Sensitivity-Enhanced, Room-Temperature Detection of NH3 with Alkalized Ti3C2Tx MXene
Abstract
:1. Introduction
2. Materials and Methods
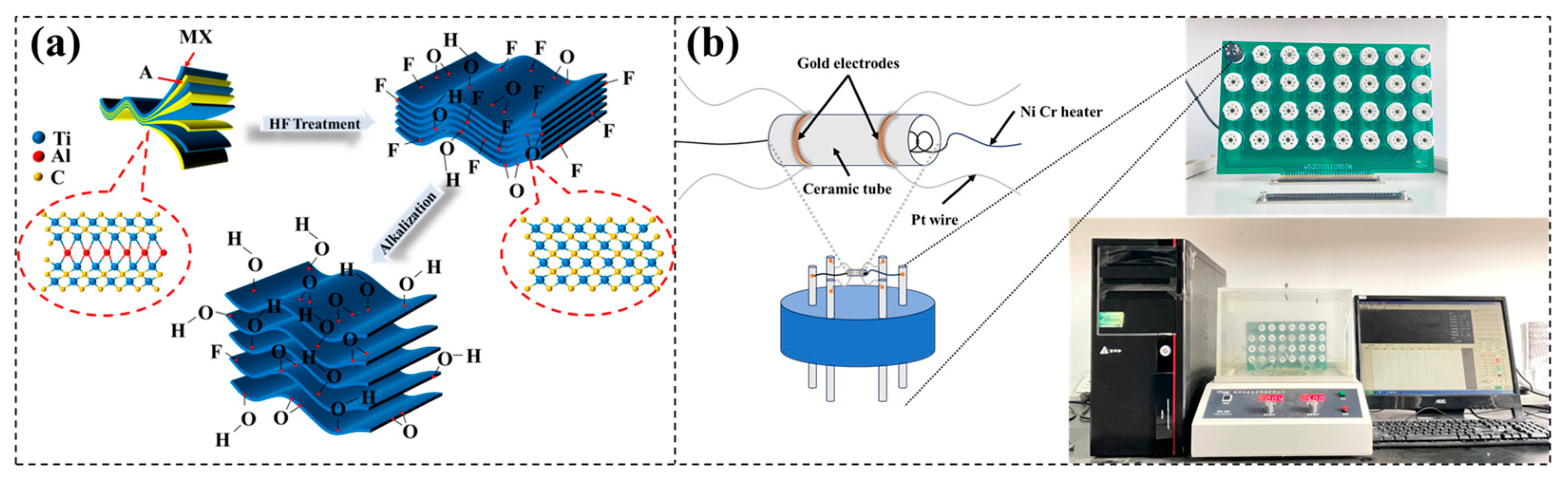
2.1. Preparation of Ti3C2Tx MXene
2.2. Preparation of Alkalized Ti3C2Tx MXene
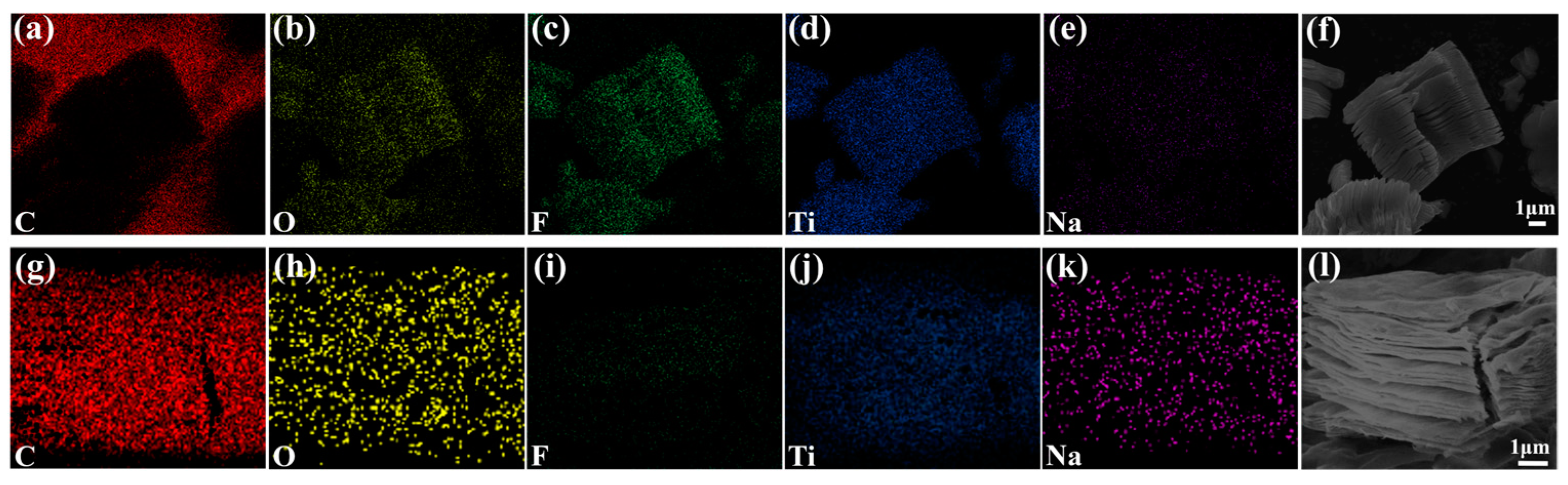
2.3. Characterization
2.4. Fabrication and Measurement of Gas Sensors
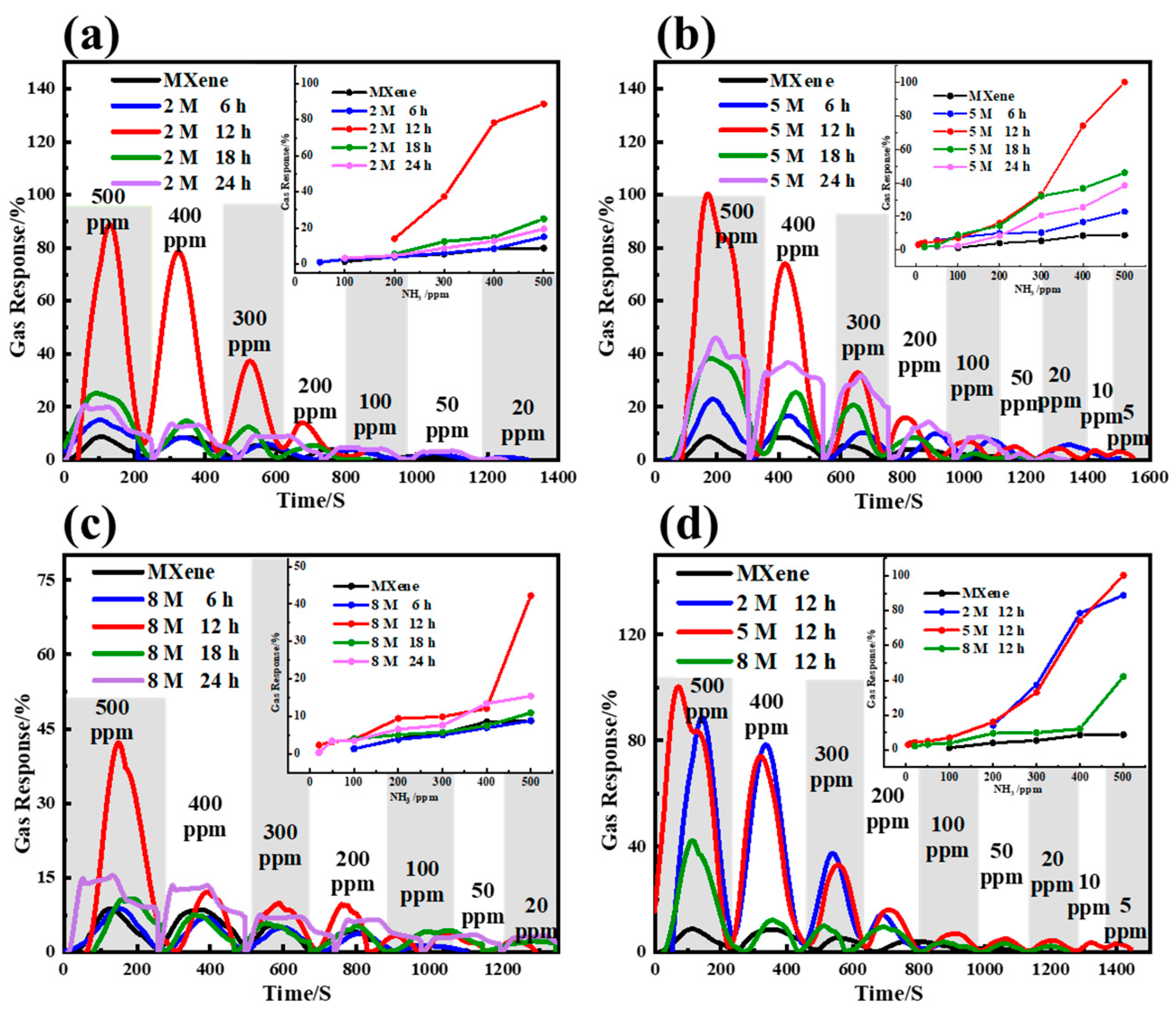
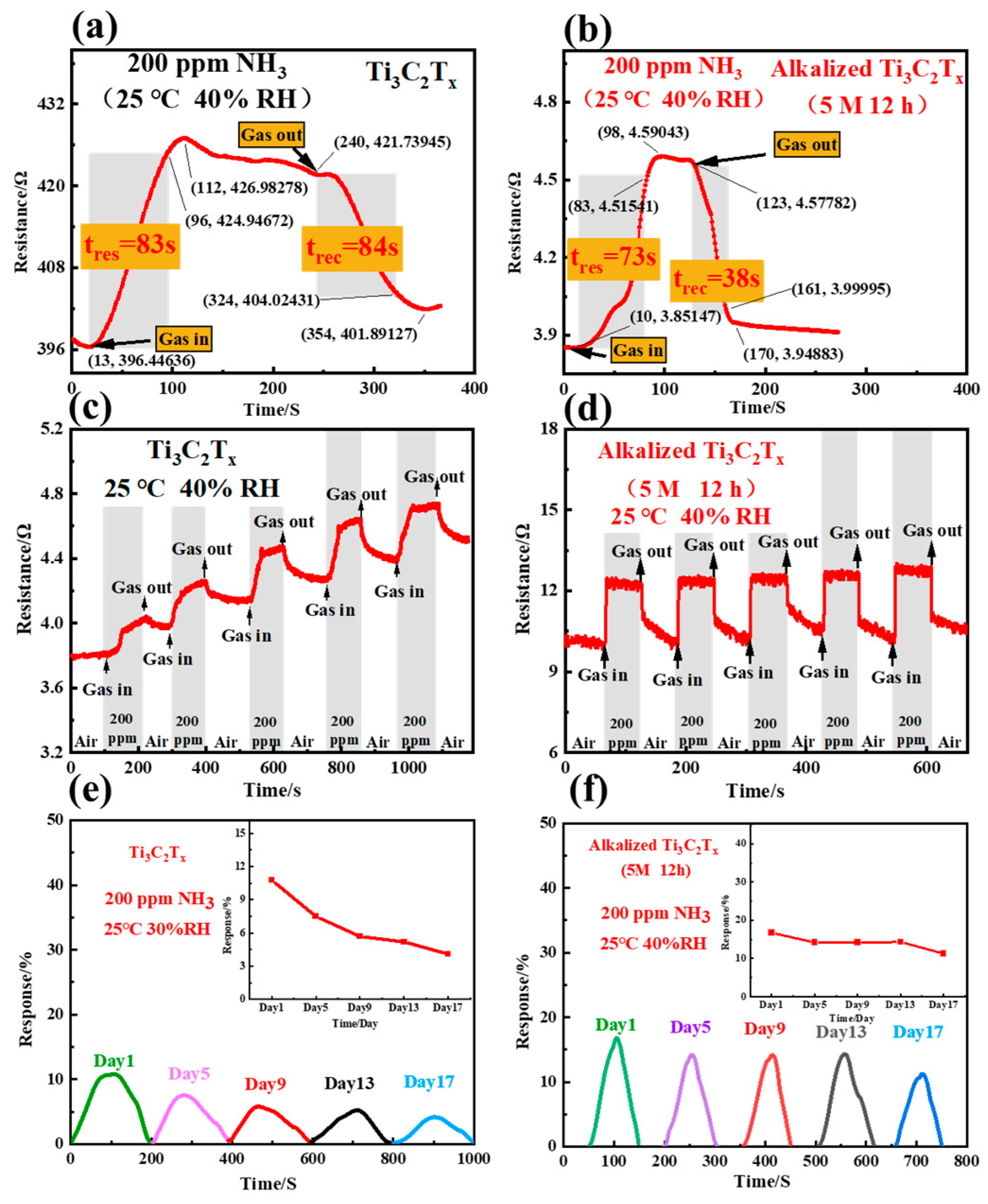
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grabowski, K.; Srivatsa, S.; Vashisth, A.; Mishnaevsky, L.; Uhl, T. Recent advances in MXene-based sensors for Structural Health Monitoring applications: A review. Measurement 2022, 189, 110575. [Google Scholar] [CrossRef]
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D.; Li, M.; Zhou, C.; Yu, D.; Lin, Y. Recent advances in flexible and wearable chemo- and bio-sensors based on two-dimensional transition metal carbides and nitrides (MXenes). Mater. Chem. B 2022, 10, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Le, M.H.; Safaeian, H.; Kim, T.-U.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Room Temperature Chemiresistive Gas Sensors Based on 2D MXenes. Sensors 2023, 23, 8829. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Liu, J.; Liu, Y.; Yang, R.; Li, L.; Zhao, Q.; Gao, J.; Zhu, G.; Zhu, B.; et al. Metal–organic framework-derived mesoporous rGO–ZnO composite nanofibers for enhanced isopropanol sensing properties. Sens. Actuators B Chem. 2023, 378, 133108. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room temperature Ggas sensing of two-dimensional titanium carbide (MXene). J. ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Zhang, H.; Zhang, D.; Zhou, P.; Huang, J. Suspended SnS2 Layers by Light Assistance for Ultrasensitive Ammonia Detection at Room Temperature. Adv. Funct. Mater. 2018, 28, 1801035. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, B.; Li, Y.; Kang, D.; Chen, Z.; Wu, Y. The effect of rigid phenoxyl substituent on the NH3-sensing properties of tetra-α-(4-tert-butylphenoxyl)-metallophthalocyanine/reduced graphene oxide hybrids. RSC Adv. 2017, 7, 22599–22609. [Google Scholar] [CrossRef]
