Improved Operation of Chloralkaline Reversible Cells with Mixed Metal Oxide Electrodes Made Using Microwaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
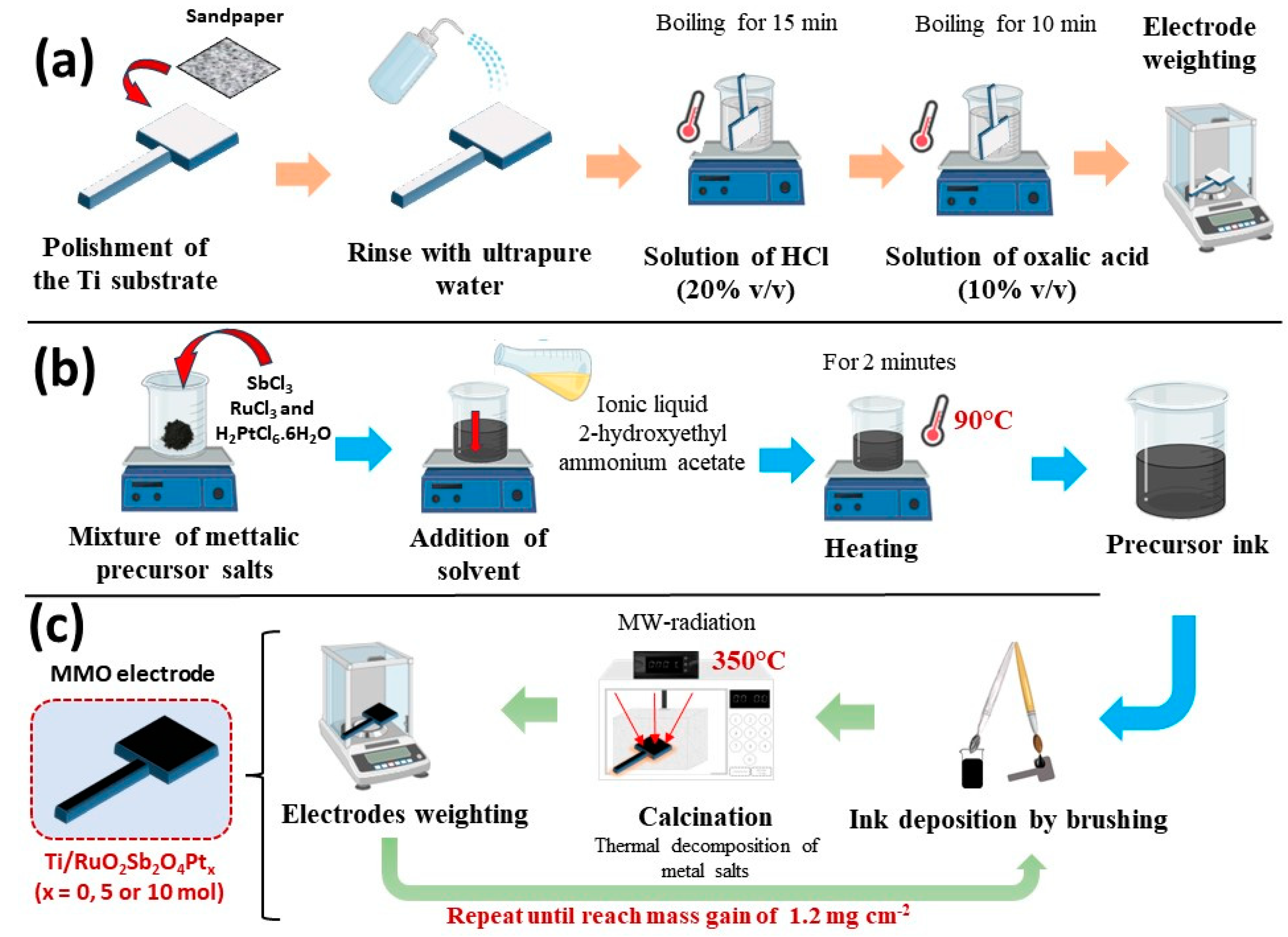
2.2. Preparation of MMO Anodes
2.3. Electrochemical Setup
2.4. Chemical Analysis
2.5. Physical Characterization of Electrodes
2.6. Electrochemical Characterization of Electrodes
3. Results and Discussion
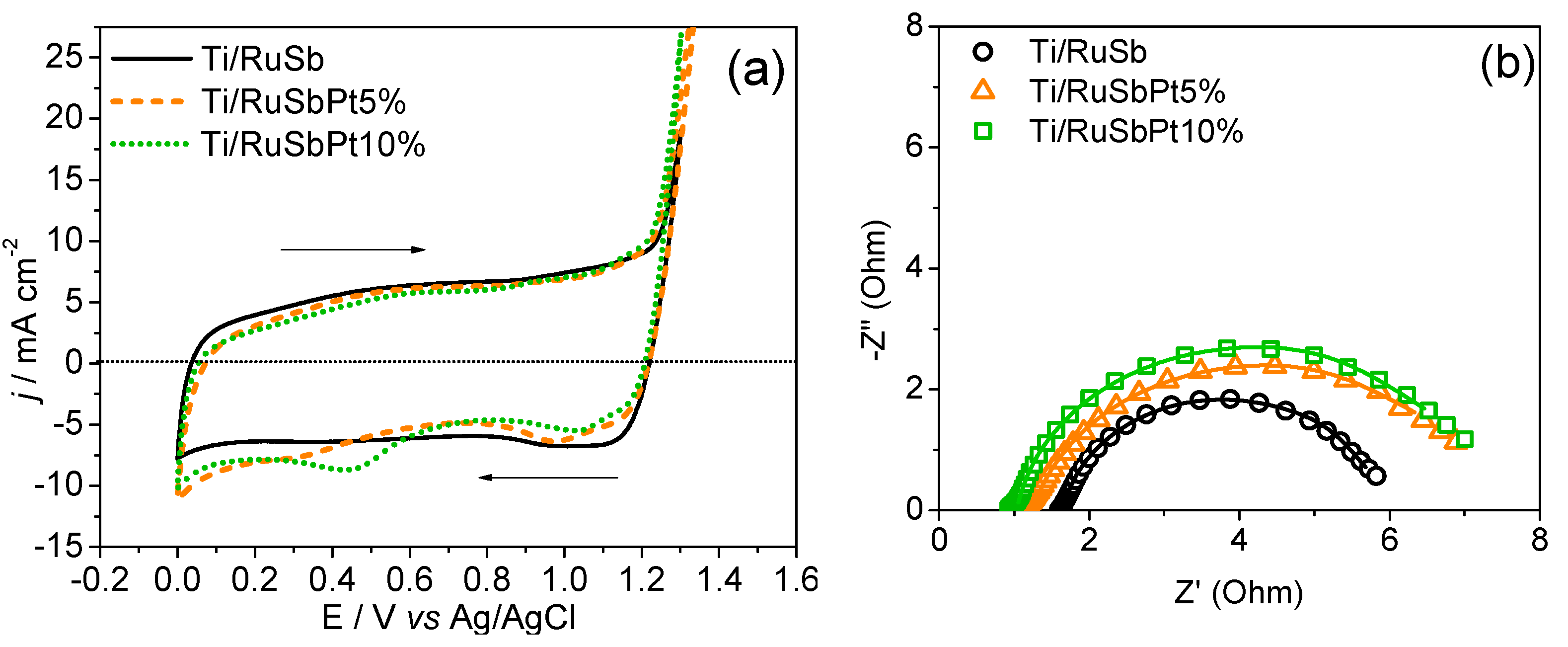
3.1. Characterization of Electrodes
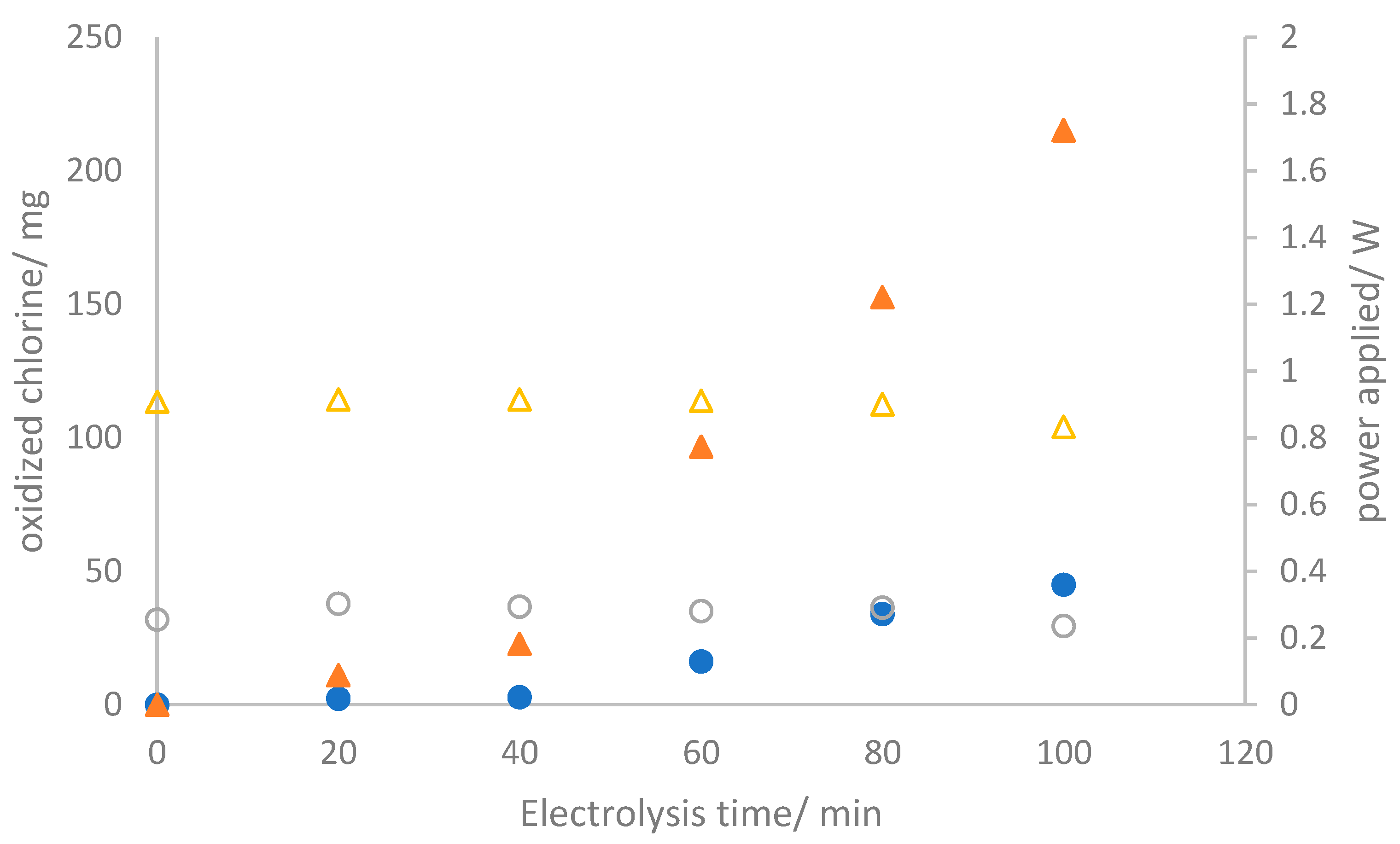
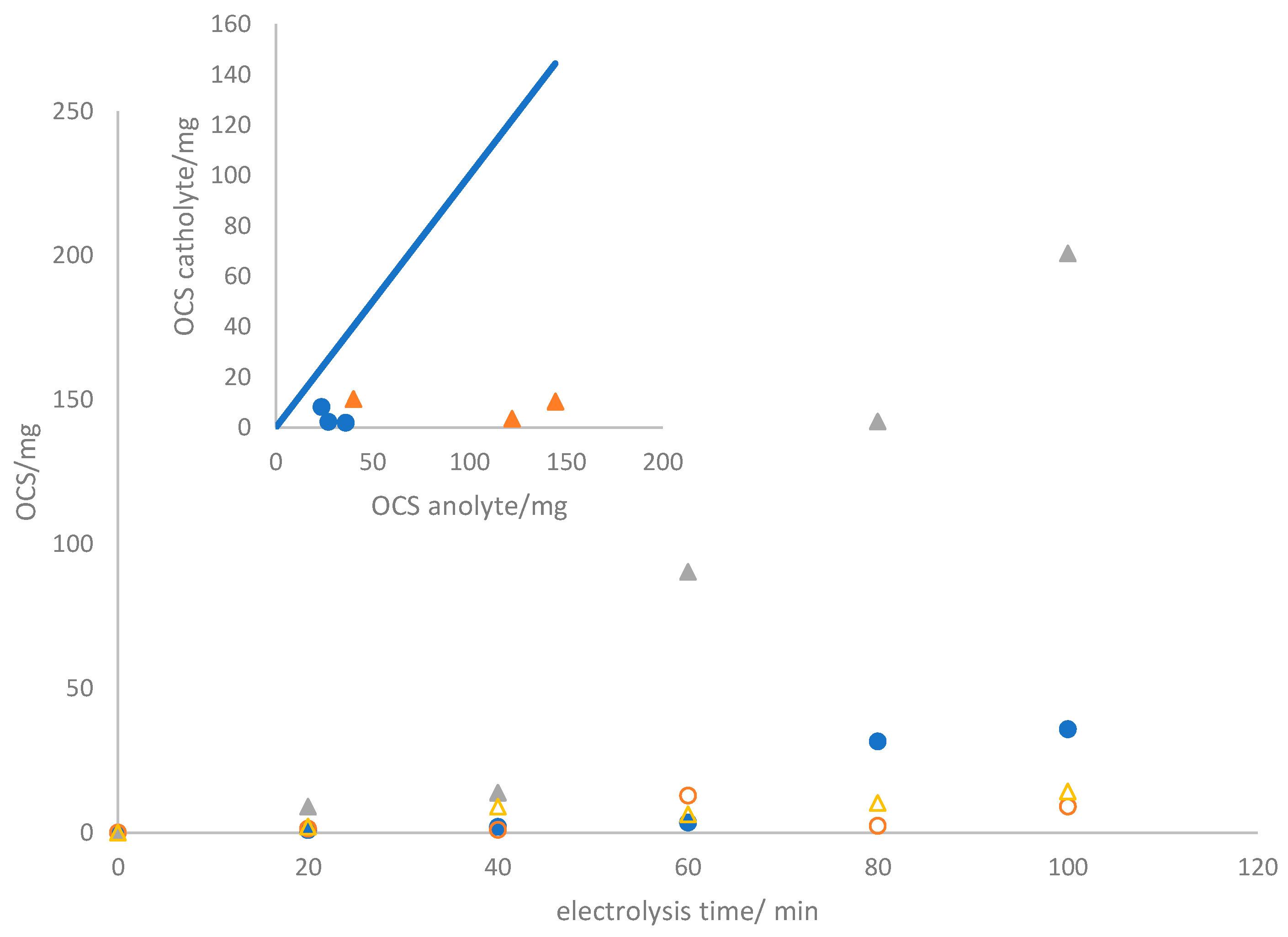
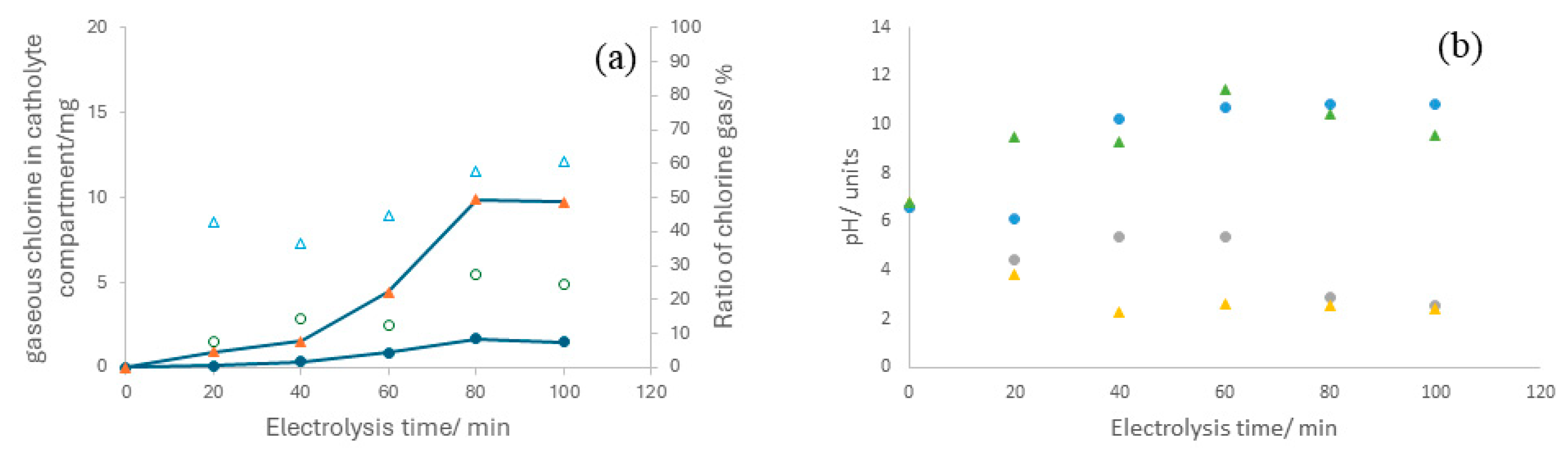
3.2. Electrolyzer Operation
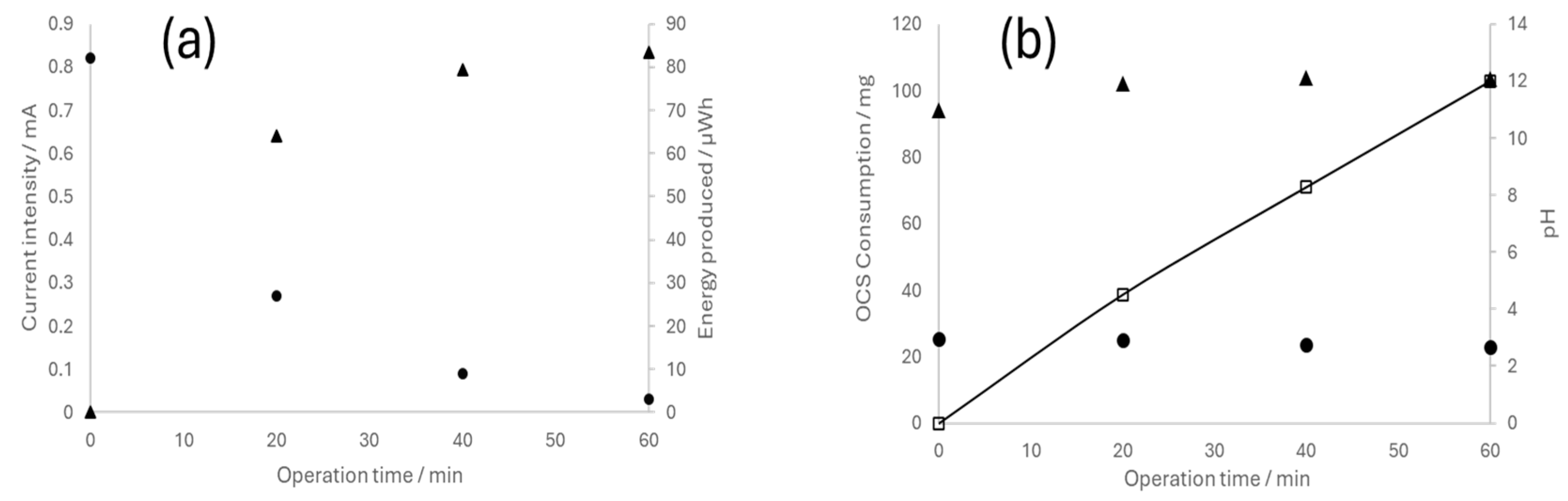
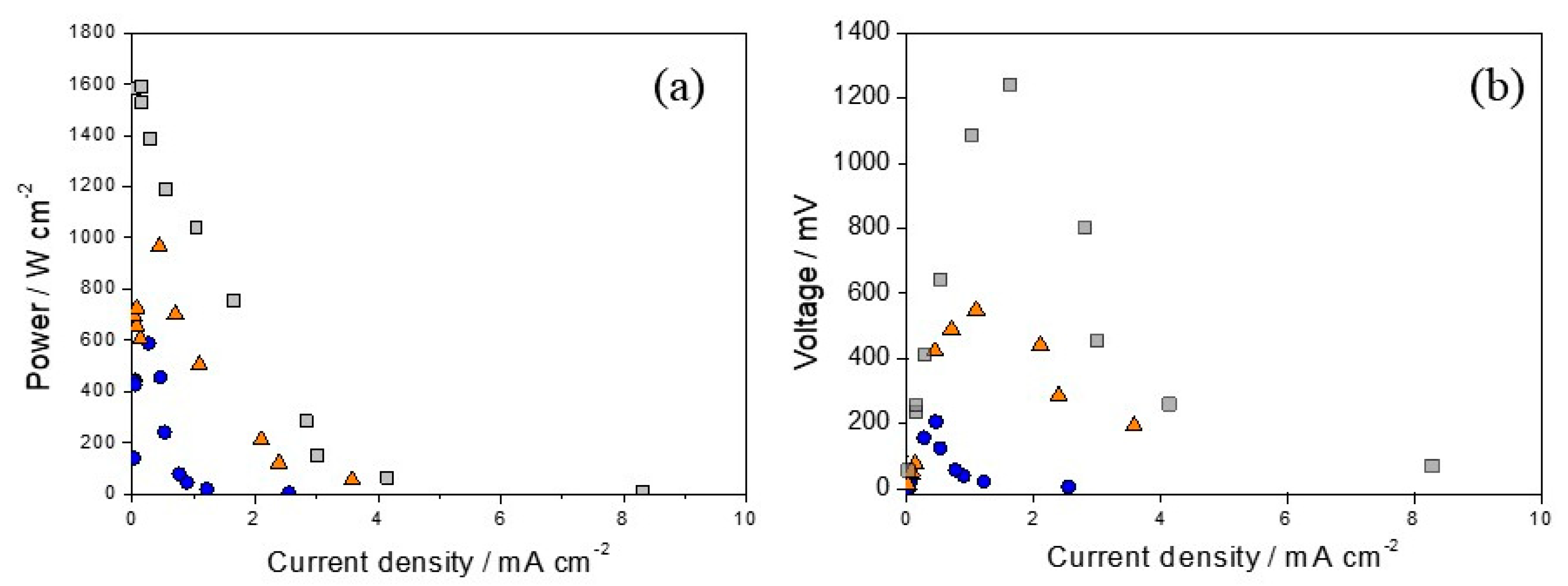
3.3. Fuel Cell Operation
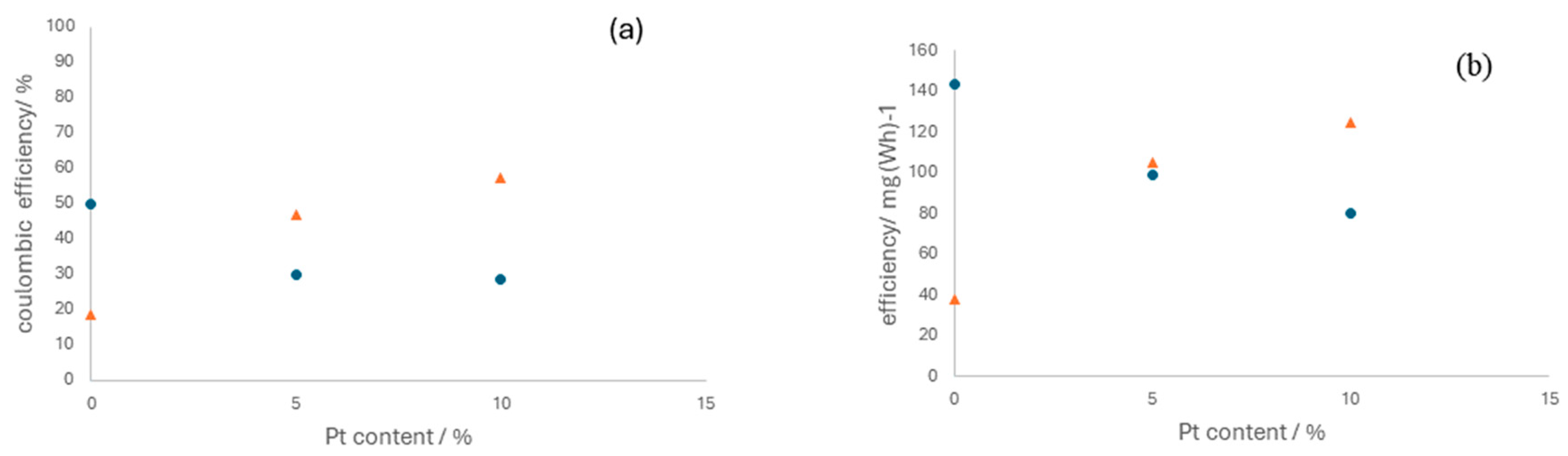
3.4. Effect of Platinum Content on Reversible Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Huang, Z.-H.; Lim, T.-T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Krstić, V.; Pešovski, B. Reviews the research on some dimensionally stable anodes (DSA) based on titanium. Hydrometallurgy 2019, 185, 71–75. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Pouladvand, I.; Khameneh Asl, S.; Hoseini, M.G.; Rezvani, M. Nanostructured Ti/TiO2–RuO2–La2O3 anodes prepared by sol–gel process and the effect of electrolyte composition on their stability. Micro Nano Lett. 2019, 14, 234–238. