 Catalyst with Superior Catalytic Performance for the Degradation of Dyes: A Borohydride-Generated Superoxide Radical Approach. Nanoscale 2015, 7, 13723–13733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, Characterization and High Photocatalytic Activity of Ag-ZnO Heterojunctions under UV-Visible Light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef] [PubMed]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Kubacka, A.; Zárate-Medina, J.; Cortés, J.A.; Fernández-García, M. Measuring and Interpreting Quantum Efficiency of Acid Blue 9 Photodegradation Using TiO2-Based Catalysts. Appl. Catal. A Gen. 2018, 550, 38–47. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 Photocatalysts with Visible-Light Photocatalytic Activity for Sulfamethoxazole Degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Tikhanova, S.M.; Nevedomskiy, V.N.; Popkov, V.I. Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics 2022, 10, 249. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yin, Z.; Barber, J.; Boey, F.Y.C.; Loo, S.C.J.; Xue, C. Preparation of Au-BiVO4 Heterogeneous Nanostructures as Highly Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 418–423. [Google Scholar] [CrossRef]
- Xu, J.; Long, K.-Z.; Wang, Y.; Xue, B.; Li, Y.-X. Fast and Facile Preparation of Metal-Doped g-C3N4 Composites for Catalytic Synthesis of Dimethyl Carbonate. Appl. Catal. A Gen. 2015, 496, 1–8. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic Materials: Recent Achievements and near Future Trends. J. Mater. Chem. A Mater. 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Ares-Dorado, A.; Alonso-Nuñez, G.; Tudela, D.; Fernández-García, M.; Kubacka, A. Role of Alkali-Cyano Group Interaction in g-C3N4 Based Catalysts for Hydrogen Photo-Production. Catal. Today 2021, 394, 25–33. [Google Scholar] [CrossRef]