- Naguib, M.; Mochali, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Kushwah, A.; Kumar, M. Two-dimensional MXenes: Recent emerging applications. J. RSC Adv. 2022, 12, 25172–25193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Zhu, X.; Jiang, Y.; Chang, X.; Sun, S. Two-dimensional transition metal MXene-based gas sensors: A review. Chin. Chem. Lett. 2024, 35, 108286. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. MXene-based gas sensors. J. Mater. Chem. C 2021, 9, 15735–15754. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Aich, S. Recent progress in surface and heterointerface engineering of 2D MXenes for gas sensing applications. Coord. Chem. Rev. 2024, 500, 215542. [Google Scholar] [CrossRef]
- Liu, Z.; Han, D.; Liu, L.; Li, D.; Han, X.; Chen, Y.; Liu, X.; Zhuo, K.; Cheng, Y.; Sang, S. Ultrasensitive ammonia gas sensor based on Ti3C2Tx/Ti3AlC2 planar composite at room temperature. Sens. Actuators B Chem. 2023, 378, 133149. [Google Scholar] [CrossRef]
- Ljubek, G.; Kralj, M.; Roković, M.K. Fluorine-free mechanochemical synthesis of MXene. Mater. Sci. Technol. 2023, 39, 1645–1649. [Google Scholar] [CrossRef]
- Báez, L.R.D.J.; Rosas, A.S.; Mahale, P.; Mallouk, T.E. Chelation-Based Route to Aluminum-Free Layered Transition Metal Carbides (MXenes). ACS Omega 2023, 8, 41969–41976. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Que, W. A novel two-dimensional accordion-like titanium carbide (MXene) for adsorption of Cr(VI) from aqueous solution. J. Adv. Dielectr. 2018, 8, 1850035. [Google Scholar] [CrossRef]
- Liang, R.; Zhong, L.; Zhang, Y.; Tang, Y.; Lai, M.; Han, T.; Wang, W.; Bao, Y.; Ma, Y.; Gan, S.; et al. Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring. Membranes 2023, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasor, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, J.; Xu, J.; Hu, H.; Ho, D. Atomic Plasma Grafting: Precise Control of Functional Groups on Ti3C2Tx MXene for Room Temperature Gas Sensors. ACS Appl. Mater. Interfaces 2023, 15, 12232–12239. [Google Scholar] [CrossRef] [PubMed]