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.K. Electrochemical oxidation of direct blue 86 dye using MMO coated Ti anode: Modelling, kinetics and degradation pathway. Chem. Eng. Process.-Process Intensif. 2022, 181, 109127. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Ghasemian, S.; Zhang, N.; Yagar, M.; De Lannoy, C.-F. Mixed metal oxide anodes used for the electrochemical degradation of a real mixed industrial wastewater. Chemosphere 2022, 286, 131600. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C.; Vercesi, G. Characterization of DSA®-type oxygen evolving electrodes: Choice of a coating. J. Appl. Electrochem. 1991, 21, 335–345. [Google Scholar] [CrossRef]
- Bravo-Yumi, N.; Espinoza-Montero, P.; Picos-Benítez, A.; Navarro-Mendoza, R.; Brillas, E.; Peralta-Hernández, J.M. Synthesis and characterization of Sb2O5-doped Ti/SnO2-IrO2 anodes toward efficient degradation tannery dyes by in situ generated oxidizing species. Electrochim. Acta 2020, 358, 136904. [Google Scholar] [CrossRef]
- Wei, S.; Xu, Y.; Tian, S.; Han, Z.; Xu, L. Effect of microwave field on microstructure and battery performance of Al-Mg-Sn-Ga anode material. Electrochim. Acta 2022, 433, 141208. [Google Scholar] [CrossRef]
- Santos, M.O.; Santos, G.O.S.; Mattedi, S.; Griza, S.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Influence of the calcination temperature and ionic liquid used during synthesis procedure on the physical and electrochemical properties of Ti/(RuO2)0.8–(Sb2O4)0.2 anodes. J. Electroanal. Chem. 2018, 829, 116–128. [Google Scholar] [CrossRef]
