Bryophyte-Bioinspired Nanoporous AAO/C/MgO Composite for Enhanced CO2 Capture: The Role of MgO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
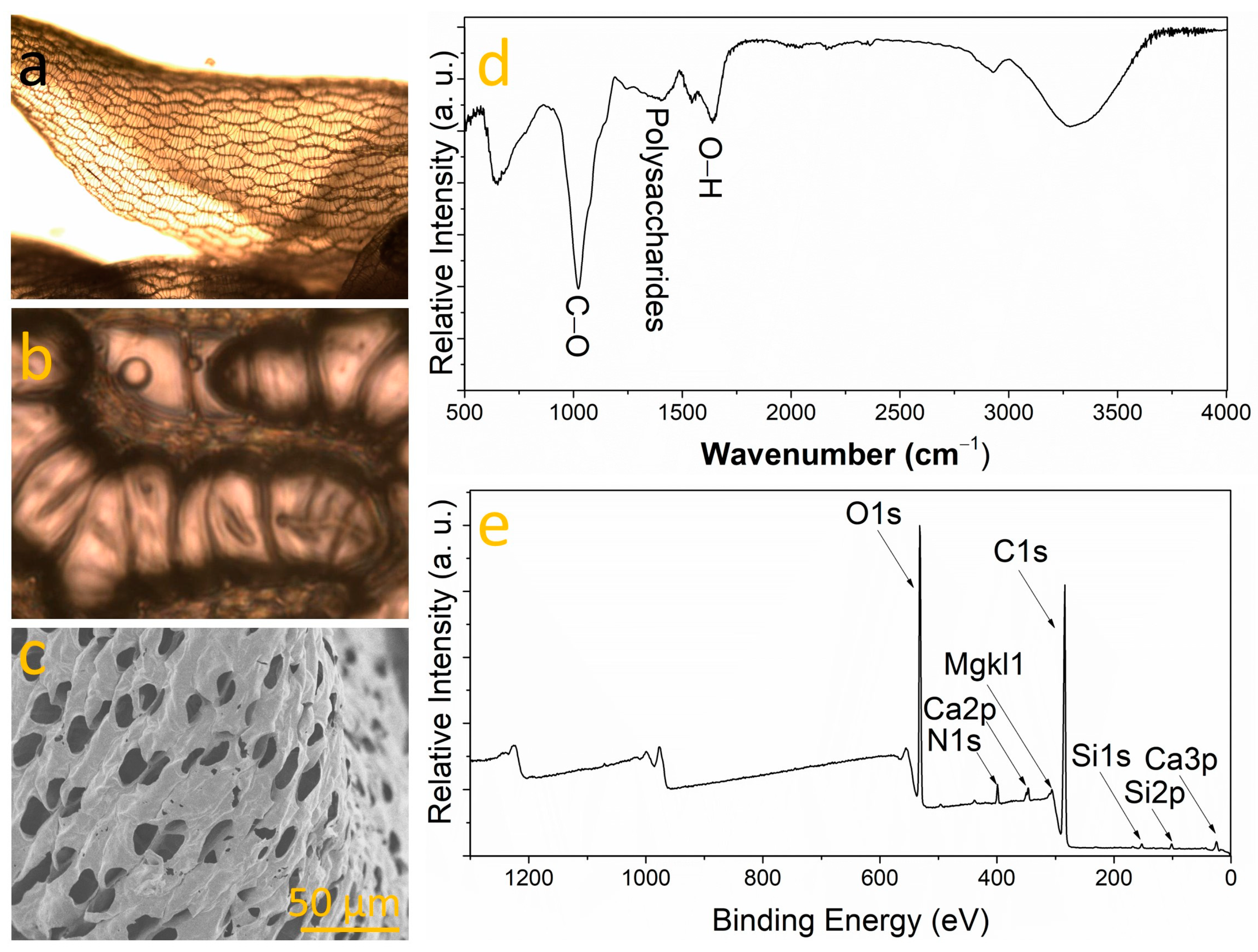
2.2. Bryophyte Study for Bioinspired Elements Selection
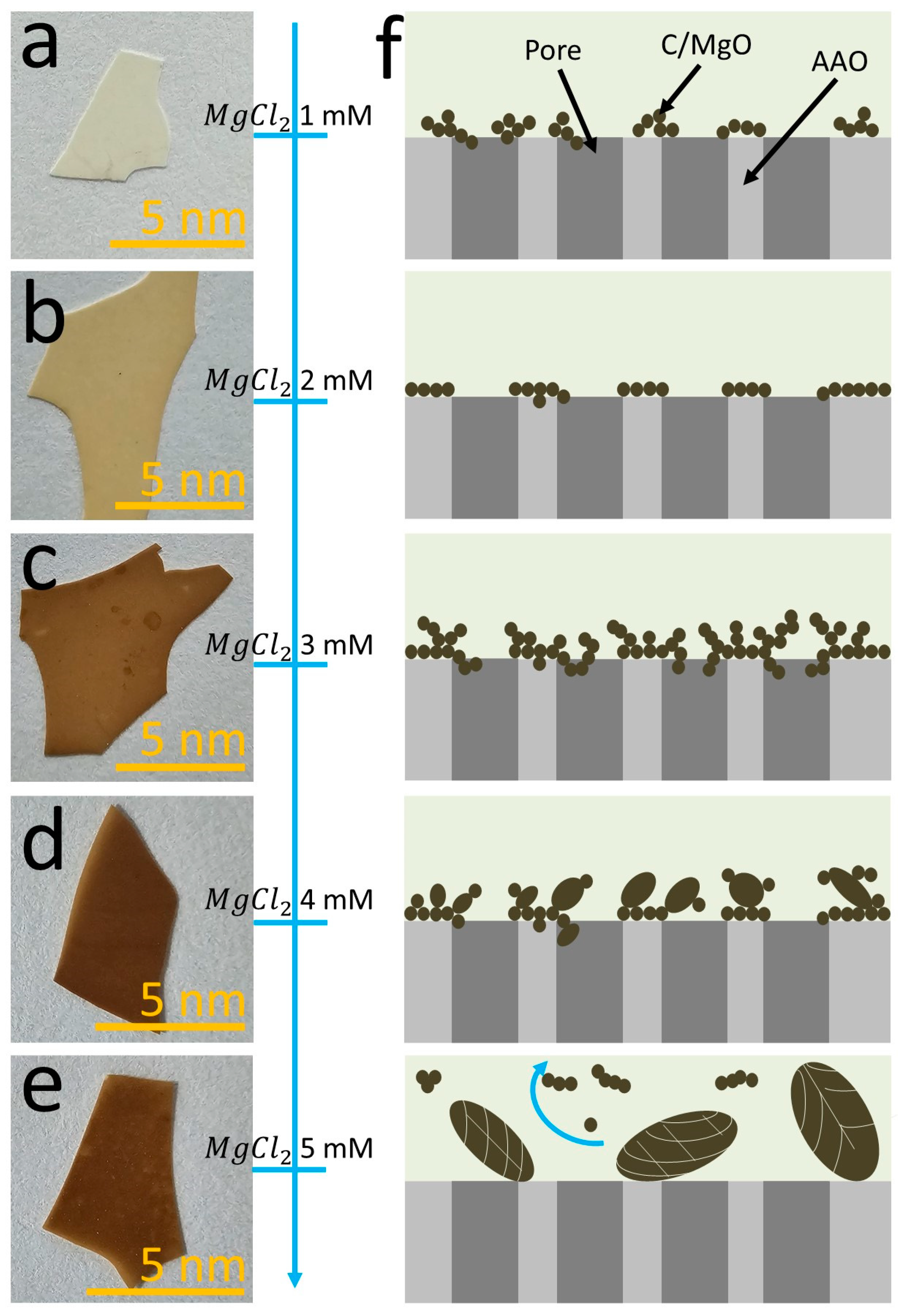
2.3. Synthesis of the Nanoporous AAO/C/MgO
2.4. Physical and Chemical Characterization
2.5. Evaluation of CO2 Capture
3. Results and Discussion
3.1. Bryophyte Characterization
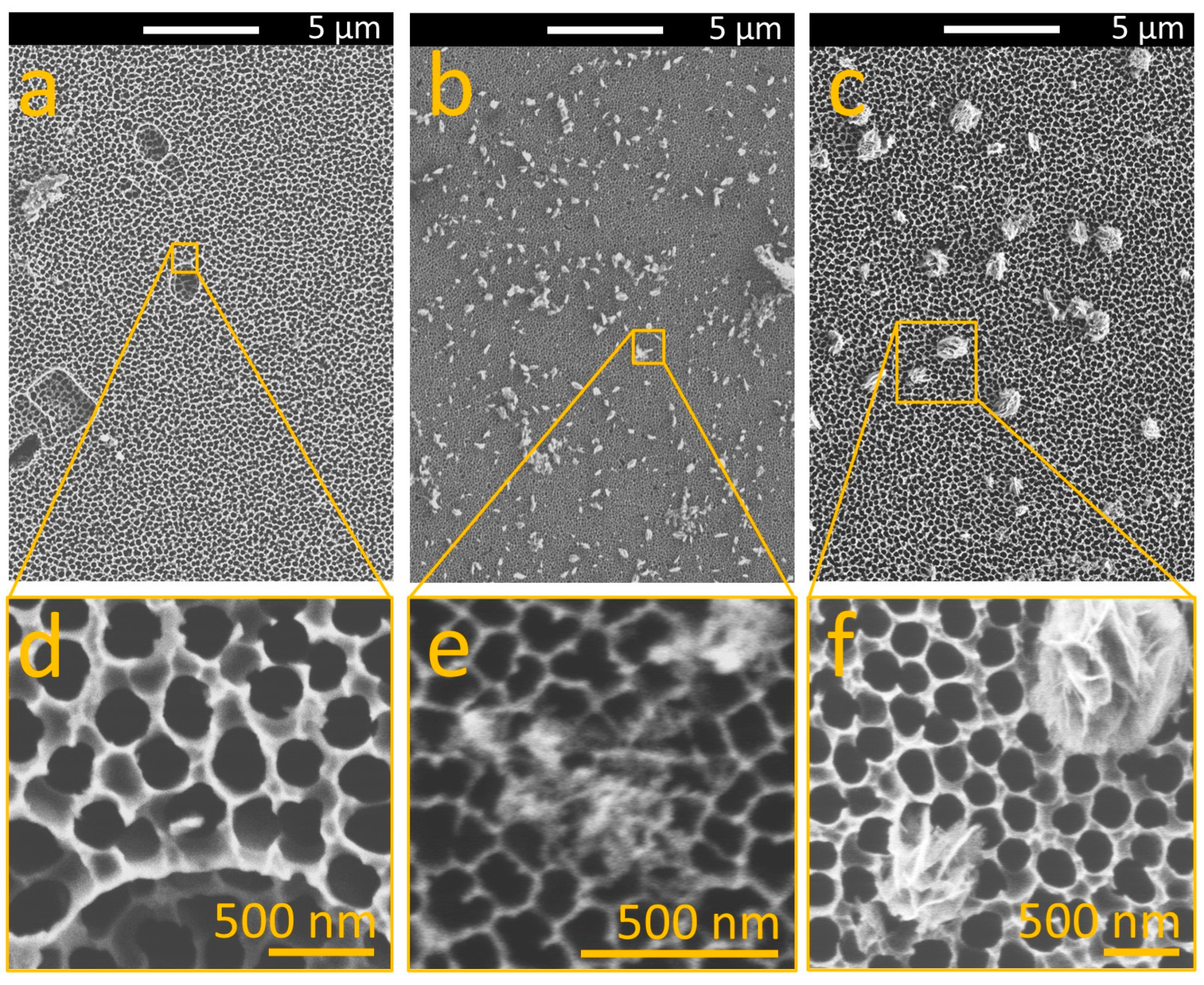
3.2. Influence of MgCl2 on the Morphology of MgO on AAO
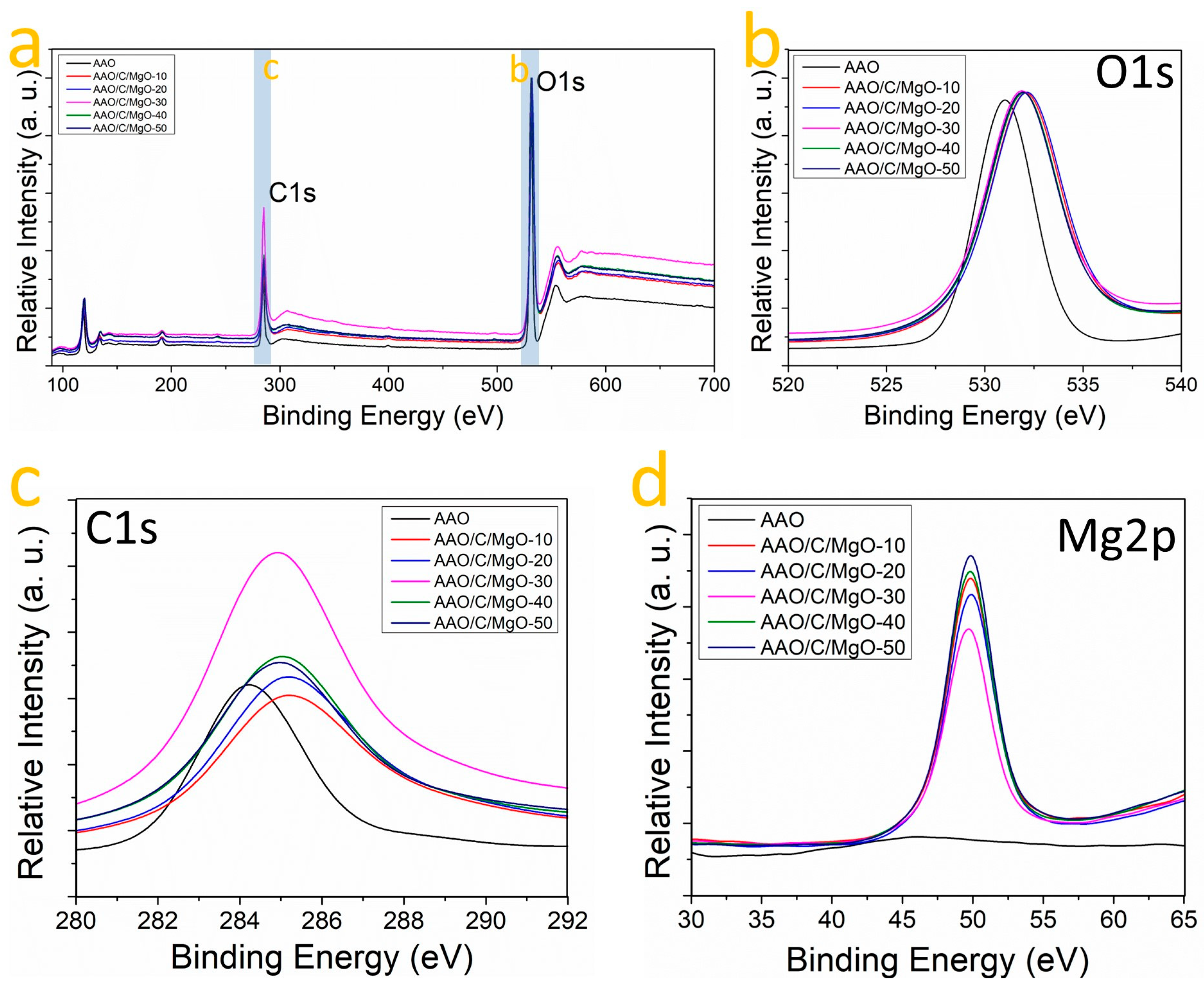
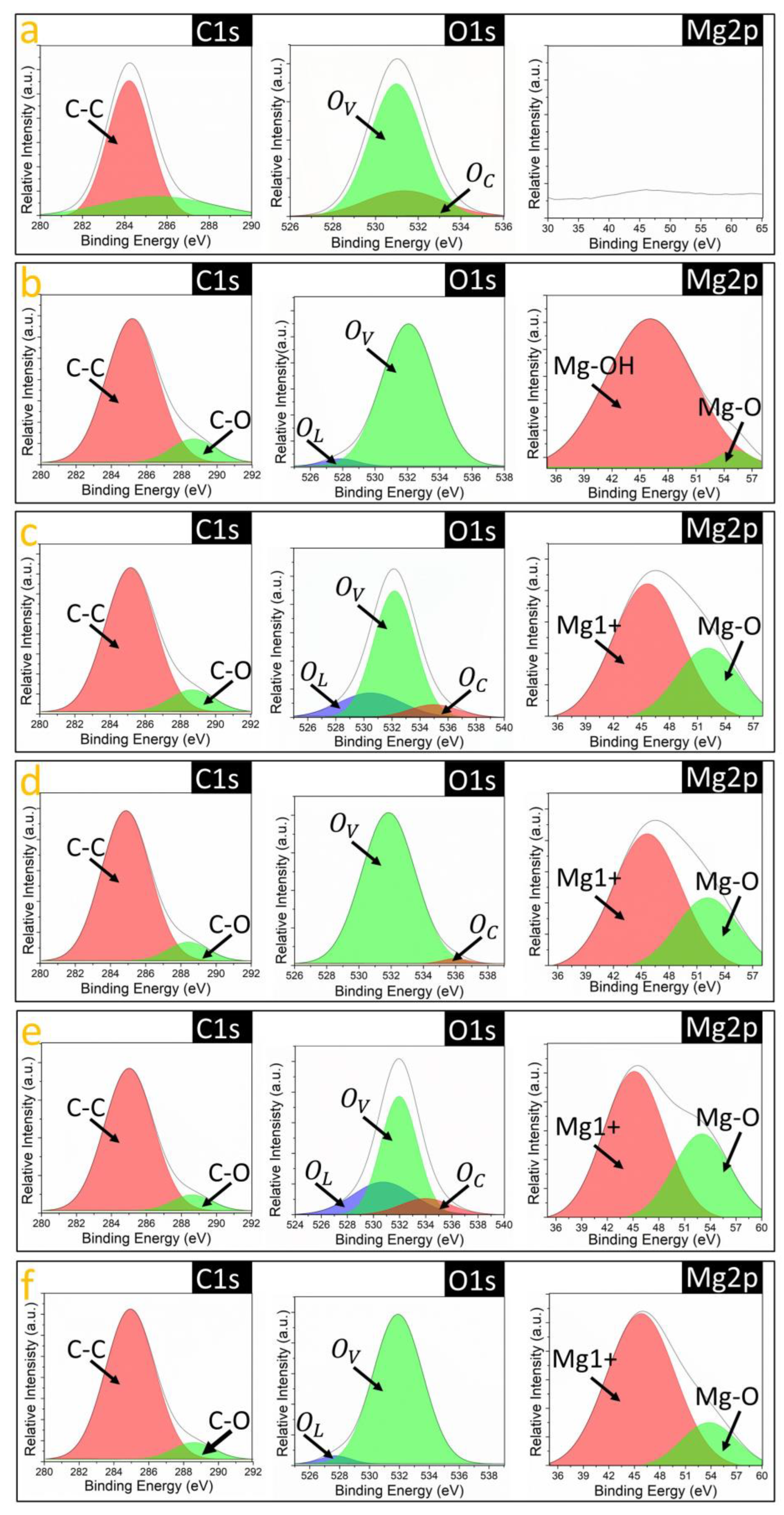
3.3. Chemical Nature of Bindings in AAO/C/MgO
3.4. CO2 Adsorption of the AAO/C/MgO
3.5. Future Prospects of the AAO/C/MgO Composite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Reis, E.A.; da Silva, G.T.S.T.; Santiago, E.I.; Ribeiro, C. Revisiting Electrocatalytic CO2 Reduction in Nonaqueous Media: Promoting CO2 Recycling in Organic Molecules by Controlling H2 Evolution. Energy Technol. 2023, 11, 2201367. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Imanova, G.; Agayev, T.; Garibov, A.; Mansimov, Z.; Kurniawan, T.A.; Habila, M.A. Hydrogen production by water spliting using (RaO)x(SiO2)y.H2O and gamma radiation. Radiat. Phys. Chem. 2024, 218, 111597. [Google Scholar] [CrossRef]
