Elastic Liposomes Containing Calcium/Magnesium Ferrite Nanoparticles Coupled with Gold Nanorods for Application in Photothermal Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Mg0.75Ca0.25Fe2O4 Nanocubes
2.2. Synthesis of Gold Nanorods
2.3. Coupling between MNPs and AuNRs
2.4. Preparation of Elastic Liposomes and Aqueous Elastic Magnetoliposomes
2.5. Structural and Magnetic Characterization
2.6. Measurement of Hyperthermia Capability
2.7. UV-Vis–NIR and Fluorescence Measurements
3. Results and Discussion
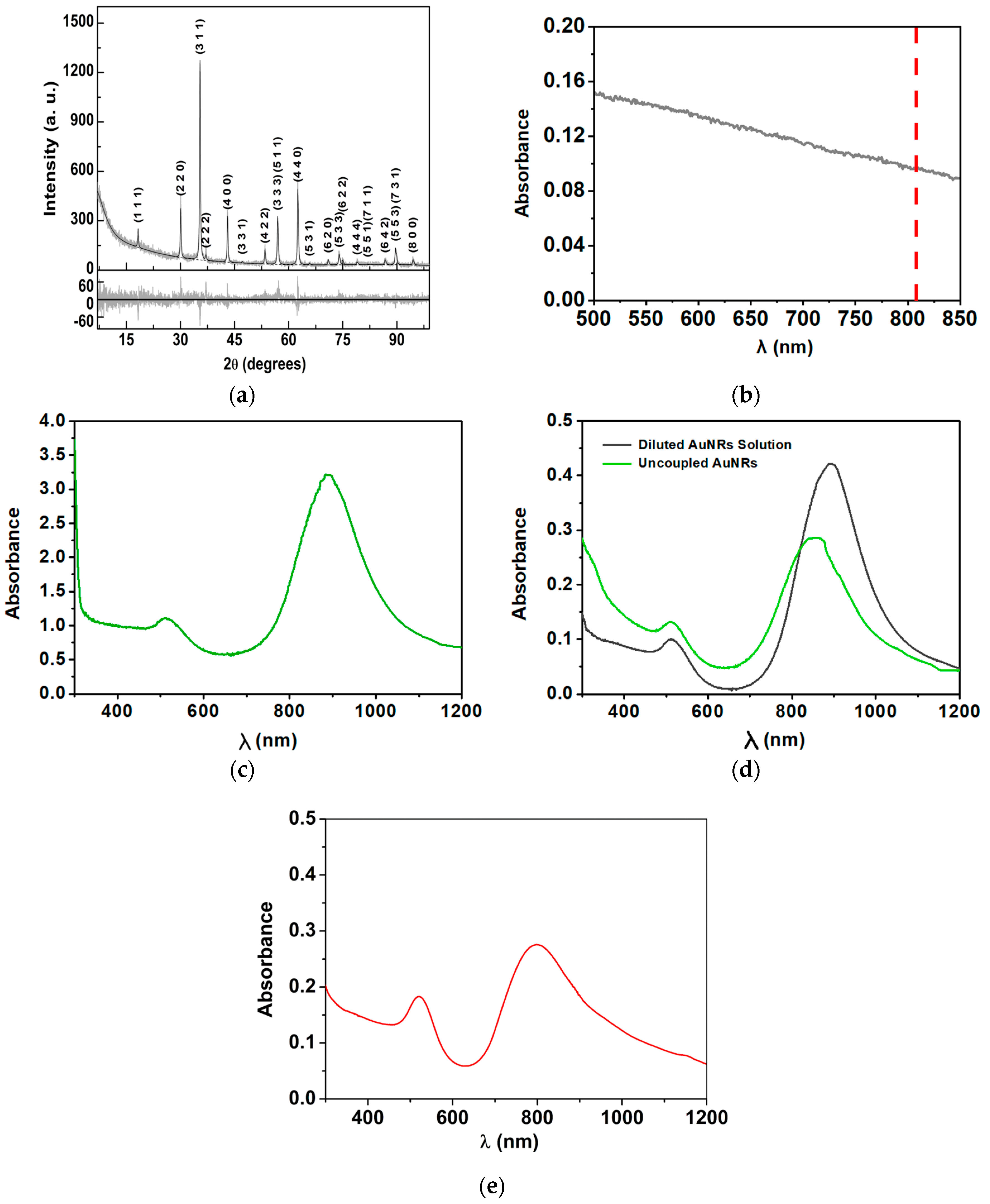
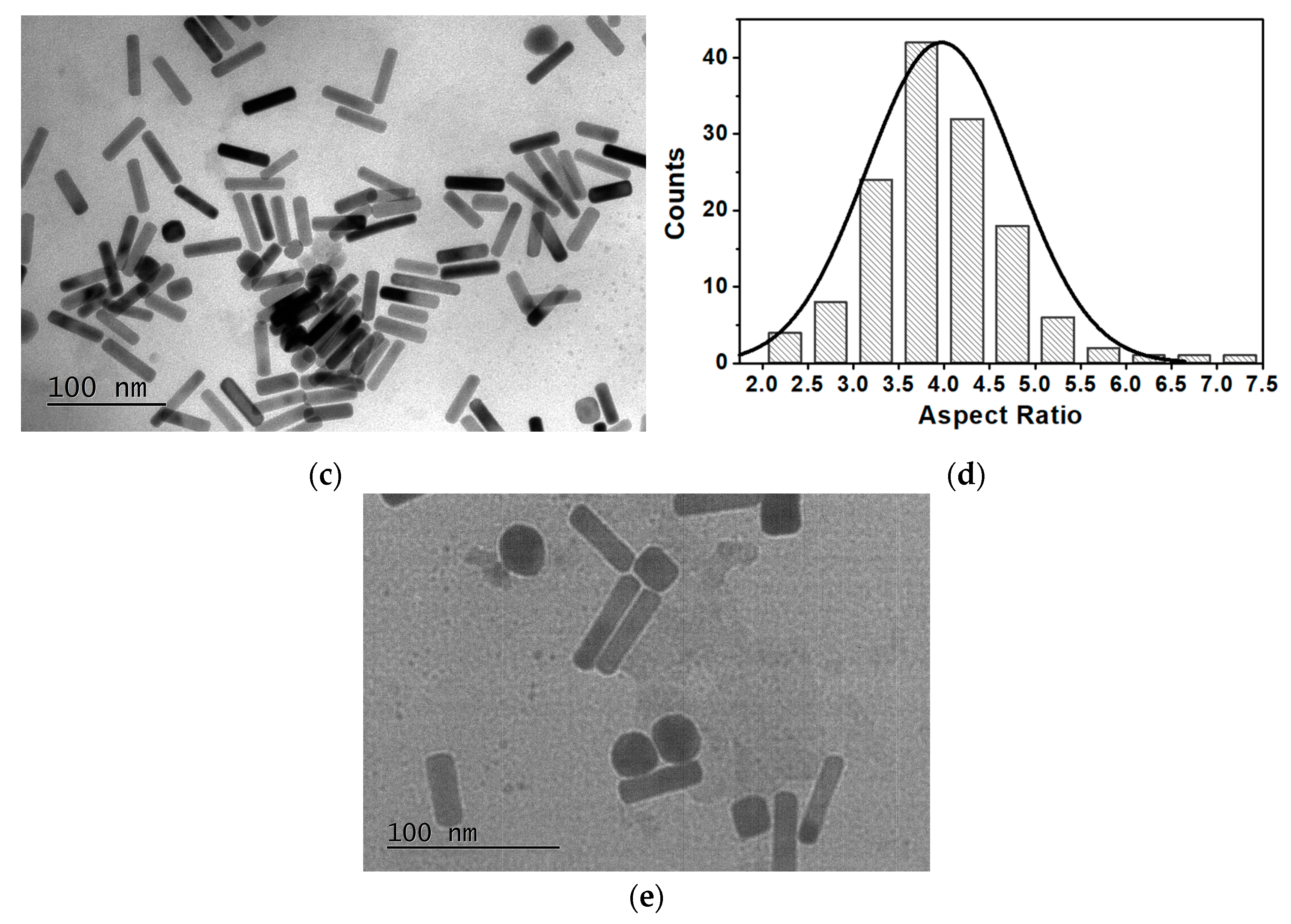
3.1. Magnetoplasmonic Nanoparticles’ Characterization
3.2. Magneto-Plasmonic Elastic Liposomes’ Characterization
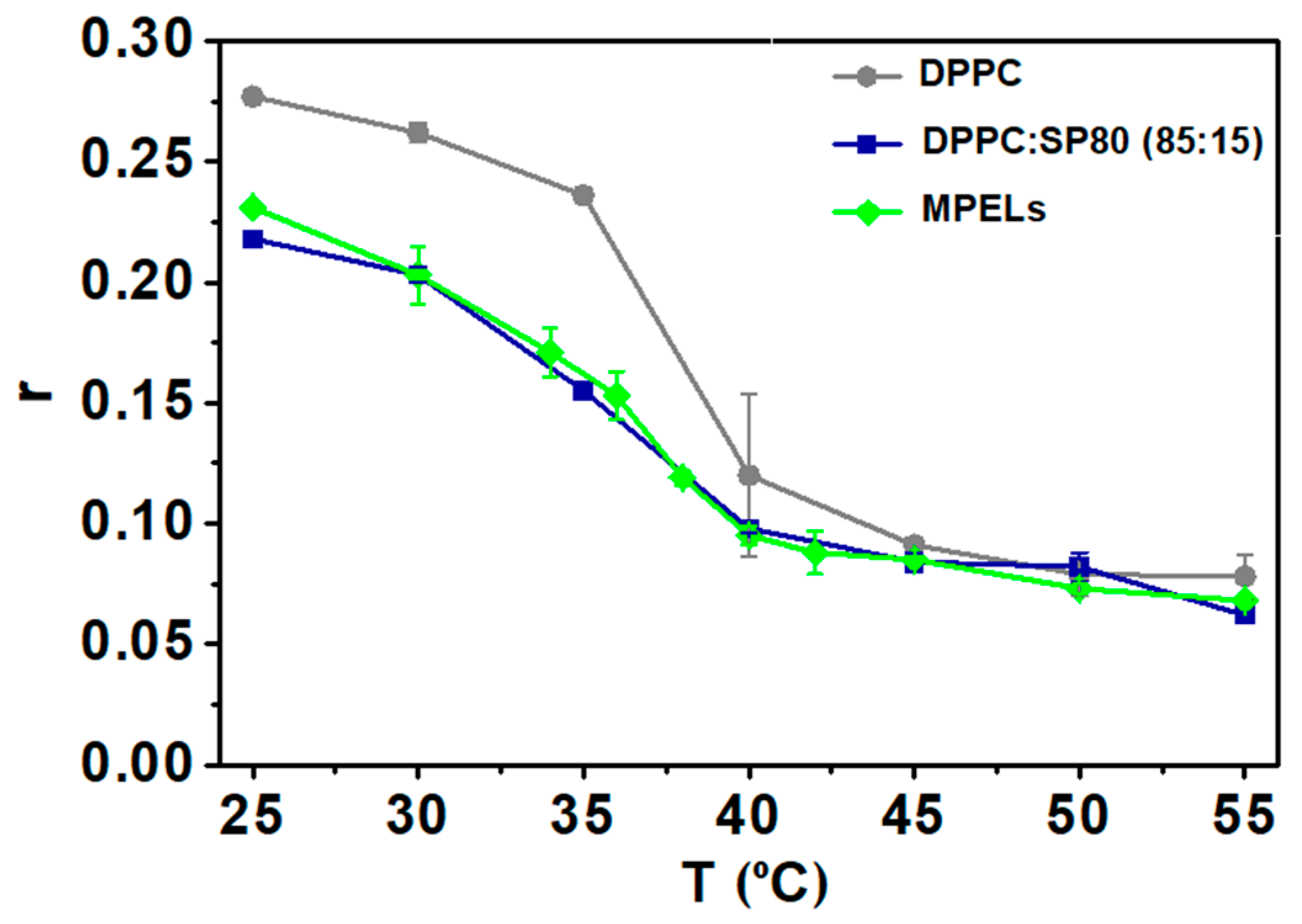
3.2.1. Elastic Properties Investigation
3.2.2. Study of the Phase Transition Temperature of MPELs
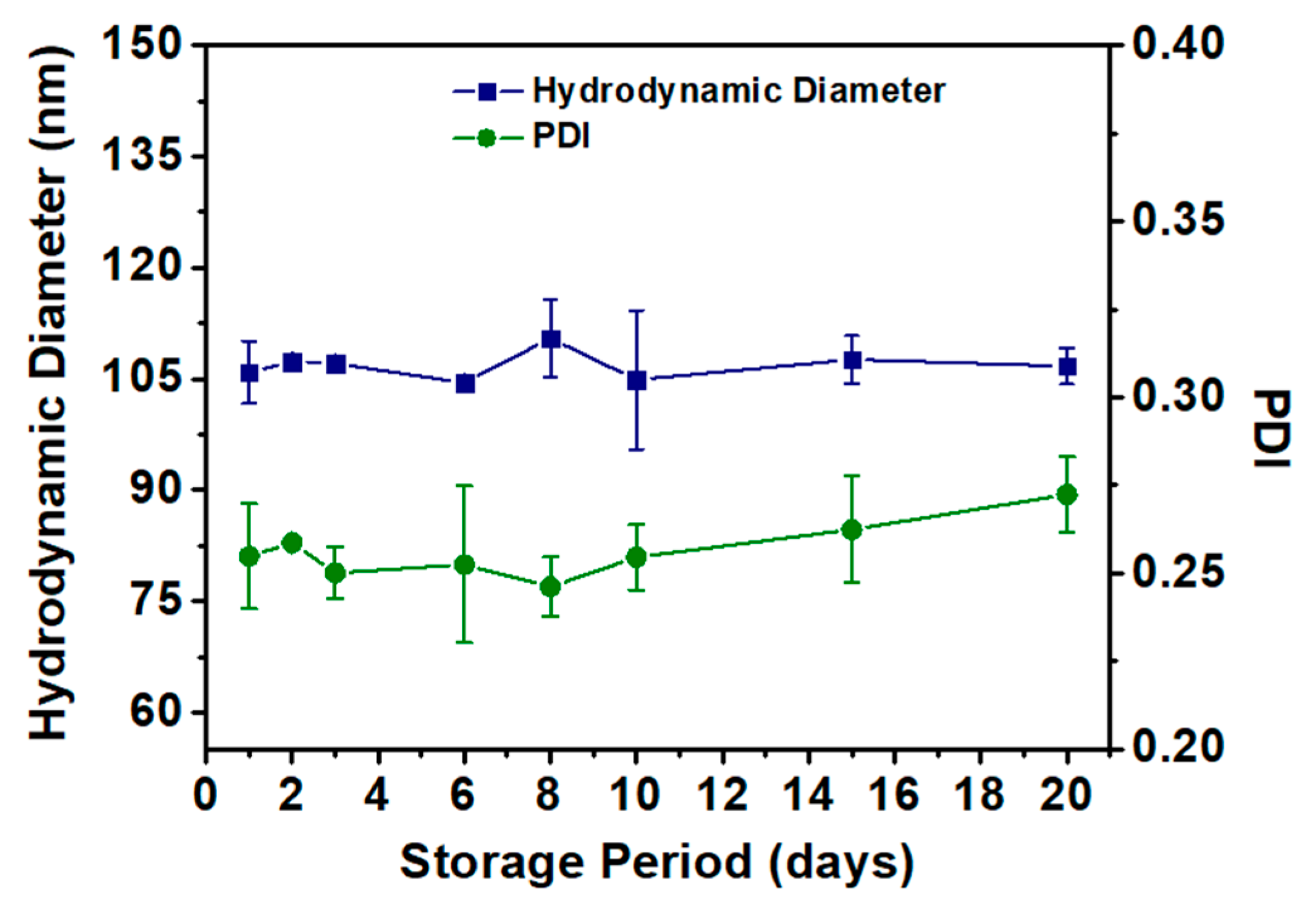
3.2.3. MPELs’ Structural Characterization and Stability
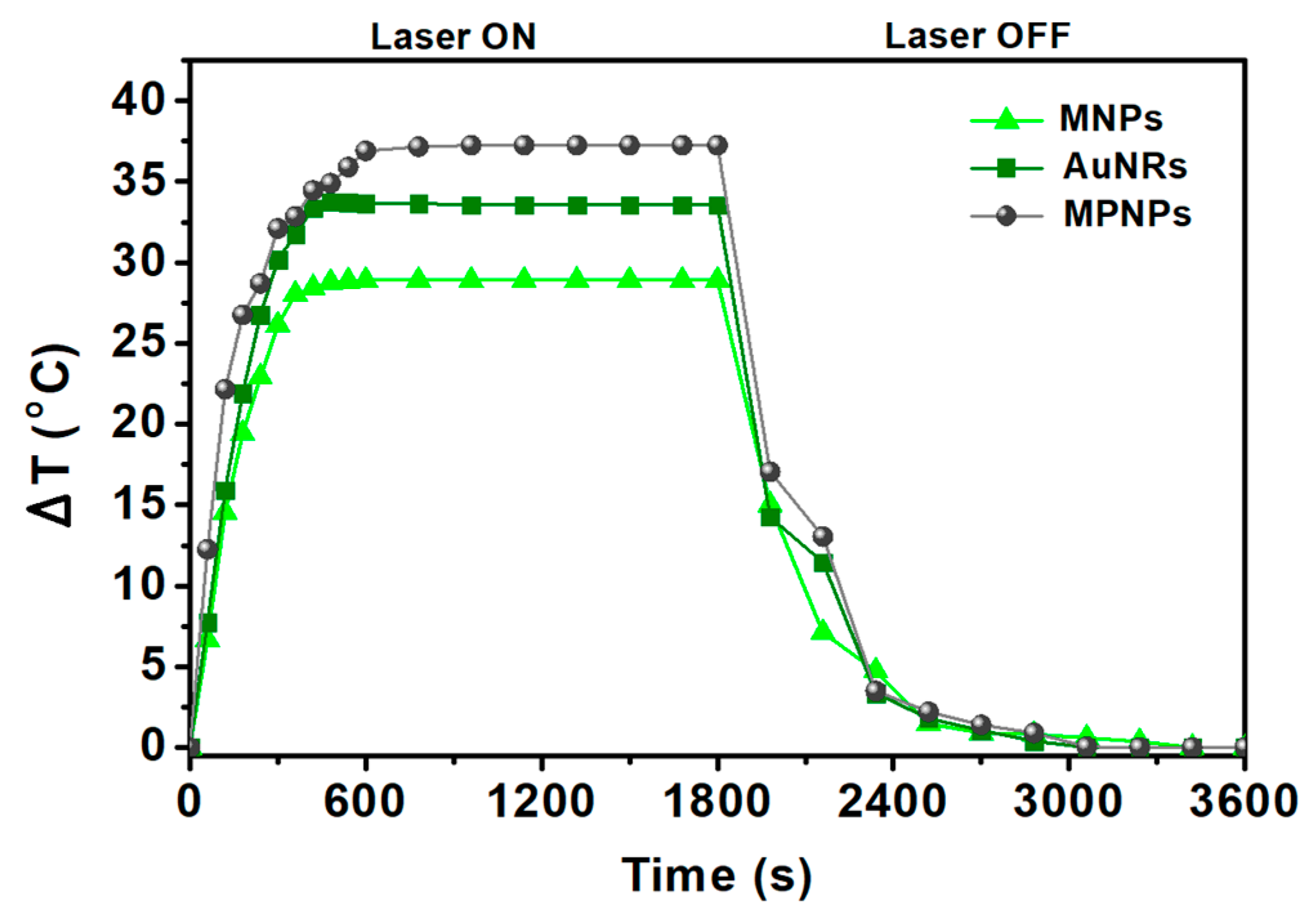
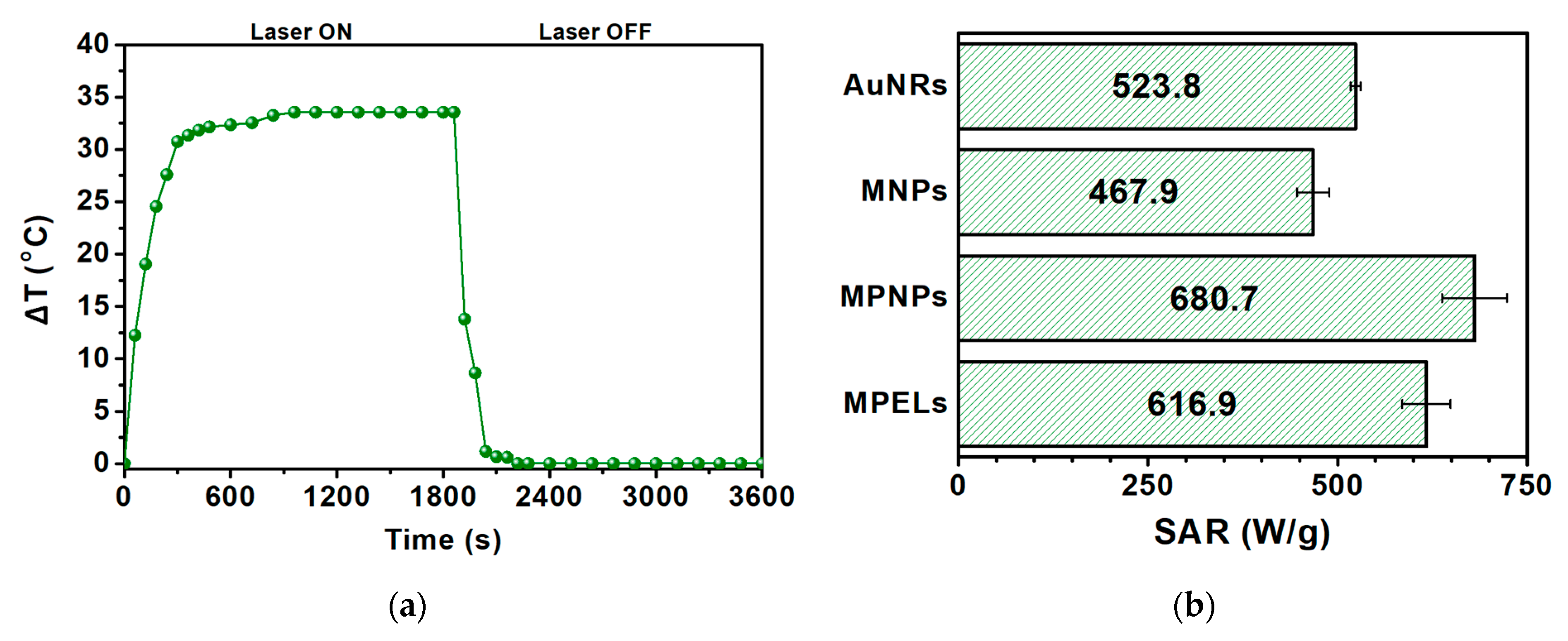
3.2.4. MPELs as Photothermal Hyperthermia Agents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.S.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Matos, C.M. Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Epidemiology and Management of Common Skin Diseases in Children in Developing Countries. Available online: https://apps.who.int/iris/bitstream/handle/10665/69229/WHO_FCH_CAH_05.12_eng.pdf (accessed on 7 August 2023).
- Dar, J.M.; Khalid, S.; Varikuti, S.; Satoskar, A.R.; Khan, G.M. Nano-elastic liposomes as multidrug carrier of sodium stibogluconate and ketoconazole: A potential new approach for the topical treatment of cutaneous Leishmaniasis. Eur. J. Pharm. Sci. 2020, 145, 105256. [Google Scholar] [CrossRef] [PubMed]