- Erden, M.; Karakilcik, M. Experimental investigation of hydrogen production performance of various salts with a chlor-alkali method. Int. J. Hydrogen Energy 2024, 52, 546–560. [Google Scholar] [CrossRef]
- Abbasi, H.M.; Jafarzadeh, K.; Mirali, S.M. An investigation of the effect of RuO2 on the deactivation and corrosion mechanism of a Ti/IrO2 + Ta2O5 coating in an OER application. J. Electroanal. Chem. 2016, 777, 67–74. [Google Scholar] [CrossRef]
- Mirseyed, S.F.; Jafarzadeh, K.; Rostamian, A.; Abbasi, H.M.; Ostadhassan, M. A new insight on the mechanisms of corrosion deactivation of a typical Ti/IrO2+ RuO2+ TiO2 coating in the presence of Ta2O5 in chlor-alkali medium. Corros. Sci. 2023, 214, 111005. [Google Scholar] [CrossRef]
- Trieu, V.; Schley, B.; Natter, H.; Kintrup, J.; Bulan, A.; Hempelmann, R. RuO2-based anodes with tailored surface morphology for improved chlorine electro-activity. Electrochim. Acta 2012, 78, 188–194. [Google Scholar] [CrossRef]
- Macounová, K.M.; Pittkowski, R.K.; Nebel, R.; Zitolo, A.; Krtil, P. Selectivity of Ru-rich Ru-Ti-O oxide surfaces in parallel oxygen and chlorine evolution reactions. Electrochim. Acta 2022, 427, 140878. [Google Scholar] [CrossRef]
