Nano-Structure Evolution and Mechanical Properties of AlxCoCrFeNi2.1 (x = 0, 0.3, 0.7, 1.0, 1.3) High-Entropy Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
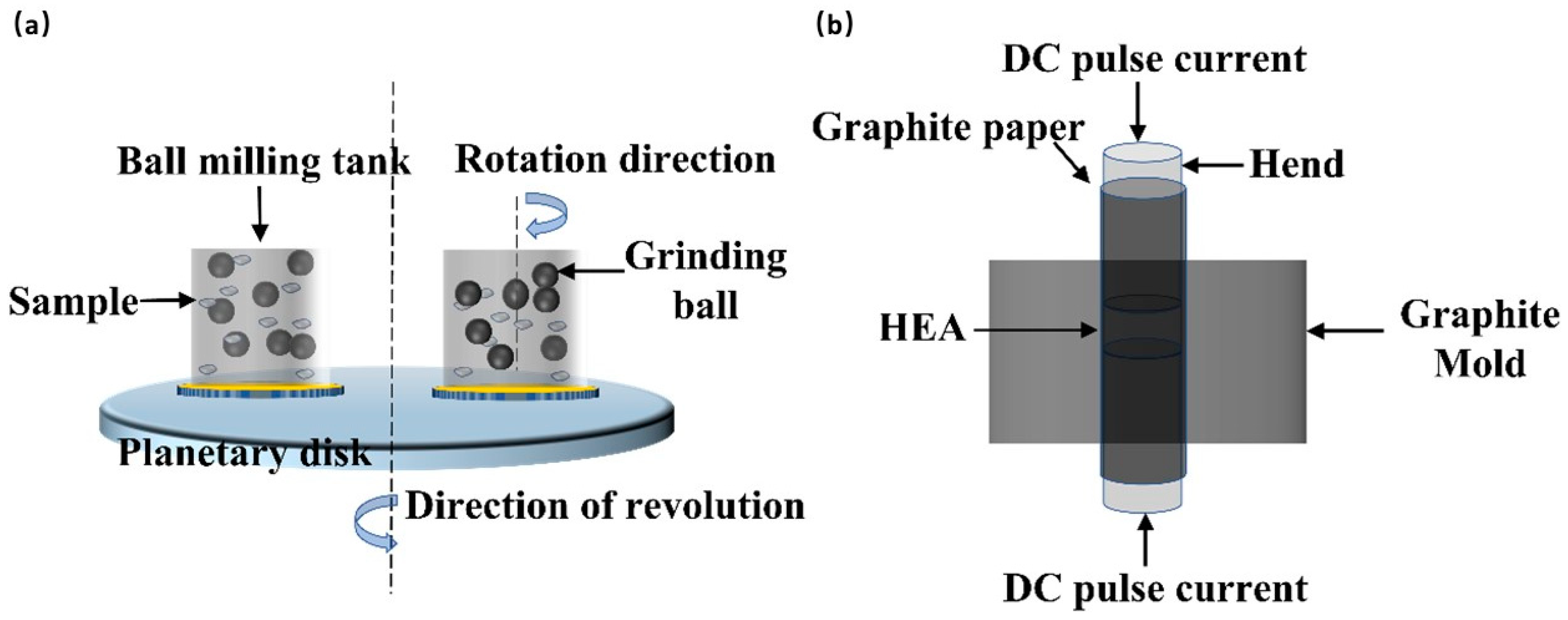
2. Experimental Details
