Investigation of Perovskite Solar Cells Using Guanidinium Doped MAPbI3 Active Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing Process
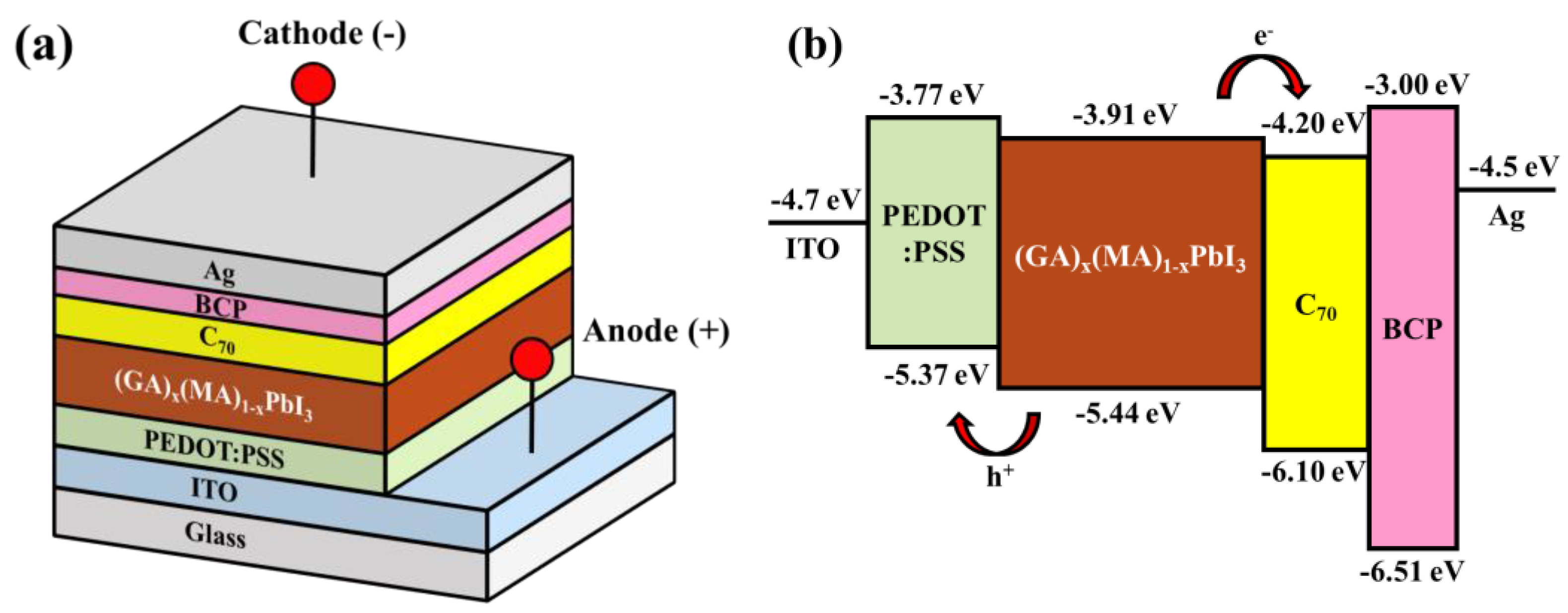
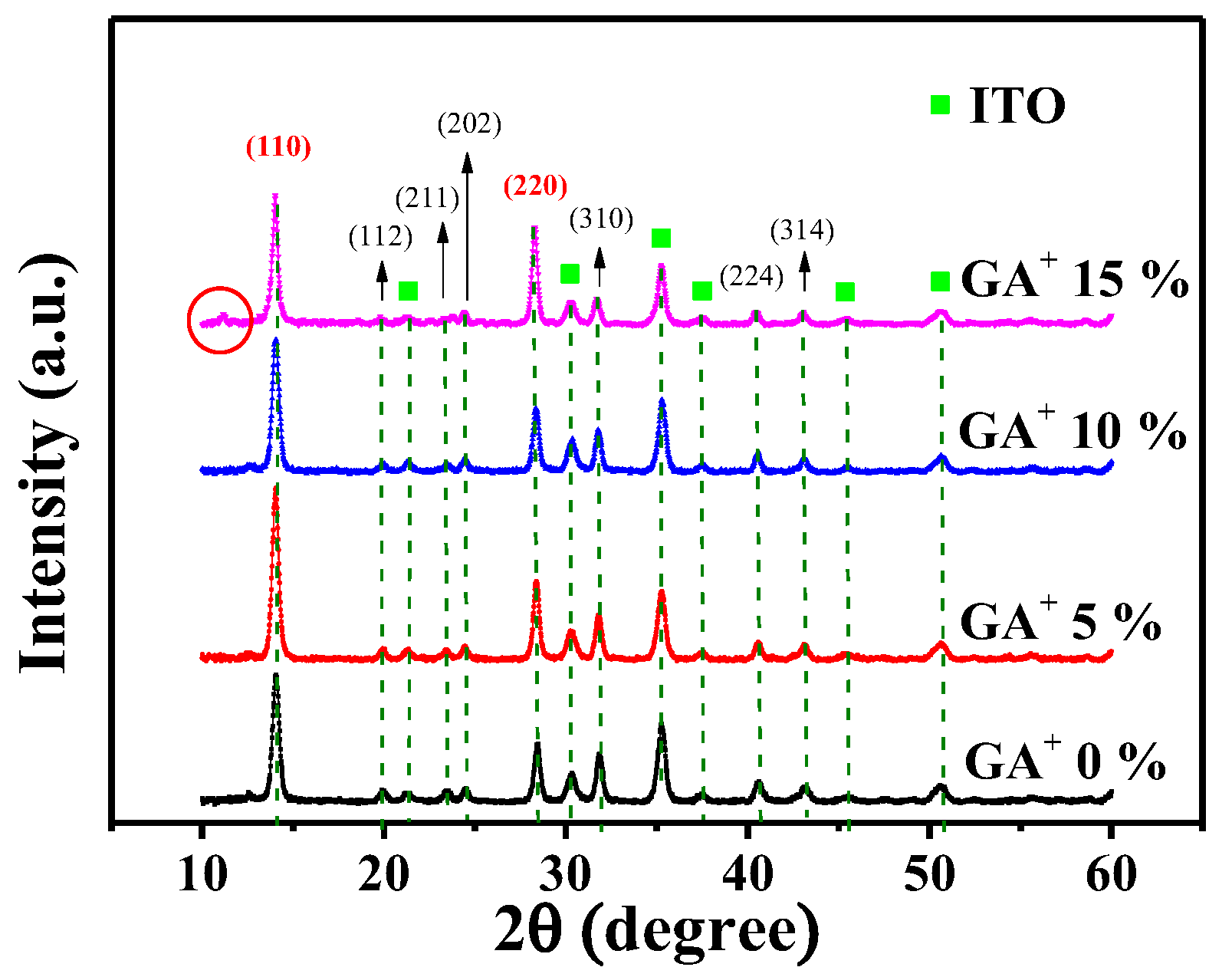
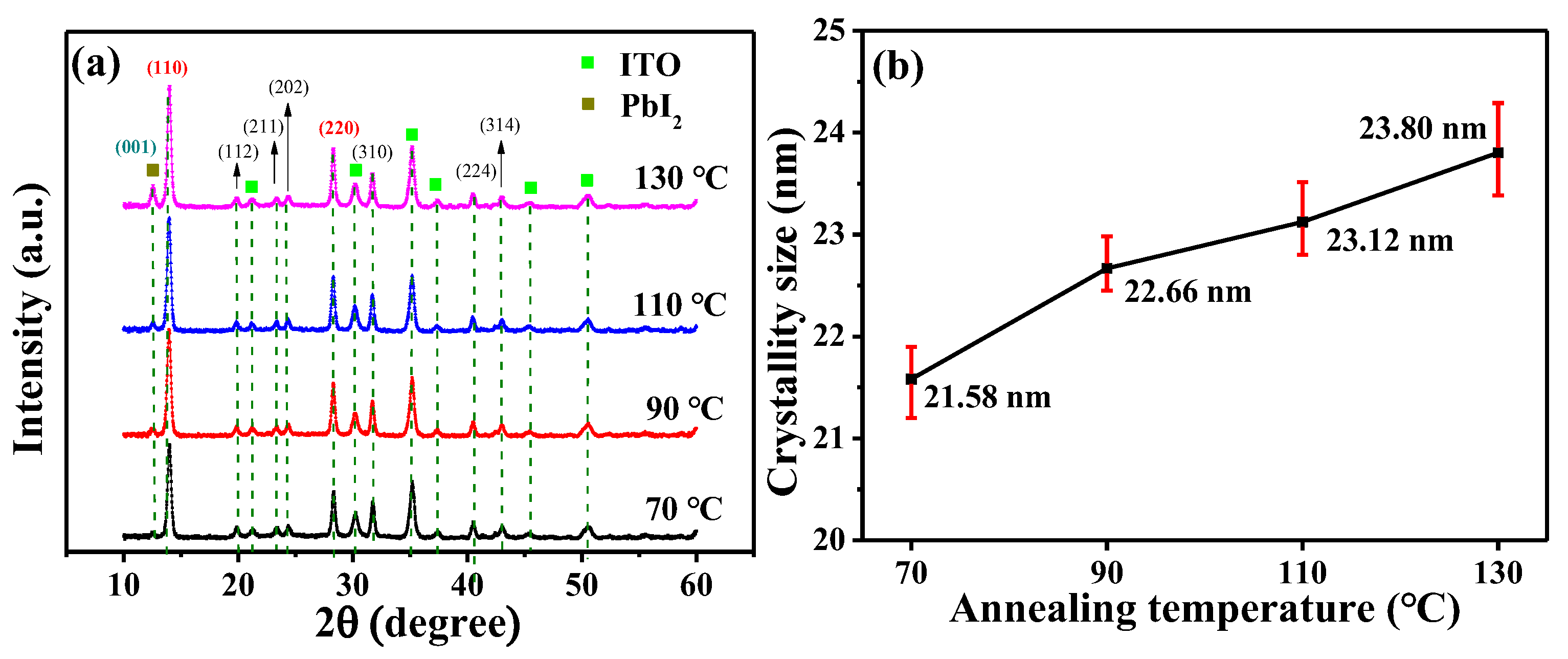
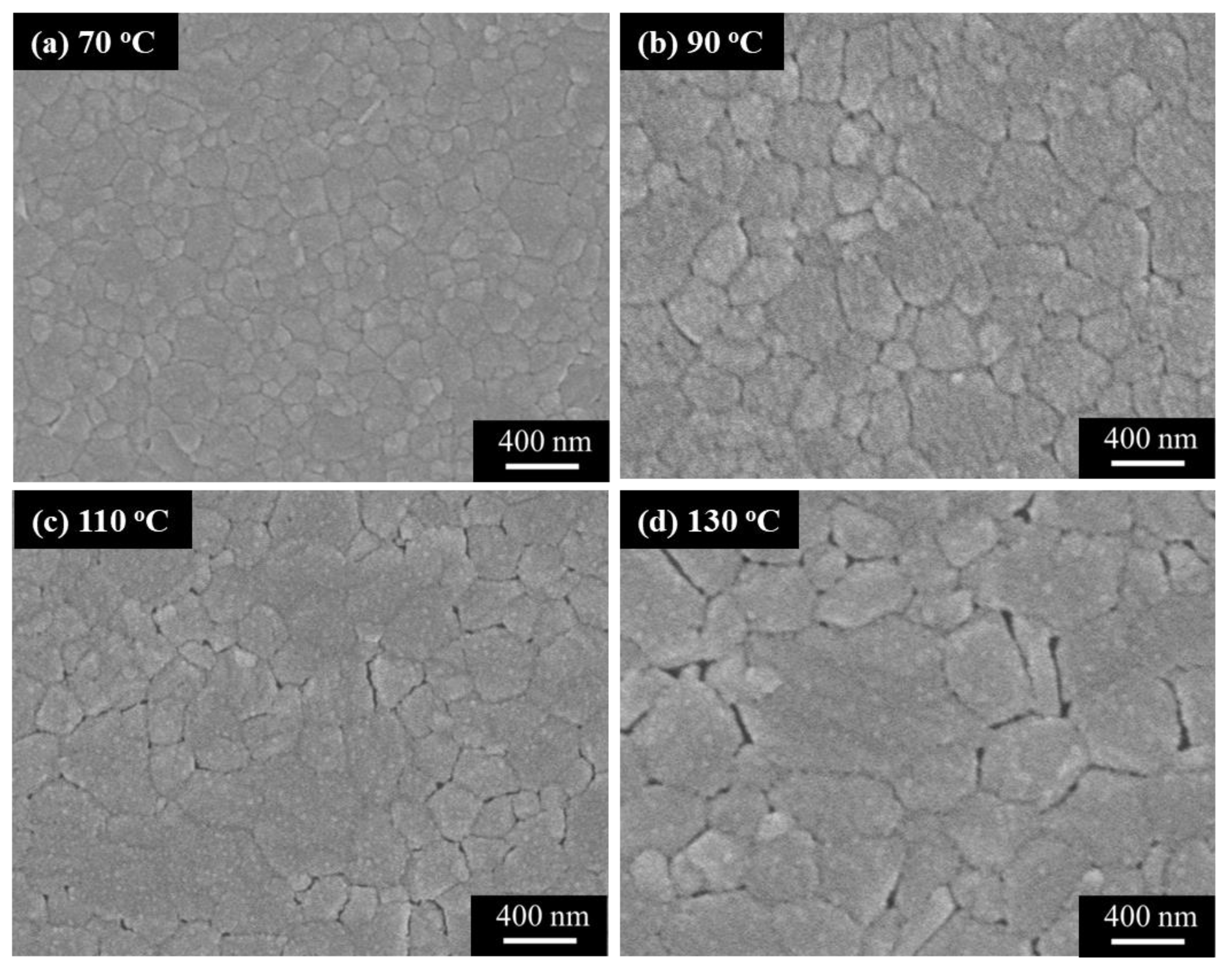
3. Experimental Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diffenbaugh, N.S.; Burke, M. Global warming has increased global economic inequality. Proc. Natl. Acad. Sci. USA 2019, 116, 9808–9813. [Google Scholar] [CrossRef]
- Martinez-Anido, C.B.; Botor, B.; Florita, A.R.; Draxl, C.; Lu, S.; Hamann, H.F.; Hodge, B.M. The value of day-ahead solar power forecasting improvement. Sol. Energy 2016, 129, 192–203. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, X.; Yao, Z.H.; Zhang, M.M. Concentrating solar power development in China. J. Sol. Energy Eng. Trans.-ASME 2010, 132, 021203. [Google Scholar] [CrossRef]
- Yun, S.N.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.D.; Han, X.G.; Hagfeldt, A. New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Lin, H.; Wang, W.L.; Liu, Y.Z.; Li, X.; Li, J.B. New trends for solar cell development and recent progress of dye sensitized solar cells. Front. Mater. Sci. 2009, 3, 345–352. [Google Scholar] [CrossRef]