- Kasian, O.; Geiger, S.; Stock, P.; Polymeros, G.; Breitbach, B.; Savan, A.; Ludwig, A.; Cherevko, S.; Mayrhofer, K.J. On the origin of the improved ruthenium stability in RuO2–IrO2 mixed oxides. J. Electrochem. Soc. 2016, 163, F3099. [Google Scholar] [CrossRef]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Gaumet, J.J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-up confined synthesis of nanorod-in-nanotube structured Sb@ N-C for durable lithium and sodium storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Yu, D.Y.; Batabyal, S.K.; Gun, J.; Sladkevich, S.; Mikhaylov, A.A.; Medvedev, A.G.; Novotortsev, V.M.; Lev, O.; Prikhodchenko, P.V. Antimony and antimony oxide@ graphene oxide obtained by the peroxide route as anodes for lithium-ion batteries. Main Group Met. Chem. 2015, 38, 43–50. [Google Scholar] [CrossRef]
- Choi, S.; Choi, W.I.; Lee, J.S.; Lee, C.H.; Balamurugan, M.; Schwarz, A.D.; Choi, Z.S.; Randriamahazaka, H.; Nam, K.T. A Reflection on Sustainable Anode Materials for Electrochemical Chloride Oxidation. Adv. Mater. 2023, 35, 2300429. [Google Scholar] [CrossRef]
- Dong, H.; Yu, W.; Hoffmann, M.R. Mixed metal oxide electrodes and the chlorine evolution reaction. J. Phys. Chem. C 2021, 125, 20745–20761. [Google Scholar] [CrossRef]
- Carvela, M.; Lobato, J.; Rodrigo, M.A. Storage of energy using a gas-liquid H2/Cl2 fuel cell: A first approach to electrochemically-assisted absorbers. Chemosphere 2020, 254, 126795. [Google Scholar] [CrossRef] [PubMed]