- Alves, G.A.S.; Pacholik, G.; Pollitt, S.; Wagner, T.; Rameshan, R.; Rameshan, C.; Föttinger, K. Mn-promoted MoS2 catalysts for CO2 hydrogenation: Enhanced methanol selectivity due to MoS2/MnOx interfaces. Catal. Sci. Technol. 2024, 14, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Xia, Z.; Zhou, B.; Wu, H.; Xue, T.; Chen, X.; Jiao, J.; Jia, S.; He, M.; Han, B. Photo-thermal coupling to enhance CO2 hydrogenation toward CH4 over Ru/MnO/Mn3O4. Nat. Commun. 2024, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa-Mayorga, O.; Neri, E.; Calderon, H.A.; Kisielowski, C. Electron Microscopy of Heterostructure for Solar Energy Recovery: ZnO Nanowires and Co3O4 Nanoparticles. Microsc. Microanal. 2017, 23, 2068–2069. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.; Cano, F.J.; Hernández-Rodríguez, M.; Cigarroa-Mayorga, O. Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture. Crystals 2022, 12, 1086. [Google Scholar] [CrossRef]
- Michael North, A. Chapter 1—What Is CO2? Thermodynamics, Basic Reactions and Physical Chemistry. In Carbon Dioxide Utilisation; Styring, P., Quadrelli, E.A., Armstrong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–17. ISBN 9780444627469. [Google Scholar] [CrossRef]
- He, T.; Ding, W.; Cheng, X.; Cai, Y.; Zhang, Y.; Xia, H.; Wang, X.; Zhang, J.; Zhang, K.; Zhang, Q. Meta-analysis shows the impacts of ecological restoration on greenhouse gas emissions. Nat. Commun. 2024, 15, 2668. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.B.; de Arellano, J.V.-G.; Rutledge-Jonker, S.; van der Graaf, S.; Zhang, J.; van Zanten, M.C. Observational relationships between ammonia, carbon dioxide and water vapor under a wide range of meteorological and turbulent conditions: RITA-2021 campaign. Biogeosciences 2024, 21, 557–574. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ge, W.; Liu, J.; Wu, H.; Zheng, L.; Wang, X.; Qin, Y.; Zhou, J.; Wang, Y.; et al. Impacts of coal use phase-out in China on the atmospheric environment: (2) public health and global radiative forcing. Atmos. Environ. 2024, 319, 120280. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). The State of Greenhouse Gases in the Atmosphere Based on Global Observations through; WMO Greenhouse Gas Bulletin: Geneva, Switzerland, 2022; Volume 19, Available online: https://library.wmo.int/idurl/4/68532 (accessed on 13 February 2024).
- Dong, X.; Qi, D.; Chen, B.; Wu, Y.; Zheng, X.; Lin, H. Differential roles of anthropogenic CO2 in mediating seasonal amplitudes of ocean acidification metrics over a coastal coral habitat. J. Mar. Syst. 2024, 241, 103910. [Google Scholar] [CrossRef]
- Allen, L.H.; Vu, J.C. Carbon dioxide and high temperature effects on growth of young orange trees in a humid, subtropical environment. Agric. For. Meteorol. 2009, 149, 820–830. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Zhang, M.; Behrens, T.; Webster, R. A warming climate will make Australian soil a net emitter of atmospheric CO2. NPJ Clim. Atmos. Sci. 2024, 7, 79. [Google Scholar] [CrossRef]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Change 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Nadiri, A.; Gündüz, V.; Adebayo, T.S. The role of financial and trade globalization in enhancing environmental sustainability: Evaluating the effectiveness of carbon taxation and renewable energy in EU member countries. Borsa Istanb. Rev. 2024, 24, 235–247. [Google Scholar] [CrossRef]