- Assadi, M.K.; Bakhoda, S.; Saidur, R.; Hanaei, H. Recent progress in perovskite solar cells. Renew. Sust. Energ. Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Xiong, S.B.; Hao, T.Y.; Sun, Y.Y.; Yang, J.M.; Ma, R.R.; Wang, J.L.; Gong, S.J.; Liu, X.J.; Ding, L.M.; Fahlman, M.; et al. Defect passivation by nontoxic biomaterial yields 21% efficiency perovskite solar cells. J. Energy Chem. 2021, 55, 265–271. [Google Scholar] [CrossRef]
- Valadi, K.; Gharibi, S.; Taheri-Ledari, R.; Akin, S.; Maleki, A.; Shalan, A.E. Metal oxide electron transport materials for perovskite solar cells: A review. Environ. Chem. Lett. 2021, 19, 2185–2207. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.W.; You, J.B. SnO2: A wonderful electron transport layer for perovskite solar cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- Yang, J.Y.; Luo, X.R.; Zhou, Y.K.; Li, Y.Y.; Qiu, Q.Q.; Xie, T.F. Recent advances in inverted perovskite solar cells: Designing and fabrication. Int. J. Mol. Sci. 2022, 23, 11792. [Google Scholar] [CrossRef]
- Zuo, C.T.; Bolink, H.J.; Han, H.W.; Huang, J.S.; Cahen, D.; Ding, L.M. Advances in perovskite solar cells. Adv. Sci. 2016, 3, 1500324. [Google Scholar] [CrossRef]
- Park, N.G.; Segawa, H. Research direction toward theoretical efficiency in perovskite solar cells. ACS Photonics 2018, 5, 2970–2977. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chuquer, A.; Han, S.H.; Gaudel, G.S.; Pham, X.H.; Kim, H.M.; Yun, W.J.; Jun, B.H.; Rho, W.Y. Optimizing the aspect ratio of nanopatterned mesoporous TiO2 thin-film layer to improve energy conversion efficiency of perovskite solar cells. Int. J. Mol. Sci. 2021, 22, 12235. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X.J. Solar cell efficiency tables (version 62). Prog. Photovolt. 2023, 31, 651–663. [Google Scholar] [CrossRef]