- Du, Z.; Guo, R.; Lan, J.; Jiang, S.; Cheng, C.; Zhao, L.; Peng, L. Preparation and Photocatalytic Activity of Bismuth Tungstate Coated Polyester Fabric. Fiber Polym. 2017, 18, 2212–2218. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Barba-Nieto, I.; Gómez-Cerezo, M.N.; Rodríguez-Castellón, E.; Fernández-García, M.; Kubacka, A. Pt/Bg-C3N4 Catalysts for Hydrogen Photo-Production: Activity Interpretation through a Spectroscopic and Intrinsic Kinetic Analysis. J. Environ. Chem. Eng. 2021, 9, 106073. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. Rationally Designed 1D Ag@ AgVO3 Nanowire/Graphene/Protonated gC3N4 Nanosheet Heterojunctions for Enhanced Photocatalysis via Electrostatic Self-Assembly and Photochemical Reduction Methods. J. Mater. Chem. A 2015, 3, 10119–10126. [Google Scholar] [CrossRef]
- Portillo-Cortez, K.; Romero-Ibarra, J.E.; Dominguez, D.; Alonso-Nuñez, G.; Caudillo-Flores, U. Photodegradation of Ceftriaxone Using g-C3N4-ZnO Nanocomposite as an Efficient Photocatalyst. J. Photochem. Photobiol. A 2023, 445, 115090. [Google Scholar] [CrossRef]
- Yan, S.C.; Lv, S.B.; Li, Z.S.; Zou, Z.G. Organic-Inorganic Composite Photocatalyst of gC3N4 and TaON with Improved Visible Light Photocatalytic Activities. Dalton Trans. 2010, 39, 1488–1491. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Omarov, S.O.; Lobinsky, A.A.; Nevedomskiy, V.N.; Popkov, V.I. Steam Exfoliation of Graphitic Carbon Nitride as Efficient Route toward Metal-Free Electrode Materials for Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 27671–27678. [Google Scholar] [CrossRef]