- Carvela, M.; Santos, G.O.S.; Gonzaga, I.M.; Eguiluz, K.I.B.; Lobato, J.; Salazar-Banda, G.R.; Rodrigo, M.A. Platinum: A key element in electrode composition for reversible chloralkaline electrochemical cells. Int. J. Hydrogen Energy 2021, 46, 32602–32611. [Google Scholar] [CrossRef]
- Huskinson, B.; Rugolo, J.; Mondal, S.K.; Aziz, M.J. A high power density, high efficiency hydrogen–chlorine regenerative fuel cell with a low precious metal content catalyst. Energy Environ. Sci. 2012, 9, 8690–8698. [Google Scholar] [CrossRef]
- Dória, A.R.; Moratalla, A.; Almeida, C.V.; Silva, R.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Rodrigo, M.A.; Saéz, C. Influence of the calcination method and anode composition on the generation of disinfectants. Sep. Purif. Technol. 2023, 319, 124053. [Google Scholar] [CrossRef]
- Yi, Z.; Kangning, C.; Wei, W.; Wang, J.; Lee, S. Effect of IrO2 loading on RuO2–IrO2–TiO2 anodes: A study of microstructure and working life for the chlorine evolution reaction. Ceram. Int. 2007, 33, 1087–1091. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Jeon, Y.-J.; Lee, J.-Y.; Lee, K.H.; Lee, J.-Y. Bifunctional technology involving RuO2-IrO2/Ti electrode decorated with reduced graphene oxide aerogel with Pd nanoparticles: Electrochemical oxidative decomposition and detection of p-nitrophenol. J. Electroanal. Chem. 2023, 940, 117471. [Google Scholar] [CrossRef]