3. Results and Discussion
3.1. Criterion of Solid Solutions of HEAs
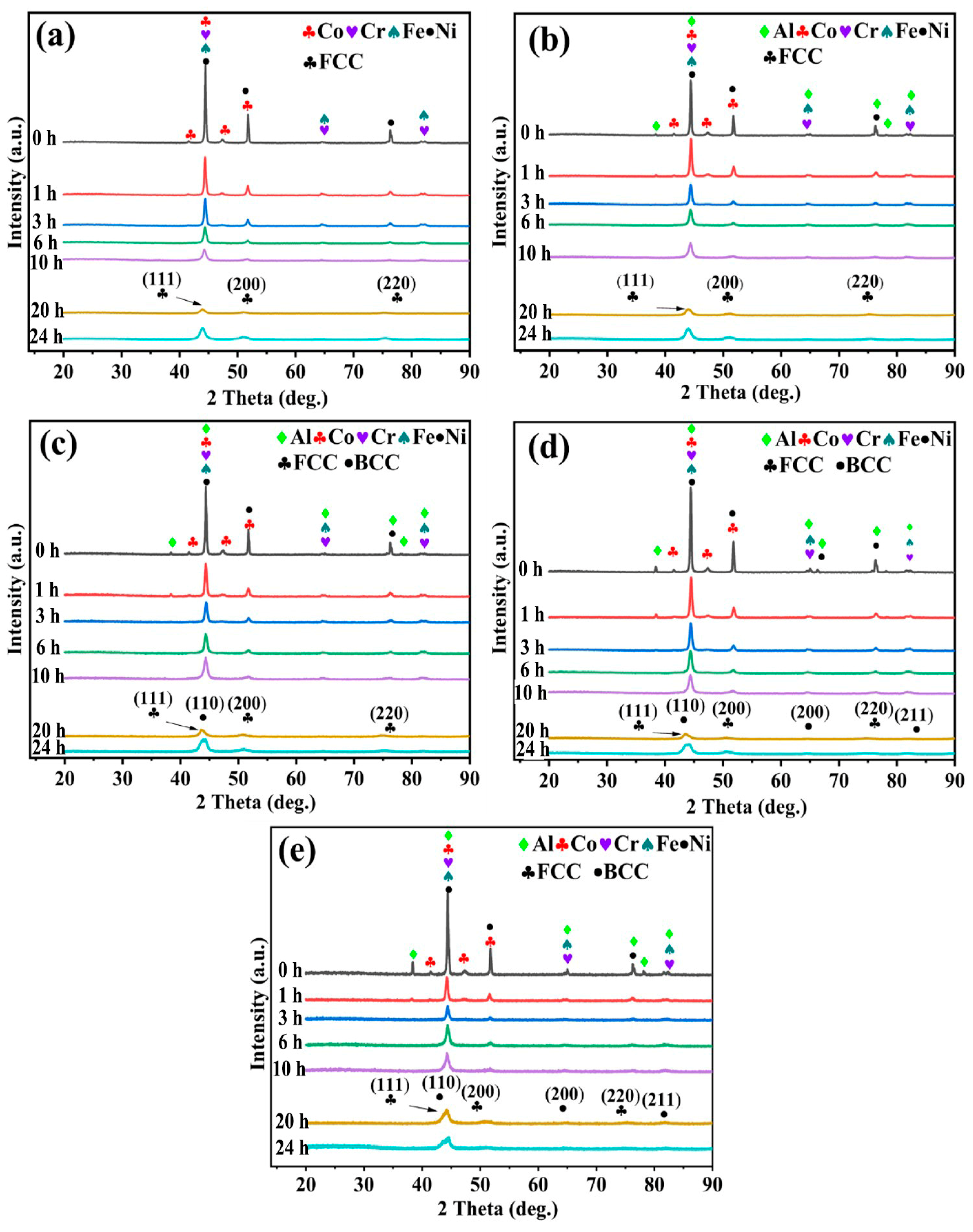
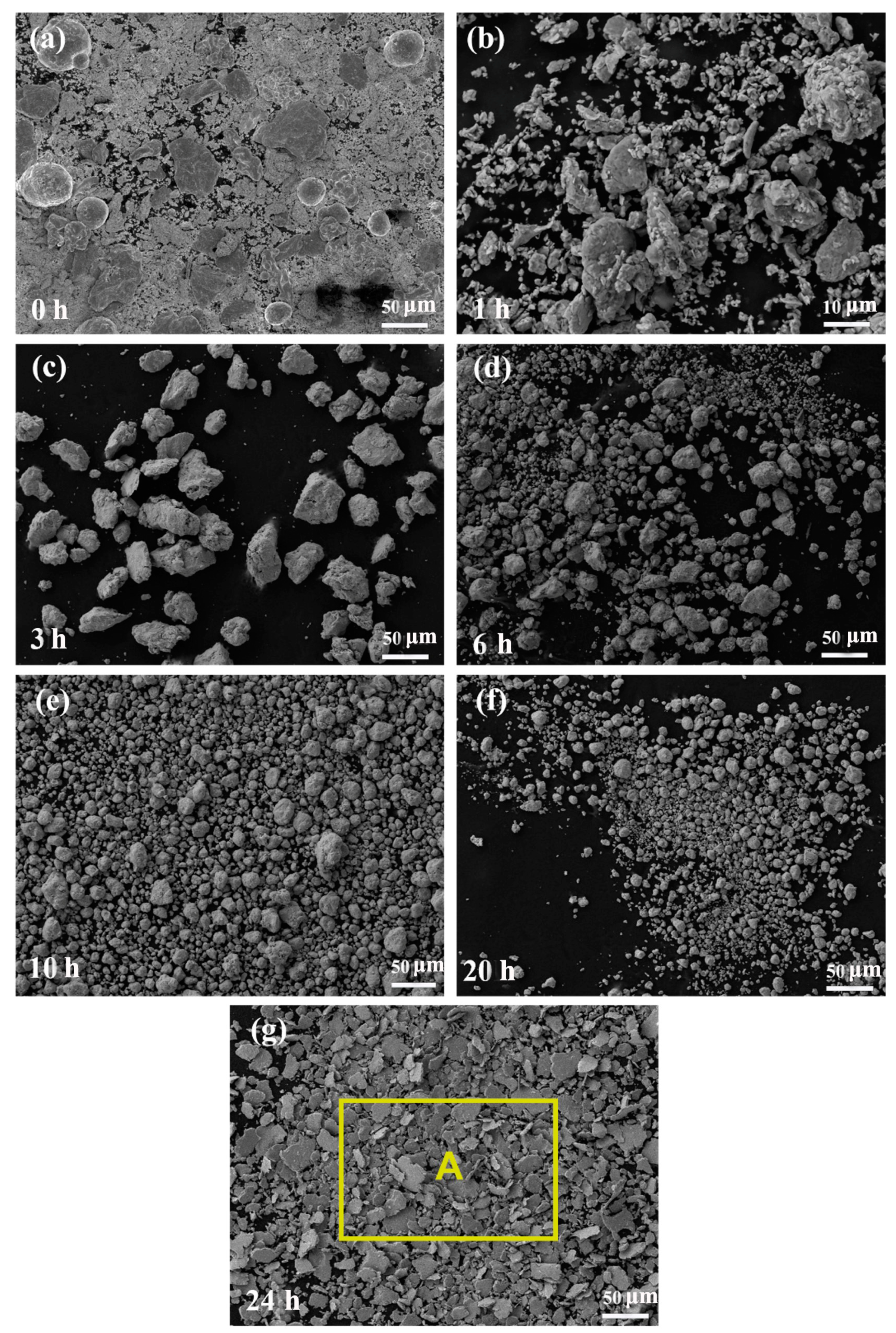
3.2. The Grain Is Refined by Ball Milling
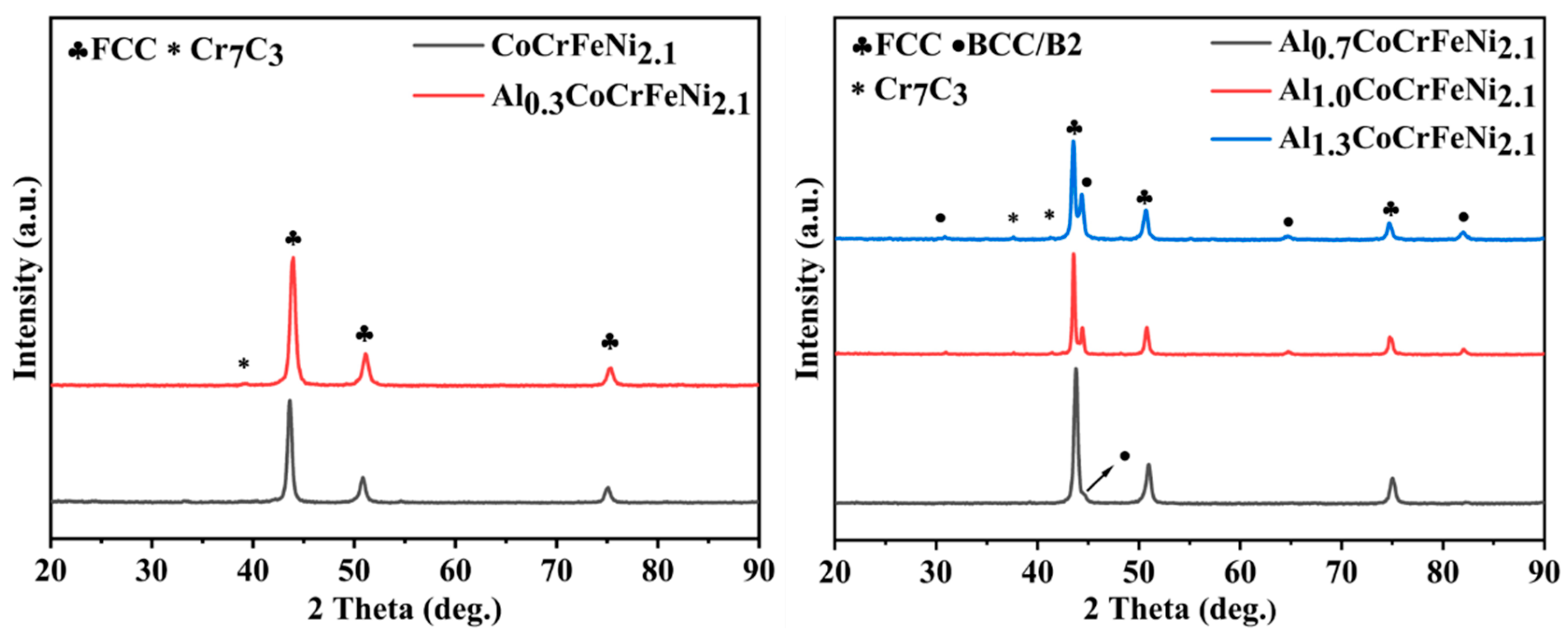
