Prednisolone Nanoprecipitation with Dean Instability Microfluidics Mixer
Abstract
1. Introduction
2. Materials and Methods
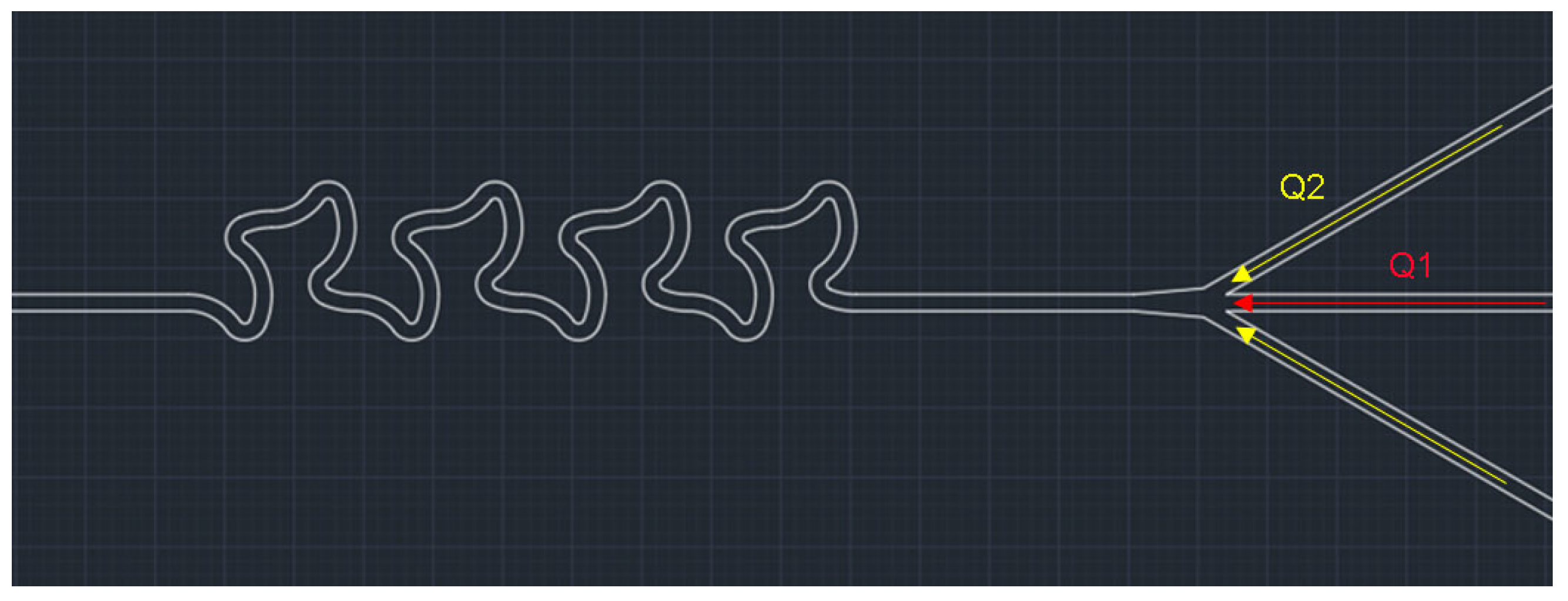
2.1. Fabrication
2.2. Sample Preparation
2.3. Experimental Setup and Data Analysis
2.4. Simulation
3. Results and Discussion
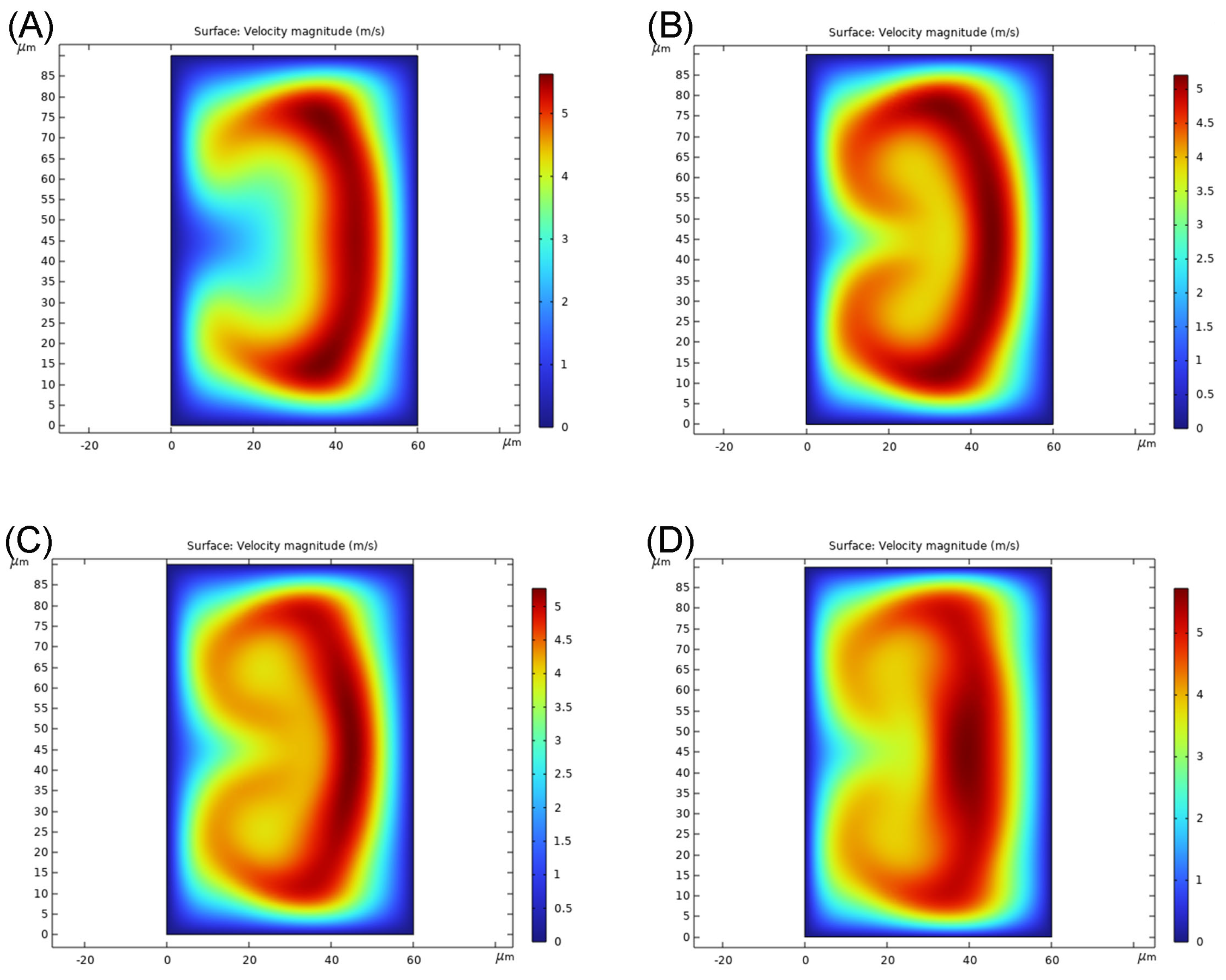
3.1. Optimization of the Channel Geometry by Simulation
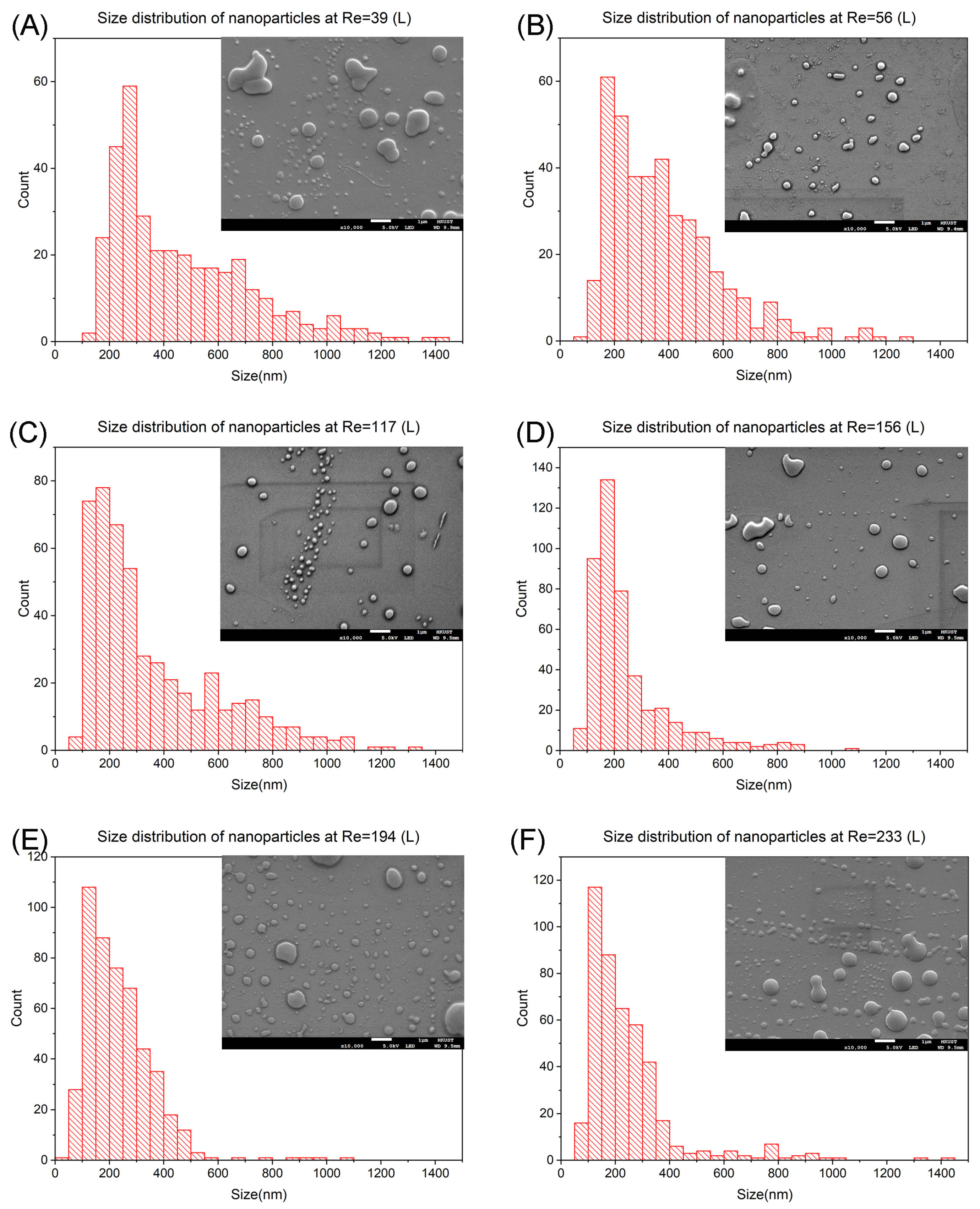
3.2. Prednisolone Nanoparticles Synthesis at Mixing Length L
3.3. Prednisolone Nanoparticles Synthesis at Mixing Length 2L
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, C.J. Sustainability as an emerging design criterion in nanoparticle synthesis and applications. J. Mater. Chem. 2008, 18, 2173–2176. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold nanoparticles: Synthesis and applications in drug delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.E.; Singh, M.K.; Kang, T.G.; Den Toonder, J.M.; Anderson, P.D. Passive and active mixing in microfluidic devices. In Proceedings of the Macromolecular Symposia, Taipei, Taiwan, 29 June–4 July 2008; Wiley Online Library: Hoboken, NJ, USA, 2009; Volume 279, pp. 201–209. [Google Scholar]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Yeh, C.P.; Tsai, C.H.; Fu, L.M. Rapid magnetic microfluidic mixer utilizing AC electromagnetic field. Electrophoresis 2009, 30, 4179–4186. [Google Scholar] [CrossRef] [PubMed]
- Frommelt, T.; Kostur, M.; Wenzel-Schäfer, M.; Talkner, P.; Hänggi, P.; Wixforth, A. Microfluidic mixing via acoustically driven chaotic advection. Phys. Rev. Lett. 2008, 100, 034502. [Google Scholar] [CrossRef]
