CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts
Abstract
:1. Introduction
2. Electrode Fabrication and Structural Analysis
2.1. Precursor and Reaction Parameter Optimizations for CoFeBP MF
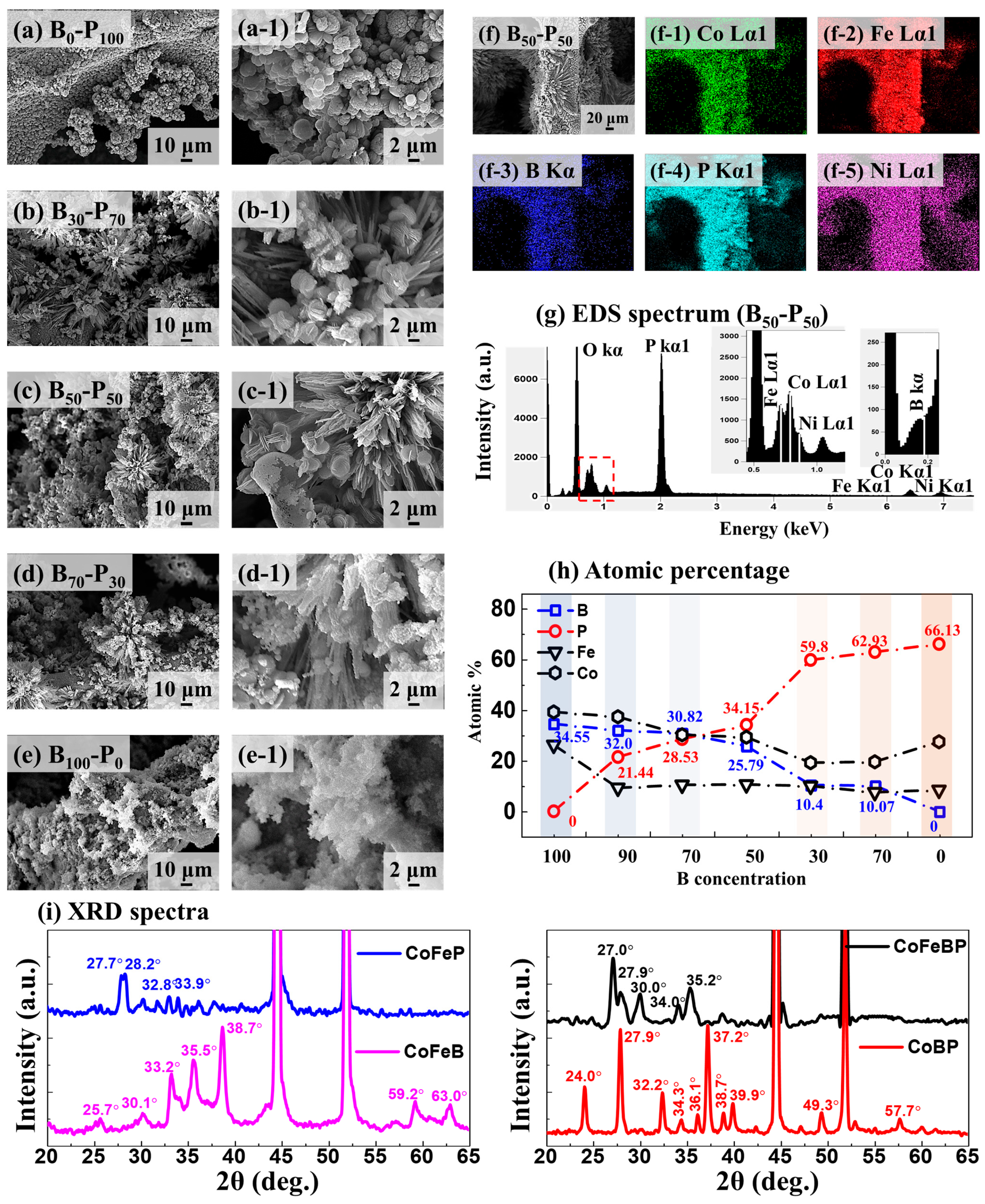
2.2. B-P Concentration Optimization for CoFeBP MF
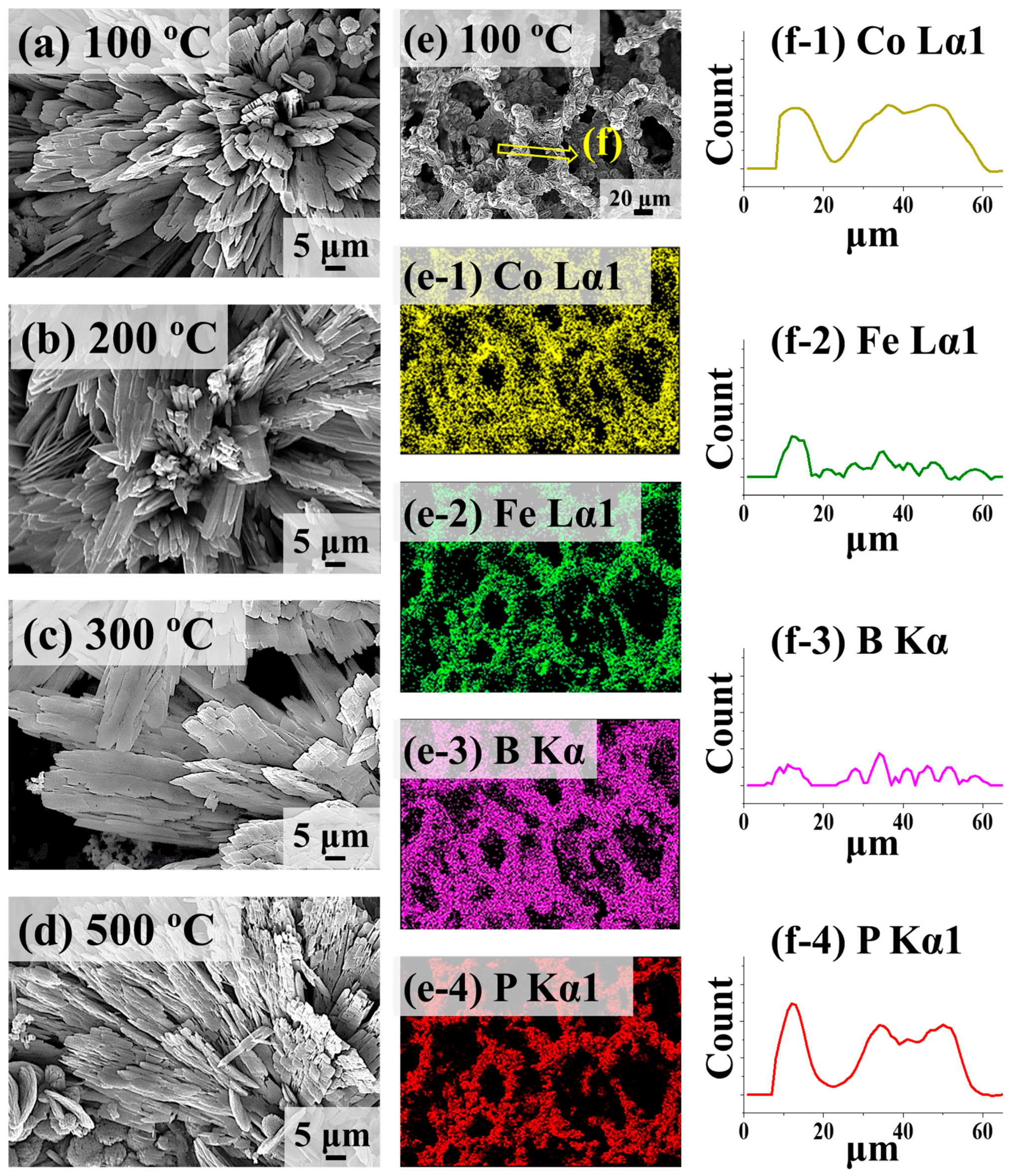
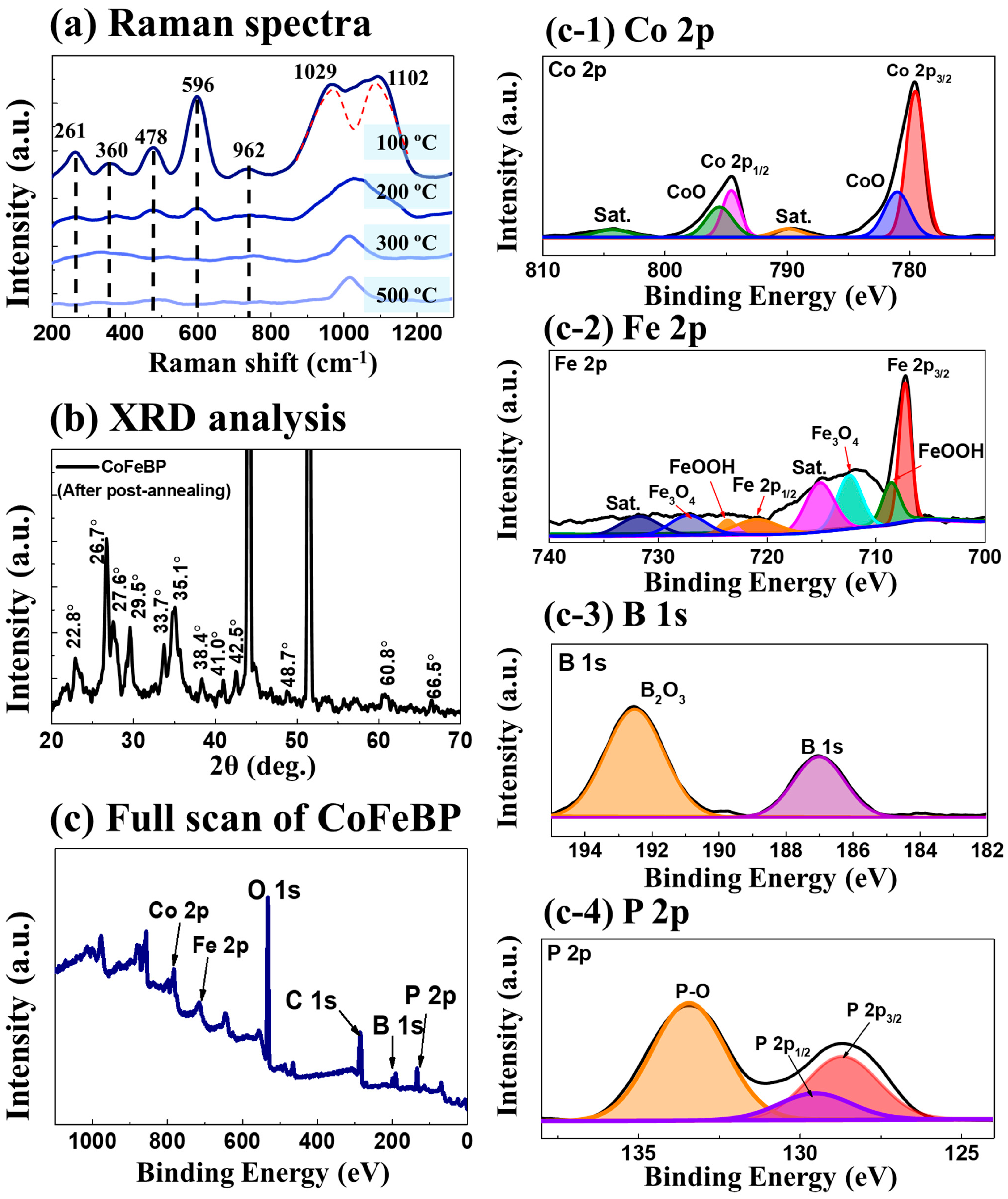
2.3. Structural Analysis of CoFeBP MF along with Post-Annealing Optimization
3. Electrochemical Analysis
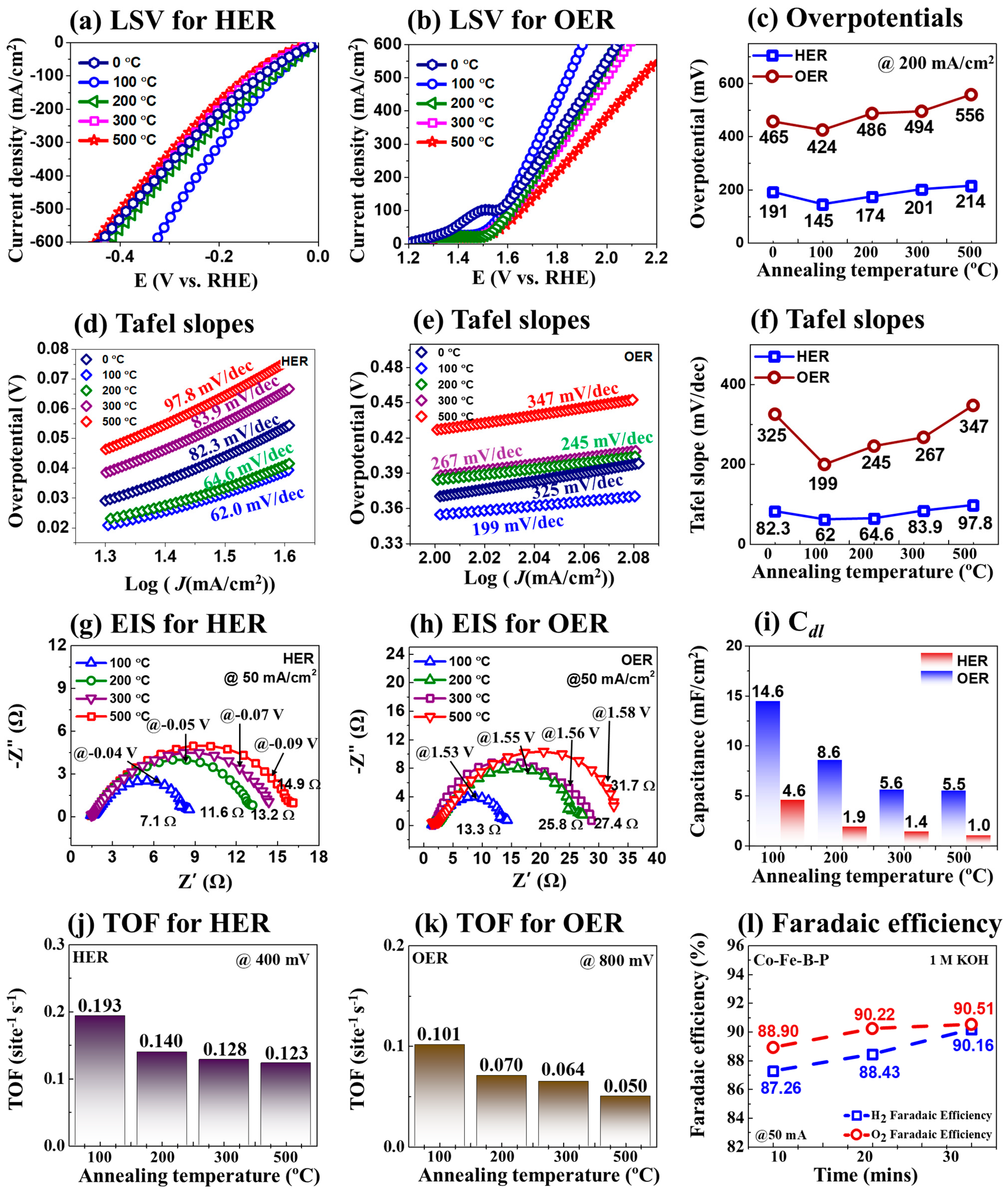
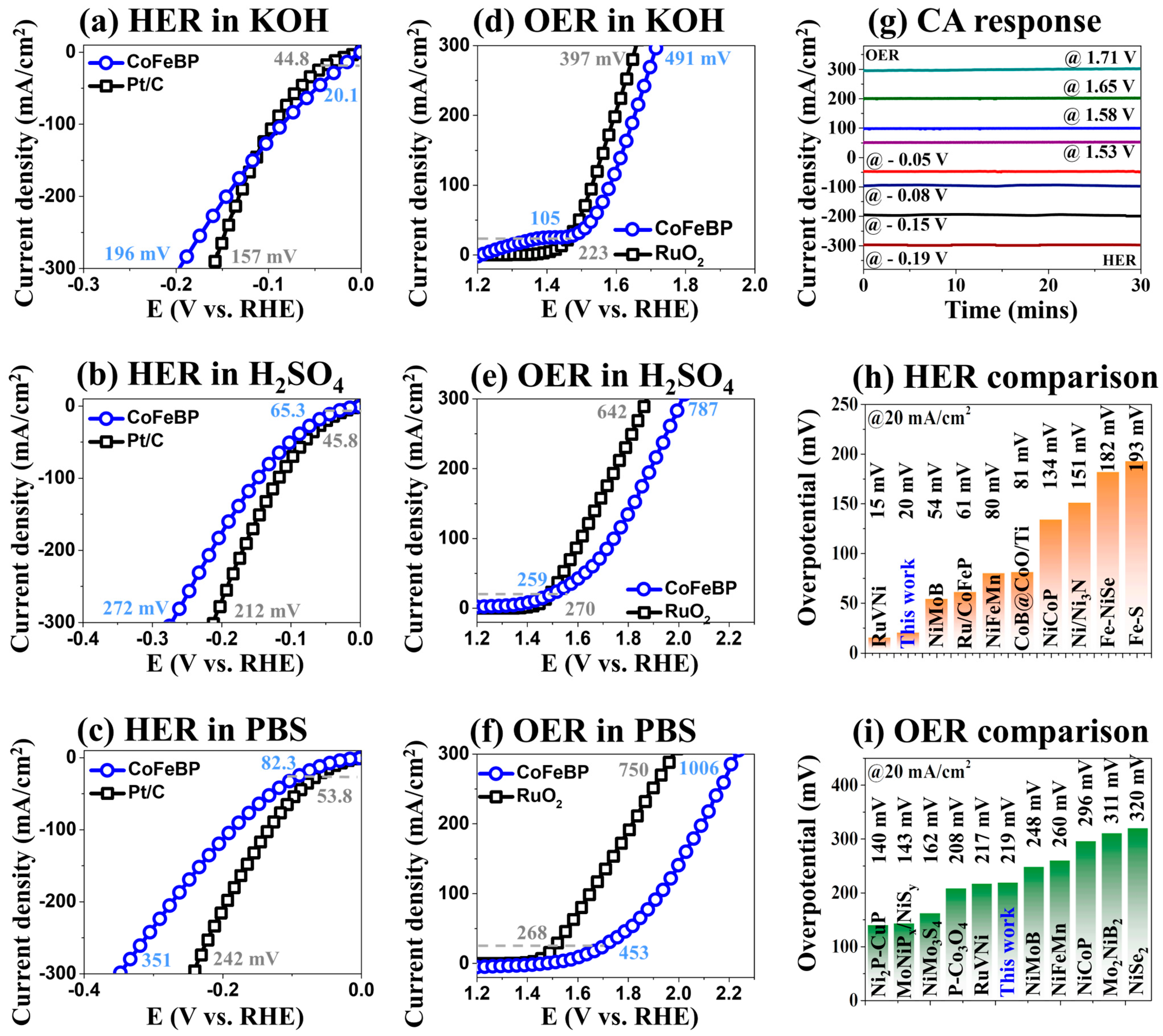
3.1. The 3-E Electrochemical Properties of CoFeBP MFs
3.2. The 3-E LSV Activity of CoFeBP MFs in Different pH Media
