Plasmonic Au@Ag Core–Shell Nanoisland Film for Photothermal Inactivation and Surface-Enhanced Raman Scattering Detection of Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Au@AgNIFs
2.3. Culture Bacteria on the Surfaces of the Au@AgNIFs for SEM Measurement
2.4. Characterizations of the Au@AgNIFs
2.5. Photothermal Assessment
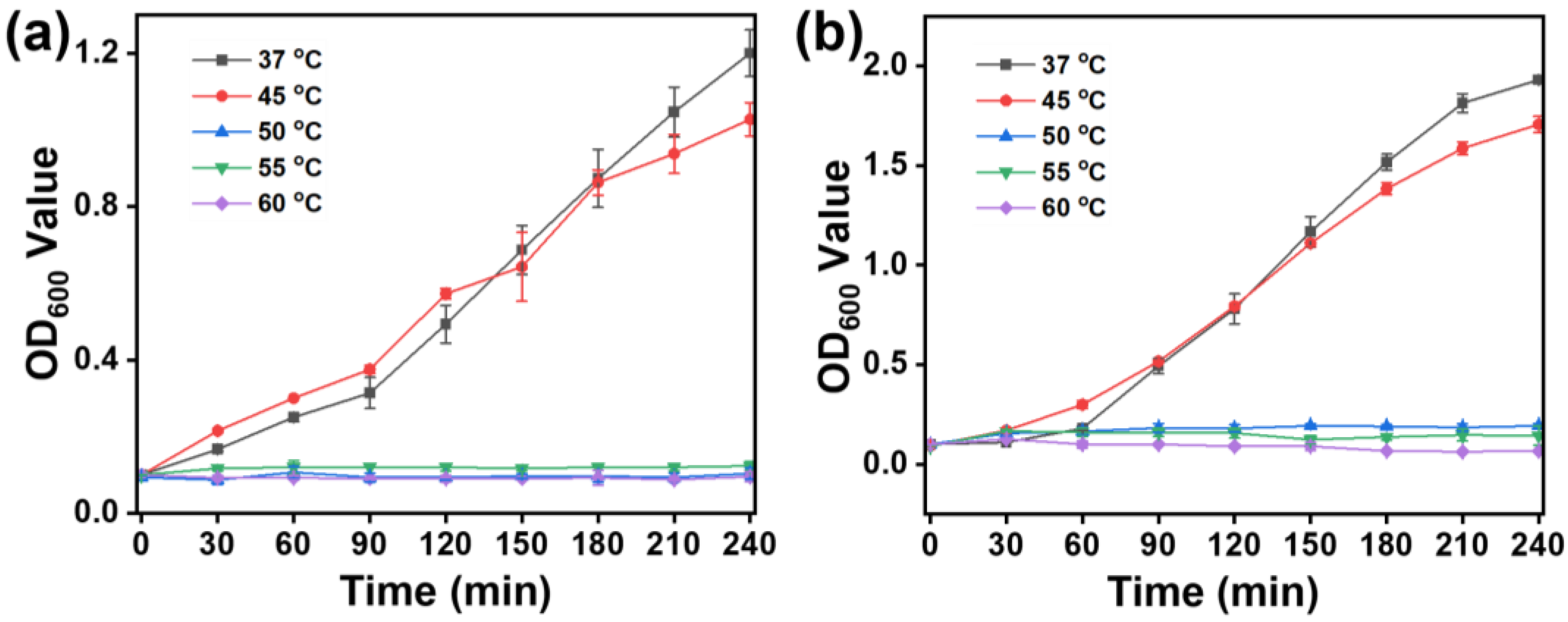
2.6. Bacterial Growth under Different Temperatures
2.7. Bacterial Inhibition by the Au@AgNIFs under Light Exposure
2.8. Raman Spectroscopy and Measurement
3. Results
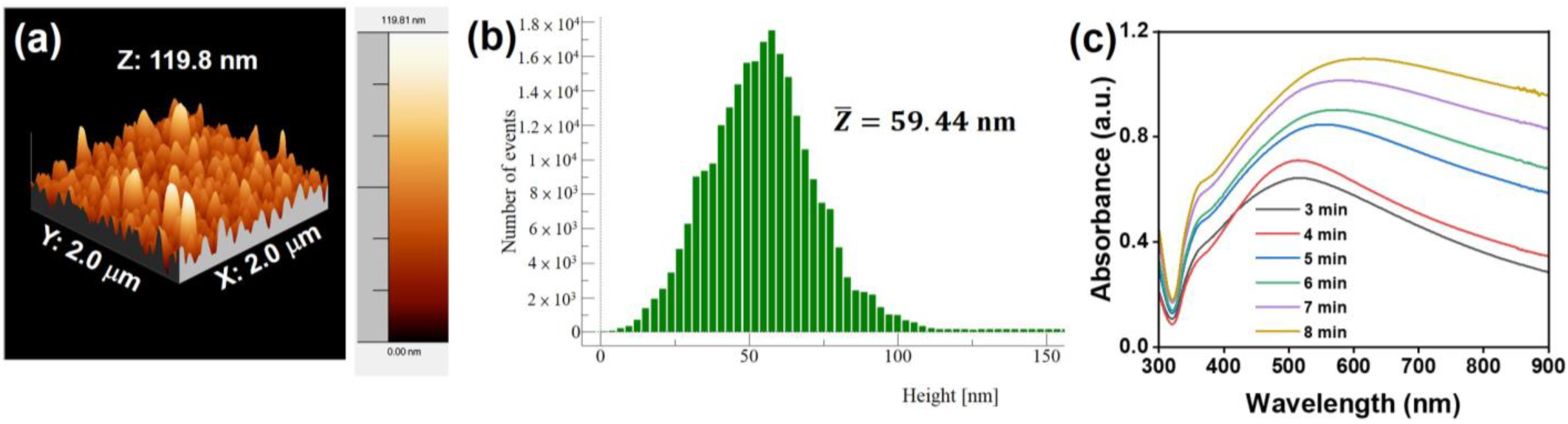
3.1. Structural and Optical Properties of the Au@AgNIFs
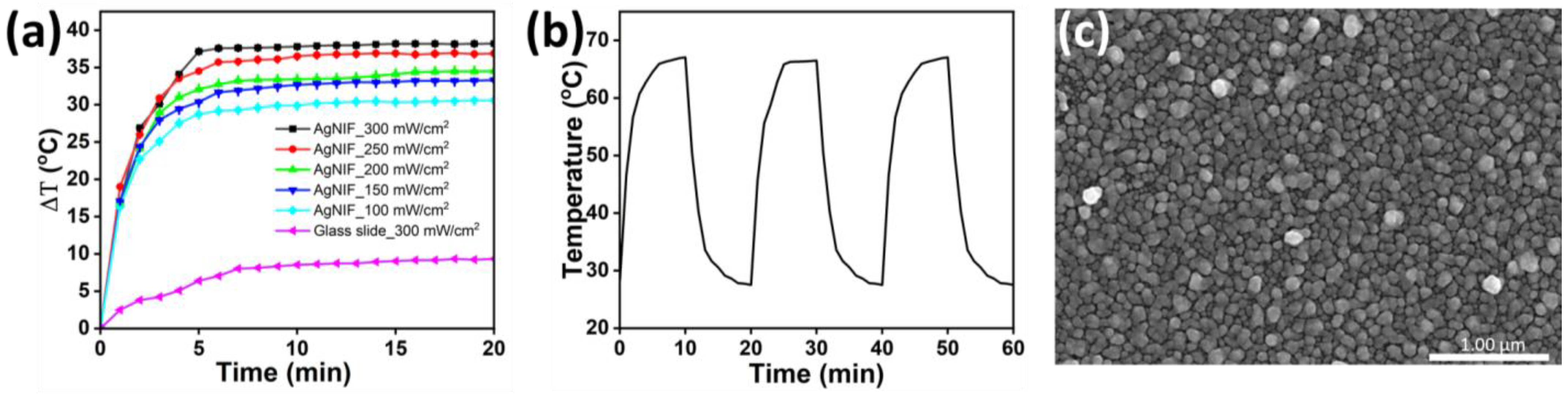
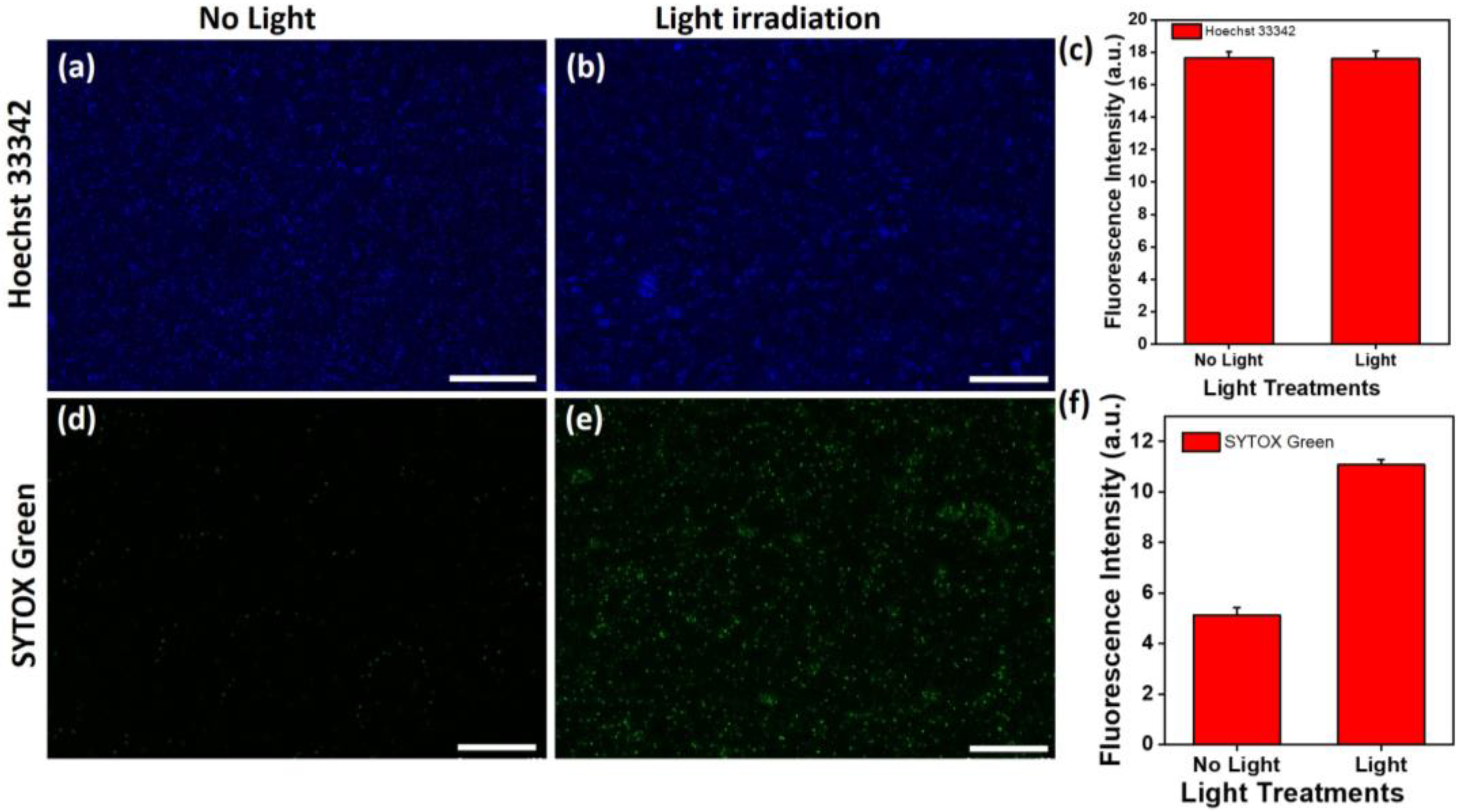
3.2. Photothermal Therapy of the Au@AgNIFs
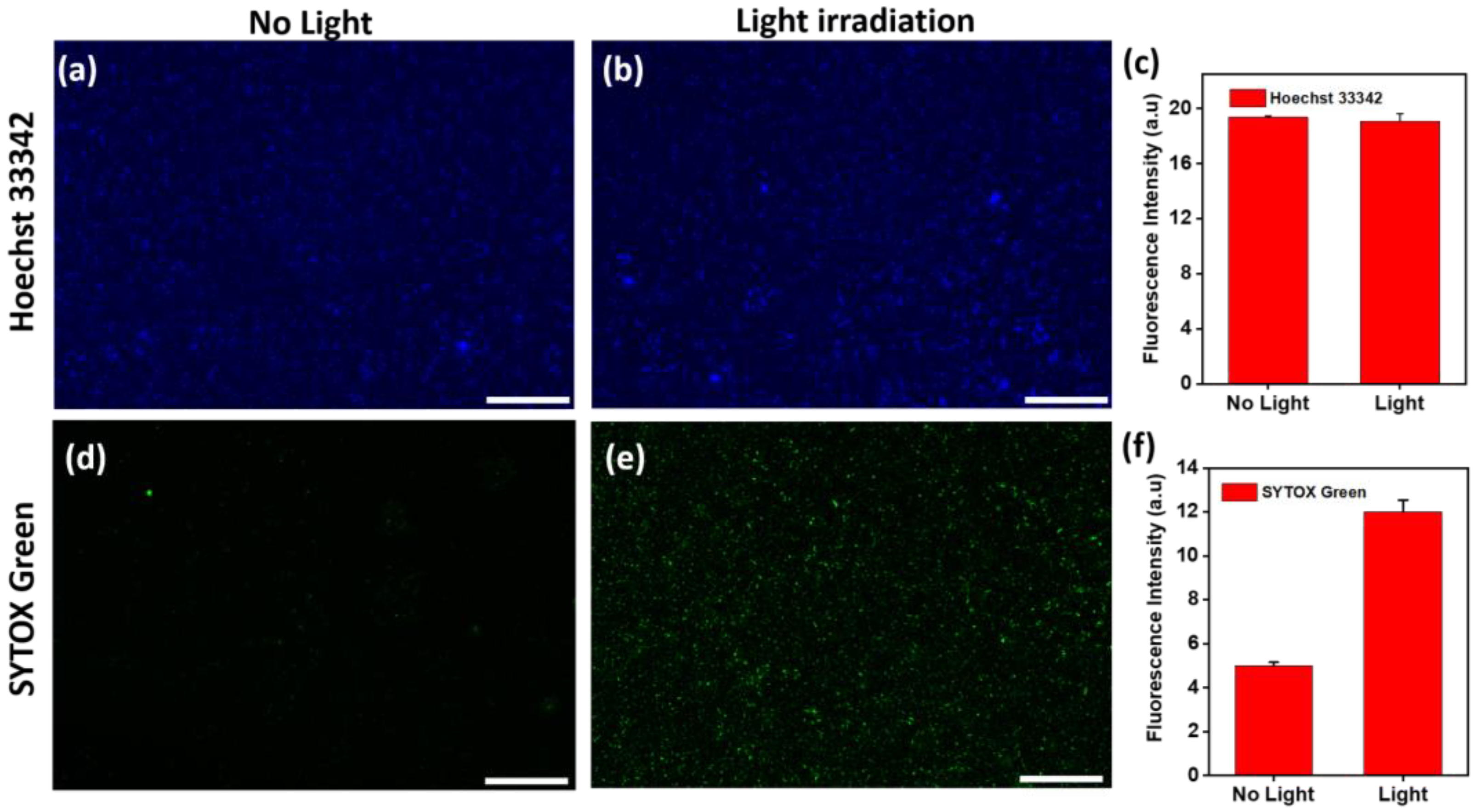
3.3. Au@AgNIFs as a SERS Substrate for Bacterial Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent Advances in Plasmonic Nanostructures for Enhanced Photocatalysis and Electrocatalysis. Adv. Mater. 2021, 33, 2000086. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Chow, T.H.; Li, N.; Bai, X.; Zhuo, X.; Shao, L.; Wang, J. Gold Nanobipyramids: An Emerging and Versatile Type of Plasmonic Nanoparticles. Acc. Chem. Res. 2019, 52, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chang, W.-H.; Lin, C.-H. Selective Growth of Patterned Monolayer Gold Nanoparticles on SU-8 through Photoreduction for Plasmonic Applications. ACS Appl. Nano Mater. 2021, 4, 229–235. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Yang, Z.-Y.; Chong, T.-W.; Liu, Y.-Y.; Pan, H.-W.; Lin, C.-H. Quantifying Cell Confluency by Plasmonic Nanodot Arrays to Achieve Cultivating Consistency. ACS Sens. 2019, 4, 1816–1824. [Google Scholar] [CrossRef]