- Anderson, J.C.; Welle, R.P. Thermally-actuated microfluidic systems. JALA: J. Assoc. Lab. Autom. 2008, 13, 65–72. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Nimafar, M.; Viktorov, V.; Martinelli, M. Experimental comparative mixing performance of passive micromixers with H-shaped sub-channels. Chem. Eng. Sci. 2012, 76, 37–44. [Google Scholar] [CrossRef]
- Hossain, S.; Ansari, M.A.; Husain, A.; Kim, K.Y. Analysis and optimization of a micromixer with a modified Tesla structure. Chem. Eng. J. 2010, 158, 305–314. [Google Scholar] [CrossRef]
- Kim, G.Y.; Han, J.I.; Park, J.K. Inertial microfluidics-based cell sorting. BioChip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Lu, X.; Chow, J.J.M.; Koo, S.H.; Tan, T.Y.; Jiang, B.; Ai, Y. Enhanced molecular diagnosis of bloodstream candida infection with size-based inertial sorting at submicron resolution. Anal. Chem. 2020, 92, 15579–15586. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A review of secondary flow in inertial microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, S.; Jiang, D.; Zhu, L.; Yang, J.; Xiang, N. Channel innovations for inertial microfluidics. Lab Chip 2020, 20, 3485–3502. [Google Scholar] [CrossRef]
- Tripathi, E.; Patowari, P.K.; Pati, S. Numerical investigation of mixing performance in spiral micromixers based on Dean flows and chaotic advection. Chem. Eng. Process.-Process Intensif. 2021, 169, 108609. [Google Scholar] [CrossRef]
- Ngo, I.; Lai, T.; Choi, H.; Le, H.; Kim, G.; Dang, T. A study on mixing performance of dean flows through spiral micro-channel under various effects. Phys. Fluids 2020, 32. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab Chip 2006, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Howell, P.B., Jr.; Mott, D.R.; Golden, J.P.; Ligler, F.S. Design and evaluation of a Dean vortex-based micromixer. Lab Chip 2004, 4, 663–669. [Google Scholar] [CrossRef]