- Fagerli, F.H.; Vullum, P.E.; Grande, T.; Wang, Z.; Selbach, S.M.; Wiik, K.; Wagner, N.P. Bulk substitution of F-terminations from Ti3C2Tx MXene by cation pillaring and gas hydrolyzation. FlatChem 2023, 38, 100470. [Google Scholar] [CrossRef]
- Chen, L.; Bi, Y.; Jing, Y.; Dai, J.; Li, Z.; Sun, C.; Men, A.; Xie, H.; Hu, M. Phosphorus Doping Strategy-Induced Synergistic Modification of Interlayer Structure and Chemical State in Ti3C2Tx toward Enhancing Capacitance. Molecules 2023, 28, 4892. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Näslund, L.-Å.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. On the organization and thermal behavior of functional groups on Ti3C2MXene surfaces in vacuum. 2D Mater. 2017, 5, 015002. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Xu, Z.; Liu, M.; Shen, C.; Meng, Q. Synthesis of amino-functionalized Ti3C2Tx MXene by alkalization-grafting modification for efficient lead adsorption. Chem. Commun. 2020, 56, 11283–11286. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, Z.; Zhang, Q.; Hu, X.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Chemically-confined mesoporous γ-Fe2O3 nanospheres with Ti3C2Tx MXene via alkali treatment for enhanced lithium storage. J. Power Sources 2021, 495, 229758. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, S.; Noh, J.-S. Control of electrical properties of Ti3C2Tx mediated by facile alkalization. Surf. Interfaces 2023, 41, 103258. [Google Scholar] [CrossRef]