3.3. SPS Synthesis of Biphasic High-Entropy Alloys
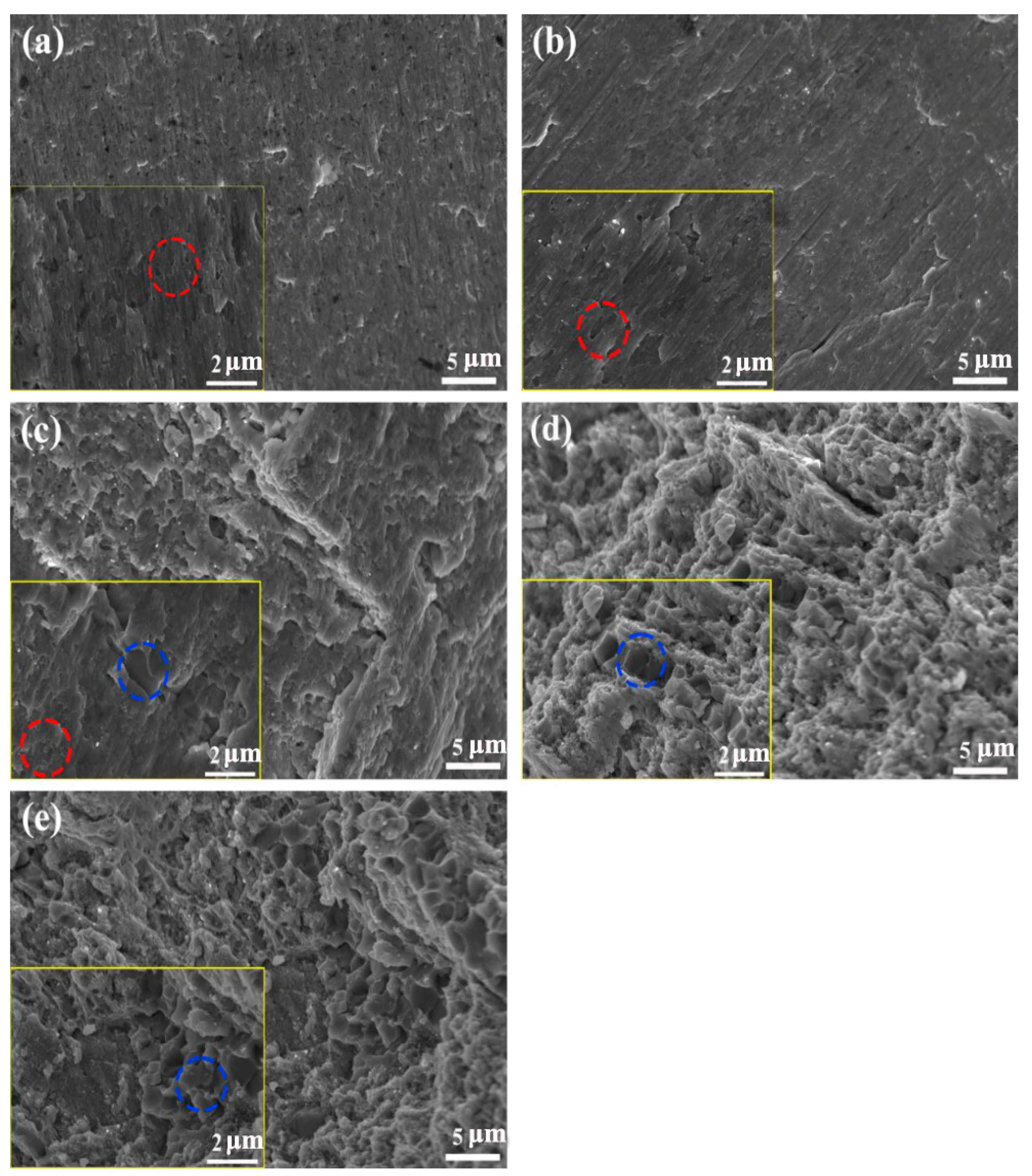
3.4. Compression Properties and Fracture Morphology of Alloys
4. Conclusions
- For the first time, the effect of Al content on AlCoCrFeNi2.1 HEAs was investigated by the combined MA-SPS technique. When Al = 0.0, 0.3, the alloy is composed of FCC nano phase, and when Al = 0.7, 1.0, 1.3, it consists of FCC-CoCrFe and BCC-AlNi nano phases.