- Ma, D.; Xie, G.; Mao, W.; Gao, J.; Yi, H.; Li, D. Comparison and Improvement of Bioinspired Mobile Algorithms to Trace the Emission Source Based on the Simulation Scenarios. Atmosphere 2022, 13, 661. [Google Scholar] [CrossRef]
- Long, Y.; Li, L.; Xu, T.; Wu, X.; Gao, Y.; Huang, J.; He, C.; Ma, T.; Ma, L.; Cheng, C.; et al. Hedgehog artificial macrophage with atomic-catalytic centers to combat Drug-resistant bacteria. Nat. Commun. 2021, 12, 6143. [Google Scholar] [CrossRef] [PubMed]
- Saldivar-Ayala, D.; Ashok, A.; Cigarroa-Mayorga, O.; Hernández-Rodríguez, Y. Tuning the plasmon resonance of Au-Ag core-shell nanoparticles: The influence on the visible light emission for inorganic fluorophores application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132359. [Google Scholar] [CrossRef]
- Hernandez-Rodríguez, E.; Delgadillo-Moya, C. The ethnobotany of bryophytes in Mexico. Bot. Sci. 2020, 99, 13–27. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Bain de, J.F. Water retention and ion exchange in the leaves of Sphagnum mosses. Plant Physiol. 1978. [Google Scholar]
- Pacheco-Cancino, P.A.; Carrillo-López, R.F.; Sepulveda-Jauregui, A.; Somos-Valenzuela, M.A. Sphagnum mosses, the impact of disturbances and anthropogenic management actions on their ecological role in CO2 fluxes generated in peatland ecosystems. Glob. Change Biol. 2024, 30, e16972. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Xu, X. Accurate descriptions of molecule-surface interactions for understanding CO2 capture by MgO-based sorbents in wet conditions. Carbon Capture Sci. Technol. 2023, 9, 100148. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.; Gallardo-Hernández, S.; Talamás-Rohana, P. Tunable Raman scattering enhancement due to self-assembled Au nanoparticles layer on porous AAO: The influence of the alumina support. Appl. Surf. Sci. 2021, 536, 147674. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E.; Talamás-Rohana, P.; Gallardo-Hernández, S. Transmission Electron Microscopy Study on the Process of Gold Nanoporous Film Formation on AAO Substrate by Thermal Treatment. Microsc. Microanal. 2023, 29 (Suppl. S1), 821–822. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Ballance, S.; Kristiansen, K.; Skogaker, N.; Tvedt, K.; Christensen, B. The localisation of pectin in Sphagnum moss leaves and its role in preservation. Carbohydr. Polym. 2012, 87, 1326–1332. [Google Scholar] [CrossRef]
- Ellis, L.T.; Wilbraham, J.; Aleffi, M.; Asthana, A.K.; Rawat, K.K.; Gupta, D.; Sahu, V.; Katiyar, P.; Asthana, G.; Srivastava, A.; et al. New national and regional bryophyte records. J. Bryol. 2018, 40, 74–97. [Google Scholar] [CrossRef]
- Glime, J. Chapter 2-1, Medical Uses: Medical Conditions. In Bryophyte Ecology; Glime, J., Ed.; Michigan Tech: Houghton, MI, USA, 2017; pp. 1–22. Available online: http://digitalcommons.mtu.edu/bryophyte-ecology/ (accessed on 13 February 2024).