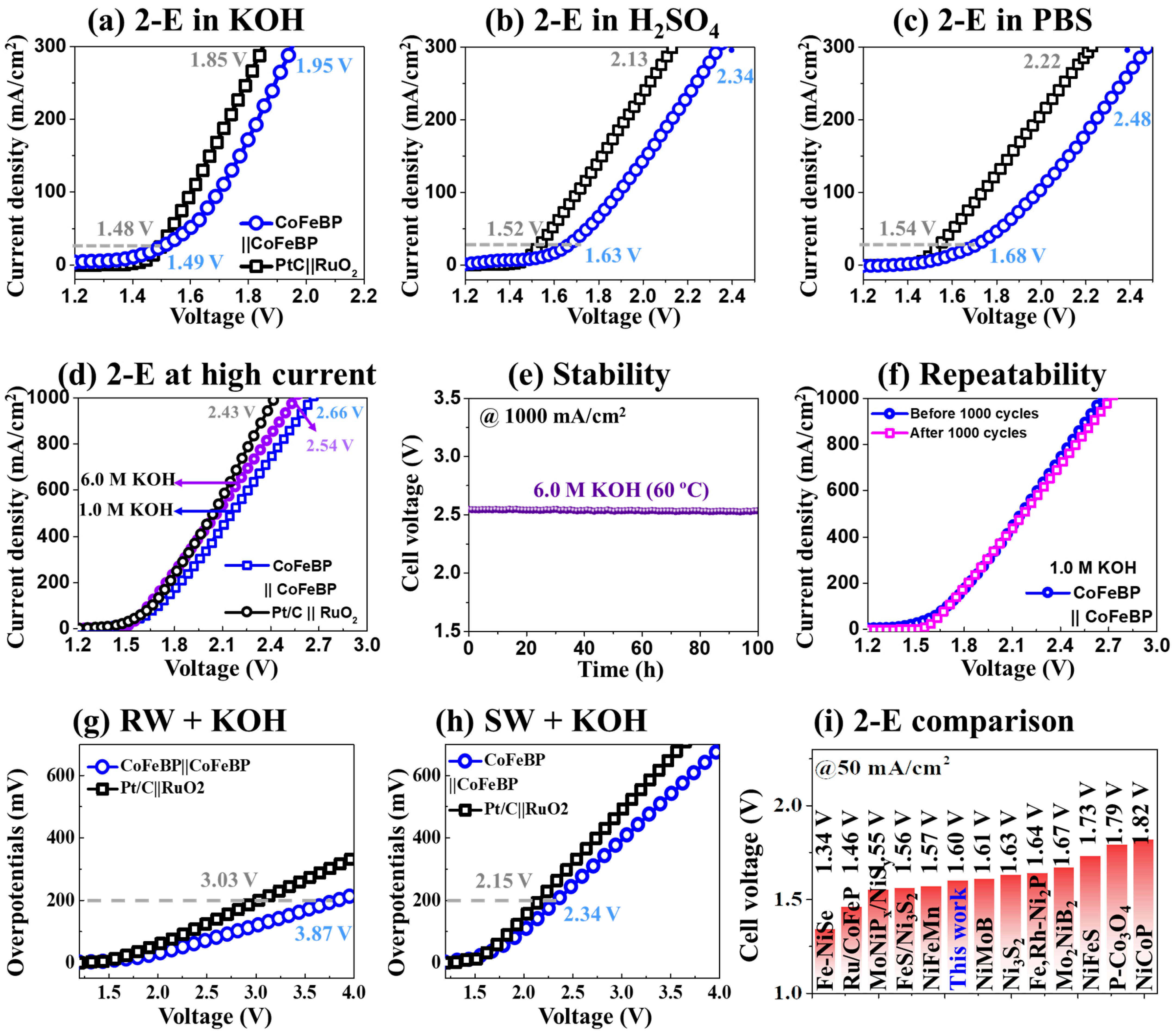
3.3. The 2-E Activity of CoFeBP MFs
3.4. Characterization after Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef]
- Saad, A.; Gao, Y.; Owusu, K.A.; Liu, W.; Wu, Y.; Ramiere, A.; Guo, H.; Tsiakaras, P.; Cai, X. Ternary Mo2NiB2 as a Superior Bifunctional Electrocatalyst for Overall Water Splitting. Small 2022, 18, 2104303. [Google Scholar] [CrossRef] [PubMed]
- Riyajuddin, S.; Azmi, K.; Pahuja, M.; Kumar, S.; Maruyama, T.; Bera, C.; Ghosh, K. Super-Hydrophilic Hierarchical Ni-Foam-Graphene-Carbon Nanotubes-Ni2P–CuP2 Nano-Architecture as Efficient Electrocatalyst for Overall Water Splitting. ACS Nano 2021, 15, 5586–5599. [Google Scholar] [CrossRef]
