1. Introduction
Transition metal ferrites with a (inverse) spinel structure represent a promising class of oxide-based materials for applications in, for example, spincaloritronics or spintronics [
1,
2,
3,
4] due to several intriguing properties, like significant magnetic saturation moments and high Curie temperatures [
5]. In the field of spintronics, inverse spinel ferrites like NiFe
2O
4 and CoFe
2O
4 are suitable for application as a spin filter [
6,
7,
8,
9], where highly spin-polarized tunneling currents are generated. The insulating and ferrimagnetic properties of nickel and cobalt ferrite with an exchange splitting of the energy levels in the conduction band result in different tunneling probabilities for the two spin orientations, which make these ferrites highly suited as spin filters [
4]. However, the spin-filter efficiency is crucially determined by the structural quality of the tunneling barrier [
8] and its interfaces [
10]. Thus, ferrites have to be prepared as thin films with low defect densities to obtain a high transmission of spin-polarized tunneling currents.
Here, MgO(001) is well suited as a substrate for NiFe2O4 films since both materials show a cubic crystalline structure and their lattice mismatch is small. Comparing the lattice constant = 833.9 pm of NiFe2O4 (inverse spinel structure) with twice the lattice constant = 421.2 pm of MgO (rocksalt structure), the lattice mismatch is only −1.0%. Hence, only small-strain and low-defect densities are expected when depositing ultrathin NiFe2O4 films on MgO(001). However, due to the almost doubled ferrite (inverse) spinel lattice constant compared to the one of the rocksalt lattice constant of MgO(001), antiphase boundaries (APBs) are likely to emerge and represent a small drawback in the use of substrates with an almost half-sized lattice constant.
Here, we like to study ultrathin films of Ni ferrite (NFO = NixFe3−xO4) with variable Ni content x. Thus, lattice matching of magnetite Fe3O4 (NFO with x = 0, inverse spinel) and NiO (NFO in the limit of no Fe content, rocksalt) have to be considered, too. Compared to MgO, the lattice mismatch of Fe3O4 ( = 839.6 pm) and NiO ( = 419.5 pm) is only −0.3% and −0.4%, respectively. Thus, it is expected that MgO(001) substrates are also well suited for epitaxy of such oxide films.
For the inverse spinel structure of NiFe2O4, on the one hand, Ni cations are in oxidation state 2+ and are octahedrally coordinated (occupation of B-sublattice sites). On the other hand, Fe cations are in oxidation state 3+. Half of them are octahedrally coordinated (occupation of B-sublattice sites), while the other half is tetrahedrally coordinated (occupation of A-sublattice sites). Due to the super exchange and double exchange between the different cations, the magnetization is effectively produced by cations. Neglecting orbital contributions due to orbital quenching, the magnetic moment of is 2 . Similar statements are also valid for Fe3O4 (magnetite) in which the role of cations is adopted by cations with magnetic moment of 4 . In NiO, Ni cations are also octahedrally coordinated, but the magnetic order is antiferromagnetic.
Thus, for the Ni ferrite Ni
xFe
3−xO
4 with varying Ni content x, the chemical composition, i.e., the cationic ratio, as well as lattice site occupation can have a significant impact on their structural, chemical, magnetic, and electronic properties [
11,
12,
13,
14]. However, in contrast to investigations focusing on the cationic Co:Fe ratio within Co
xFe
3−xO
4 films [
12,
13], a systematic study on the influence of the cationic stoichiometry in Ni
xFe
3−xO
4 films is still lacking. Therefore, we report on our preparation of Ni
xFe
3−xO
4 thin films on MgO(001) via reactive molecular beam epitaxy (RMBE) and investigate the influence of the cationic stoichiometry on the structural, chemical, and magnetic properties.
