On-Line pH Measurement Cation Exchange Kinetics of Y3+-Exchanged Alginic Acid for Y2O3 Nanoparticles Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
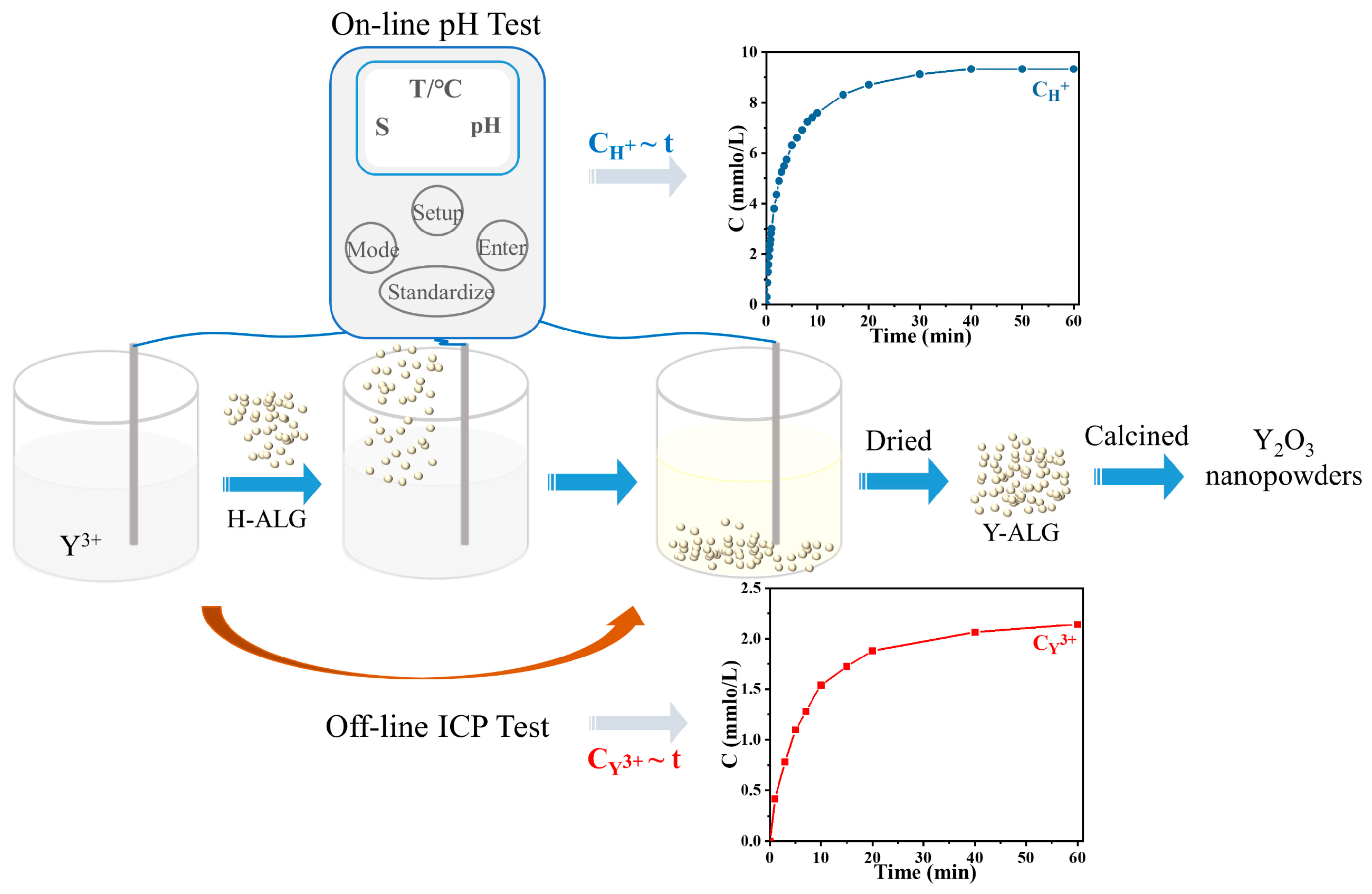
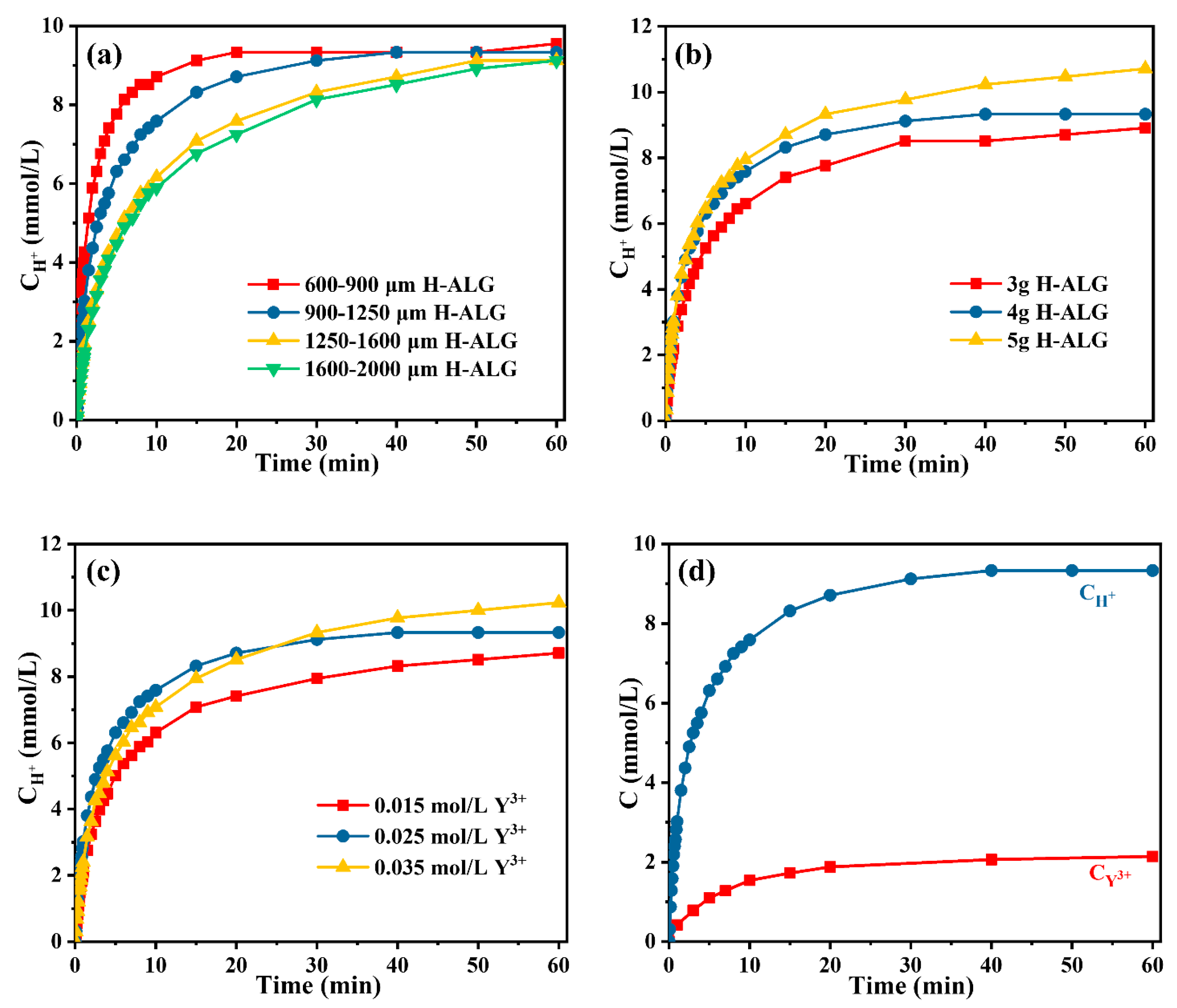
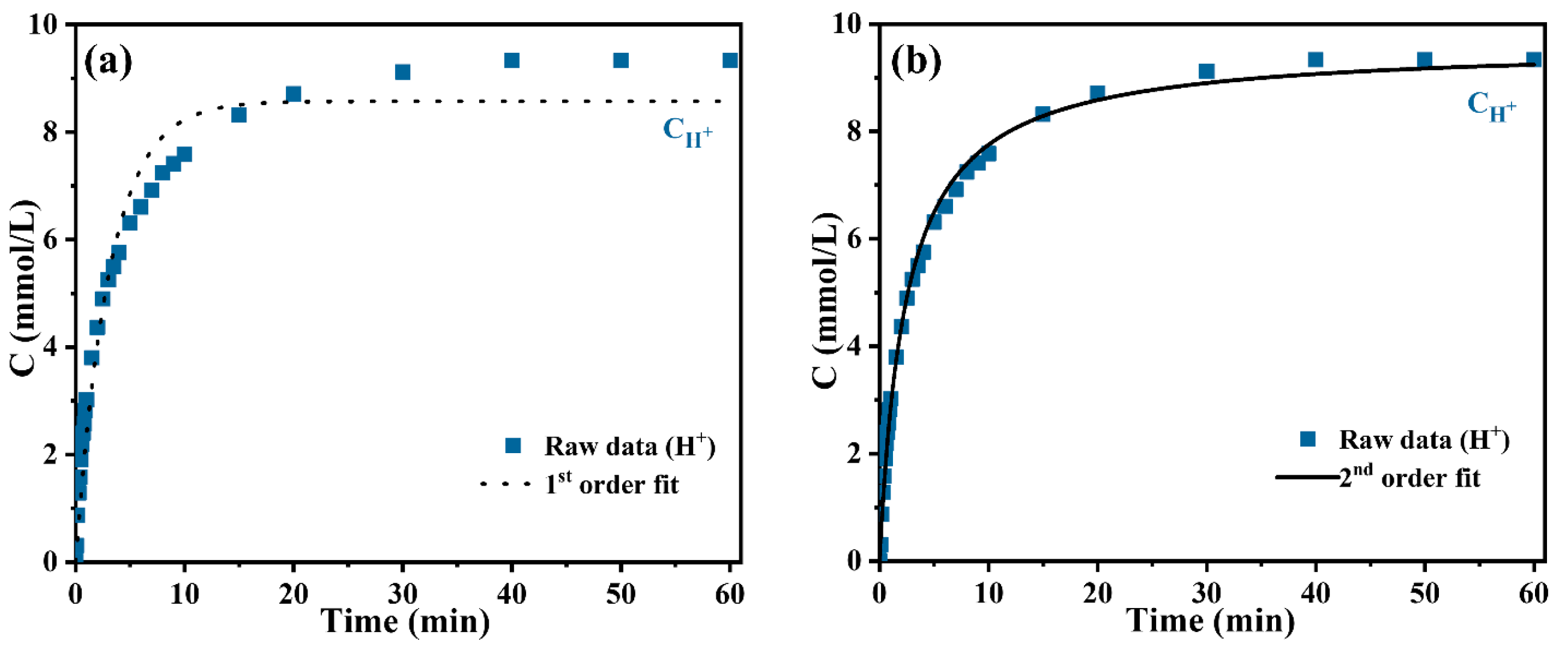
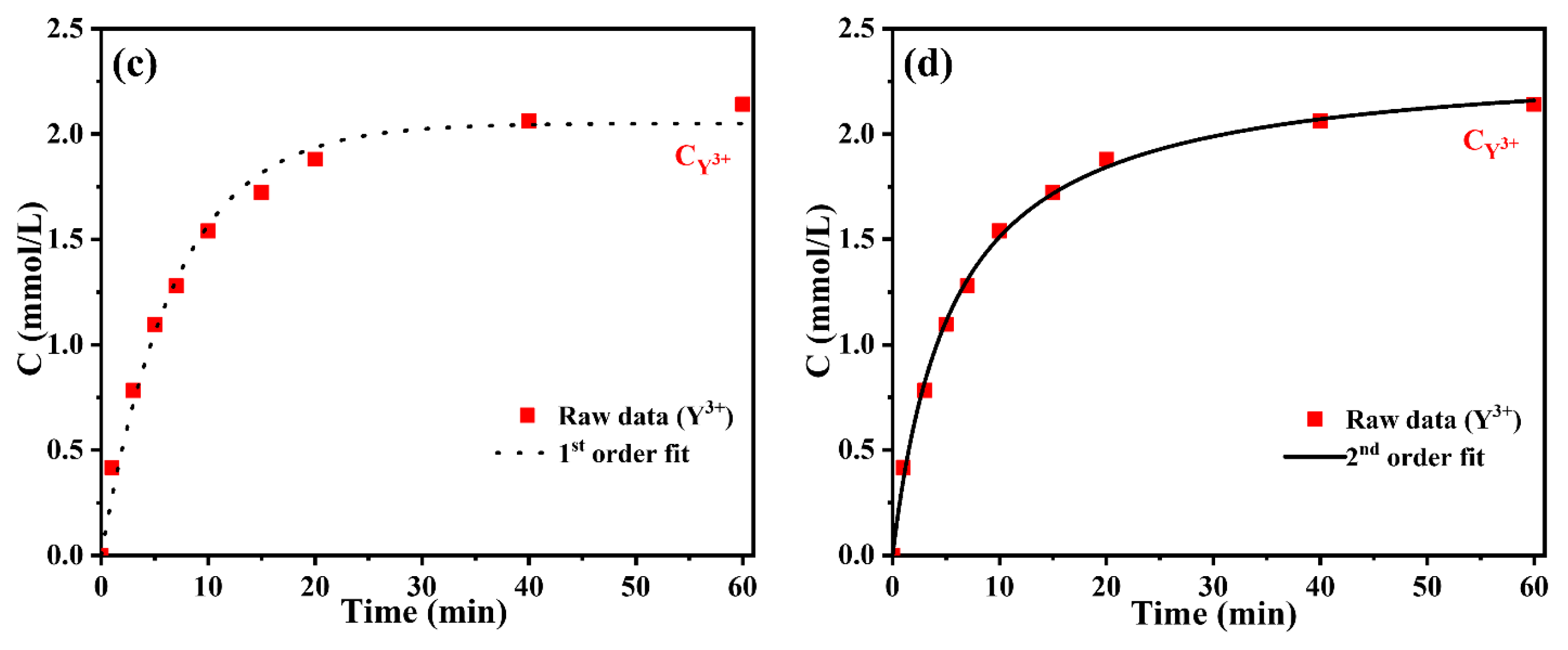
3.1. The Cation Exchange Reaction Kinetics of H+~Y3+
3.2. TGA/DSC of Y-ALG
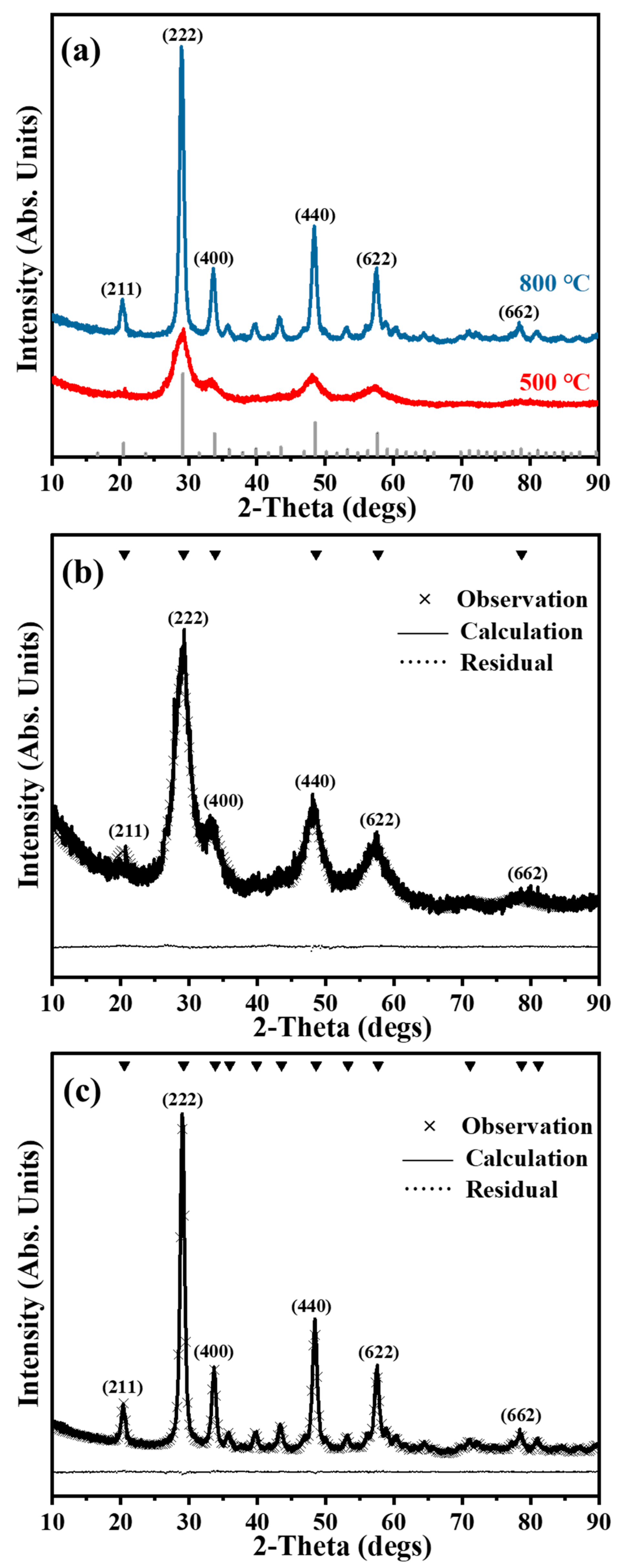
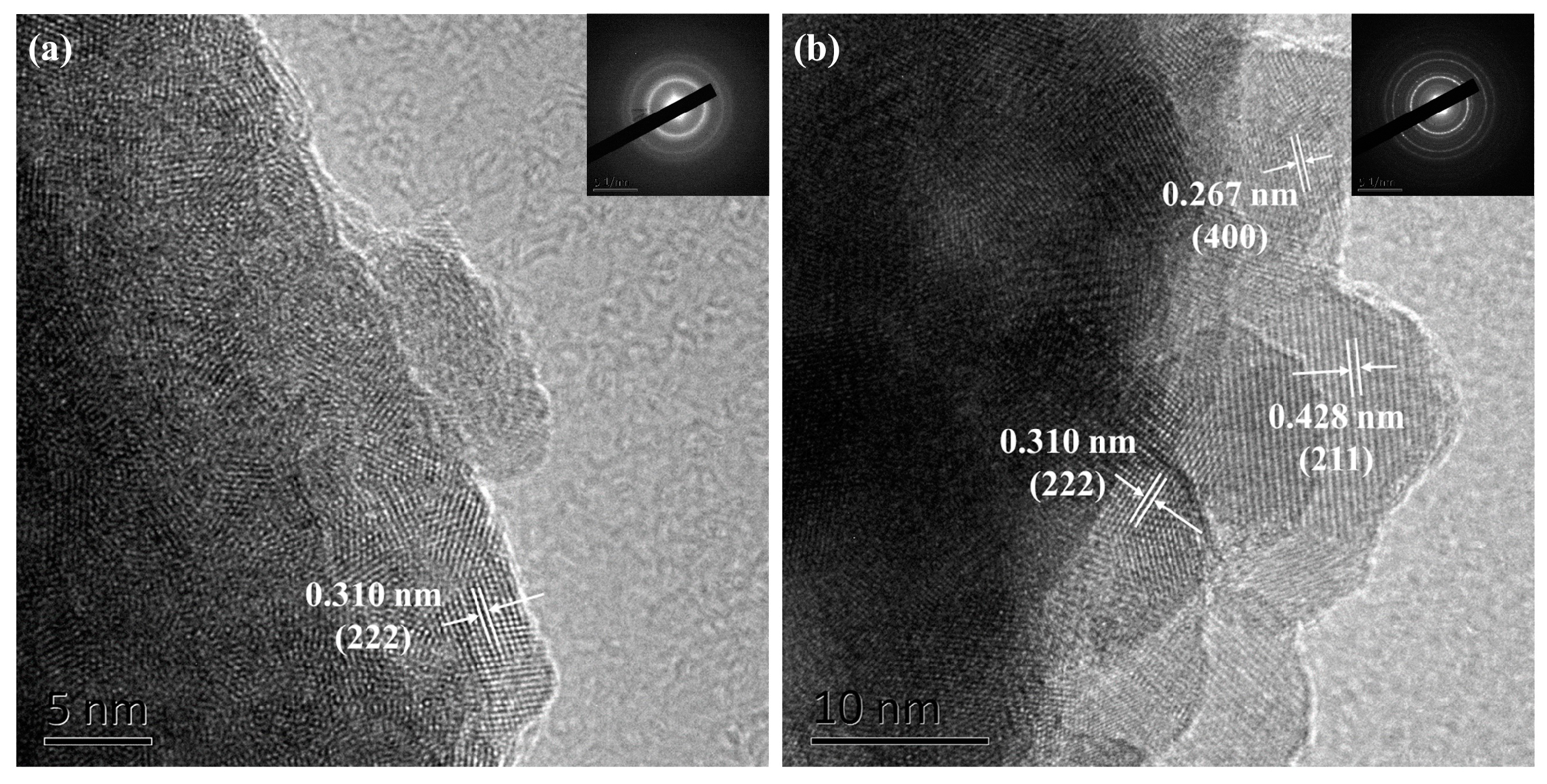
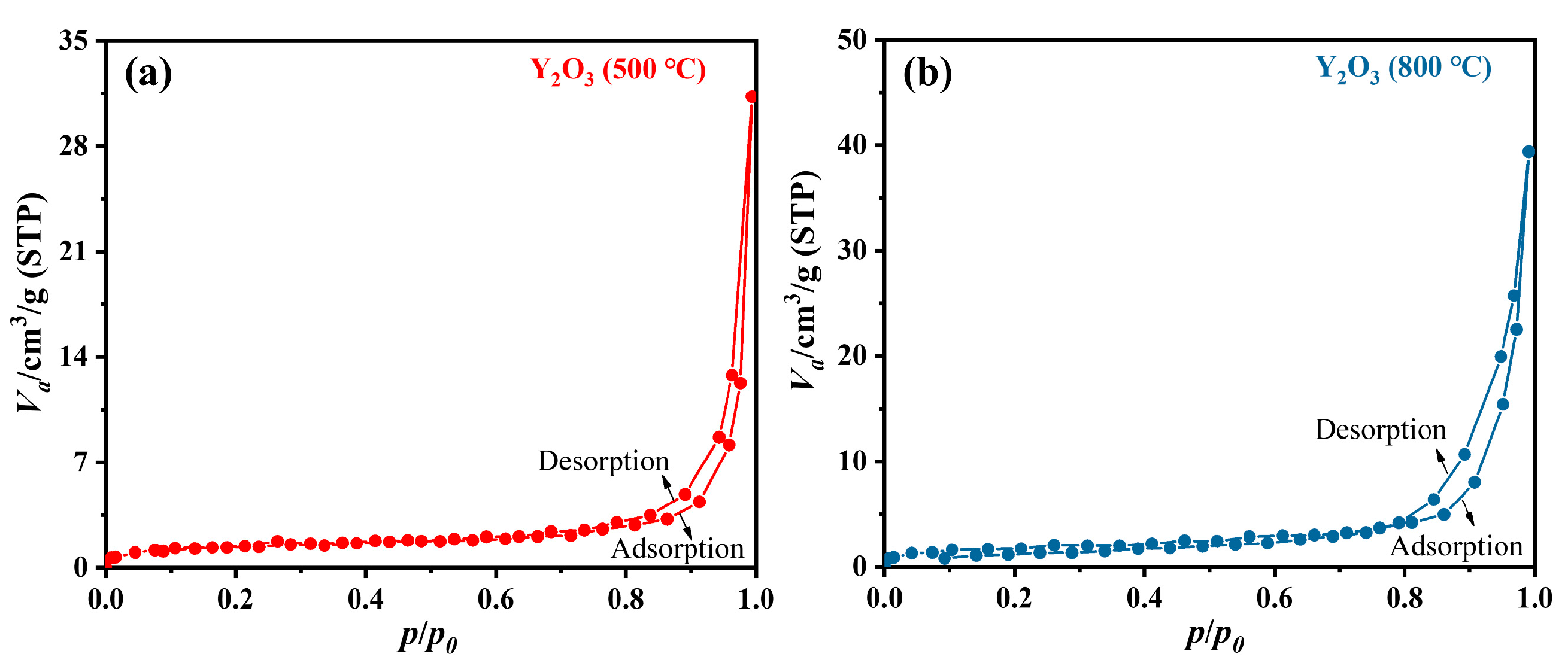
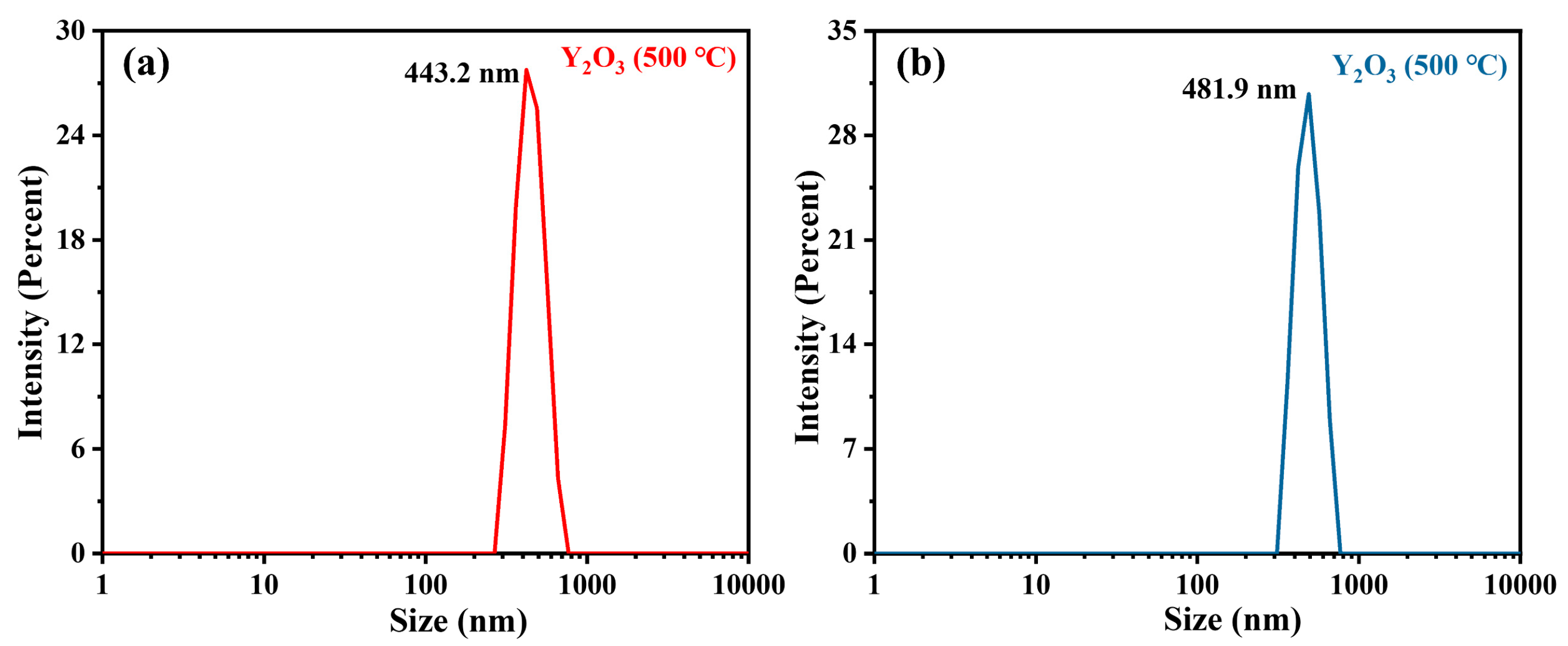
3.3. Characterization of Y2O3
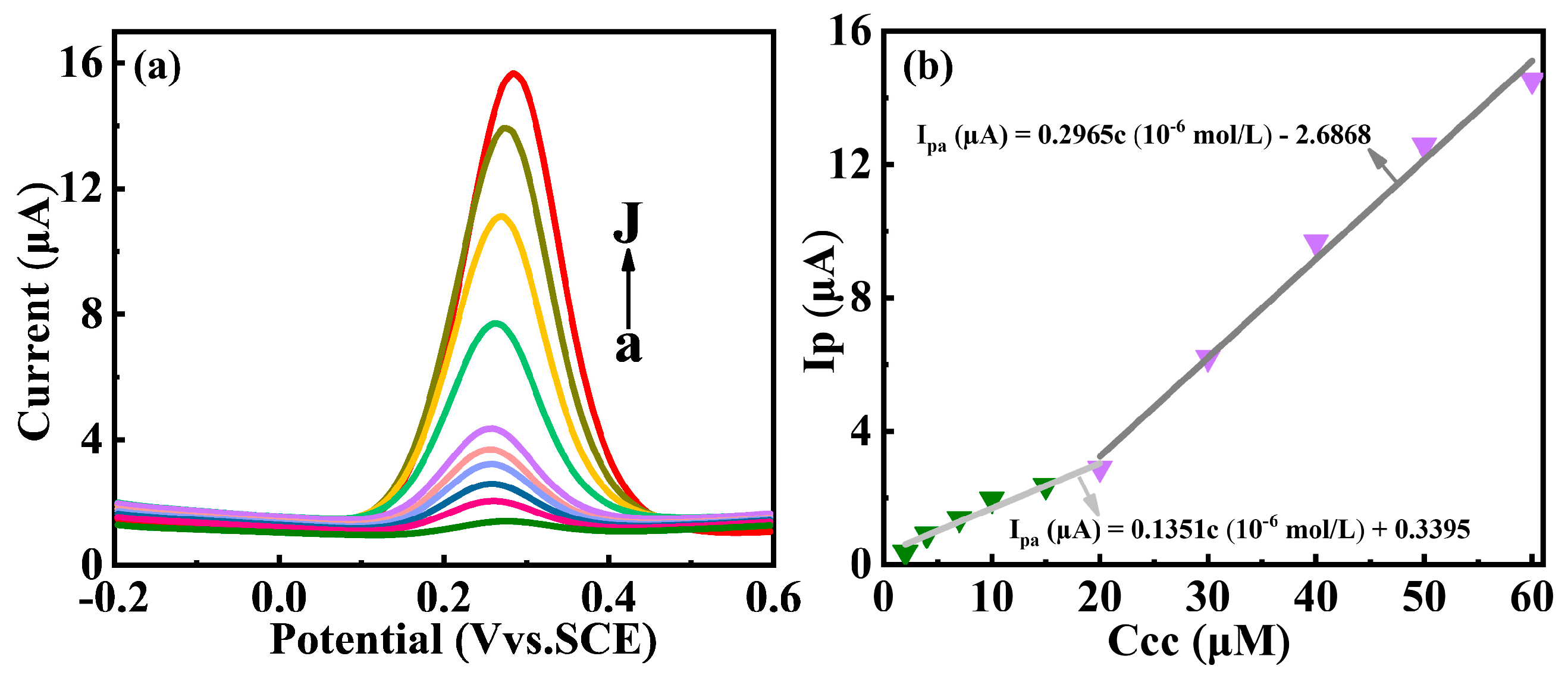
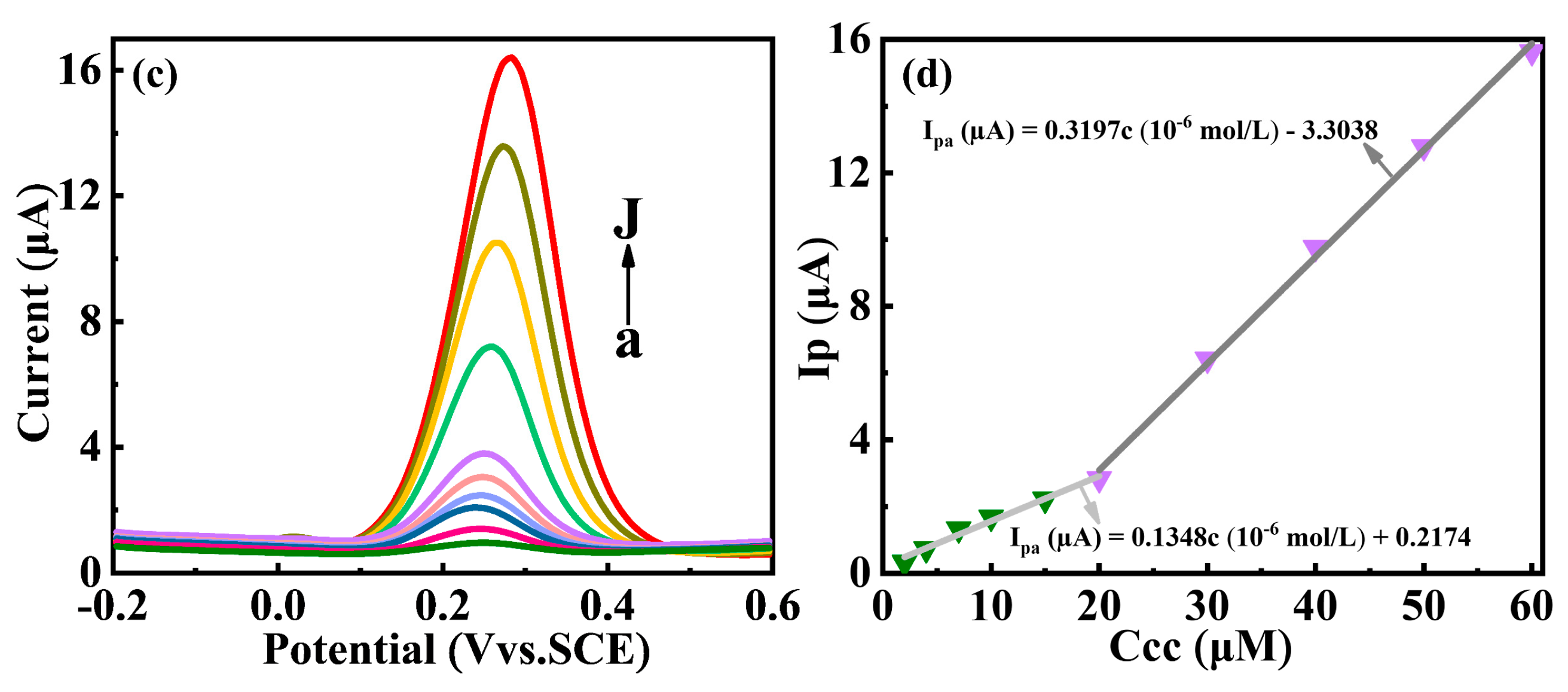
3.4. Electrochemical Determation of Catechol at the Y2O3/GCE Electrode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pezeshkpour, S.; Abdullah, A.Z.; Salamatinia, B.; Amini Horri, B. Ionic-gelation synthesis of gadolinium doped ceria (Ce0.8Gd0.2O1.90) nanocomposite powder using sodium-alginate. Ceram. Int. 2017, 43, 7123–7135. [Google Scholar] [CrossRef]