- Yu, X.; Andreo, J.; Walden, M.; Del Campo, J.F.; Basabe-Desmonts, L.; Benito-Lopez, F.; Burg, T.P.; Wuttke, S. The Importance of Dean Flow in Microfluidic Nanoparticle Synthesis: A ZIF-8 Case Study. Small Methods 2024, 8, 2300603. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Knauer, A.; Malsch, D.; Csáki, A.; Henkel, T.; Köhler, J.M.; Fritzsche, W. Combination of microfluidic high-throughput production and parameter screening for efficient shaping of gold nanocubes using Dean-flow mixing. Lab Chip 2017, 17, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.R. Fluid motion in a curved channel. Proc. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1928, 121, 402–420. [Google Scholar]
- Reid, W. On the stability of viscous flow in a curved channel. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1958, 244, 186–198. [Google Scholar]
- Peng, K.; Xu, F.; Yang, L.; Yao, C.; Chen, G. Dean instability and vortex-induced mixing for two miscible fluids in T-micromixers. Chem. Eng. Process.-Process Intensif. 2022, 176, 108975. [Google Scholar] [CrossRef]
- Nivedita, N.; Ligrani, P.; Papautsky, I. Dean flow dynamics in low-aspect ratio spiral microchannels. Sci. Rep. 2017, 7, 44072. [Google Scholar] [CrossRef]
- Wong, Y.C.; Dai, C.; Xian, Q.; Yan, Z.; Zhang, Z.; Wen, W. Flow study of Dean’s instability in high aspect ratio microchannels. Sci. Rep. 2023, 13, 17896. [Google Scholar] [CrossRef]