- Yin, Q.; Tan, L.; Lang, Q.; Ke, X.; Bai, L.; Guo, K.; Qiao, R.; Bai, S. Plasmonic molybdenum oxide nanosheets supported silver nanocubes for enhanced near-infrared antibacterial activity: Synergism of photothermal effect, silver release and photocatalytic reactions. Appl. Catal. B Environ. 2018, 224, 671–680. [Google Scholar] [CrossRef]
- Wang, G.; Hao, C.; Ma, W.; Qu, A.; Chen, C.; Xu, J.; Xu, C.; Kuang, H.; Xu, L. Chiral Plasmonic Triangular Nanorings with SERS Activity for Ultrasensitive Detection of Amyloid Proteins in Alzheimer’s Disease. Adv. Mater. 2021, 33, 2102337. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, Y.; Wang, Y.; Du, T.; Li, C.; Wang, X.; Jiang, H. Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord. Chem. Rev. 2021, 449, 214218. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal Membrane Distillation for Seawater Desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-loaded pegylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Yu, Y.; Mei, L.; Shi, Y.; Zhang, X.; Cheng, K.; Cao, F.; Zhang, L.; Xu, J.; Li, X.; Xu, Z. Ag-Conjugated graphene quantum dots with blue light-enhanced singlet oxygen generation for ternary-mode highly-efficient antimicrobial therapy. J. Mater. Chem. B 2020, 8, 1371–1382. [Google Scholar] [CrossRef]