- Pezeshkpour, S.; Salamatinia, B.; Amini Horri, B. Synthesis and characterization of nanocrystalline NiO-GDC via sodium alginate-mediated ionic sol-gel method. Ceram. Int. 2018, 44, 3201–3210. [Google Scholar] [CrossRef]
- Sardar, S.; Kale, G.; Ghadiri, M.; Cespedes, O. Structural study of holmium zirconate nanoparticles obtained through carbon neutral sol-gel process. Thermochim. Acta 2019, 676, 120–129. [Google Scholar] [CrossRef]
- Liu, L.Y.; Li, C.; Wang, Z.H.; Wang, X.Z. Structurally related electromagnetic properties of NixFeyO4 nanomaterials synthesized by granulated sodium alginate. J. Alloys Compd. 2021, 858, 157641. [Google Scholar] [CrossRef]
- Liu, L.Y.; Wang, Z.H.; Wang, X.Z. CoFe2O4 nanopowder synthesis by a solid-liquid cation-exchange process and their magnetic properties. Ceram. Int. 2021, 47, 14418–14426. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kale, G.M.; Ghadiri, M. Synthesis and characterization of CexGd1−xO2−δ nanopowders employing an alginate mediated ion-exchange process. Chem. Eng. J. 2012, 198, 149–153. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.; Kale, G.M.; Ghadiri, M. Ion-exchange kinetics and thermal decomposition characteristics of Fe2+-exchanged alginic acid membrane for the formation of iron oxide nanoparticles. J. Mater. Sci. 2014, 49, 7151–7155. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjak-Bræk, G.; Smidsrød, O. Alginate-based new materials. Int. J. Biol. Macromol. 1997, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; Kwon, O.J.; Kim, D.H.; Park, J.S. Characterization of the polyurethane foam using alginic acid as a polyol. Fiber. Polym. 2007, 8, 257–262. [Google Scholar] [CrossRef]
- Jayasankar, K.; Pandey, A.; Mishra, B.K.; Das, S. Mixed fuel synthesis of Y2O3 nanopowder and their applications as dispersoid in ODS steel. Adv. Powder Technol. 2015, 26, 1306–1313. [Google Scholar] [CrossRef]
- Ma, S.; Li, A.; Zhou, S.Q.; Yang, Y.J.; Wang, S.L.; Liu, M.B. Microstructure and mechanical properties of nickel strengthened by Y2O3 through rock-milling and spark plasma sintering. J. Alloys Compd. 2018, 750, 911–916. [Google Scholar] [CrossRef]
- Shruthi, J.; Jayababu, N.; Ghosal, P.; Ramana Reddy, M.V. Ultrasensitive sensor based on Y2O3-In2O3 nanocomposites for the detection of methanol at room temperature. Ceram. Int. 2019, 45, 21497–21504. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, E.P.; Glumov, O.V.; Mel’nikova, N.A.; Murin, I.V.; Kalinina, M.V.; Shilova, O.A.; Sevastyanov, V.G.; et al. Microstructural, electrophysical and gas-sensing properties of CeO2-Y2O3 thin films obtained by the sol-gel process. Ceram. Int. 2020, 46, 121–131. [Google Scholar] [CrossRef]
- Liu, H.M.; He, D.H. Properties of Ni/Y2O3 and its catalytic performance in methane conversion to syngas. Int. J. Hydrog. Energ. 2011, 36, 14447–14454. [Google Scholar] [CrossRef]
- Sun, G.B.; Hidajat, K.; Wu, X.S.; Kawi, S. A crucial role of surface oxygen mobility on nanocrystalline Y2O3 support for oxidative steam reforming of ethanol to hydrogen over Ni/Y2O3 catalysts. Appl. Catal. B Environ. 2008, 81, 303–312. [Google Scholar] [CrossRef]
- Tian, L.L.; Xu, J.C. Electrodeposition and characterization of Ni-Y2O3 composite. Appl. Surf. Sci. 2011, 257, 7615–7620. [Google Scholar] [CrossRef]
- Hou, X.R.; Zhou, S.M.; Li, W.J.; Li, Y.K. Study on the effect and mechanism of zirconia on the sinterability of yttria transparent ceramic. J. Eur. Ceram. Soc. 2010, 30, 3125–3129. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Z.G.; Yao, Q.; Wang, Z.Y.; Zhang, Y.K.; Wei, S.; Zhou, T.Y.; Ben, Y.; Sun, R.; Chen, H. Stirring speed assisted homogenization of precipitation reaction for enhanced optical performance of Y2O3 transparent ceramics. Ceram. Int. 2018, 44, 4947–4972. [Google Scholar] [CrossRef]
- Lin, L.L.; Starostin, S.A.; Li, S.R.; Khan, S.A.; Hessel, V. Synthesis of yttrium oxide nanoparticles via a facile microplasma-assisted process. Chem. Eng. Sci. 2018, 178, 157–166. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Qin, M.L.; Huang, M.; Wu, T.; Jia, B.R.; Wu, H.Y.; Ma, J.D.; Chen, G.; Qu, X.H. Magnetic properties of evenly mixed Fe-Y2O3 nanocomposites synthesized by a facile wet-chemical based route. J. Magn. Magn. Mater. 2019, 491, 165576. [Google Scholar] [CrossRef]
- Foo, Y.T.; Abdullah, A.Z.; Horri, B.A.; Salamatinia, B. Synthesis and characterisation of Y2O3 using ammonia oxalate as a precipitant in distillate pack co-precipitation process. Ceram. Int. 2018, 44, 18693–18702. [Google Scholar] [CrossRef]