- Yang, Z.; Li, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Su, P.; et al. Improvement of gas and humidity sensing properties of organ-like MXene by alkaline treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yue, S.; Zhang, J.; Qian, L.; Guo, X. Three-Dimensional Vanadium and Nitrogen Dual-Doped Ti3C2 Film with Ultra-High Specific Capacitance and High Volumetric Energy Density for Zinc-Ion Hybrid Capacitors. Nanomaterials 2024, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Alothman, A.A.; Khan, M.R.; Albaqami, M.D.; Mohandoss, S.; Alothman, Z.A.; Ahmad, N.; Alqahtani, K.N. Ti3C2-MXene/NiO Nanocomposites-Decorated CsPbI3 Perovskite Active Materials under UV-Light Irradiation for the Enhancement of Crystal-Violet Dye Photodegradation. Nanomaterials 2023, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Fang, L.; Wu, F.; Zhang, S.; Ruan, H.; Luo, H.; Zhang, H.; Li, W.; Long, X.; Hu, B.; et al. 3D crinkled alk-Ti3C2 MXene based flexible piezoresistive sensors with ultra-high sensitivity and ultra-wide pressure range. J. Adv. Mater. Technol. 2021, 6, 2001157. [Google Scholar] [CrossRef]
- Sui, J.; Chen, X.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. MXene derivatives: Synthesis and applications in energy convention and storage. RSC Adv. 2021, 11, 16065–16082. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.; Pai, R.; Wilson, O.; Gadasu, E.; Badr, H.; Karmakar, A.; Magenau, A.J.D.; Kalra, V.; Barsoum, M.W. Effect of base/nucleophile treatment on interlayer ion intercalation, surface terminations, and osmotic swelling of Ti3C2Tz MXene multilayers. Chem. Mater. 2022, 34, 678–693. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, Y.C.; Koo, C.M.; Kim, S.J. Ti3C2Tx MXene Nanolaminates with Ionic Additives for Enhanced Gas-Sensing Performance. ACS Appl. Nano Mater. 2022, 5, 11997–12005. [Google Scholar] [CrossRef]
- Lee, S.H.; Eom, W.; Shin, H.; Ambade, R.B.; Bang, J.H.; Kim, H.W.; Han, T.H. Room-Temperature, Highly Durable Ti3C2Tx MXene/Graphene Hybrid Fibers for NH3 Gas Sensing. ACS Appl. Mater. Interfaces 2020, 12, 10434–10442. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, K.; Peng, H.; Li, F.; Yin, F. A flexible VOCs sensor based on a 3D Mxene framework with a high sensing performance. J. Mater. Chem. A 2018, 6, 18116–18124. [Google Scholar] [CrossRef]