The chemical composition and structure of the NixFe3−xO4 film surfaces were characterized in-situ by means of laboratory-based X-ray photoelectron spectroscopy (XPS) and low-energy electron diffraction (LEED), respectively. Further, the bulk structure was analyzed by synchrotron-based high-resolution X-ray diffraction (HR-XRD), whereas film thicknesses were determined by analysis of X-ray reflectivity (XRR) measurements. The chemical composition and cationic valence states in deeper layers were investigated by hard X-ray photoelectron spectroscopy (HAXPES). In addition, angle-resolved HAXPES (AR-HAXPES) measurements reveal information about the depth-dependent cationic stoichiometry. X-ray absorption spectroscopy (XAS) measurements were complementarily conducted to obtain information about the cationic valence states and site occupancies by comparing the data to charge-transfer multiplet (CTM) calculations. Magnetic properties were studied by means of a superconducting quantum interference device (SQUID).
2. Experimental Details
A considerable number of NixFe3−xO4 film with film thicknesses 16 nm and varying Ni content x (0 ≤ x ≤ 2.07) were prepared on MgO(001) substrates via RMBE in an ultra high vacuum (UHV) chamber. Prior to film growth, the substrates were annealed at 400 °C for 1 h in a molecular oxygen atmosphere of 1 × 10−4 mbar to obtain clean and well-ordered substrate surfaces. The chemical purity and surface structure were controlled after substrate annealing by means of laboratory-based in situ XPS and LEED, respectively. Ultrathin NixFe3−xO4 films were deposited on the cleaned substrates via thermal co-evaporation of Ni and Fe via electron bombardment of the respective pure metal rods in a molecular oxygen atmosphere of 5 × 10−6 mbar, while the substrate temperature was controlled to 250 °C during film growth. The Ni:Fe ratio in the ferrite films was varied by tuning individually the flux from each evaporation source in a way that the total flux from both sources was kept constant. We obtained a deposition rate of 1.2 () nm/min by this procedure. The chemical composition, i.e., the Ni:Fe ratio in the ferrite films as well as the surface structure were again controlled in situ by means of surface sensitive XPS and LEED, respectively. For XPS measurements, Mg radiation with a photon energy of = 1253.6 eV was used.
After film preparation and in situ surface characterization (XPS, LEED), film thicknesses were determined ex situ by means of X-ray reflectivity (XRR) in a Philips X’Pert Pro diffractometer at Bielefeld University. Here, Cu
radiation with a photon energy of
= 8048.0 eV was used. XRR data were analyzed using an in-house-developed fitting tool, which is based on the Parratt algorithm [
15] and the Névot–Croce roughness model [
16].
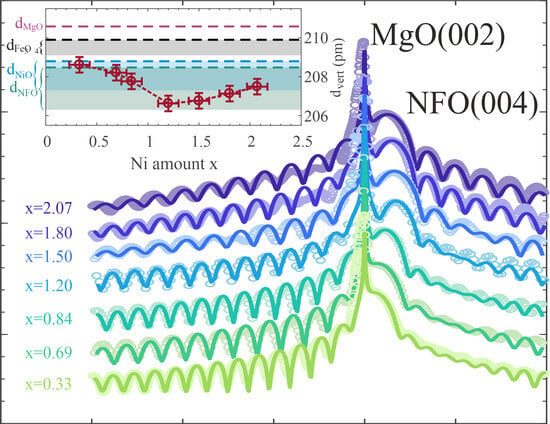
Additionally, the ferrite ultrathin film structure was characterized by high resolution X-ray diffraction (HR-XRD) measurements at beamline P08 of PETRA III at the Deutsches Elektronen-Synchrotron (DESY), Germany. Here, a photon energy of = 18 keV was used. The sample and detector were positioned with respect to the incident beam by using a six-circle (4S+2D-type) diffractometer (KOHZU NZD-3), while data acquisition was conducted by using a two-dimensional detector (PILATUS 100k or DECTRIS EIGER 1M). The HR-XRD measurements were conducted in - geometry to perform out-of-plane scans along the (00L) crystal truncation rod (CTR) across the MgO(002) and NFO(004) Bragg reflections. Analysis of HR-XRD data was performed by using an in-house-developed fitting tool, which employs full kinematic diffraction theory.