- Kalaivani, S.; Ponnilavan, V.; Kumar, P.N.; Kannan, S. Structural, charge density and bond length variations in c-Y2O3 influenced by progressive cerium additions. CrystEngComm 2019, 21, 5332–5343. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tao, S.; Li, X.L.; Wang, X.G.; Wei, Y.; Jia, Y.L.; Ju, J.; Cheng, Y.H.; Wang, H.S.; Gong, S.W.; Yao, X.J.; et al. Facile synthesis of hierarchical nanosized single-crystal aluminophosphate molecular sieves from highly homogeneous and concentrated precursors. Angew. Chem. Int. Ed. 2020, 59, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, P.; Priamushko, T.; Patil, P.; Florek, J.; Garstenauer, D.; Mautner, A.; Shin, J.W.; Ryoo, R.; Pichler, C.M.; Kleitz, F. Low-Temperature controlled synthesis of nanocast mixed metal oxide spinels for enhanced OER activity. J. Colloid. Interf. Sci. 2024, 661, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, R.; Venkatesan, R.; Gangan, A.; Samal, R.; Chakraborty, B.; Late, D.J.; Rout, C.S. Experimental and density functional theory investigations of catechol sensing properties of ZnO/RGO nanocomposites. Appl. Surf. Sci. 2019, 495, 143588. [Google Scholar] [CrossRef]