- Lin, S.; Chen, H.; Wang, R.; Jiang, T.; Wang, R.; Yu, F. Hollow silver–gold alloy nanoparticles for enhanced photothermal/photodynamic synergetic therapy against bacterial infection and acceleration of wound healing. Biomater. Sci. 2023, 11, 4874–4889. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.-C.; Wang, D.-Y.; Kuo, T.-R. Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Zhang, S.; Gao, L.; Lin, F.; Dai, H. Self-reduced MXene-Metal interaction electrochemiluminescence support with synergistic electrocatalytic and photothermal effects for the bimodal detection of ovarian cancer biomarkers. J. Colloid Interface Sci. 2024, 661, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, S.; Chen, W.; Hu, X.; Liu, Y.; Jiang, L.; Luo, L.; Luo, W.; Ma, Y.; Jiang, X.; et al. Shape-stabilized nanosilver-modified grapefruit peel-based porous carbon composite phase change material with high thermal conductivity, photothermal conversion performance and thermal management capability. J. Energy Storage 2024, 83, 110819. [Google Scholar] [CrossRef]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C.B. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Darius, E.; Lien, M.C.; Yeh, I.H.; Shi, H.F.; Huang, Y.H.; Chen, C.H.; Chen, H.W.; Su, C.Y.; Hsu, R.Y.; et al. Two-Dimensional Cs2AgBiBr6-Based Biosensor for Selective and Sensitive Detection of Cardiac Biomarker Troponin I. ACS Appl. Nano Mater. 2023, 6, 23022–23028. [Google Scholar] [CrossRef]
- Ren, H.; Sun, Y.; Wang, J.; Qiu, H.; Zhang, S.; Zhang, Y.; Yu, X.; Hu, J.; Hu, Y. Regulated synthesis of an Au NB-DT@Ag bimetallic core-molecule-shell nanostructure for reliable SERS detection. Anal. Methods 2023, 15, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Xu, Y.; Peng, F.; Ma, W.; Zhao, Y. Plasmonic metal NP-bismuth composite film with amplified SERS activity for multiple detection of pesticides and veterinary drugs. Chem. Eng. J. 2023, 474, 145933. [Google Scholar] [CrossRef]