- In terms of microstructure, Al = 0.0 and Al = 0.3 alloys exhibit a light-colored FCC nanocrystalline structure. The microstructure of Al = 0.7 shows that the light-colored FCC phase is the matrix, and the brown BCC phase appears in an irregular form. Al = 1.0 microstructure shows that BCC phases are point-like and clustered in an irregular shape and FCC phases are cross-distributed. In the microstructure of the Al = 1.3 alloy, the BCC nano phase is irregular and densely distributed in the FCC nano phase.
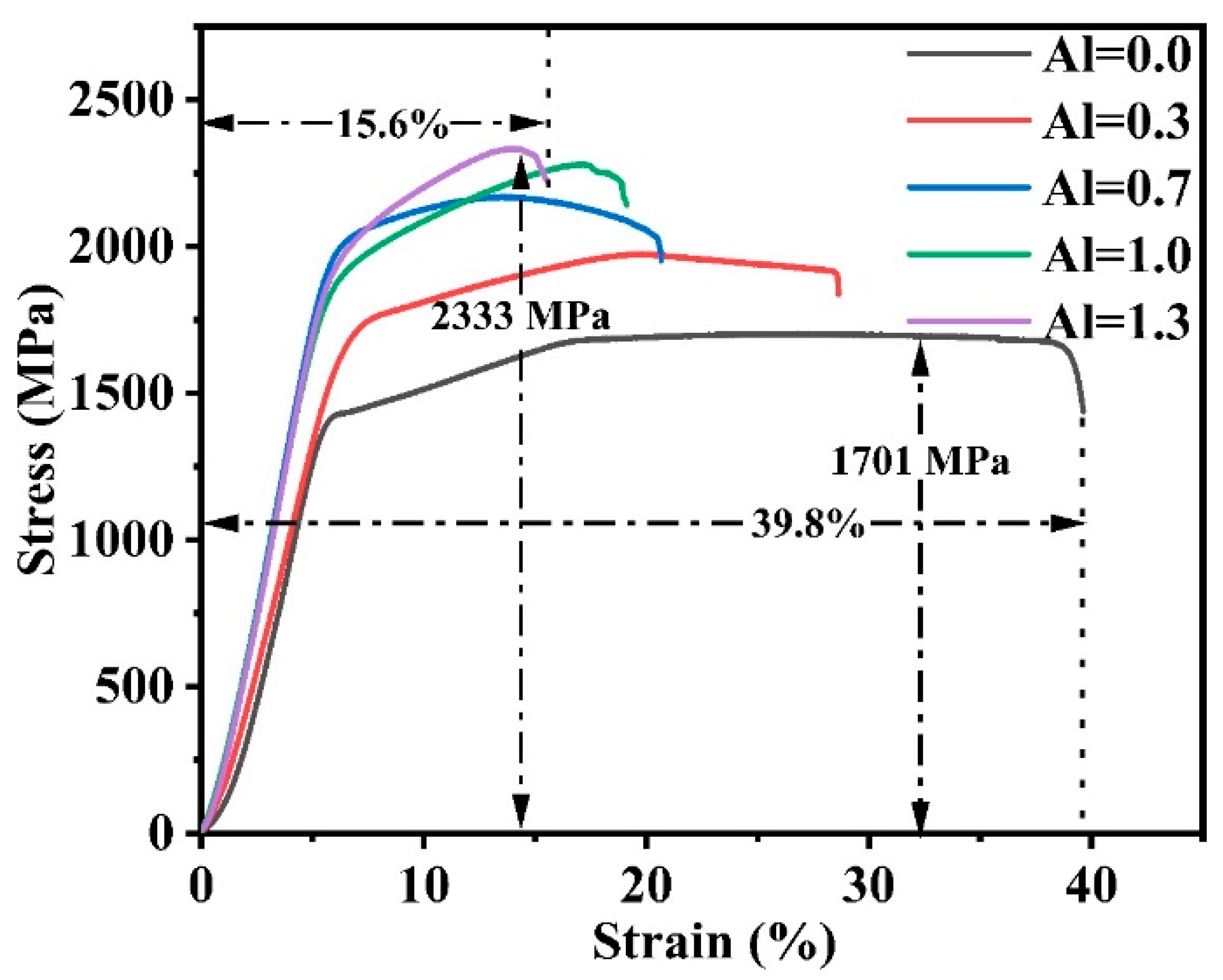
- The HEA prepared by MA-SPS has high hardness and strength. In this paper, with the increase in Al content, the hardness and strength of the alloy gradually increase, and the strain gradually decreases. When Al = 0.0, the maximum compressive strain is 39.8%, and the fracture strength and hardness are the lowest, which are 1701 MPa and 433 HV, respectively. When Al = 1.3, the highest fracture strength and hardness are 2333 MPa and 563 HV, but the compressive strain is only 15.6%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lnoue, A.; Wang, X.M. Bulk amorphous FC20 (Fe-C-Si) alloys with small amounts of b and their crystallized structure and mechanical properties. Acta Mater. 2000, 48, 1383–1395. [Google Scholar]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys-a new era of exploitation. Adv. Struct. Mater. 2007, 560, 1–9. [Google Scholar]
- Wu, W.H.; Yang, C.C.; Yeh, J.W. Industrial development of high entropy alloys. Eur. J. Control 2006, 31, 737–747. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, A375–A377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J. Solid solution formation criteria for high entropy alloys. Mater. Sci. Forum 2007, 561–565, 1337–1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Greer, A.L. Confusion by design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhy, A.; Somsen, C. The influence of temperature and mircrostucture on the tensile properties of a CoCrFeMnNi high entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N.; Scott, J.M.; Wilks, G.