- Majumdar, S. The effects of crystallite size, surface area and morphology on the sensing properties of nanocrystalline SnO2 based system. Ceram. Int. 2015, 41, 14350–14358. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Wang, J.; Hou, S.J.; Mukherjee, S.; Fischer, R.A. Room temperature synthesis mediated porphyrinic nanoMOF enables benchmark electrochemical biosensing. Small 2023, 19, 2301933. [Google Scholar] [CrossRef]

| C–t | 1st Order Fit | 2nd Order Fit | ||||||
|---|---|---|---|---|---|---|---|---|
| r2 | SD | r2 | SD | |||||
| CH+–t | 0.3217 | 8.5741 | 0.9656 | 32.39% | 0.04339 | 9.6124 | 0.9963 | 3.48% |
| CY3+–t | 0.1445 | 2.0504 | 0.9898 | 0.59% | 0.07523 | 2.3619 | 0.9981 | 0.11% |
| Diff. Temp. for 2 h | 500 °C | 800 °C |
|---|---|---|
| Lattice parameter (Å) | 10.6997 | 10.6101 |
| Size (nm) | 3.67 (2) | 15.26 (9) |
| Micro Strain | 0.75 (1) | 0.21 (8) |
| Rwp (%) | 10.23 | 7.94 |
| Rexp (%) | 7.52 | 7.53 |
| GOF | 1.36 | 1.05 |
| (hkl) | Ref. a d (Å) | TEM d (Å) | XRD d (Å) |
|---|---|---|---|
| (211) | 4.33 | 4.28 (6) | 4.37 (2) |
| (222) | 3.06 | 3.10 (3) | 3.08 (2) |