- Al-Badwy, A.H.; Khalil, A.M.; Bashal, A.H.; Kebeish, R. Polysaccharides from Spirulina platensis (PSP): Promising biostimulants for the green synthesis of silver nanoparticles and their potential application in the treatment of cancer tumors. Microb. Cell Fact. 2023, 22, 247. [Google Scholar] [CrossRef]
- Addoun, N.; Boual, Z.; Delattre, C.; Chouana, T.; Gardarin, C.; Dubessay, P.; Benaoun, F.; Addaoud, S.; El Hadj, M.D.O.; Michaud, P.; et al. Beneficial Health Potential of Algerian Polysaccharides Extracted from Plantago ciliata Desf. (Septentrional Sahara) Leaves and Seeds. Appl. Sci. 2021, 11, 4299. [Google Scholar] [CrossRef]
- Yehuda, N.; Gheber, L.A.; Kushmaro, A.; Arad, S. Complexes of Cu–Polysaccharide of a Marine Red Microalga Produce Spikes with Antimicrobial Activity. Mar. Drugs 2022, 20, 787. [Google Scholar] [CrossRef] [PubMed]
- Grande-Tovar, C.D.; Castro, J.I.; Tenorio, D.L.; Zapata, P.A.; Florez-López, E.; Valencia-Llano, C.H. Chitosan–Polyvinyl Alcohol Nanocomposites for Regenerative Therapy. Polymers 2023, 15, 4595. [Google Scholar] [CrossRef] [PubMed]
- Buğday, N.; Ates, M.N.; Duygulu, O.; Deng, W.; Ji, X.; Altin, S.; Yaşar, S. ZIF-12-derived N-doped Fe/Co/S/@C nanoparticles as high-performance composite anode electrode materials for lithium-ion batteries. J. Alloys Compd. 2022, 928, 167037. [Google Scholar] [CrossRef]
- Desrues, A.; De Vito, E.; Boismain, F.; Alper, J.P.; Haon, C.; Herlin-Boime, N.; Franger, S. Electrochemical and X-ray Photoelectron Spectroscopic Study of Early SEI Formation and Evolution on Si and Si@C Nanoparticle-Based Electrodes. Materials 2022, 15, 7990. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Wang, W.; Hu, X.; Deng, Y.; Wang, H.; Wu, Y. The generation of carbon/oxygen double defects in FeP/CoP-N-C enhanced by β particles for photic driving degradation of levofloxacin. Sep. Purif. Technol. 2022, 303, 122186. [Google Scholar] [CrossRef]
- Gbe, J.-L.K.; Ravi, K.; Singh, M.; Neogi, S.; Grafouté, M.; Biradar, A.V. Hierarchical porous nitrogen-doped carbon supported MgO as an excellent composite for CO2 capture at atmospheric pressure and conversion to value-added products. J. CO2 Util. 2022, 65, 102222. [Google Scholar] [CrossRef]
- Li, Y.; Shuai, X.-X.; Zhang, M.; Ma, F.-Y.; Chen, J.; Qiao, J.; Chen, R.-H.; Du, L.-Q. Preparation of ethylenediamine-modified pectin/alginate/Fe3O4 microsphere and its efficient Pb2+ adsorption properties. Int. J. Biol. Macromol. 2022, 223, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Khalid, M.; Ahmad, M.S.; Kamal, S.; Shahid, M. Effect of structural variation on enzymatic activity in tetranuclear (Cu4) clusters with defective cubane core. J. Biomol. Struct. Dyn. 2022, 40, 9067–9080. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Wang, X.; Pang, Y.; Ge, C.; Xu, Y.; Chen, L.; Li, S.; Wang, L. Porous Au/AAO: A simple and feasible SERSsubstrate for dynamic monitoring and mechanism analysis of DNA oxidation. Appl. Surf. Sci. 2022, 606, 154842. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Referencing to adventitious carbon in X-ray photoelectron spectroscopy: Can differential charging explain C 1s peak shifts? Appl. Surf. Sci. 2022, 606, 154855. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, D.; Cheng, X.; Xu, Q.; Zhang, L.; Huang, L.; Tu, Y.; Yu, X.; Zhang, T.; Li, Y.; et al. Adsorption and Oxidation of CO on Co3O4/Ir(100) Thin Films. J. Phys. Chem. C 2022, 126, 21638–21649. [Google Scholar] [CrossRef]