- Yu, H.; Dai, L.; Liu, Y.; Zhou, Y.; Fan, P.; Luo, J.; Zhong, A. Ti3C2Tx MXene-SnO2 nanocomposite for superior room temperature ammonia gas sensor. J. Alloys Compd. 2023, 962, 171170. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Jiang, Y.; He, Z.; Liu, B.; Xie, H.; Li, H.; Li, Z.; Wang, Y.; Tai, H. Toward Agricultural Ammonia Volatilization Monitoring: A Flexible Polyaniline/Ti3C2T Hybrid Sensitive Films Based Gas Sensor. Sens. Actuators B Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chu, L.; Li, Z.; Sun, C.; Shi, Y.; Ma, J. 2H-MoS2/Ti3C2Tx MXene composites for enhanced NO2 gas sensing properties at room temperature. Sens. Actuators Rep. 2022, 4, 100103. [Google Scholar] [CrossRef]
- Ma, J.; Zhai, H.; Zhang, Z.; Di, M.; Sun, Z.; Zhang, K.; Hu, J.; Fan, H. Three-dimensional alkalized V2CTx MXene/NH2-Graphene heterojunction nanocomposites for ammonia detection at room temperature. ACS Appl. Nano Mater. 2023, 6, 19797–19806. [Google Scholar]
- Liu, M.; Ding, Y.; Lu, Z.; Song, P.; Wang, Q. Layered Ti3C2Tx MXene/CuO spindles composites for NH3 detection at roomtemperature. J. Alloys Compd. 2023, 938, 168563. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Thomas, A.M.; Thomas, A.M.; Vidyasagar, D.; Rao, V.N.; Yoon, S.-G.; Kim, Y.-H.; Kim, S.-G.; Kim, M.-D. ZnO@Ti3C2Tx MXene Hybrid Composite-Based Schottky-Barrier-Coated SAW Sensor for Effective Detection of Sub-ppb-Level NH3 at Room Temperature under UV Illumination. ACS Mater. Lett. 2023, 5, 2739–2746. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, P.; Tian, W.; Qin, X.; Zhang, Y.; Sun, Z. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater. Chem. Phys. 2018, 206, 270–276. [Google Scholar]
- Wang, R.; Cao, H.; Yao, C.; Peng, C.; Qiu, J.; Dou, K.; Tsidaeva, N.; Wang, W. Construction of alkalized MXene-supported CoFe2O4/CS composites with super-strong adsorption capacity to remove toxic dyes from aqueous solution. J. Appl. Surf. Sci. 2023, 624, 157091. [Google Scholar] [CrossRef]