Complementary to laboratory-based surface sensitive XPS, HAXPES measurements were conducted at beamline P09 of PETRA III at DESY. In contrast to XPS, the higher used photon energy of = 6 keV for HAXPES measurements excites photoelectrons with higher kinetic energy and, thus, higher inelastic mean free paths (IMFPs) and consequently higher information depths . Thus, HAXPES is used to determine the chemical composition and cationic valencies not only in the near-surface region (as probed by XPS) but also in deeper subsurface layers. The endstation is equipped with a SPECS Phoibos 225 HV hemispherical analyzer with a delay line detector to record HAXPES spectra at beamline P09.
Additionally, a wide-angle lens with ±30° angular acceptance was used to record angle-resolved HAXPES (AR-HAXPES) spectra of photoelectrons with different off-normal emission angles
. The angular-dependent information depth
, from which 95% of photoelectrons (detected at the off-normal emission angle
) originate, is given by
with
as the IMFP of the respective photoelectrons as determined by the Tanuma, Powell, and Penn (TPP-2M) algorithm [
17]. As a consequence, varying the detection angle
in AR-HAXPES measurements allows for depth-dependent photoelectron detection (cf.
Table 1). For this purpose, the incident angle
between the incident beam and surface plane was kept constant, and the data detected within the acceptance angle sections of
were integrated for better statistics.
Complementarily, XAS measurements were performed to probe the cationic valence states and the lattice site occupancies within the film lattice. These measurements were performed at the Superconducting Vector Magnet Endstation at beamline 4.0.2 of the Advanced Light Source (ALS). All samples were measured at room temperature with an incident angle of
towards the [100] direction of the ferrite films. The XAS spectra were measured across the Fe
(690–750 eV) and Ni
(835–890 eV) absorption edges in the total electron yield (TEY) mode, which is surface sensitive with a probing depth of ∼5 nm in, e.g., Fe
3O
4 [
18,
19].
In order to quantify the cationic lattice site occupancies in the ferrite film, XAS measurements were analyzed by comparing the data to spectra obtained by charge transfer multiplet (CTM) calculations. In these calculations, a molecule complex composed of the respective cation in a given ligand crystal field is taken as a basis, and charge transfer is considered between the cation and ligand. The total CTM calculated spectrum is obtained by a weighted linear superposition of the individual spectra of each cation. By comparing the calculation with the experimental XAS spectra, quantitative information about the site occupancies of each cation is determined. In addition to crystal field effects and the charge transfer interaction, the CTM models include 100% spin–orbit coupling. Further, the Slater integrals
,
, and
, which account for
d-
d and
p-
d Coulomb and exchange interactions [
20], were each reduced to 80%, which is consistent within ±10% with previous studies on Fe
3O
4 [
21,
22,
23,
24,
25]. Charge transfer is considered by setting the O 2
p-Fe 3
d charge transfer energies
and
of the initial and final states, respectively. The best results for these parameters as well as for the crystal field energies 10Dq were obtained for the values listed in
Table 2 for the respective cations in a given ligand field, which are similar to the values used in former studies on Fe
3O
4 [
25,
26].
Additionally, the CTM calculated spectra were compared to the data by assuming a Gaussian instrumental broadening of 0.25 eV and a Lorentzian lifetime broadening of 0.3 eV (0.6 eV) for () edges.
Magnetic properties of the NixFe3−xO4 films were characterized by using a SQUID of type S700X from CRYOGENIC. Here, the magnetization M was recorded at room temperature as a function of applied magnetic field H, which was tuned from +7 T to −7 T and back again to obtain M vs. H curves, which show hysteretic behavior for ferrimagnetic or ferromagnetic material.
4. Discussion
As revealed by HAXPES, the overstoichiometric NixFe3−xO4 films with x > 1 exhibit almost only cations, while spectra obtained from understoichiometric films with x < 1 also show contributions due to cations. On the one hand, this result agrees well with the assumption that cations are gradually substituted by cations for increasing Ni content. On the other hand, the rising high binding energy shoulder as well as the altered satellite structure and the shift to lower binding energies of the Ni 2 photoemission spectrum for overstoichiometric films with x > 1 show that an increasing fraction of the cations is located in the NiO rocksalt configuration instead of the NFO inverse spinel configuration.