- Ramos-Álvarez, D.; Hernández-Rodríguez, Y.; Vega-Gómez, J.; Cigarroa-Mayorga, O. Influence of copper support on the charge transfer enhancement of zinc oxide nanoflakes. Mater. Lett. 2023, 349, 134875. [Google Scholar] [CrossRef]
- Mudgal, D.; Yadav, N.; Singh, J.; Srivastava, G.K.; Mishra, V. Xanthan gum-based copper nano-magnetite doped carbon aerogel: A promising candidate for environmentally friendly catalytic dye degradation. Int. J. Biol. Macromol. 2023, 253, 127491. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-H.; Huang, J.-M.; Chang, Y.-S.; Chan, C.-T.; Hu, H.-J. Fabrication and Characterization of MgO-Based Enzymatic Glucose Biosensors. IEEE Sens. J. 2023, 23, 28587–28596. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Richeson, D.; Li, Y. A comparison increasing the photodegradation power of a Ag/g–C3N4/CoNi–LDH nanocomposite: Photocatalytic activity toward water treatment. J. Environ. Sci. 2025, 148, 437–450. [Google Scholar] [CrossRef]
- Briggs, D.D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopies, 1st ed.; IM Publications and Surface Spectra: Trowbridge, UK, 2003; Available online: https://scholar.google.com/scholar_lookup?title=Surface+Analysis+by+Auger+and+X-ray+Photoelectron+Spectroscopies&author=Briggs,+D.&author=Grant,+J.T.&publication_year=2003 (accessed on 9 February 2024).
- Banger, K.; Yamashita, Y.; Mori, K.; Peterson, R.L.; Leedham, T.; Rickard, J.; Sirringhaus, H. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol–gel on chip’ process. Nat. Mater. 2011, 10, 45–50. Available online: https://www.nature.com/articles/nmat2914 (accessed on 9 March 2024). [CrossRef] [PubMed]
- Liu, X.; Huo, Y.-Q.; Yan, L.-K.; Fan, N.; Cai, K.-Z.; Su, Z.-M. Hollow porous MnFe2O4 sphere grown on elm-money-derived biochar towards energy-saving full water electrolysis. Chem. Eur. J. 2020, 26, 14397. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/chem.202002134?src=getftr (accessed on 10 March 2024). [CrossRef] [PubMed]
- Sharmila, A.; Selvaraj, C.I. Phyto-synthesized MgO nanoparticles using Scutia myrtina Kurz extract: Promising insights into photocatalytic degradation, antioxidant potential, cytotoxicity and toxicity assessment. J. Mol. Struct. 2024, 1304, 137698. [Google Scholar] [CrossRef]
- Mohan, T.; Kumar, J.; Roy, I. Iron selenide nanorods for light-activated anticancer and catalytic applications. New J. Chem. 2023, 47, 2527–2535. [Google Scholar] [CrossRef]
- Lin, L.; Han, S.; Meng, F.; Li, J.; Chen, K.; Hu, E.; Jiang, J. The influence of pore size and pore structure of silica-based material on the amine-modified adsorbent for CO2 capture. Sep. Purif. Technol. 2024, 340, 126735. [Google Scholar] [CrossRef]