- Thorat, N.; Borade, S.; Varma, R.; Yadav, A.; Gupta, S.; Fernandes, R.; Sarawade, P.; Bhanage, B.M.; Patel, N. High Surface Area Nanoflakes of P- gC3N4 Photocatalyst Loaded with Ag Nanoparticle with Intraplanar and Interplanar Charge Separation for Environmental Remediation. J. Photochem. Photobiol. A 2021, 408, 113098. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Zhang, J.; Wei, H.; Dang, L. Integrating g-C3N4 Nanosheets with MOF-Derived Porous CoFe2O4 to Form an S-Scheme Heterojunction for Efficient Pollutant Degradation via the Synergy of Photocatalysis and Peroxymonosulfate Activation. Environ. Res. 2024, 241, 117653. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Gupta, S.; Bhagat, B.R.; Yadav, M.; Dashora, A.; Varma, R.S.; Thorat, N.; Patel, R.; Patel, N. Unraveling the Synergy between Oxygen Doping and Embedding Fe Nanoparticles in gC3N4 towards Enhanced Photocatalytic Rates. Appl. Surf. Sci. 2022, 603, 154404. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Liu, R.; Huang, Y.; Liang, Y.; Hu, C.; Wang, M.; Liu, Z.; Tai, Y.; Liu, J. Synergetic Effect of the Interface Electric Field and the Plasmon Electromagnetic Field in Au-Ag Alloy Mediated Z-Type Heterostructure for Photocatalytic Hydrogen Production and CO2 Reduction. Appl. Catal. B Environ. 2023, 331, 122700. [Google Scholar] [CrossRef]