- Chang, T.C.; Liao, C.Y.; Lee, C.T.; Lee, H.Y. Investigation of the performance of perovskite solar cells with ZnO-covered PC61BM electron transport layer. Materials 2023, 16, 5061. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.S.; Chen, Y.C.; Lee, C.T.; Lee, H.Y. Performance improvement of perovskite solar cells using electron and hole transport layers. Sol. Energy 2018, 174, 897–900. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alenzi, S.M.; Alsolami, A.; Alsofyani, N.; Alfahd, A.; Alzahrani, Y.A.; Aljuwayr, A.; Abduljawad, M. High-performance and stable perovskite solar cells using carbon quantum dots and upconversion nanoparticles. Int. J. Mol. Sci. 2022, 23, 14441. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Zaidi, A.; Hussain, A.; Ul Hassan, N.; Ali, J.; Ahmed, F.; Khan, M.U.; Iqbal, N.; Elnasr, T.A.S.; Helal, M.H. Single- and multilayered perovskite thin films for photovoltaic applications. Nanomaterials 2022, 12, 3208. [Google Scholar] [CrossRef]
- Chang, K.C.; Yeh, T.H.; Lee, H.Y.; Lee, C.T. High performance perovskite solar cells using multiple hole transport layer and modulated FAxMA1−xPbI3 active layer. J. Mater. Sci. Mater. Electron. 2020, 31, 4135–4141. [Google Scholar] [CrossRef]