This (qualitative) result can be quantified better analyzing XAS data by CTM calculations. The analysis provides an enhanced : ratio compared to the understoichiometric regime. However, a small amount of octahedrally coordinated is still observed. More remarkably, also a crucial decrease in tetrahedrally coordinated is determined for x > 1, which can be assigned to a decreasing amount of inverse spinel-type structures in the overstoichiometric NFO films.
Figure 12 shows the fraction of the oxide film assuming that
can be identified with the film fraction with an inverse spinel structure. Obviously, films have an inverse spinel structure for low Ni contents x≪1. For x≲1, however, there is an excess of octahedrally coordinated cations that cannot all be incorporated into the inverse spinel part of the film. Since these cations are octahedrally coordinated, it can be concluded that they are incorporated in some rocksalt-like structure, which may be of the type
O (Fe-doped NiO). It has to be emphasized that the fraction of
cations and
cations incorporated in the inverse spinel structure or in the rocksalt structure cannot be determined.
For x > 1, the major parts of the films have a rocksalt structure and the inverse spinel part is strongly suppressed. In addition, there is also an excess of octahedrally coordinated
cations compared to the fraction of tetrahedrally coordinated
(cf.
Figure 10). Thus, there have to be cation vacancies in the rocksalt part of the film due to charge neutrality. Therefore, this deficient rocksalt structure may be denoted by
with oxygen excess
as discussed for cation ordering in Fe
3O
4 [
42].
XRR and AR-HAXPES studies, however, give no indications of layers of additional or separated phases neither in the conversion from Fe
3O
4 to NiFe
2O
4 for x < 1 nor from NiFe
2O
4 to an NiO-like rocksalt phase for x > 1. In fact, both measurement techniques point to single crystalline films with homogeneous cation and valence state depth distributions. Thus, in particular, both inverse spinel parts as well as rocksalt parts are randomly distributed within the ferrite film without forming distinctly separated layers. The latter has been reported by Kuschel et al., where NiFe
2O
4 films were prepared by the interdiffusion of Fe
3O
4/NiO bilayers on
(001) induced by post-deposition annealing (PDA) [
32]. The final film stack of the PDA treatment exhibits a segregated NiO layer on top of the NiFe
2O
4 film, when the initial Ni:Fe ratio exceeds the stoichiometric ratio of 1:2. Note that the same behavior is noticed for similar PDA treatment of Fe
3O
4/CoO bilayers, where an ultrathin CoO layer segregates to the top of the formed CoFe
2O
4 film [
43]. As a consequence, the co-evaporation method as performed in this study suppresses the NiO segregation to the surface due to the significantly lower sample temperature of 250 °C during film growth compared to at least 600 °C for the alternate PDA preparation method.
The finding of single crystalline films with homogeneous cationic distributions throughout the whole range of Ni content x is confirmed by the observation of only single diffraction peaks in HR-XRD measurements. Two strictly separated but coexisting phases, e.g., agglomeration in inverse spinel and rocksalt clusters with different lattice constants, would also result in different (vertical) layer distances and, therefore, in the observation of separated diffraction peaks, if the crystalline regions of each phase are sufficiently large. Finally, the Ni-Fe oxide film has an alloy-like structure mixing the locally inverse spinel and rocksalt structures without long-range order. In addition, the cation distribution in these different local structures is undetermined, and the overall structure may be denoted by [NixFe3−xO4]1−z []z.
The in situ LEED measurements on the surface structure of the ultrathin NixFe3−xO4 films reveal structural changes with varying Ni content x of the films. First, the diffraction pattern of an inverse spinel surface with a superstructure is observed for Ni content x ≪ 1. This superstructure is characteristic for Fe3O4(001) surfaces and can be observed up to a Ni contents x ≤ 0.7.
For intermediate Ni contents x ≲ 1, the superstructure vanishes, but the fundamental (1×1)S spinel pattern remains. This pattern is characteristic for NiFe2O4(001). This behavior comes along with a decreasing vertical layer distance as determined by HR-XRD analysis, which is also expected considering the decrease in bulk layer distances from Fe3O4 to NiFe2O4. These observations made in LEED and XRD therefore indicate a subsequent conversion from Fe3O4 to NiFe2O4 when increasing the Ni content x fro, x = 0 to x = 1. Further, within this conversion for x < 1, HAXPES measurements reveal a decrease in the relative content accompanied by an increase in the relative amount within the Fe cations. However, the valency remains unchanged, which indicates that the increase in the Ni content can be associated with the expected (but incomplete) exchange of by cations. This replacement with increasing x is reinforced by the quantitative analysis of XAS spectra by means of CTM calculations (see above).