- Gupta, P.; Mishra, K.; Mittal, A.K.; Handa, N.; Paul, M.K. Current Expansion of Silver and Gold Nanomaterials towards Cancer Theranostics: Development of Therapeutics. Curr. Nanosci. 2024, 20, 356–372. [Google Scholar] [CrossRef]
- Zhang, W.; Zi, X.; Bi, J.; Liu, G.; Cheng, H.; Bao, K.; Qin, L.; Wang, W. Plasmonic Nanomaterials in Dark Field Sensing Systems. Nanomaterials 2023, 13, 2027. [Google Scholar] [CrossRef] [PubMed]
- Fendi, F.W.S.; Mukhtar, W.M.; Abdullah, M. Surface plasmon resonance sensor for COVID-19 detection: A review on plasmonic materials. Sens. Actuator A Phys. 2023, 362, 114617. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. Two-dimensional materials and their role in sensitivity enhancement of surface plasmon resonance based biosensor. TrAC Trends Anal. Chem. 2024, 171, 117497. [Google Scholar] [CrossRef]
- Chia, Z.-C.; Chen, Y.-L.; Chuang, C.-H.; Hsieh, C.-H.; Chen, Y.-J.; Chen, K.-H.; Huang, T.-C.; Chen, M.-C.; Huang, C.-C. Polyphenol-assisted assembly of Au-deposited polylactic acid microneedles for SERS sensing and antibacterial photodynamic therapy. Chem. Commun. 2023, 59, 6339–6342. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Chia, Z.C.; Hsieh, Y.-T.; Tsai, H.-C.; Tai, Y.; Yu, T.-T.; Huang, C.-C. A facile wet-chemistry approach to engineer an Au-based SERS substrate and enhance sensitivity down to ppb-level detection. Nanoscale 2021, 13, 3991–3999. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-T.; Hsu, I.L.; Cheng, T.-Y.; Wu, W.-J.; Lee, C.-W.; Li, T.-J.; Cheung, C.I.; Chin, Y.-C.; Chen, H.-C.; Chiu, Y.-C.; et al. Off-Resonance SERS Nanoprobe-Targeted Screen of Biomarkers for Antigens Recognition of Bladder Normal and Aggressive Cancer Cells. Anal. Chem. 2019, 91, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, Y.C.; Wang, C.I.; Shen, T.H.; Wang, H.Y.; Pan, X.Y.; Wang, D.Y.; Liou, C.C.; Chang, Y.H.; Chen, Y.C.; et al. Highly oriented Langmuir-Blodgett film of silver cuboctahedra as an effective matrix-free sample plate for surface-assisted laser desorption/ionization mass spectrometry. Nanoscale 2017, 9, 11119–11125. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Huang, Y.-H.; Cheng, H.-Y.; Kuo, T.-R.; Chang, Y.-M. Statistical analysis of cuboctahedral silver nanocrystals Langmuir–Blodgett film SERS substrate. J. Raman Spectrosc. 2018, 49, 792–799. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle-Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-enhanced light–matter interactions and applications. npj Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Tabakman, S.M.; Chen, Z.; Casalongue, H.S.; Wang, H.; Dai, H. A New Approach to Solution-Phase Gold Seeding for SERS Substrates. Small 2011, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Tabakman, S.M.; Welsher, K.; Chen, Z.; Robinson, J.T.; Wang, H.; Zhang, B.; Dai, H. Near-infrared-fluorescence-enhanced molecular imaging of live cells on gold substrates. Angew. Chem. Int. Ed. 2011, 50, 4644–4648. [Google Scholar] [CrossRef]