- Wu, H.T.; Cheng, Y.T.; Leu, C.C.; Wu, S.H.; Shih, C.F. Improving two-step prepared CH3NH3PbI3 perovskite solar cells by Co-doping potassium halide and water in PbI2 layer. Nanomaterials 2019, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.K.; Wang, Q.; Zhou, X.F.; Li, Z.H.; Wang, M.; Lei, Y.T.; Zhang, H.; Wang, H.X.; Jin, Z.W. Guanidium-assisted crystallization engineering for highly efficient CsPbI3 solar cells. J. Mater. Chem. C 2022, 10, 8234–8240. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Yang, X.Y.; Su, R.; Wu, P.; Gong, Q.H.; Zhu, R. High-performance CsPbIxBr3-x all-inorganic perovskite solar cells with efficiency over 18% via spontaneous interfacial manipulation. Adv. Funct. Mater. 2020, 30, 2000457. [Google Scholar] [CrossRef]
- Nam, J.E.; Jo, H.J.; Kang, J.K.; Woo, S.; Hwang, D.K. Optimization of electrolyte components on the performance of organic-dye-sensitized solar cells. J. Nanosci. Nanotechnol. 2017, 17, 8100–8104. [Google Scholar] [CrossRef]
- Jeanbourquin, X.A.; Li, X.E.; Law, C.; Barnes, P.R.F.; Humphry-Baker, R.; Lund, P.; Asghar, M.I.; O’Regan, B.C. Rediscovering a key interface in dye-sensitized solar cells: Guanidinium and iodine competition for binding sites at the dye/electrolyte surface. J. Am. Chem. Soc. 2014, 136, 7286–7294. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive effects of guanidinium iodide on CH3NH3PbI3 perovskite solar cells. Phys. Status Solidi A Appl. Mat. 