| (400) | 2.65 | 2.67 (1) | 2.66 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhou, F.; Zhang, Y.; Sun, Y.; Zhang, S.; Cai, K.; Qiu, R.; Lin, Y.; Fa, W.; Wang, Z. On-Line pH Measurement Cation Exchange Kinetics of Y3+-Exchanged Alginic Acid for Y2O3 Nanoparticles Synthesis. Nanomaterials 2024, 14, 696. https://doi.org/10.3390/nano14080696
Liu L, Zhou F, Zhang Y, Sun Y, Zhang S, Cai K, Qiu R, Lin Y, Fa W, Wang Z. On-Line pH Measurement Cation Exchange Kinetics of Y3+-Exchanged Alginic Acid for Y2O3 Nanoparticles Synthesis. Nanomaterials. 2024; 14(8):696. https://doi.org/10.3390/nano14080696
Chicago/Turabian StyleLiu, Lingyu, Fengchen Zhou, Yuxiang Zhang, Yanhua Sun, Shixing Zhang, Kun Cai, Ruichong Qiu, Yi Lin, Wenjun Fa, and Zihua Wang. 2024. "On-Line pH Measurement Cation Exchange Kinetics of Y3+-Exchanged Alginic Acid for Y2O3 Nanoparticles Synthesis" Nanomaterials 14, no. 8: 696. https://doi.org/10.3390/nano14080696
APA StyleLiu, L., Zhou, F., Zhang, Y., Sun, Y., Zhang, S., Cai, K., Qiu, R., Lin, Y., Fa, W., & Wang, Z. (2024). On-Line pH Measurement Cation Exchange Kinetics of Y3+-Exchanged Alginic Acid for Y2O3 Nanoparticles Synthesis. Nanomaterials, 14(8), 696. https://doi.org/10.3390/nano14080696