For intermediate Ni contents x ≳ 1, the apparent fundamental (1×1)S spinel pattern can be observed although XAS analysis points to an increasing fraction of local rocksalt structures. However, the existing rocksalt-related LEED pattern may be hidden by the spinel-related LEED pattern since the reciprocal surface unit cell of the latter has half the size of the reciprocal rocksalt unit cell. Clear conversion of the surface structure from inverse spinel to rocksalt is observed for x > 1.5.
Depending on Ni content x,
Figure 13 presents an overview of different phases of ultrathin Ni
xFe
3−xO
4 films as determined by different experimental techniques.
This transition is in accordance with the subsequent increase in the vertical layer distance when increasing x in the overstoichiometric regime as determined by HR-XRD. This is also according to the slight increase when comparing the bulk layer distances of NiFe2O4 and NiO. In addition, due to the larger bulk NiO layer distance compared to the one of NiFe2O4, NiO exhibits a smaller lattice mismatch to the MgO substrate. This should also result in smaller vertical compressive strain, which could further explain the increased layer distances for increasing x in the overstoichiometric regime. Moreover, a significant decrease in the interface distance for x > 1.20 as determined by HR-XRD analysis also points to the formation of a different structure at the interface. However, the oxide film surface is still extremely smooth, and its roughness is only slightly increased for the overstoichiometric films compared to the understoichiometric regime.
The out-of-plane lattice constants obtained by HR-XRD are all compressively strained as can be explained by an at least partial adaption of the film to the substrate lattice (pseudomorphic growth mode) resulting in lateral expansion and vertical compression. Based on elastic theory for the continuum and implying a homogeneous tetragonally strained film structure due to in-plane tensile stress, the expected vertical layer distance can be estimated by
with
as the Poisson ratio of the film material [
44]. Here,
c and
a are the bulk vertical and lateral lattice constants, whereas
and
denote the respective differences between strained and bulk lattice constants. Following Equation (
3) and taking the Poisson ratios for the stoichiometric cases of Fe
3O
4 (
= 0.356 [
45]), NiFe
2O
4 (
= 0.338 [
45]), and NiO (
= 0.297 [
45]) into account, compressed vertical layer distances of
= 209.1 pm,
= 206.3 pm, and
= 207.3 pm are obtained for the three respective stoichiometric oxides, assuming complete pseudomorphic adaption of the lateral lattice constants to the lattice constant of the MgO substrate. These estimated values [according to the lower limits of the filled regions in
Figure 5a] match very well with the evolution of the measured strained vertical layer distances, which in turn confirms complete adaption of the film to the substrate lattice without any hint of strain relaxation. As a consequence thereof, all prepared ferrite films are assumed to exhibit negligible amounts of defects like misfit dislocations, which would release the applied strain. This assumption is confirmed by the complete vertical crystallinity and high crystalline quality of the ferrite films with very smooth interfaces/surfaces independent of the Ni content as determined by HR-XRD analysis.
Further, the assumed absence of strain releasing defects can be followed by estimating the theoretical critical thickness D
above which misfit dislocations are incorporated into the film to release strain. Using the model of Matthews and Blakeslee [
46], the critical thickness
for the initial cooperation of dislocations is given by
Here,
b is the magnitude of the Burgers vector,
f is the modulus of the lattice mismatch,
= 90° is the angle between the Burgers vector and the dislocation line, and
= 45° is the angle between the Burgers vector and the direction normal to the dislocation line and within the plane of the interface. For Fe
3O
4 and NiFe
2O
4, the magnitudes of the Burgers vectors for the different involved oxides are
/4 = 296.8 pm and
/4 = 294.8 pm (
: cubic bulk lattice constants) [
47]. For NiO, the magnitude of the Burgers vector is given by
/2 = 295.3 pm. Taking the respective Poisson ratios into account (see above), critical thicknesses of 103.9 nm, 27.4 nm, and 34.8 nm are obtained for Fe
3O
4, NiFe
2O
4, and NiO, respectively. All values are significantly larger than the prepared film thicknesses within this study (up to 18.6 nm) and are therefore consistent with the observed homogeneous strain. Moreover, this observation agrees with the results obtained for the off-stoichiometric NFO films in a former study [
48].