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Pan, W.C.; Lin, J.M.; Wu, J.H.; Rong, B.; Zhang, X.P.; Chen, Q.; Zhang, M.; Wang, S.B.; Sun, W.H.; Wang, X.B.; et al. Interface modification by formamidine acetate for efficient perovskite solar cells. Sol. Energy 2022, 232, 304–311. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; De Miguel, G.; Nazeeruddin, M.K. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Wang, Y.F.; Chu, Y.M.; Yang, K.; Wang, X.D.; Gao, C.X.; Wang, Q.F.; Du, J.K.; Li, S.; et al. Improving the performance of perovskite solar cells via a novel additive of N,1-fluoroformamidinium iodide with electron-withdrawing fluorine group. Adv. Funct. Mater. 2021, 31, 2010603. [Google Scholar] [CrossRef]
- Li, W.; Thirumurugan, A.; Barton, P.T.; Lin, Z.S.; Henke, S.; Yeung, H.H.M.; Wharmby, M.T.; Bithell, E.G.; Howard, C.J.; Cheetham, A.K. Mechanical tunability via hydrogen bonding in metal–organic frameworks with the perovskite architecture. J. Am. Chem. Soc. 2014, 136, 7801–7804. [Google Scholar] [CrossRef]
- De Marco, N.; Zhou, H.P.; Chen, Q.; Sun, P.Y.; Liu, Z.H.; Meng, L.; Yao, E.P.; Liu, Y.S.; Schiffer, A.; Yang, Y. Guanidinium: A route to enhanced carrier lifetime and open-circuit voltage in hybrid perovskite solar cells. Nano Lett. 2016, 16, 1009–1016. [Google Scholar] [CrossRef]
- Hoang, M.T.; Pannu, A.S.; Yang, Y.; Madani, S.; Shaw, P.; Sonar, P.; Tesfamichael, T.; Wang, H.X. Surface treatment of inorganic CsPbI3 nanocrystals with guanidinium iodide for efficient perovskite light-emitting diodes with high brightness. Nano-Micro Lett. 2022, 14, 69. [Google Scholar] [CrossRef]