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A promising new class of high tenmperature alloys: Entectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhou, Y.; Zhang, L. A novel Fe20Co20Ni41Al19 eutectic high entropy alloy with excellent tensile properties. Mater. Lett. 2018, 216, 144–146. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L. A new CrFeNi2ALeutectic high entropy alloy system with excellent mechanical properties. J. Alloys Compd. 2019, 770, 655–661. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid solition phase or amorphous phase. Prog. Nat. Sci. Mater. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Am, Jr.; Ian, L.G. Handbook of Materials; Beijing Industry Press: Beijing, China, 2008. [Google Scholar]
- Chen, Y.L.; Hu, Y.H.; Hsieh, C.A. Compertitiong between elements during meachanical alloying in an octonary multi-principal-element alloy system. J. Alloys Compd. 2009, 481, 768–775. [Google Scholar] [CrossRef]
- Karia, R.C.; Bruno, D.S.L.; Leronarda, D.S.V. Meachanial alloying and spark plasma sintering of AlCrCuFeZn high entropy alloys. Mater. Sci. Technol. 2020, 36, 1861–1869. [Google Scholar]
- Singh, N.; Shadangi, Y.; Goud, G.S. MgAlSiCrFeNi low-density high entropy alloy processed by mechanical alloying and spark plasma sintering: Effect on phase evolution and thermal stability. J. Alloys Compd. 2021, 875, 159923. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Maurice, D.; Courtney, T. Modeling of mechanical alloying: Part I deformation, coalescence, bdand fragmentation mechanisms. Metall. Mater. Trans. A 1994, 25, 147–158. [Google Scholar] [CrossRef]
- Vaidya, M.; Karati, A.; Marshal, A.; Pradeep, K.G.; Murty, B.S. Phase evolution and stability of nanocrystalline CoCrFeNi and CoCrFeMnNi high entropy alloys. J. Alloys Compd. 2019, 770, 1004–1015. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