- Tabakman, S.M.; Lau, L.; Robinson, J.T.; Price, J.; Sherlock, S.P.; Wang, H.; Zhang, B.; Chen, Z.; Tangsombatvisit, S.; Jarrell, J.A. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat. Commun. 2011, 2, 466. [Google Scholar] [CrossRef]
- Lee, C.-W.; Lin, Z.-C.; Chiang, Y.-C.; Li, S.-Y.; Ciou, J.-J.; Liu, K.-W.; Lin, Y.-C.; Huang, B.-J.; Peng, K.-T.; Fang, M.-L.; et al. AuAg nanocomposites suppress biofilm-induced inflammation in human osteoblasts. Nanotechnology 2023, 34, 165101. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-C.; Kuo, T.-R.; Huang, M.-H.; Huang, H.-K.; Chen, C.-C. Design of Fluorescence-Enhanced Silver Nanoisland Chips for High-Throughput and Rapid Arsenite Assay. ACS Omega 2020, 5, 19771–19777. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Yen, H.-C.; Lee, M.-W.; Sheu, Y.-L.; Chen, P.-C.; Dai, H.; Chen, C.-C. Large-Scale Inhomogeneous Fluorescence Plasmonic Silver Chips: Origin and Mechanism. Chem 2020, 6, 3396–3408. [Google Scholar] [CrossRef]
- Tan, S.-H.; Yougbaré, S.; Tao, H.-Y.; Chang, C.-C.; Kuo, T.-R. Plasmonic Gold Nanoisland Film for Bacterial Theranostics. Nanomaterials 2021, 11, 3139. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.S.; Gut, I.M.; Scheeler, S.M.; Lyman, M.E.; McNutt, P.M. Compatibility of SYTO 13 and Hoechst 33342 for longitudinal imaging of neuron viability and cell death. BMC Res. Notes 2012, 5, 437. [Google Scholar] [CrossRef]
- Arvidsson, M.; Rashed, S.K.; Aits, S. An annotated high-content fluorescence microscopy dataset with Hoechst 33342-stained nuclei and manually labelled outlines. Data Brief 2023, 46, 108769. [Google Scholar] [CrossRef] [PubMed]