- Sarkar, K.; Mukherjee, S.; Mukherjee, S.; Mitra, M.K. Synthesis, characterization and studies on optical, dielectric and magnetic properties of undoped and cobalt doped nanocrystalline bismuth ferrite. J. Inst. Eng. India Ser. D 2014, 95, 135–143. [Google Scholar] [CrossRef]
- He, K.; Chen, N.F.; Wang, C.J.; Wei, L.S.; Chen, J.K. Method for determining crystal grain size by X-ray diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Choi, Y.; Kim, T.; Lim, S.; Park, T. A review on reducing grain boundaries and morphological improvement of perovskite solar cells from methodology and material-based perspectives. Small Methods 2020, 4, 1900569. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, T.L.; Li, J.L.; Zhang, L.; Li, H.T.; Lu, Z.L.; Wang, G. All-inorganic perovskite solar cells with both high open-circuit voltage and stability. Front. Mater. 2020, 6, 330. [Google Scholar] [CrossRef]
- Kazemi, M.A.A.; Jamali, A.; Sauvage, F. A holistic study on the effect of annealing temperature and time on CH3NH3PbI3-based perovskite solar cell characteristics. Front. Energy Res. 2021, 9, 732886. [Google Scholar] [CrossRef]
- Xia, B.; Wu, Z.X.; Dong, H.; Xi, J.; Wu, W.; Lei, T.; Xi, K.; Yuan, F.; Jiao, B.; Xiao, L.X.; et al. Formation of ultrasmooth perovskite films toward highly efficient inverted planar heterojunction solar cells by micro-flowing anti-solvent deposition in air. J. Mater. Chem. A 2016, 4, 6295–6303. [Google Scholar] [CrossRef]