| Element | Al | Co | Cr | Fe | Ni |
|---|---|---|---|---|---|
| Al | 0 | −19 | −10 | −11 | −22 |
| Co | 0 | −4 | −1 | 0 | |
| Cr | 0 | −1 | −7 | ||
| Fe | 0 | −2 | |||
| Ni | 0 |
| Element | Atomic Number | Crystal Structure | Atomic Radius (Å) | Fusing Point (°C) | Electron-Egativity (Pauling) | (VEC) | Molar Mass |
|---|---|---|---|---|---|---|---|
| Al | 13 | FCC | 1.432 | 660 | 1.61 | 3 | 26.98 |
| Cr | 24 | BCC | 1.249 | 1857 | 1.66 | 6 | 51.99 |
| Fe | 26 | BCC, FCC | 1.241 | 1535 | 1.83 | 8 | 55.85 |
| Co | 27 | HCP, FCC | 1.251 | 1495 | 1.88 | 9 | 58.93 |
| Ni | 28 | FCC | 1.246 | 1453 | 1.91 | 10 | 58.69 |
| Alloys | Crystalline Size (nm) | |
|---|---|---|
| FCC | BCC | |
| Al00 | 7.5 | - |
| Al03 | 6.5 | - |
| Al07 | 5.7 | 20.5 |
| Al10 | 3.5 | 8.8 |
| Al13 | 6.1 | 15.2 |
| Area | Al | Co | Cr | Fe | Ni |
|---|---|---|---|---|---|
| Nominal | 16.39 | 16.39 | 16.39 | 16.39 | 34.42 |
| HEAs | 13.43 | 16.95 | 16.45 | 17.48 | 35.69 |
| Alloys | FCC (%) | BCC (%) |
|---|---|---|
| Al00 | 100 | - |
| Al03 | 100 | - |
| Al07 | 93.5 | 6.5 |
| Al10 | 71.2 | 28.8 |
| Al13 | 67.8 | 32.2 |
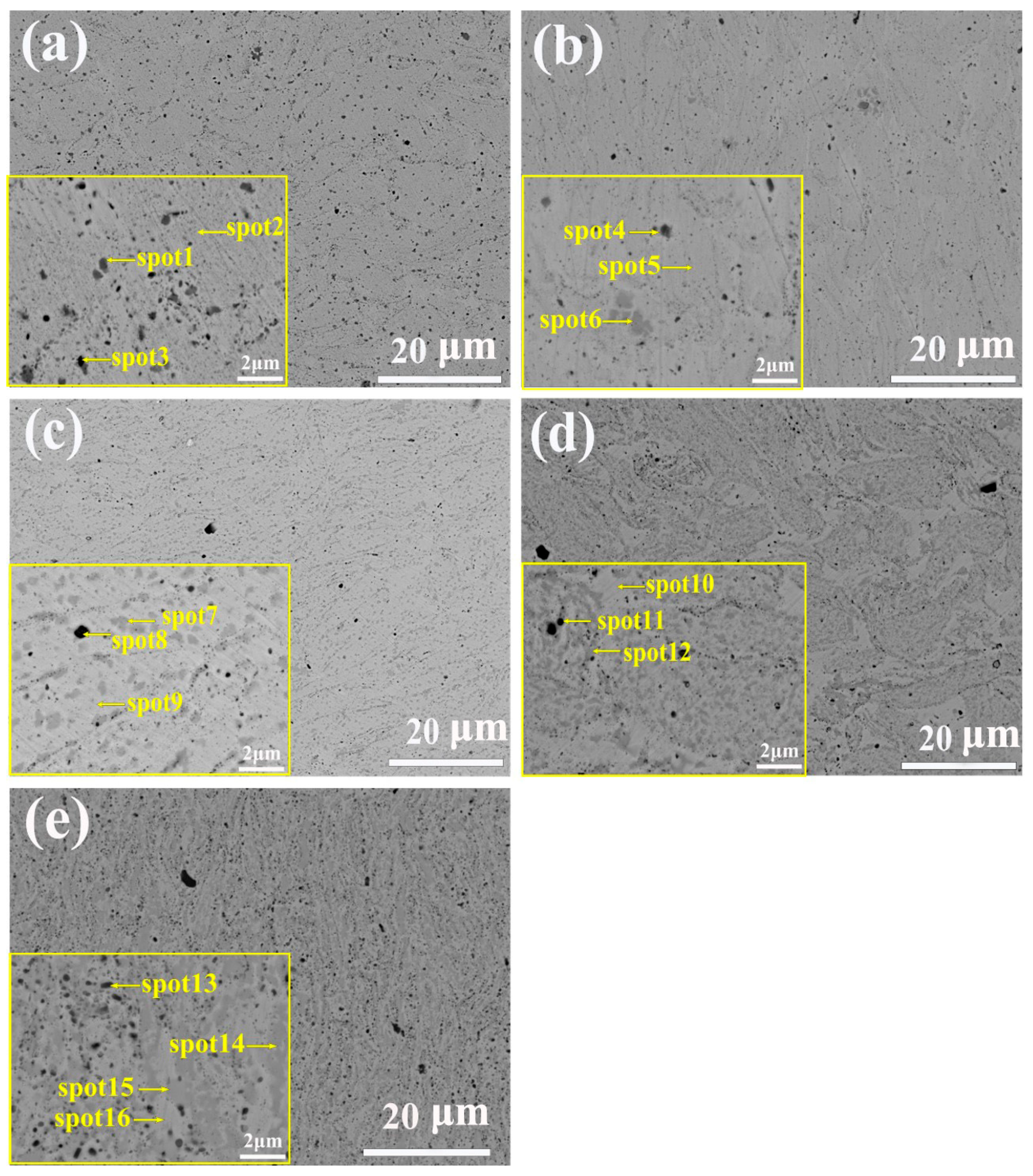
| Alloys | Region | Al | Co | Cr | Fe | Ni | C | O |
|---|---|---|---|---|---|---|---|---|
| Al00 | Nominal | - | 19.6 | 19.6 | 19.6 | 41.2 | - | - |
| Spot1 | - | 7.2 | 31.4 | 7.9 | 13.7 | 39.8 | - | |
| Spot2 | - | 18.5 | 19.7 | 20.1 | 41.8 | - | - | |
| Spot3 | 11.1 | 10.9 | 18.3 | 11.6 | 22.4 | - | 25.7 | |
| Al03 | Nominal | 5.5 | 18.5 | 18.5 | 18.5 | 39.0 | - | - |
| Spot4 | 12.8 | 13.8 | 14.2 | 13.8 | 28.7 | - | 16.6 | |
| Spot5 | 6.5 | 18.7 | 17.3 | 18.6 | 38.9 | - | - | |
| Spot6 | 3.9 | 4.1 | 43.9 | 5.0 | 5.6 | 37.4 | - | |
| Al07 | Nominal | 12.0 | 17.2 | 17.2 | 17.2 | 36.2 | - | - |
| Spot7 | 25.5 | 11.1 | 9.1 | 11.2 | 43.1 | - | - | |
| Spot8 | 29.2 | 5.3 | 6.1 | 12.8 | 10.2 | - | 36.4 | |
| Spot9 | 10.2 | 19.6 | 18.4 | 20.3 | 34.5 | - | - | |