- Sadabad, R.K.; Xia, A.; Benkafadar, N.; Faniku, C.; Preciado, D.; Yang, S.; Valdez, T.A. Topical Delivery of Elastic Liposomal Vesicles for Treatment of Middle and Inner Ear Diseases. ACS Appl. Bio Mater. 2022, 5, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Haque, M.W.; Singh, S.K.; Ahmed, F.J. Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using Design Expert: Part II. Drug Deliv. 2016, 23, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.P.; Cai, B.C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomedicine 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta BBA-Rev. Biomembr. 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Bierman, W. The temperature of the skin surface. J. Am. Med. Assoc. 1936, 106, 1158. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Vattikuti, S.V.P.; Manivasagan, P.; Jang, E.S.; Chintagumpala, K.; Kim, K.H. Ca-Doped MgFe2O4 Nanoparticles for Magnetic Hyperthermia and Their Cytotoxicity in Normal and Cancer Cell Lines. ACS Appl. Nano Mater. 2022, 5, 5847–5856. [Google Scholar] [CrossRef]
- Hirazawa, H.; Kusamoto, S.; Aono, H.; Naohara, T.; Mori, K.; Hattori, Y.; Maehara, T.; Watanabe, Y. Preparation of fine Mg1−XCaXFe2O4 powder using reverse coprecipitation method for thermal coagulation therapy in an ac magnetic field. J. Alloys Compd. 2008, 461, 467–473. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Rodrigues, A.R.O.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Castanheira, E.M.S.; Coutinho, P.J.G. Stealth Magnetoliposomes Based on Calcium-Substituted Magnesium Ferrite Nanoparticles for Curcumin Transport and Release. Int. J. Mol. Sci. 2020, 21, 3641. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Fernandes, D.E.M.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Rodrigues, A.R.O.; Castanheira, E.M.S. Magnetoliposomes with Calcium-Doped Magnesium Ferrites Anchored in the Lipid Surface for Enhanced DOX Release. Nanomaterials 2023, 13, 2597. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin. Pharmaceutics 2021, 13, 1248. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.; Khramtsov, M.; Garanina, A.; Mogilnikov, P.; Sviridenkova, N.; Shchetinin, I.; Savchenko, A.; Abakumov, M.; Majouga, A. Synthesis of iron oxide nanorods for enhanced magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 469, 443–449. [Google Scholar] [CrossRef]
- Mona, L.P.; Songca, S.P.; Ajibade, P.A. Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy. Nanotechnol. Rev. 2021, 11, 176–190. [Google Scholar] [CrossRef]
- Lisjak, D.; Mertelj, A. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater. Sci. 2018, 95, 286–328. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Chung, P.F.; Krisnawati, D.I.; Rinawati, F.; Irawan, H.; Kristanto, H.; Kuo, T.R. Gold Nanorod-Decorated Metallic MoS2 Nanosheets for Synergistic Photothermal and Photodynamic Antibacterial Therapy. Nanomaterials 2021, 11, 3064. [Google Scholar] [CrossRef]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagnosis Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.; Han, H.W.; Lim, Y.R.; Manivasagan, P.; Jang, E.S. Triphenylphosphonium-Functionalized Gold Nanorod/Zinc Oxide Core–Shell Nanocomposites for Mitochondrial-Targeted Phototherapy. Pharmaceutics 2024, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Casero, I.M.; de la Presa, P.; Mille, N.; Carrey, J.; Garcia, M.A.; Hernando, A.; Herrasti, P. Hybrid nanoparticles for magnetic and plasmonic hyperthermia. Phys. Chem. Chem. Phys. 2018, 20, 24065–24073. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Santos, L.C.A.; Macedo, D.O.; Rio, I.S.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Plasmonic/magnetic liposomes based on nanoparticles with multicore-shell architecture for chemo/thermotherapy. J. Phys. D Conf. Ser. 2022, 2407, 012051. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of multifunctional liposomes containing magnetic/plasmonic MnFe₂O₄/Au core/shell nanoparticles. Pharmaceutics 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, Q.; Revia, R.; Wang, K.; Tian, B.; Lin, G.; Lee, W.; Hong, Y.-K.; Zhang, M. Iron oxide-carbon core-shell nanoparticles for dual-modal imaging-guided photothermal therapy. J. Control. Release 2018, 289, 70–78. [Google Scholar] [CrossRef]
- Pacheco, A.R.F.; Cardoso, B.D.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Carvalho, V.M.; Rodrigues, R.O.; Coutinho, P.J.G.; Castelo-Grande, T.; Augusto, P.A.; et al. Development of pH-Sensitive Magnetoliposomes Containing Shape Anisotropic Nanoparticles for Potential Application in Combined Cancer Therapy. Nanomaterials 2023, 13, 1051. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H. Oleic acid as the capping agent in the synthesis of noble metal nanoparticles in imidazolium-based ionic liquids. Chem. Commun. 2006, 24, 2545–2547. [Google Scholar] [CrossRef] [PubMed]
- López-Ramón, M.V.; Moreno-Castilla, C.; Fontecha-Cámara, M.A. Removal of the oleylamine capping agent from MnFe2O4 hollow spheres prepared by an Ostwald ripening mechanism. Appl. Surf. Sci. 2023, 612, 155796. [Google Scholar] [CrossRef]
- Salavatov, N.A.; Dement’eva, O.V.; Mikhailichenko, A.I.; Rudoy, V.M. Some Aspects of Seedless Synthesis of Gold Nanorods. Colloid J. 2018, 80, 541–549. [Google Scholar] [CrossRef]
- Ribeiro, B.C.; Alvarez, C.A.R.; Alves, B.C.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives. Materials 2022, 15, 1737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Niu, H.; Zhang, Y.; Liu, J.; Shi, Y.; Zhang, X.; Cai, Y. Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatic hydrocarbons from environmental water and milk samples. J. Chromatogr. A 2012, 1238, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Friedel, P.; Kleeberg, R. IUCr Commission on Powder Diffraction. Newsletter 1998, 20, 5–8. [Google Scholar]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Poojari, C.; Wilkosz, N.; Lira, R.B.; Dimova, R.; Jurkiewicz, P.; Petka, R.; Kepczynski, M.; Róg, T. Behavior of the DPH fluorescence probe in membranes perturbed by drugs. Chem. Phys. Lipids 2019, 223, 104784. [Google Scholar] [CrossRef]
- Pandey, B.N.; Mishra, K.P. Radiation induced oxidative damage modification by cholesterol in liposomal membrane. Radiat. Phys. Chem. 1999, 54, 481–489. [Google Scholar] [CrossRef]
- Pailhé, N.; Wattiaux, A.; Gaudon, M.; Demourgues, A. Correlation between structural features and vis–NIR spectra of α-Fe2O3 hematite and AFe2O4 spinel oxides (A = Mg, Zn). J. Solid State Chem. 2008, 181, 1040. [Google Scholar] [CrossRef]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tang, J.; Zheng, R.; Guo, G.; Donga, A.; Wang, Y.; Yang, W. Nuclear-Targeted Multifunctional Magnetic Nanoparticles for Photothermal Therapy. Adv. Healthc. Mater. 2017, 6, 1601289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, G.; Wen, X.; Li, F.; Ji, X.; Li, Q.; Wu, M.; Cheng, Q.; Yu, Y.; Tang, J.; et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J. Controlled Release 2020, 326, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Mohapatra, J.; Beatty, J.; Elkins, J.; Pandey, N.K.; Chalise, A.; Chen, W.; Jin, M.; Liu, J.P. Iron-based magnetic nanoparticles for multimodal hyperthermia heating. J. Alloys Compd. 2021, 871, 159475. [Google Scholar] [CrossRef]