- Nukunudompanich, M.; Budiutama, G.; Suzuki, K.; Hasegawa, K.; Ihara, M. Dominant effect of the grain size of the MAPbI3 perovskite controlled by the surface roughness of TiO2 on the performance of perovskite solar cells. Crystengcomm 2020, 22, 2718–2727. [Google Scholar] [CrossRef]
- Liu, D.Y.; Gangishetty, M.K.; Kelly, T.L. Effect of CH3NH3PbI3 thickness on device efficiency in planar heterojunction perovskite solar cells. J. Mater. Chem. A 2014, 2, 19873–19881. [Google Scholar] [CrossRef]
- Momblona, C.; Malinkiewicz, O.; Roldan-Carmona, C.; Soriano, A.; Gil-Escrig, L.; Bandiello, E.; Scheepers, M.; Edri, E.; Bolink, H.J. Efficient methylammonium lead iodide perovskite solar cells with active layers from 300 to 900 nm. APL Mater. 2014, 2, 081504. [Google Scholar] [CrossRef]
- Xiao, M.D.; Huang, F.Z.; Huang, W.C.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem.-Int. Edit. 2014, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Wong, L.H.; Etgar, L. Effect of perovskite thickness on electroluminescence and solar cell conversion efficiency. J. Phys. Chem. Lett. 2020, 11, 8189–8194. [Google Scholar] [CrossRef] [PubMed]