| Al10 | Nominal | 16.4 | 16.4 | 16.4 | 16.4 | 34.4 | - | - |
| Spot10 | 8.9 | 18.5 | 18.5 | 19.9 | 33.9 | - | - | |
| Spot11 | 33.8 | 4.9 | 5.6 | 5.0 | 10.9 | - | 39.9 | |
| Spot12 | 21.6 | 13.7 | 12.9 | 13.5 | 38.3 | - | - | |
| Al13 | Nominal | 20.4 | 15.6 | 15.6 | 15.6 | 32.8 | - | - |
| Spot13 | 26.2 | 9.4 | 9.5 | 10.0 | 14.35 | - | 30.6 | |
| Spot14 | 31.12 | 10.85 | 6.3 | 9.8 | 41.9 | - | - | |
| Spot15 | 8.75 | 20.0 | 19.1 | 20.6 | 31.6 | - | - | |
| Spot16 | 10.9 | 5.6 | 30.1 | 6.6 | 15.4 | - | - |
| Alloys | (J/mol K) | δ (%) | Ω | VEC | |
|---|---|---|---|---|---|
| Al00 | −3.83 | 11.00 | 1.02 | 4.47 | 8.63 |
| Al03 | −6.97 | 12.17 | 3.22 | 2.63 | 8.32 |
| Al07 | −10.14 | 12.73 | 4.43 | 1.82 | 7.95 |
| Al10 | −11.51 | 12.90 | 4.97 | 1.58 | 7.70 |
| Al13 | −13.38 | 12.96 | 5.36 | 1.33 | 7.49 |
| Alloys | Hardness/HV | Yield Strength/MPa | Fracture Strength/MPa | Strain/% |
|---|---|---|---|---|
| Al00 | 433 | 1246 | 1701 | 39.8 |
| Al03 | 472 | 1778 | 1974 | 28.6 |
| Al07 | 564 | 2075 | 2169 | 20.6 |
| Al10 | 538 | 1998 | 2279 | 19.0 |
| Al13 | 563 | 2099 | 2333 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Lu, Z.; Zhang, X. Nano-Structure Evolution and Mechanical Properties of AlxCoCrFeNi2.1 (x = 0, 0.3, 0.7, 1.0, 1.3) High-Entropy Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Nanomaterials 2024, 14, 641. https://doi.org/10.3390/nano14070641
Liu G, Lu Z, Zhang X. Nano-Structure Evolution and Mechanical Properties of AlxCoCrFeNi2.1 (x = 0, 0.3, 0.7, 1.0, 1.3) High-Entropy Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Nanomaterials. 2024; 14(7):641. https://doi.org/10.3390/nano14070641
Chicago/Turabian StyleLiu, Guiqun, Ziteng Lu, and Xiaoli Zhang. 2024. "Nano-Structure Evolution and Mechanical Properties of AlxCoCrFeNi2.1 (x = 0, 0.3, 0.7, 1.0, 1.3) High-Entropy Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering" Nanomaterials 14, no. 7: 641. https://doi.org/10.3390/nano14070641