- Qiu, E.; Chen, X.; Yang, D.P.; Regulacio, M.D.; Ramos, R.M.C.R.; Luo, Z.; Wu, Y.L.; Lin, M.; Li, Z.; Loh, X.J.; et al. Fabricating Dual-Functional Plasmonic–Magnetic Au@MgFe2O4 Nanohybrids for Photothermal Therapy and Magnetic Resonance Imaging. ACS Omega 2022, 7, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Ashokkumar, S.; Manohar, A.; Joe, A.; Han, H.W.; Seo, S.H.; Thambi, T.; Duong, H.S.; Kaushik, N.K.; Kim, K.H.; et al. Biocompatible Calcium Ion-Doped Magnesium Ferrite Nanoparticles as a New Family of Photothermal Therapeutic Materials for Cancer Treatment. Pharmaceutics 2023, 15, 1555. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for antitumor drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef]
- Encarnación, C.; Jungwirth, F.; Vila-Liarte, D.; Renero-Lecuna, C.; Kavak, S.; Orue, I.; Wilhelm, C.; Bals, S.; Henriksen-Lacey, M.; Aberasturi, D.J.; et al. Hybrid Core–Shell Nanoparticles for Cell-Specific Magnetic Separation and Photothermal Heating. J. Mater. Chem. B 2023, 11, 5574–5585. [Google Scholar] [CrossRef]
- Brullot, W.; Verbiest, T. Optical Properties of Magnetic-Plasmonic Nanoparticle Multilayers. Proc. SPIE 2014, 9163, 362–374. [Google Scholar] [CrossRef]
- Guo, J.; Shi, L.; Wu, L.; Pan, S.; Yuan, X.; Zhao, J. Spin-Polarized Electron Transport in Highly Reduced MgFe2O4-δ. Mater. Res. Express 2018, 5, 126301. [Google Scholar] [CrossRef]
- Cabanac, M.; Massonnet, B. Thermoregulatory responses as a function of core temperature in humans. J. Physiol. 1977, 265, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.; Oikonomou, S.; Konstantinou, C.; Makris, K.C. A randomized cross-over trial investigating differences in 24-h personal air and skin temperatures using wearable sensors between two climatologically contrasting settings. Sci. Rep. 2021, 11, 22020. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.R. Membrane “fluidity” as detected by diphenylhexatriene probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Pereira, M.; Rodrigues, A.R.O.; Amaral, L.; Côrte-Real, M.; Santos-Pereira, C.; Castanheira, E.M.S. Bovine Lactoferrin-Loaded Plasmonic Magnetoliposomes for Antifungal Therapeutic Applications. Pharmaceutics 2023, 15, 2162. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Biophys. Acta BBA-Rev. Biomembr. 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Shi, X.; Perry, H.L.; Wilton-Ely, J.D.E.T. Strategies for the functionalization of gold nanorods to reduce toxicity and aid clinical translation. Nanotheranostics 2021, 5, 155–165. [Google Scholar] [CrossRef]
- Teixeira, P.V.; Adega, F.; Martins-Lopes, P.; Machado, R.; Lopes, C.C.; Lúcio, M. pH-Responsive Hybrid Nanoassemblies for Cancer Treatment: Formulation Development, Optimization, and In Vitro Therapeutic Performance. Pharmaceutics 2023, 15, 326. [Google Scholar] [CrossRef] [PubMed]


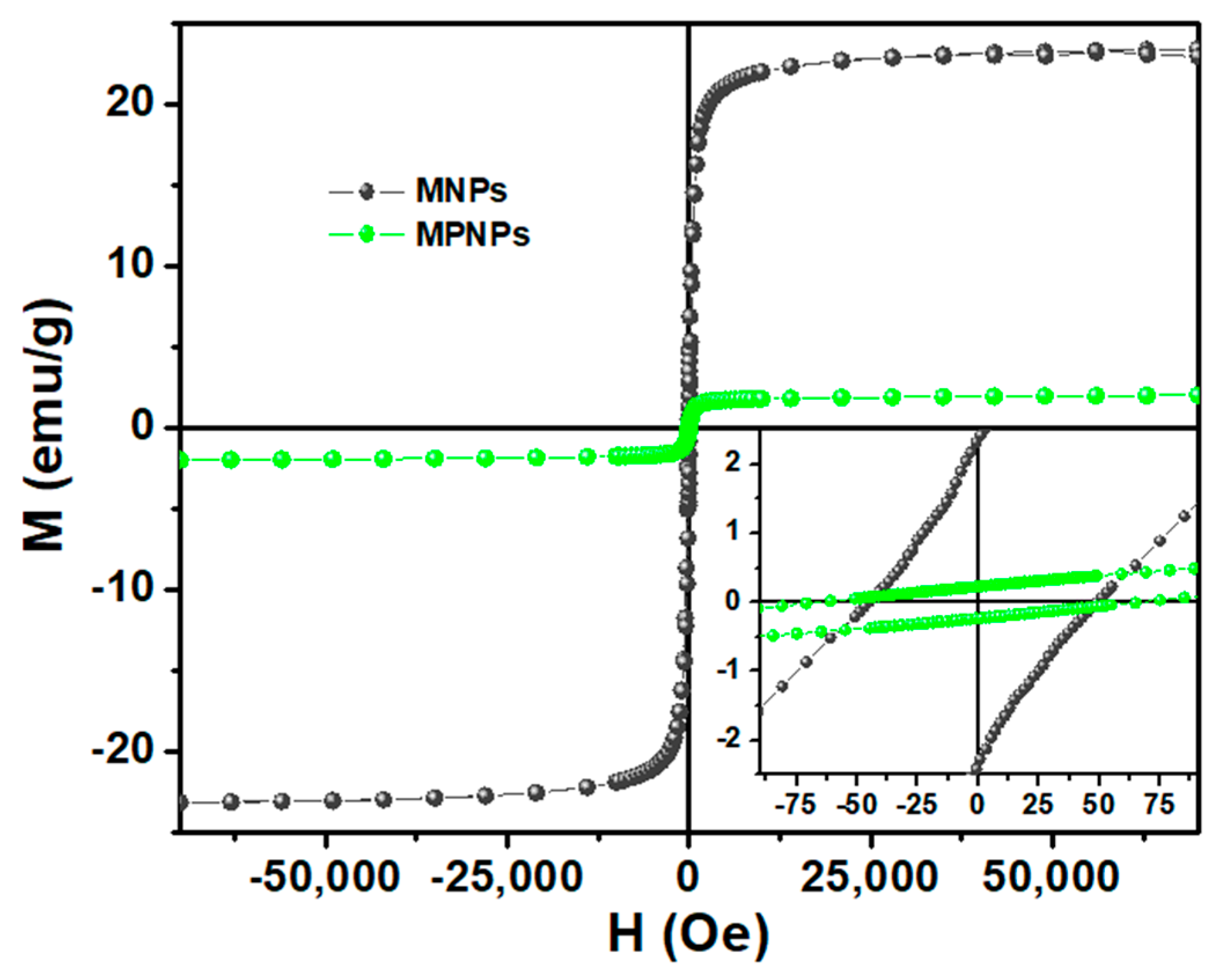

| Samples | Hc (Oe) | Ms (emu/g) | Mr (emu/g) | Mr/Ms |
|---|---|---|---|---|
| MNPs | 49.0 | 23.5 | 2.4 | 0.102 |
| MPNPs | 67.2 | 2.20 | 0.23 | 0.105 |
| Sample | SAR (W/g) | ΔT (°C) |
|---|---|---|
| AuNRs | 523.8 ± 6.7 | 33.6 |
| MNPs | 467.9 ± 21 | 28.9 |
| MPNPs | 680.7 ± 43 | 37.2 |
| Temperature | |||||
|---|---|---|---|---|---|
| 25 °C | 37 °C | ||||
| Formulation | Ratio | r | η (P) | r | η (P) |
| DPPC | --- | 0.277 ± 0.003 | 7.9 ± 0.3 | 0.236 ± 0.003 | 4.5 ± 0.2 |
| DPPC:SP60 | 70:30 | 0.261 ± 0.015 | 6.4 ± 1.2 | 0.209 ± 0.020 | 3.3 ± 0.8 |
| 80:20 | 0.249 ± 0.014 | 5.5 ± 1 | 0.242 ± 0.005 | 4.9 ± 0.3 | |
| 85:15 | 0.252 ± 0.005 | 5.5 ± 0.3 | 0.232 ± 0.014 | 4.4 ± 0.7 | |
| DPPC:SP65 | 70:30 | 0.222 ± 0.004 | 3.8 ± 0.2 | 0.197 ± 0.004 | 2.9 ± 0.1 |
| 80:20 | 0.243 ± 0.003 | 4.9 ± 0.2 | 0.223 ± 0.019 | 3.9 ± 0.8 | |
| 85:15 | 0.230 ± 0.004 | 4.2 ± 0.2 | 0.226 ± 0.008 | 4.0 ± 0.5 | |
| DPPC:SP80 | 70:30 | 0.225 ± 0.015 | 4.0 ± 0.6 | 0.135 ± 0.002 | 1.4 ± 0.03 |
| 80:20 | 0.248 ± 0.007 | 5.3 ± 0.5 | 0.144 ± 0.002 | 1.6 ± 0.04 | |
| 85:15 * | 0.218 ± 0.003 | 3.63 ± 0.01 | 0.125 ± 0.018 | 1.3 ± 0.3 | |