- Lian, P.; Dong, Y.; Wu, Z.; Zheng, S.; Wang, X.; Wang, S.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Qin, W.; Li, K.; Feng, L.; Chen, J.; Qiao, H.; Yang, M.; Tian, Z.; Li, X.; Gu, C.; et al. A high-sensitivity, fast-response and high-stability humidity sensor of curly flake Ti3C2Tx MXene prepared by electrolytic intercalation of NaOH solution. J. Mater. Chem. A 2022, 10, 22278–22288. [Google Scholar] [CrossRef]
- Xiao, B.; Li, Y.; Yu, X.; Cheng, J. MXenes: Reusable materials for NH3 sensor or capturer by controlling the charge injection. Sens. Actuators B Chem. 2016, 235, 103–109. [Google Scholar] [CrossRef]
- Näslund, L.-Å.; Persson, I. XPS spectra curve fittings of Ti3C2Tx based on first principles thinking. Appl. Surf. Sci. 2022, 593, 153442. [Google Scholar] [CrossRef]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Célérier, S.; Barsoum, M.W. A critical analysis of the X-ray photoelectron spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Ghidiu, M.; Halim, J.; Kota, S.; Bish, D.; Gogotsi, Y.; Barsoum, M.W. Ion-exchange and cation solvation reactions in Ti3C2 MXene. Chem. Mater. 2016, 28, 3507–3514. [Google Scholar] [CrossRef]
- Ma, S.; Guo, J.; Zhang, H.; Shao, X.; Zhang, D. A Room Temperature Trimethylamine Gas Sensor Based on Electrospinned Molybdenum Oxide Nanofibers/Ti3C2Tx MXene Heterojunction. Nanomaterials 2024, 14, 537. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tan, J.; Cheng, P.; Ai, Y.; Xu, Y.; Ye, N. 3D porous MXene (Ti3C2Tx) prepared by alkaline-induced flocculation for supercapacitor electrodes. Materials 2022, 15, 925. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, H.; Li, T.; Wang, L.; Zhang, L.; Yu, Y.; Qiao, Z.; Liu, W. Research about the capacitance properties of ion-induced multilayer and self-assembled monolayer Ti3C2Tx. RSC Adv. 2022, 12, 3554–3560. [Google Scholar] [CrossRef]