- Hakimi-Tehrani, M.J.; Hasanzadeh-Tabrizi, S.A.; Koupaei, N.; Saffar, A.; Rafiei, M. Synthesis of g-C3N4/ZnO/WO3 Nanocomposite as a Highly Efficient Antibacterial Adsorbent for Water Treatment. Diam. Relat. Mater. 2022, 130, 109506. [Google Scholar] [CrossRef]
- Abbas, K.K.; AbdulkadhimAl-Ghaban, A.M.H.; Rdewi, E.H. Synthesis of a Novel ZnO/TiO2-Nanorod MXene Heterostructured Nanophotocatalyst for the Removal Pharmaceutical Ceftriaxone Sodium from Aqueous Solution under Simulated Sunlight. J. Environ. Chem. Eng. 2022, 10, 108111. [Google Scholar] [CrossRef]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharma, A. ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasichenko, D.B.; Berdyugin, S.N.; Gerasimov, E.Y.; Saraev, A.A.; Cherepanova, S.V.; Kozlova, E.A. Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light. Nanomaterials 2023, 13, 2176. [Google Scholar] [CrossRef]
- Vijayarangan, R.; Mohan, S.; Selvaraj, M.; Assiri, M.A.; Ilangovan, R. Surface-Engineered Anisotropic g-C3N4 Photocatalysts via Green-Exfoliation for Visible Light-Driven Water Remediation. Surf. Interfaces 2024, 44, 103782. [Google Scholar] [CrossRef]
- Jiao, J.; Wan, J.; Ma, Y.; Wang, Y. Facile Formation of Silver Nanoparticles as Plasmonic Photocatalysts for Hydrogen Production. RSC Adv. 2016, 6, 106031–106034. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and Interpreting Quantum Efficiency for Hydrogen Photo-Production Using Pt-Titania Catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Topchiyan, P.; Vasilchenko, D.; Tkachev, S.; Sheven, D.; Eltsov, I.; Asanov, I.; Sidorenko, N.; Saraev, A.; Gerasimov, E.; Kurenkova, A. Highly Active Visible Light-Promoted Ir/g-C3N4 Photocatalysts for the Water Oxidation Reaction Prepared from a Halogen-Free Iridium Precursor. ACS Appl. Mater. Interfaces 2022, 14, 35600–35612. [Google Scholar] [CrossRef]