| DPPC:SP85 | 70:30 | 0.207 ± 0.005 | 3.2 ± 0.2 | 0.112 ± 0.004 | 1.6 ± 0.05 |
| 80:20 | 0.230 ± 0.010 | 4.2 ± 0.5 | 0.107 ± 0.002 | 1.3 ± 0.03 | |
| 85:15 | 0.231 ± 0.018 | 4.4 ± 0.9 | 0.135 ± 0.024 | 1.5 ± 0.4 | |
| DPPC:TW80 | 70:30 | 0.274 ± 0.006 | 7.5 ± 0.7 | 0.251 ± 0.002 | 5.4 ± 0.1 |
| 80:20 | 0.282 ± 0.003 | 8.5 ± 0.4 | 0.236 ± 0.011 | 4.5 ± 0.5 | |
| 85:15 | 0.269 ± 0.015 | 7.2 ± 1 | 0.224 ± 0.008 | 3.9 ± 0.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, A.R.F.; Barros, A.M.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Rodrigues, A.R.O.; Castanheira, E.M.S. Elastic Liposomes Containing Calcium/Magnesium Ferrite Nanoparticles Coupled with Gold Nanorods for Application in Photothermal Therapy. Nanomaterials 2024, 14, 679. https://doi.org/10.3390/nano14080679
Pacheco ARF, Barros AM, Amorim CO, Amaral VS, Coutinho PJG, Rodrigues ARO, Castanheira EMS. Elastic Liposomes Containing Calcium/Magnesium Ferrite Nanoparticles Coupled with Gold Nanorods for Application in Photothermal Therapy. Nanomaterials. 2024; 14(8):679. https://doi.org/10.3390/nano14080679
Chicago/Turabian StylePacheco, Ana Rita F., Ana Margarida Barros, Carlos O. Amorim, Vítor S. Amaral, Paulo J. G. Coutinho, Ana Rita O. Rodrigues, and Elisabete M. S. Castanheira. 2024. "Elastic Liposomes Containing Calcium/Magnesium Ferrite Nanoparticles Coupled with Gold Nanorods for Application in Photothermal Therapy" Nanomaterials 14, no. 8: 679. https://doi.org/10.3390/nano14080679
APA StylePacheco, A. R. F., Barros, A. M., Amorim, C. O., Amaral, V. S., Coutinho, P. J. G., Rodrigues, A. R. O., & Castanheira, E. M. S. (2024). Elastic Liposomes Containing Calcium/Magnesium Ferrite Nanoparticles Coupled with Gold Nanorods for Application in Photothermal Therapy. Nanomaterials, 14(8), 679. https://doi.org/10.3390/nano14080679