- Limbu, T.B.; Chitar, B.; Orlando, J.D.; Cervantes, M.Y.G.; Kumari, S.; Li, Q.; Tang, Y.G.; Yan, F. Green synthesis of reduced Ti3C2Tx MXene nanosheets with enhanced conductivity, oxidation stability, and SERS activity. J. Mater. Chem. C 2020, 8, 4722–4731. [Google Scholar] [CrossRef]




| Materials | NH3 (ppm) | Response (%) | Response/Recovery Time | Ref. |
|---|---|---|---|---|
| Ti3C2Tx | 100 | 0.21 | - | [9] |
| Ti3C2Tx | 100 | 0.8 | - | [37] |
| Ti3C2Tx | 100 | 0.12 | 6.8/13.3 min (100 ppm NH3) | [38] |
| Ti3C2Tx/Graphene | 100 | 6.77 | 3/12 min (100 ppm NH3) | [39] |
| 3D Ti3C2Tx | 100 | 0.8 | 1.5/1.7 min (5 ppm NH3) | [40] |
| Ti3C2Tx/SnO2 | 100 | 3.1 | 109/342 s (100 ppm NH3) | [41] |
| MXene/TiO2/cellulose | 100 | 6.84 | 76/62 s (100 ppm NH3) | [42] |
| Alkalized Ti3C2Tx | 100 | 7.04 | 73/38 s (200 ppm NH3) | This work |
| Single-layer Ti3C2Tx | 500 | 6.13 | 45/94 s (25 ppm NH3) | [43] |
| Ti3C2Tx MXene/MoS2 | 500 | 5.8 | - | [44] |
| Alkalized Ti3C2Tx | 500 | 78 | - | [31] |
| Alkalized Ti3C2Tx | 500 | 100.3 | 73/38 s (200 ppm NH3) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Xu, J.; Li, Q.; Zhang, W.; Lu, C.; Song, X.; Liu, L.; Chen, Y. Sensitivity-Enhanced, Room-Temperature Detection of NH3 with Alkalized Ti3C2Tx MXene. Nanomaterials 2024, 14, 680. https://doi.org/10.3390/nano14080680
Tan Y, Xu J, Li Q, Zhang W, Lu C, Song X, Liu L, Chen Y. Sensitivity-Enhanced, Room-Temperature Detection of NH3 with Alkalized Ti3C2Tx MXene. Nanomaterials. 2024; 14(8):680. https://doi.org/10.3390/nano14080680
Chicago/Turabian StyleTan, Yi, Jinxia Xu, Qiliang Li, Wanting Zhang, Chong Lu, Xingjuan Song, Lingyun Liu, and Ying Chen. 2024. "Sensitivity-Enhanced, Room-Temperature Detection of NH3 with Alkalized Ti3C2Tx MXene" Nanomaterials 14, no. 8: 680. https://doi.org/10.3390/nano14080680
APA StyleTan, Y., Xu, J., Li, Q., Zhang, W., Lu, C., Song, X., Liu, L., & Chen, Y. (2024). Sensitivity-Enhanced, Room-Temperature Detection of NH3 with Alkalized Ti3C2Tx MXene. Nanomaterials, 14(8), 680. https://doi.org/10.3390/nano14080680