Apart from the structural and electronic characterization, magnetization M vs. H measurements reveal a decrease in saturation magnetizations as it is expected for increasing Ni content x. For x = 0 (Fe3O4), the measured saturation magnetization matches the expected value of 4 /f.u. with only a slight deviation to a lower value. The subsequent decrease in for increasing x is stronger than expected for the gradual substitution of cations by cations, causing the magnetization of 2 /f.u. for stoichiometric bulk NiFe2O4 (x = 1). Here, the saturation magnetization drops below the value of 2 /f.u. already for Ni content x ≥ 0.87.
Partly, this low saturation magnetization may be due to the coexistence of a ferrimagnetic NFO spinel structure with antiferromagnetic rocksalt structures. However, major parts of this deviation could be ascribed to the presence of APBs in the ferrite films [
49], which are typically formed during the film growth of the ferrite with a double-sized lattice constant compared to the one of the MgO substrate. This discrepancy forces that the islands or layers formed during film growth with nucleation centers that are non-integer multiples of the ferrite lattice constant apart from each other are out-of-phase upon merging and, thus, form APBs. Strong antiferromagnetic coupling across the APBs as it is known for, for example, Fe
3O
4 on MgO(001) [
50], could consequently result in a lower saturation magnetization as it is observed here for all prepared ferrite films.
Further, the observed discrepancy to lower values for x > 1 could be ascribed to the decreasing : ratio and the consequently decreasing content of inverse spinel-type structures. The relative excess compared to the spinel-type structures could possibly couple antiferromagnetically in the rock-salt-like structures and would therefore not give a ferromagnetic contribution to the magnetization and give rise to an enhanced discrepancy of to lower values.
5. Conclusions
Within this study, a structural, chemical, and magnetic characterization was conducted on NixFe3−xO4 ultrathin films with thickness 16 ± 2 nm and varying Ni content x. The films were prepared by RMBE on lattice-matched MgO(001). All films are compressively strained in the vertical direction due to lateral tensile strain, resulting from an adaption of the film to the substrate lattice. As a consequence, the films are assumed to exhibit very low defect densities (except for APBs, see below) as the results and theoretical predictions show no evidence of released strain by defects like, for example, misfit dislocations. The films are completely crystalline from the interface to the surface with extremely low interface and surface roughnesses, independent of the cationic stoichiometry.
On the one hand, for understoichiometric Ni content x < 1, a successive conversion from Fe3O4 to (almost) stoichiometric NiFe2O4 with increasing x is observed while conserving the inverse spinel structure. Within this regime, octahedrally coordinated cations are replaced by cations, according to the decreasing : ratio and a constant valency as observed in HAXPES and XAS. On the other hand, for the overstoichiometric regime x > 1, a crucial decrease in tetrahedrally coordinated cations (attributed to the inverse spinel structure of NFO) is noticed and mostly and cations are present within the films, indicating a decrease in inverse spinel-type structures. Parts of the films show a local rocksalt structure for mixed Fe-Ni monoxide. However, these parts are homogeneously distributed within the films and not strictly separated from the ferrite-like NFO parts.
The saturation magnetization of the films decreases, converting Fe3O4 to NiFe2O4. However, the decrease is stronger than expected for this conversion. This result can be related to the presence of APBs, whereas the discrepancy in the overstoichiometric regime (x > 1) can be related to the presence of antiferromagnetic rocksalt structures.
Apart from the presence of APBs, all films exhibit high crystalline quality with low defect densities and sharp interfaces and surfaces, which is crucial for the implementation in spin filter applications [
8,
10]. Hence, from the results in the presented study, high applicability in spintronics not only for stoichiometric NiFe
2O
4 but also for NFO films with off-stoichiometric cationic ratios could be expected.