- Olesik, J.W. Inductively Coupled Plasma Mass Spectrometers. In Treatise on Geochemistry, 2nd ed.; Elsevier Inc.: New York, NY, USA, 2013; Volume 15, pp. 309–336. ISBN 9780080983004. [Google Scholar]
- Brenner, I.J. Inductively Coupled Plasma Mass Spectrometry Applications. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: New York, NY, USA, 2016; pp. 229–235. ISBN 9780128032244. [Google Scholar]
- Wu, K.; Wu, P.; Zhu, J.; Liu, C.; Dong, X.; Wu, J.; Meng, G.; Xu, K.; Hou, J.; Liu, Z. Synthesis of Hollow Core-Shell CdS@ TiO2/Ni2P Photocatalyst for Enhancing Hydrogen Evolution and Degradation of MB. Chem. Eng. J. 2019, 360, 221–230. [Google Scholar] [CrossRef]
- Ismael, M. The Photocatalytic Performance of the ZnO/g-C3N4 Composite Photocatalyst toward Degradation of Organic Pollutants and Its Inactivity toward Hydrogen Evolution: The Influence of Light Irradiation and Charge Transfer. Chem. Phys. Lett. 2020, 739, 136992. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Q.; Xia, Y.; Wang, J.; Chen, H.; Xu, Q.; Liu, J.; Feng, W.; Chen, S. Preparation and Characterization of Cu-Doped TiO2 Nanomaterials with Anatase/Rutile/Brookite Triphasic Structure and Their Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Wu, P.; Yelemulati, H.; Zhu, S.; Peng, B.; Li, H.; Ye, H.; Hou, J.; Wu, K.; Liu, Z. Amorphous-Crystalline Junction Oxygen Vacancy-Enriched CoPi/ZnIn2S4/AB-TiO2 Catalyst for Oxygen Evolution Reaction. Chem. Eng. J. 2023, 466, 143252. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Hang, H.; Zhang, D.; Zhang, N.; Xiao, Z.; Tao, H. Self-Assembly Method Assisted Synthesis of g-C3N4/ZnO Heterostructure Nanocomposites with Enhanced Photocatalytic Performance. Opt. Mater. 2019, 96, 109266. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Ansari, F.; Bachiller-Baeza, B.; Colón, G.; Fernández-García, M.; Kubacka, A. (NH4)4[NiMo6O24H6]. 5H2O/g-C3N4 Materials for Selective Photo-Oxidation of CO and C=C Bonds. Appl. Catal. B Environ. 2020, 278, 119299. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of Cobalt Ferrite and Graphene: High-Performance and Recyclable Visible-Light Photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Zhao, Z.; Liu, J.; Luo, X.; Yuan, X.; Luo, K.; He, J.; Yu, D.; Zhao, Y. From Triazine to Heptazine: Origin of Graphitic Carbon Nitride as a Photocatalyst. ACS Omega 2020, 5, 12557–12567. [Google Scholar] [CrossRef]
- Jin, J.; Liang, Q.; Ding, C.; Li, Z.; Xu, S. Simultaneous Synthesis-Immobilization of Ag Nanoparticles Functionalized 2D g-C3N4 Nanosheets with Improved Photocatalytic Activity. J. Alloys Compd. 2017, 691, 763–771. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag Modified g-C3N4 Composites with Enhanced Visible-Light Photocatalytic Activity for Diclofenac Degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Liu, R.; Yang, W.; He, G.; Zheng, W.; Li, M.; Tao, W.; Tian, M. Ag-Modified g-C3N4 Prepared by a One-Step Calcination Method for Enhanced Catalytic Efficiency and Stability. ACS Omega 2020, 5, 19615–19624. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally Sustainable Fabrication of Ag@g-C3N4 Nanostructures and Their Multifunctional Efficacy as Antibacterial Agents and Photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Pandurangan, M.; Vattikuti, S.V.P.; Tettey, C.O.; Sreekanth, T.V.M.; Shim, J. Enhanced Photocatalytic Activity of Ag/g-C3N4 Composite. Sep. Purif. Technol. 2017, 188, 228–237. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Liu, F.; Yuan, X.; Guo, Y.; Zhang, S.; Guo, W.; Huo, M. Preparation and Enhanced Visible-Light Photocatalytic Activity of Silver Deposited Graphitic Carbon Nitride Plasmonic Photocatalyst. Appl. Catal. B Environ. 2013, 142, 828–837. [Google Scholar] [CrossRef]

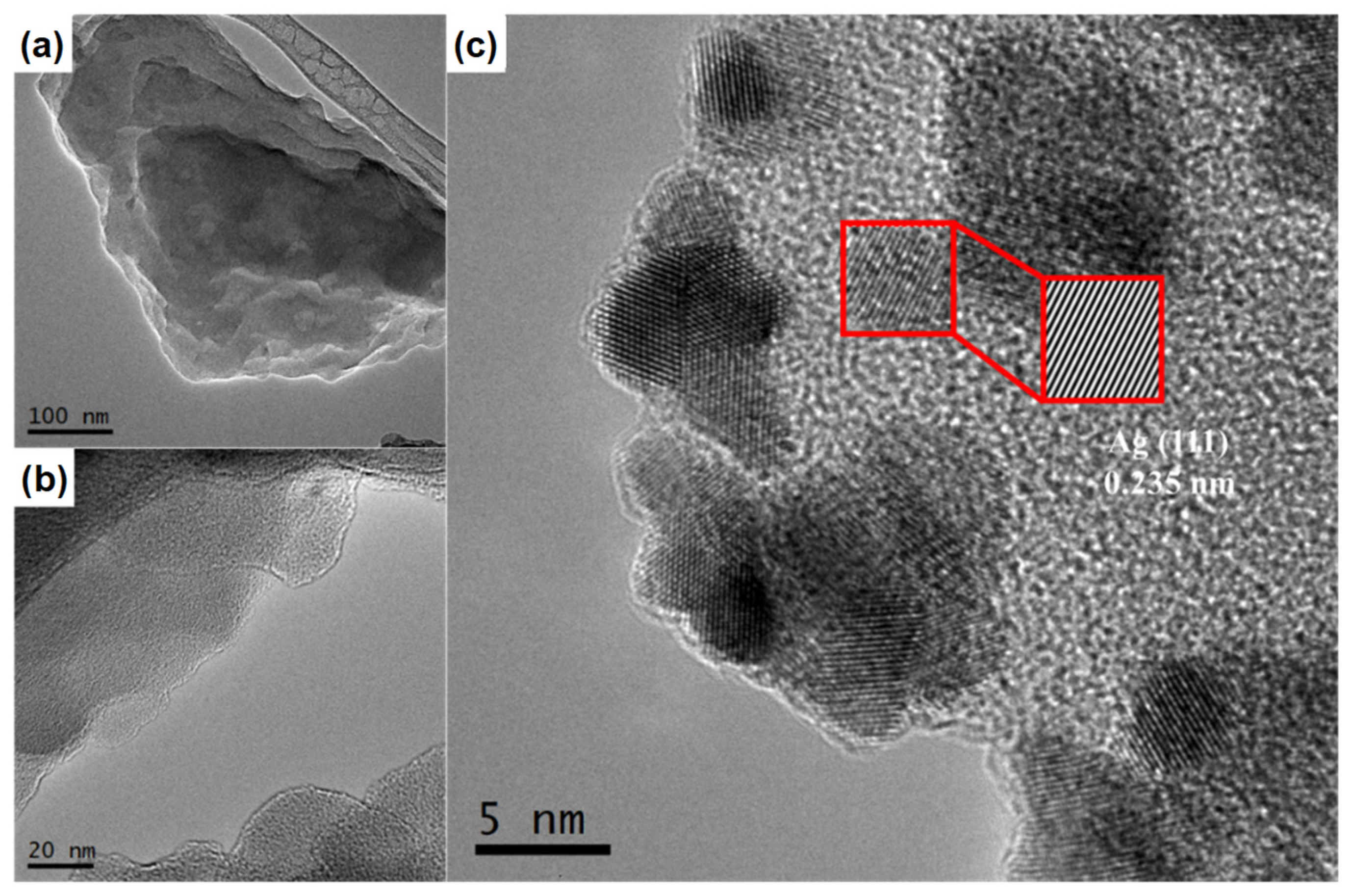
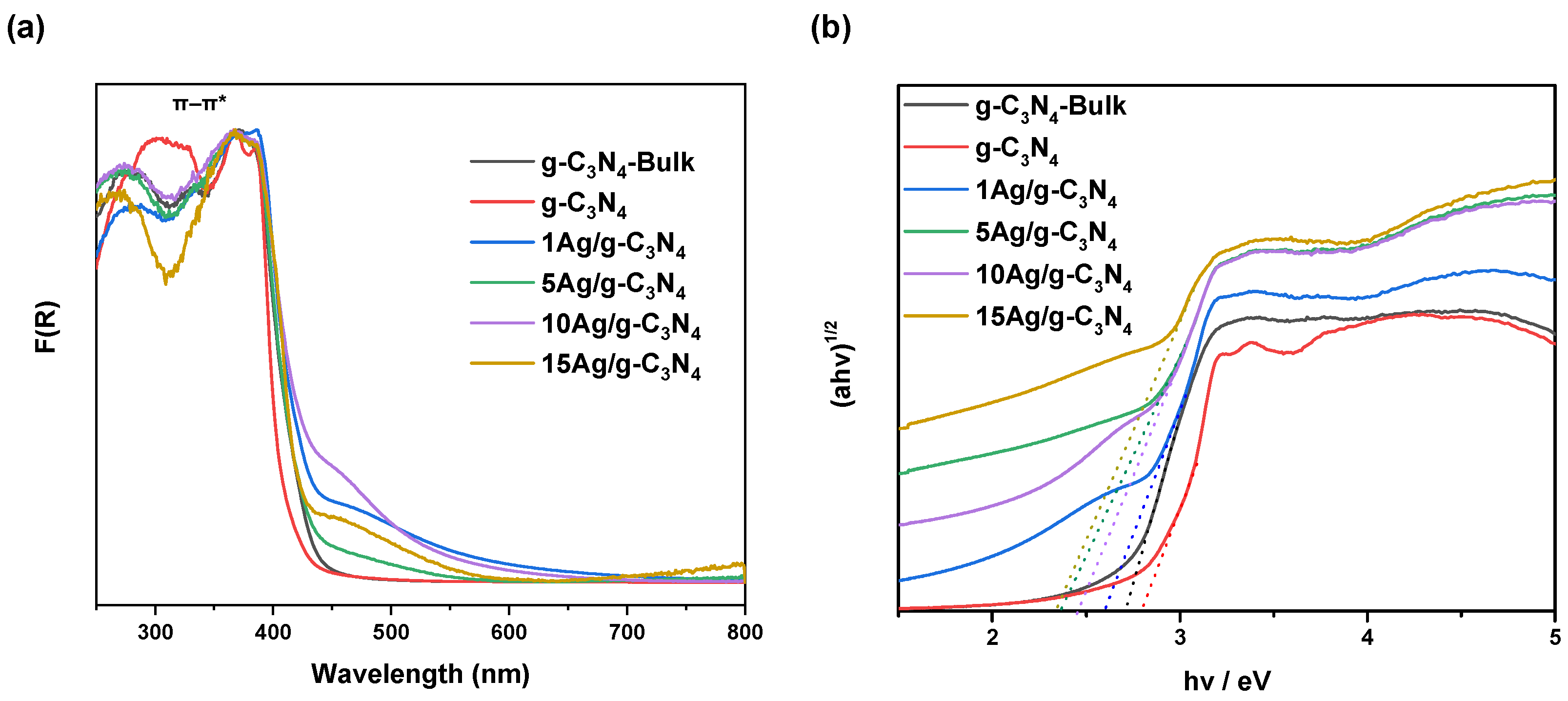
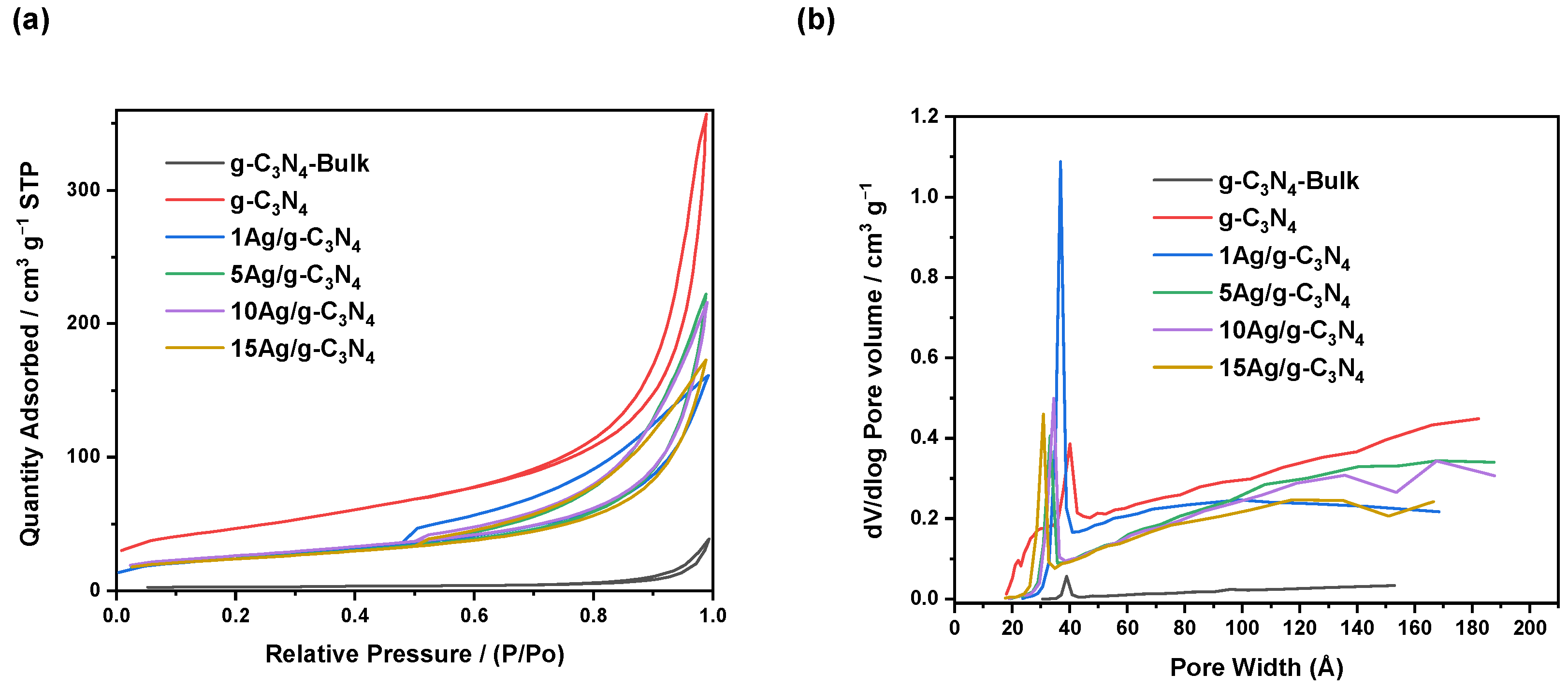
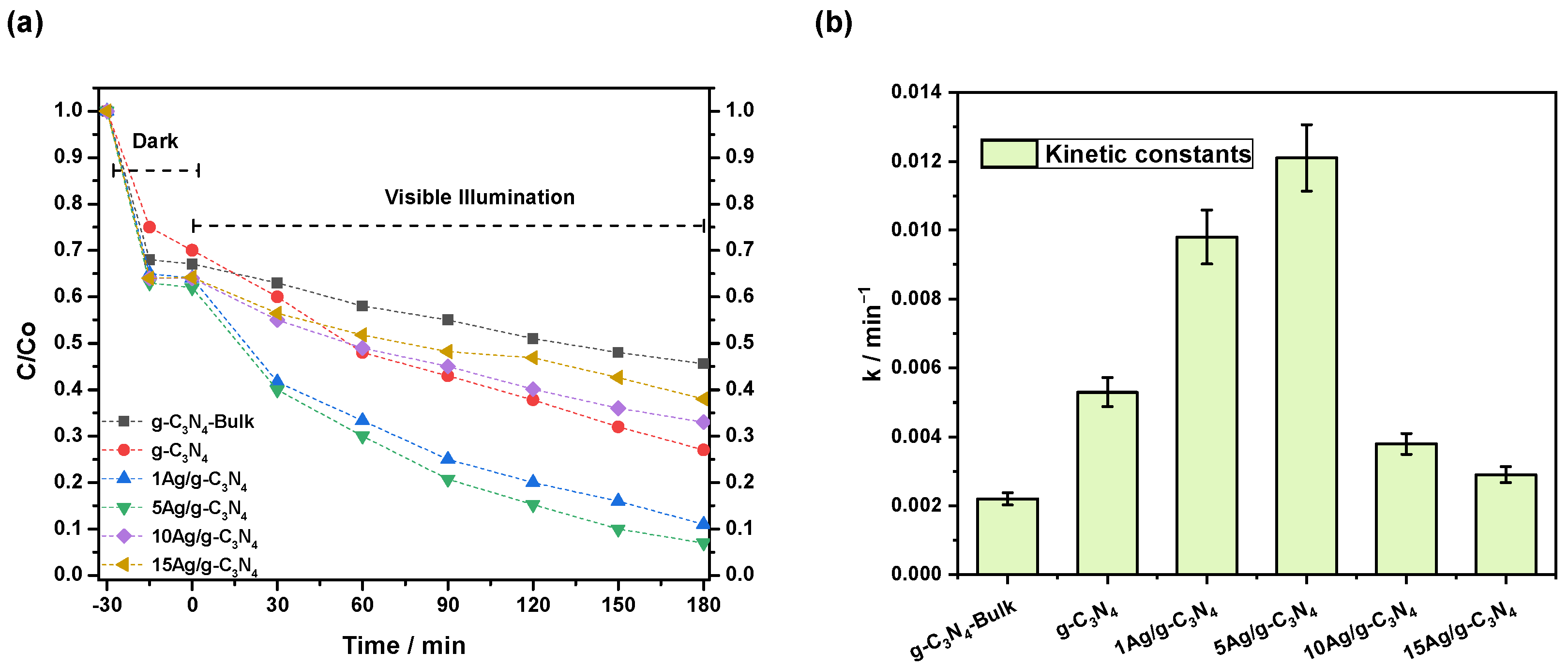
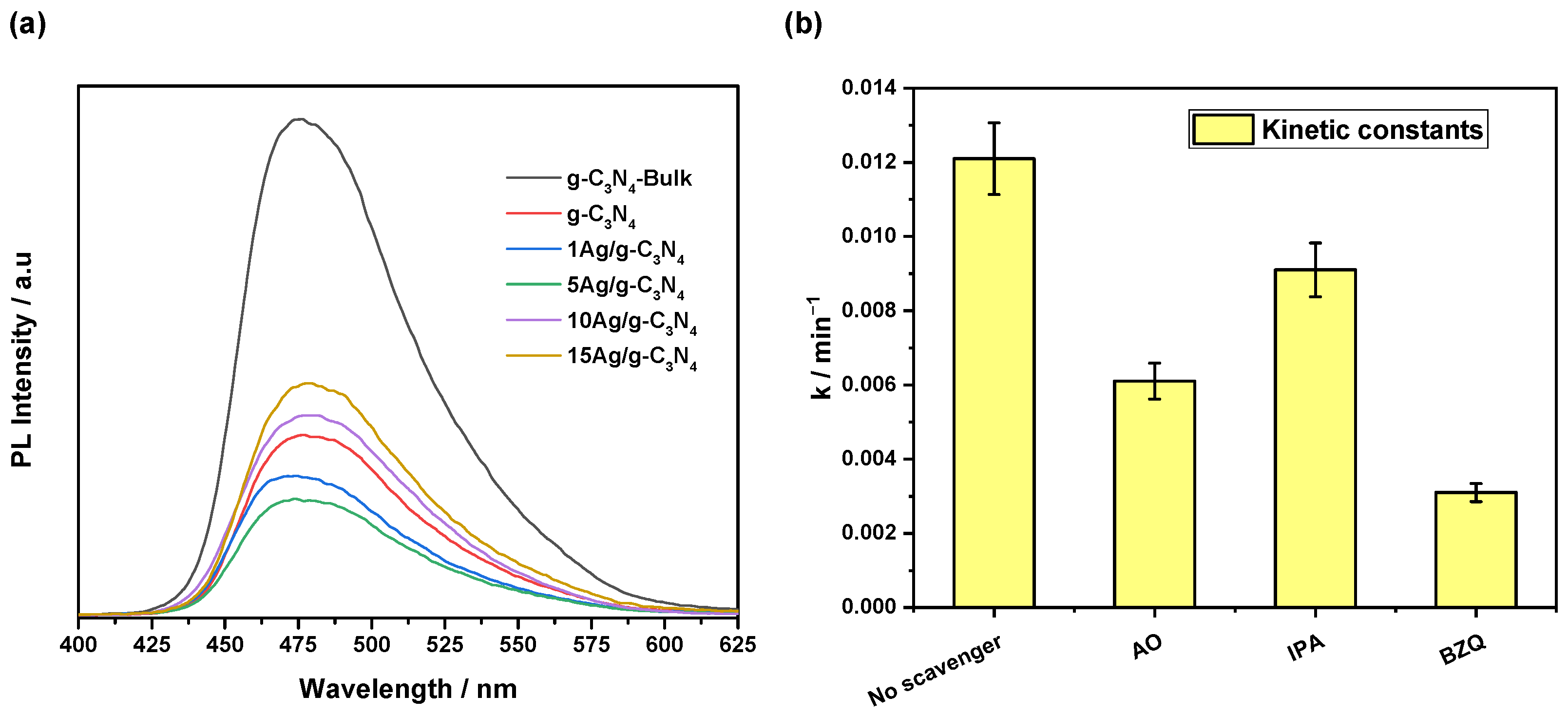
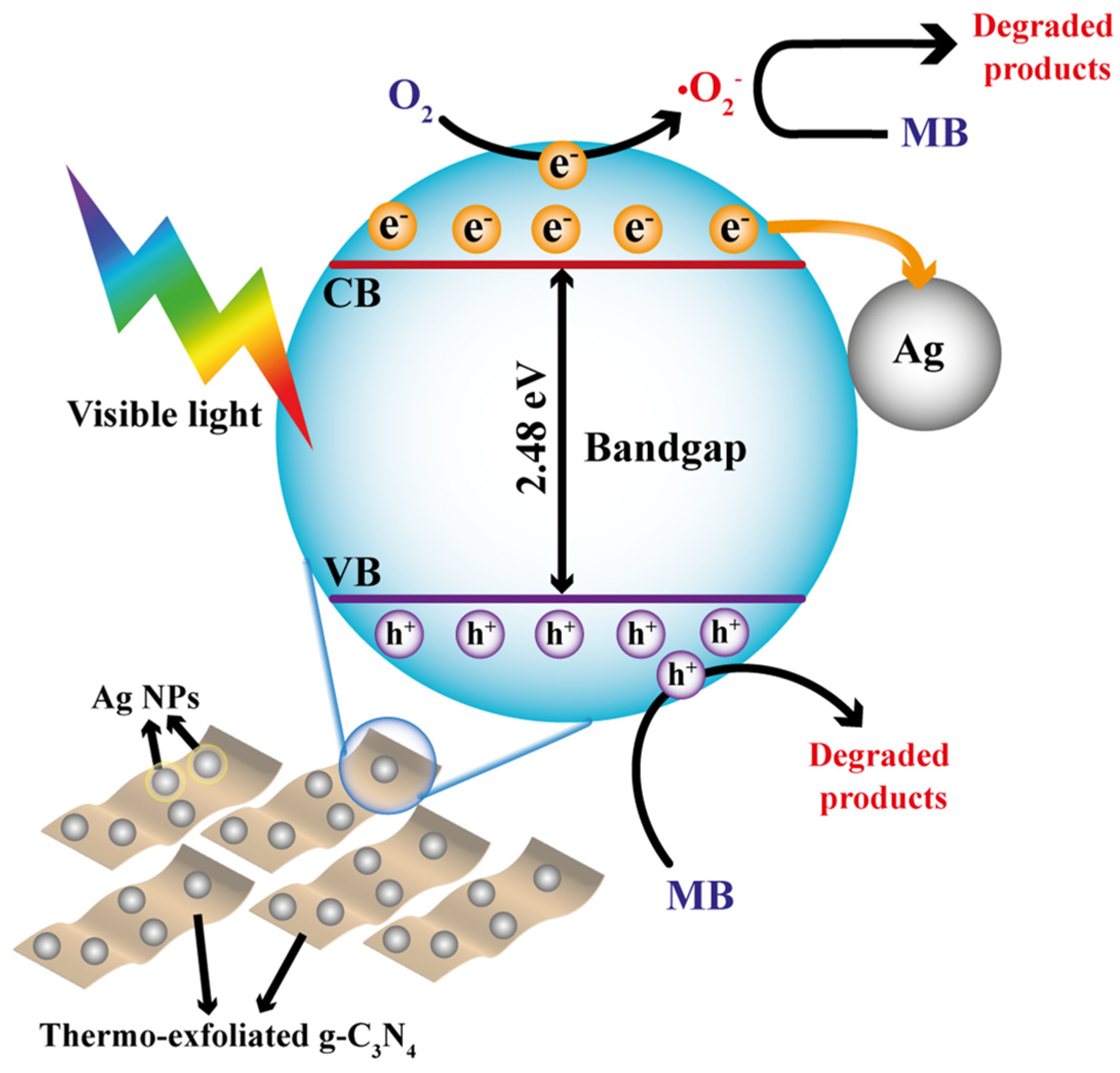
| Catalyst | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | Bandgap (eV) |
|---|---|---|---|---|
| g-C3N4-Bulk | 9.2 | 0.013 | 6.9 | 2.74 |
| g-C3N4 | 143.8 | 0.257 | 5.2 | 2.78 |
| 1Ag/g-C3N4 | 85.9 | 0.189 | 5.6 | 2.63 |
| 5Ag/g-C3N4 | 88.9 | 0.186 | 6.3 | 2.48 |
| 10Ag/g-C3N4 | 89.9 | 0.180 | 5.9 | 2.43 |
| 15Ag/g-C3N4 | 87.8 | 0.179 | 6.1 | 2.36 |
| Sample | Methodology | Surface Area [m2 g−1] | Application | Light Source | Ref. |
|---|---|---|---|---|---|
| Ag-Modified g-C3N4 | One-step calcination at 550 °C for 8 h) | 31.5 | MO degradation (98.7% in 2 h) | Visible (300 W Xe lamp with a 420 nm filter) | [45] |
| Ag@g-C3N4 | Single-step process at 550 °C for 4 h | 38.1 | MB (100%, 210 min) and RhB degradation (~89%, 250 min) | Visible (3M, 400 W, λ > 500 nm) | [46] |