- Yang, J.; Gao, D.; Zhang, H.; Yi, Q. Construction of ZIF-8 and amino functionalized porous ionic liquids for efficient CO2 capture. Fuel 2024, 366, 131351. [Google Scholar] [CrossRef]
- Aliyu, M.; Yusuf, B.O.; Abdullahi, A.S.; Bakare, A.I.; Umar, M.; Hakeem, A.S.; Ganiyu, S.A. Ti2C-MXene/activated carbon nanocomposite for efficient CO2 capture: Insights into thermodynamics properties. Sep. Purif. Technol. 2024, 340, 126737. [Google Scholar] [CrossRef]
- Yuan, H.; Li, P.; Sun, X.; Cen, D.; Luo, D.; Yan, X.; Lei, G.; Zheng, W.; Hu, Z.; Yang, R.T. Amine-grafted on boron modified SBA-15 for direct air capture of CO2. Sep. Purif. Technol. 2024, 341, 126720. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Huang, Q.; Yu, L.; He, Z.; Lu, H.; Li, Q. Tetraethylenepentamine-modified Cu2(OH)PO4 for efficient CO2 capture. Sep. Purif. Technol. 2024, 341, 126884. [Google Scholar] [CrossRef]
- An, M.; Guo, T.; Guo, Q. Facile preparation of coal-based ultramicroporous carbon microspheres for selective CO2 capture. Carbon Resour. Convers. 2024, 7, 100205. [Google Scholar] [CrossRef]
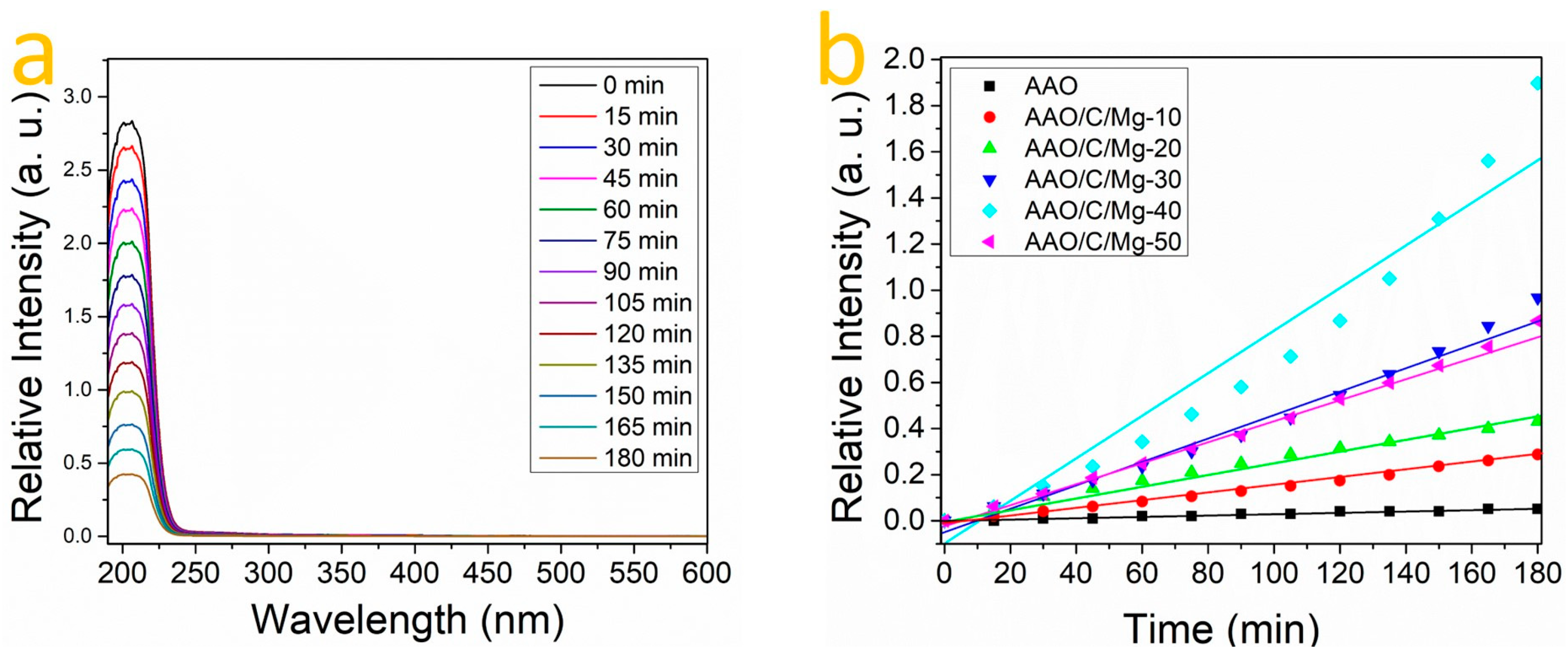
| Sample | K Constant |
|---|---|
| AAO | 0.00028 |
| AAO/C/MgO-10 | 0.00159 |
| AAO/C/MgO-20 | 0.00239 |
| AAO/C/MgO-30 | 0.00537 |
| AAO/C/MgO-40 | 0.10531 |
| AAO/C/MgO-50 | 0.00481 |
| Material | Amount of CO2 Adsorption | Time | Reference |
|---|---|---|---|
| ZIF-8-W/[TEPA][MIm] | 2.22 mol/mol ILs | 65 min | [55] |
| Ti2C-MXene/activated carbon nanocomposite | 67.83 cm3/g | 6 h | [56] |
| Amine-grafted on boron-modified SBA-15 | 0.79 mmol/g | 300 min | [57] |
| Tetraethylenepentamine-modified Cu2(OH)PO4 | 0.67 molCO2/molN | 22 min | [58] |
| Ultramicroporous carbon microspheres | 0.24 cm3/g | 6 h | [59] |
| AAO/C/MgO-40 | 1.66 mmol/g | 180 min | Reported in this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Valadez, P.J.; Baños-López, E.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Bryophyte-Bioinspired Nanoporous AAO/C/MgO Composite for Enhanced CO2 Capture: The Role of MgO. Nanomaterials 2024, 14, 658. https://doi.org/10.3390/nano14080658
Cortés-Valadez PJ, Baños-López E, Hernández-Rodríguez YM, Cigarroa-Mayorga OE. Bryophyte-Bioinspired Nanoporous AAO/C/MgO Composite for Enhanced CO2 Capture: The Role of MgO. Nanomaterials. 2024; 14(8):658. https://doi.org/10.3390/nano14080658
Chicago/Turabian StyleCortés-Valadez, Paulina Jaqueline, Esperanza Baños-López, Yazmín Mariela Hernández-Rodríguez, and Oscar Eduardo Cigarroa-Mayorga. 2024. "Bryophyte-Bioinspired Nanoporous AAO/C/MgO Composite for Enhanced CO2 Capture: The Role of MgO" Nanomaterials 14, no. 8: 658. https://doi.org/10.3390/nano14080658
APA StyleCortés-Valadez, P. J., Baños-López, E., Hernández-Rodríguez, Y. M., & Cigarroa-Mayorga, O. E. (2024). Bryophyte-Bioinspired Nanoporous AAO/C/MgO Composite for Enhanced CO2 Capture: The Role of MgO. Nanomaterials, 14(8), 658. https://doi.org/10.3390/nano14080658