- Basak, M.; Rahman, M.L.; Ahmed, M.F.; Biswas, B.; Sharmin, N. The use of X-ray diffraction peak profile analysis to determine the structural parameters of cobalt ferrite nanoparticles using Debye-Scherrer, Williamson-Hall, Halder-Wagner and Size-strain plot: Different precipitating agent approach. J. Alloys Compd. 2022, 895, 162694. [Google Scholar] [CrossRef]
- Da Silva, L.; De Faria, L.; Boodts, J. Determination of the morphology factor of oxide layers. Electrochim. Acta 2001, 47, 395–403. [Google Scholar] [CrossRef]
- Audichon, T.; Napporn, T.W.; Canaff, C.; Morais, C.; Comminges, C.; Kokoh, B. IrO2 Coated on RuO2 as Efficient and Stable Electroactive Nanocatalysts for Electrochemical Water Splitting. J. Phys. Chem. 2016, 120, 2562–2573. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Li, H.; Zhang, S.; Martin, J.; Wang, H. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef]
- Carvela, M.; Lobato, J.; Rodrigo, M.A. Chloralkali low temperature PEM reversible electrochemical cells. Electrochim. Acta 2021, 387, 138542. [Google Scholar] [CrossRef]
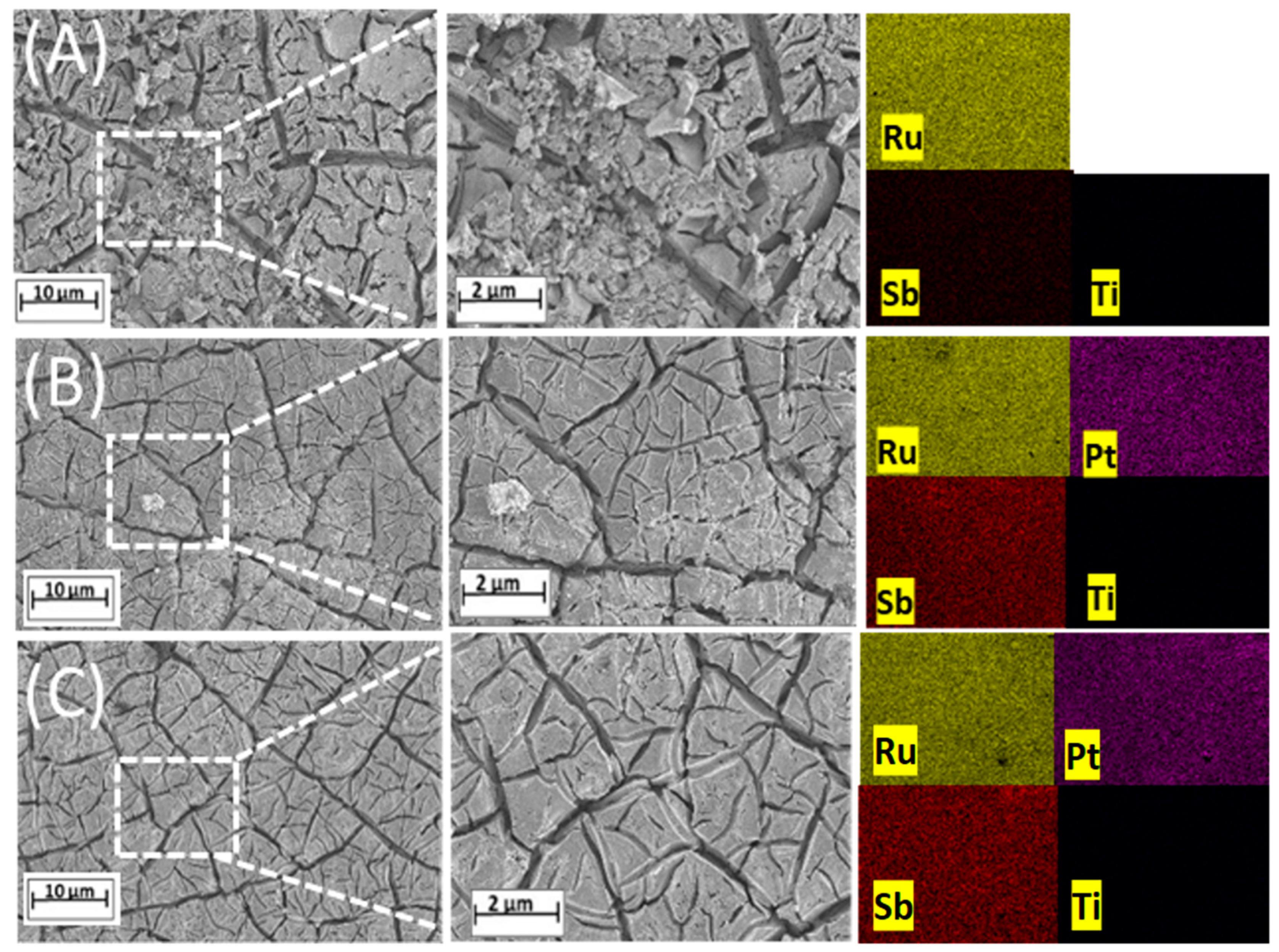

| Electrode | Nominal Composition (mol%) | Experimental Composition from EDS (mol%) | Experimental Composition from ICP (mol%) | XRD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ru | Sb | Pt | Ru | Sb | Pt | Ru | Sb | Pt | FWHM (rad) | Crystallite Size (nm) | |