- Ifkovits, Z.P.; Evans, J.M.; Meier, M.C.; Papadantonakis, K.M.; Lewis, N.S. Decoupled Electrochemical Water-Splitting Systems: A Review and Perspective. Energy Environ. Sci. 2021, 14, 4740–4759. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Chen, S.; Zhou, Y.; Li, H.; Chen, Y.; Zhang, R.; Wei, G.; Kang, Y. Hierarchical Mesoporous MXene-NiCoP Electrocatalyst for Water-Splitting. ACS Appl. Mater. Interfaces 2020, 12, 18570–18577. [Google Scholar] [CrossRef]
- Debata, S.; Patra, S.; Banerjee, S.; Madhuri, R.; Sharma, P.K. Controlled Hydrothermal Synthesis of Graphene Supported NiCo2O4 Coral-like Nanostructures: An Efficient Electrocatalyst for Overall Water Splitting. Appl. Surf. Sci. 2018, 449, 203–212. [Google Scholar] [CrossRef]
- Kumaravel, S.; Karthick, K.; Sam Sankar, S.; Karmakar, A.; Madhu, R.; Bera, K.; Kundu, S. Recent Progresses in Engineering of Ni and Co Based Phosphides for Effective Electrocatalytic Water Splitting. ChemElectroChem 2021, 8, 4638–4685. [Google Scholar] [CrossRef]
- Habib, M.A.; Mandavkar, R.; Lin, S.; Burse, S.; Khalid, T.; Joni, M.H.; Jeong, J.-H.; Lee, J. Ni-BP Micro Spheres for Superior Water Splitting OER Electrocatalyst Satisfying Industrial Operational Requirement. Chem. Eng. J. 2023, 462, 142177. [Google Scholar]