| Ag/g-C3N4 composite | Thermal treatment at 550 °C for 4 h | 7.3 | MG degradation (80%, 100 min) | UV (40 W, Philips) | [47] |
| Ag/g-C3N4 | Thermal polymerization at 250 °C for 1 h, 350 °C for 2 h, and 550 °C for 2 h | 66.0 | MO degradation (92%, 120 min) | Visible (Xe lamp, 300 W with UV and IR filters) | [48] |
| Ag/g-C3N4 | Thermal exfoliation at 500° for 2 h, and 500 °C for 2 h | 90.0 | MB degradation (94%, 180 min) | Visible (8 W, Tecnolite) | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portillo-Cortez, K.; Caudillo-Flores, U.; Sánchez-López, P.; Smolentseva, E.; Dominguez, D.; Fuentes-Moyado, S. Photocatalytic Activity of Ag Nanoparticles Deposited on Thermoexfoliated g-C3N4. Nanomaterials 2024, 14, 623. https://doi.org/10.3390/nano14070623
Portillo-Cortez K, Caudillo-Flores U, Sánchez-López P, Smolentseva E, Dominguez D, Fuentes-Moyado S. Photocatalytic Activity of Ag Nanoparticles Deposited on Thermoexfoliated g-C3N4. Nanomaterials. 2024; 14(7):623. https://doi.org/10.3390/nano14070623
Chicago/Turabian StylePortillo-Cortez, Karina, Uriel Caudillo-Flores, Perla Sánchez-López, Elena Smolentseva, David Dominguez, and Sergio Fuentes-Moyado. 2024. "Photocatalytic Activity of Ag Nanoparticles Deposited on Thermoexfoliated g-C3N4" Nanomaterials 14, no. 7: 623. https://doi.org/10.3390/nano14070623
APA StylePortillo-Cortez, K., Caudillo-Flores, U., Sánchez-López, P., Smolentseva, E., Dominguez, D., & Fuentes-Moyado, S. (2024). Photocatalytic Activity of Ag Nanoparticles Deposited on Thermoexfoliated g-C3N4. Nanomaterials, 14(7), 623. https://doi.org/10.3390/nano14070623