| Ti/(RuO2)70-(Sb2O4)30 | 70 | 30 | - | 75.5 | 24.5 | - | 69.4 | 30.6 | - | 0.2362 | 17.3 |
| Ti/(RuO2)66.5-(Sb2O4)28.5-Pt5 | 66.5 | 28.5 | 5 | 67.0 | 27.5 | 5.5 | 84.9 | 12.5 | 2.6 | 0.3936 | 20.8 |
| Ti/(RuO2)63-(Sb2O4)27-Pt10 | 63 | 27 | 10 | 66.2 | 23.6 | 10.2 | 60.8 | 22.7 | 16.5 | 0.1968 | 41.6 |
| Electrode | q*(mC cm−2) | Cd (mF cm−2) | Cd,e (mF cm−2) | Cd,i (mF cm−2) | φ (Cd,i/Cd) |
|---|---|---|---|---|---|
| Ti/(RuO2)70-(Sb2O4)30 | 298.1 | 46.1 | 17.3 | 39.1 | 0.84 |
| Ti/(RuO2)66.5-(Sb2O4)28.5-Pt5 | 290.4 | 52.9 | 20.8 | 46.44 | 0.88 |
| Ti/(RuO2)63-(Sb2O4)27-Pt10 | 281.6 | 47.03 | 41.6 | 42.03 | 0.89 |
| Electrode | RΩ/Ω | QCPE(dl)/F | Rtc/Ω | η | χ2 |
|---|---|---|---|---|---|
| Ti/(RuO2)70-(Sb2O4)30 | 1.65 | 0.28 | 4.3 | 0.90 | 0.0006 |
| Ti/(RuO2)66.5-(Sb2O4)28.5-Pt5 | 1.25 | 0.19 | 6.1 | 0.84 | 0.0006 |
| Ti/(RuO2)63-(Sb2O4)27-Pt10 | 1.03 | 0.18 | 6.3 | 0.84 | 0.0007 |
| Electrode | Power Density (W cm−2) | Maximum Cell Voltage (mV) | Reference |
|---|---|---|---|
| Ti/(RuO2)63-(Sb2O4)27-Pt10 | 1.6 | 1300 | This work |
| (Ru0.09Co0.91)3O4 | 0.4 | 1000 | [25] |
| Ti/Ru0.5Ir0.5O2 | 0.006 | − | [24] |
| Pt/C | 0.007 | − | [34] |
| Platinum Content (%) | |||
|---|---|---|---|
| Parameter | 0 | 5 | 10 |
| a/V | 2053.22 | 1411.94 | 1454.15 |
| b/V dec−1 | 1141.01 | 483.07 | 131.13 |