- Little, A.C.; Kovalenko, I.; Goo, L.E.; Hong, H.S.; Kerk, S.A.; Yates, J.A.; Purohit, V.; Lombard, D.B.; Merajver, S.D.; Lyssiotis, C.A. High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions. Commun. Biol. 2020, 3, 271. [Google Scholar] [CrossRef]
- Purschke, M.; Rubio, N.; Held, K.D.; Redmond, R.W. Phototoxicity of Hoechst 33342 in time-lapse fluorescence microscopy. Photochem. Photobiol. Sci. 2010, 9, 1634–1639. [Google Scholar] [CrossRef]
- Harhala, M.; Gembara, K.; Miernikiewicz, P.; Owczarek, B.; Kaźmierczak, Z.; Majewska, J.; Nelson, D.C.; Dąbrowska, K. DNA Dye Sytox Green in Detection of Bacteriolytic Activity: High Speed, Precision and Sensitivity Demonstrated with Endolysins. Front. Microbiol. 2021, 12, 752282. [Google Scholar] [CrossRef]
- Roger, L.M.; Adarkwa Darko, Y.; Bernas, T.; White, F.; Olaosebikan, M.; Cowen, L.; Klein-Seetharaman, J.; Lewinski, N.A. Evaluation of fluorescence-based viability stains in cells dissociated from scleractinian coral Pocillopora damicornis. Sci. Rep. 2022, 12, 15297. [Google Scholar] [CrossRef]
- Wei, C.; Li, M.; Zhao, X. Surface-enhanced Raman scattering (SERS) with silver nano substrates synthesized by microwave for rapid detection of foodborne pathogens. Front. Microbiol. 2018, 9, 2857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef] [PubMed]
- You, S.-M.; Luo, K.; Jung, J.-Y.; Jeong, K.-B.; Lee, E.-S.; Oh, M.-H.; Kim, Y.-R. Gold nanoparticle-coated starch magnetic beads for the separation, concentration, and SERS-based detection of E. coli O157: H7. ACS Appl. Mater. Interfaces 2020, 12, 18292–18300. [Google Scholar] [CrossRef] [PubMed]