- Ali, H.S.; Blagden, N.; York, P.; Amani, A.; Brook, T. Artificial neural networks modelling the prednisolone nanoprecipitation in microfluidic reactors. Eur. J. Pharm. Sci. 2009, 37, 514–522. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, P.; Zhou, Q. Adhesion promotion between PDMS and glass by oxygen plasma pre-treatment. J. Adhes. Sci. Technol. 2014, 28, 1046–1054. [Google Scholar] [CrossRef]
- Squire, H.B.; Winter, K. The secondary flow in a cascade of airfoils in a nonuniform stream. J. Aeronaut. Sci. 1951, 18, 271–277. [Google Scholar] [CrossRef]

| 2nd Radius of Curvature [m] | 3rd Radius of Curvature [m] | Mixing Index (cor. to 3 sig. fig.) |
|---|---|---|
| 50 | 200 | 0.988 |
| 60 | 260 | 0.987 |
| 60 | 210 | 0.986 |
| 50 | 190 | 0.986 |
| 60 | 190 | 0.985 |
| 40 | 160 | 0.985 |
| 70 | 200 | 0.981 |
| 70 | 220 | 0.981 |
| 60 | 170 | 0.980 |
| 70 | 230 | 0.979 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, Y.C.; Yang, S.; Wen, W. Prednisolone Nanoprecipitation with Dean Instability Microfluidics Mixer. Nanomaterials 2024, 14, 652. https://doi.org/10.3390/nano14080652
Wong YC, Yang S, Wen W. Prednisolone Nanoprecipitation with Dean Instability Microfluidics Mixer. Nanomaterials. 2024; 14(8):652. https://doi.org/10.3390/nano14080652
Chicago/Turabian StyleWong, Yu Ching, Siyu Yang, and Weijia Wen. 2024. "Prednisolone Nanoprecipitation with Dean Instability Microfluidics Mixer" Nanomaterials 14, no. 8: 652. https://doi.org/10.3390/nano14080652
APA StyleWong, Y. C., Yang, S., & Wen, W. (2024). Prednisolone Nanoprecipitation with Dean Instability Microfluidics Mixer. Nanomaterials, 14(8), 652. https://doi.org/10.3390/nano14080652