| c/Ω | −2801.24 | −641.32 | −404.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.Y.C.; Santos, G.O.S.; Dória, A.R.; Requena, I.; Lanza, M.R.V.; Salazar-Banda, G.R.; Eguiluz, K.I.B.; Lobato, J.; Rodrigo, M.A. Improved Operation of Chloralkaline Reversible Cells with Mixed Metal Oxide Electrodes Made Using Microwaves. Nanomaterials 2024, 14, 693. https://doi.org/10.3390/nano14080693
Ribeiro JYC, Santos GOS, Dória AR, Requena I, Lanza MRV, Salazar-Banda GR, Eguiluz KIB, Lobato J, Rodrigo MA. Improved Operation of Chloralkaline Reversible Cells with Mixed Metal Oxide Electrodes Made Using Microwaves. Nanomaterials. 2024; 14(8):693. https://doi.org/10.3390/nano14080693
Chicago/Turabian StyleRibeiro, Jamylle Y. C., Gessica O. S. Santos, Aline R. Dória, Iñaki Requena, Marcos R. V. Lanza, Giancarlo R. Salazar-Banda, Katlin I. B. Eguiluz, Justo Lobato, and Manuel A. Rodrigo. 2024. "Improved Operation of Chloralkaline Reversible Cells with Mixed Metal Oxide Electrodes Made Using Microwaves" Nanomaterials 14, no. 8: 693. https://doi.org/10.3390/nano14080693
APA StyleRibeiro, J. Y. C., Santos, G. O. S., Dória, A. R., Requena, I., Lanza, M. R. V., Salazar-Banda, G. R., Eguiluz, K. I. B., Lobato, J., & Rodrigo, M. A. (2024). Improved Operation of Chloralkaline Reversible Cells with Mixed Metal Oxide Electrodes Made Using Microwaves. Nanomaterials, 14(8), 693. https://doi.org/10.3390/nano14080693