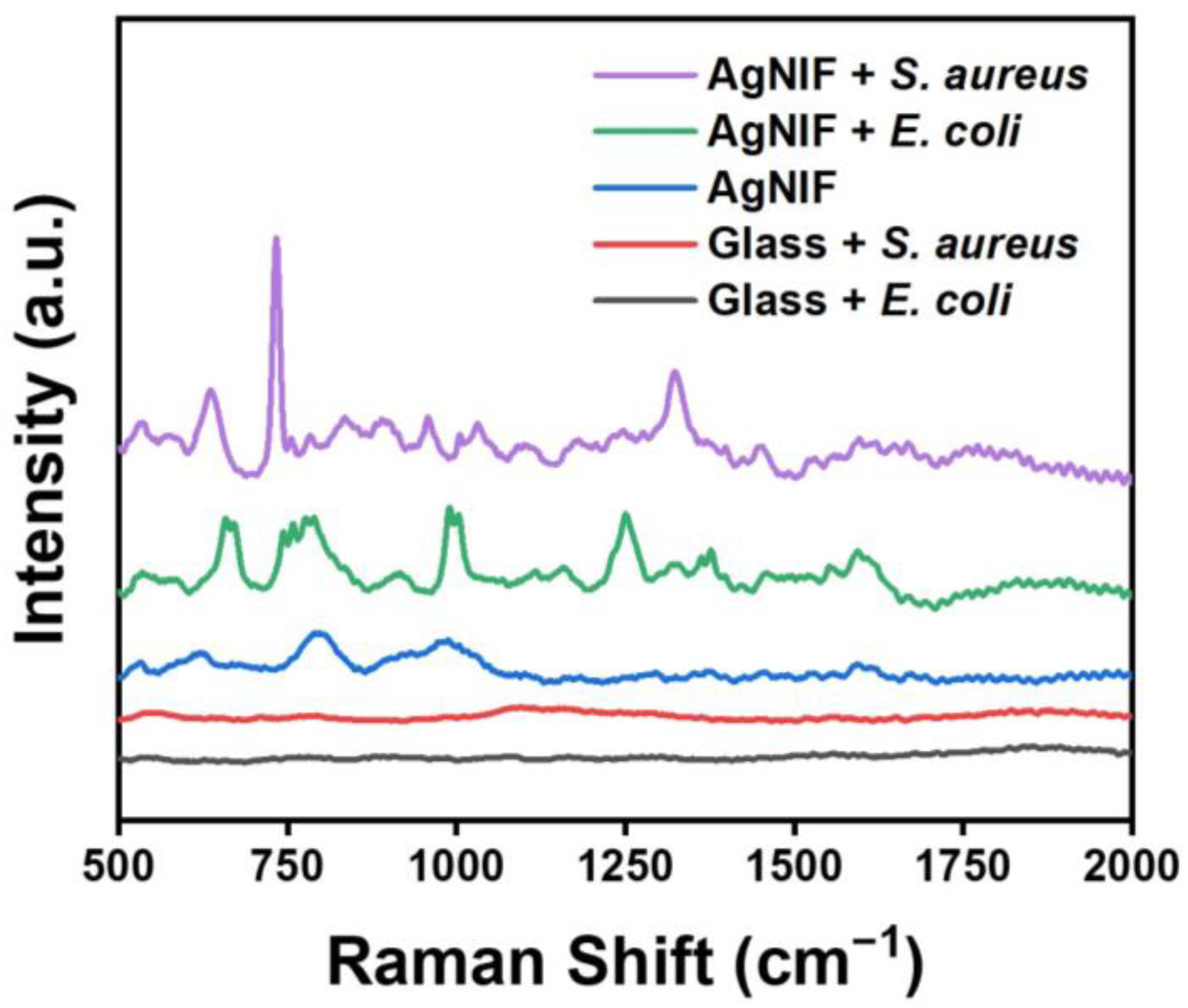
| Peak Position (cm−1) | Peak Assignment | |
|---|---|---|
| E. coli | S. aureus | |
| 634 | C–C twisting | |
| 733 | 742 | Adenine, glycosidic ring mode |
| 834 | Tyrosine | |
| 957 | Bending (C=C) or tyrosine | |
| 1002 | 990 | Phenylalanine or glucose |
| 1032 | C-H deformation | |
| 1168 | 1160 | 12-Methylpalmitic acid or aceto acetate |
| 1247 | Amide III | |
| 1250 | Cytosine, adenine | |
| 1322 | 1322 | Stretching (NH2) adenine, polyadenine, DNA |
| 1448 | 1452 | CH2 deformation mode of proteins |
| 1593 | 1592 | CN stretching of Amide II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, S.; Mutalik, C.; Yougbaré, S.; Chen, C.-Y.; Kuo, T.-R. Plasmonic Au@Ag Core–Shell Nanoisland Film for Photothermal Inactivation and Surface-Enhanced Raman Scattering Detection of Bacteria. Nanomaterials 2024, 14, 695. https://doi.org/10.3390/nano14080695
Husain S, Mutalik C, Yougbaré S, Chen C-Y, Kuo T-R. Plasmonic Au@Ag Core–Shell Nanoisland Film for Photothermal Inactivation and Surface-Enhanced Raman Scattering Detection of Bacteria. Nanomaterials. 2024; 14(8):695. https://doi.org/10.3390/nano14080695
Chicago/Turabian StyleHusain, Sadang, Chinmaya Mutalik, Sibidou Yougbaré, Chun-You Chen, and Tsung-Rong Kuo. 2024. "Plasmonic Au@Ag Core–Shell Nanoisland Film for Photothermal Inactivation and Surface-Enhanced Raman Scattering Detection of Bacteria" Nanomaterials 14, no. 8: 695. https://doi.org/10.3390/nano14080695