- Cerdán-Pasarán, A.; López-Luke, T.; Esparza, D.; Zarazúa, I.; De la Rosa, E.; Fuentes-Ramírez, R.; Alatorre-Ordaz, A.; Sánchez-Solís, A.; Torres-Castrod, A.; Zhang, J.Z. Photovoltaic properties of multilayered quantum dot/quantum rod-sensitized TiO2 solar cells fabricated by SILAR and electrophoresis. Phys. Chem. Chem. Phys. 2015, 17, 18590–18599. [Google Scholar] [CrossRef] [PubMed]



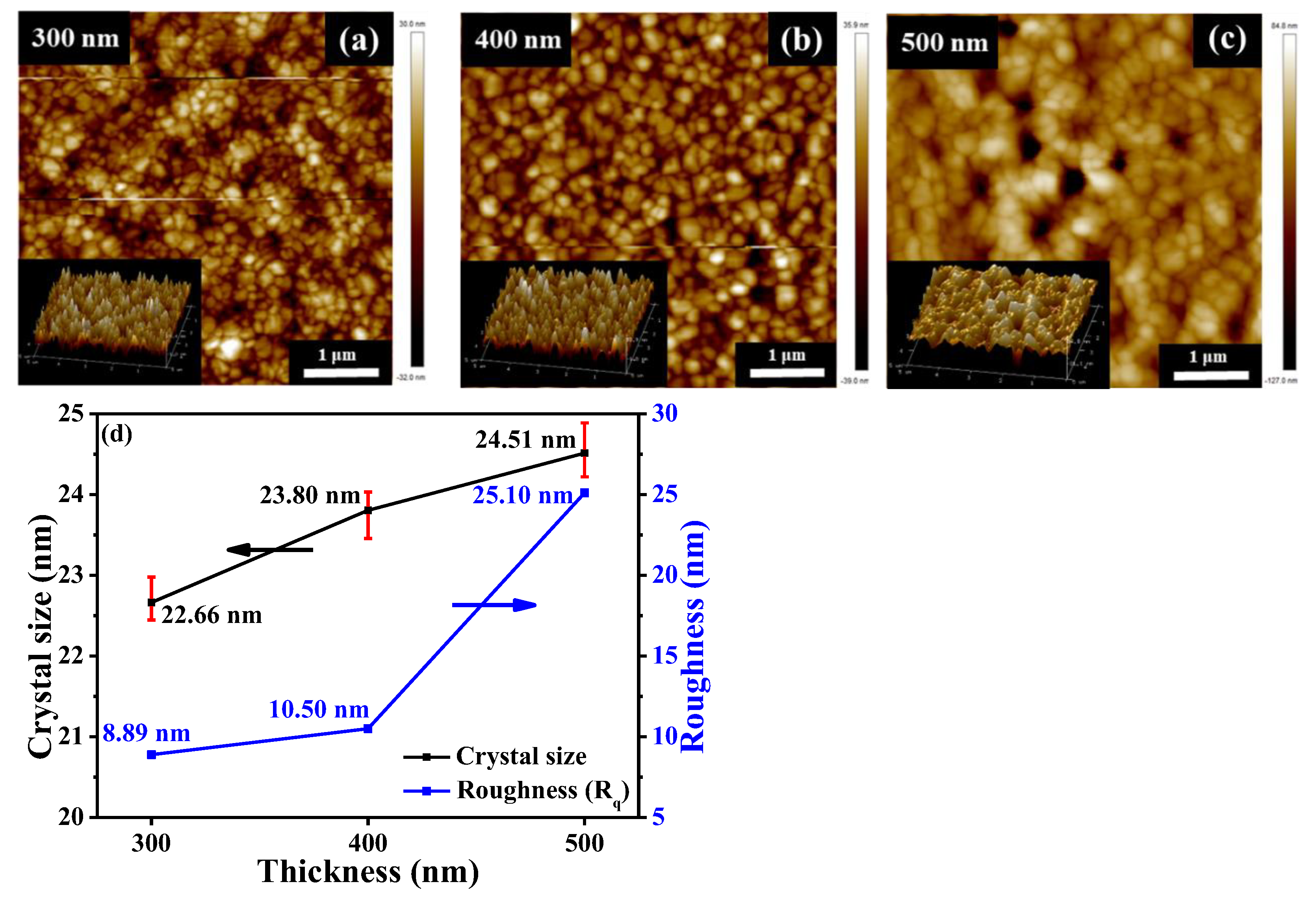

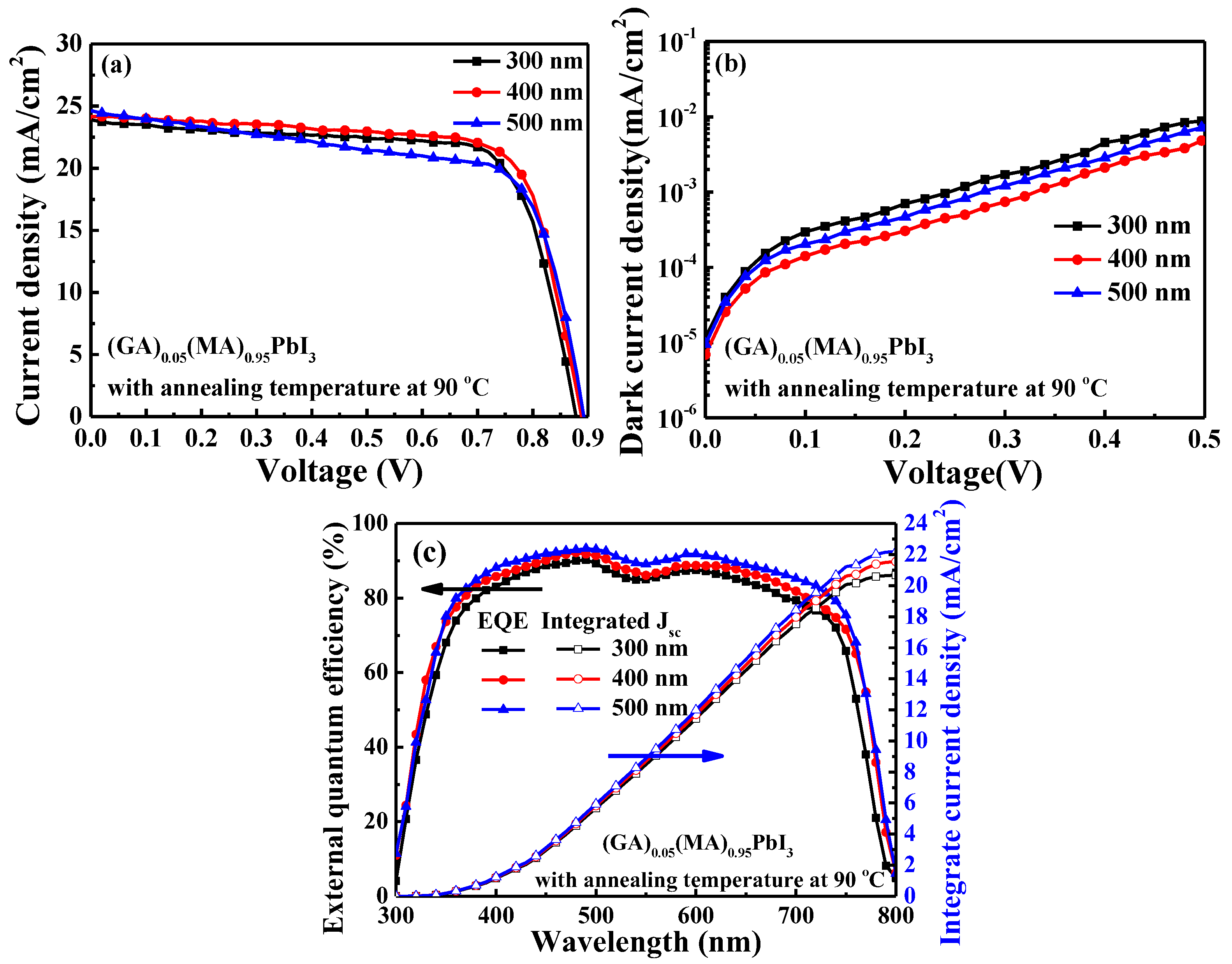

| Perovskite Thickness (nm) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Integrated Jsc (mA/cm2) |
|---|---|---|---|---|---|
| 300 | 0.879 | 23.88 | 73.1 | 15.36 | 20.69 |
| 400 | 0.891 | 24.21 | 73.1 | 15.78 | 21.55 |
| 500 | 0.894 | 24.75 | 66.6 | 14.73 | 22.21 |
| Perovskite Active Layer | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Integrated Jsc (mA/cm2) |
|---|---|---|---|---|---|
| MAPbI3 | 0.783 | 19.71 | 65.0 | 10.03 | 17.05 |
| (GA)0.05(MA)0.95PbI3 | 0.891 | 24.21 | 73.1 | 15.78 | 21.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-C.; Lee, C.-T.; Lee, H.-Y. Investigation of Perovskite Solar Cells Using Guanidinium Doped MAPbI3 Active Layer. Nanomaterials 2024, 14, 657. https://doi.org/10.3390/nano14080657
Chang T-C, Lee C-T, Lee H-Y. Investigation of Perovskite Solar Cells Using Guanidinium Doped MAPbI3 Active Layer. Nanomaterials. 2024; 14(8):657. https://doi.org/10.3390/nano14080657
Chicago/Turabian StyleChang, Ting-Chun, Ching-Ting Lee, and Hsin-Ying Lee. 2024. "Investigation of Perovskite Solar Cells Using Guanidinium Doped MAPbI3 Active Layer" Nanomaterials 14, no. 8: 657. https://doi.org/10.3390/nano14080657