- Battiato, S.; Bruno, L.; Pellegrino, A.L.; Terrasi, A.; Mirabella, S. Optimized Electroless Deposition of NiCoP Electrocalysts for Enhanced Water Splitting. Catal. Today 2023, 423, 113929. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced Electrocatalysts with Unusual Active Sites for Electrochemical Water Splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Loni, E.; Shokuhfar, A.; Siadati, M.H. Cobalt-Based Electrocatalysts for Water Splitting: An Overview. Catal. Surv. Asia 2021, 25, 114–147. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of Hierarchical Ni-Co-P Hollow Nanobricks with Oriented Nanosheets for Efficient Overall Water Splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Burse, S.; Kulkarni, R.; Mandavkar, R.; Habib, M.A.; Lin, S.; Chung, Y.-U.; Jeong, J.-H.; Lee, J. Vanadium-Doped FeBP Microsphere Croissant for Significantly Enhanced Bi-Functional HER and OER Electrocatalyst. Nanomaterials 2022, 12, 3283. [Google Scholar] [CrossRef] [PubMed]
- Barati Darband, G.; Aliofkhazraei, M.; Rouhaghdam, A.S. Facile Electrodeposition of Ternary Ni-Fe-Co Alloy Nanostructure as a Binder Free, Cost-Effective and Durable Electrocatalyst for High-Performance Overall Water Splitting. J. Colloid Interface Sci. 2019, 547, 407–420. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Jeong, D.S.; Singh, A.N.; Jana, A.; Im, H.; Nguyen, D.A.; Kim, K.S. Crystalline-Amorphous Interface of Mesoporous Ni2P@FePOxHy for Oxygen Evolution at High Current Density in Alkaline-Anion-Exchange-Membrane Water-Electrolyzer. Appl. Catal. B Environ. 2022, 306, 121127. [Google Scholar] [CrossRef]
- Mohili, R.; Hemanth, N.R.; Jin, H.; Lee, K.; Chaudhari, N. Emerging High Entropy Metal Sulphides and Phosphides for Electrochemical Water Splitting. J. Mater. Chem. A 2023, 11, 10463–10472. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Ge, L.; Peng, C.; Zhu, L.; Zou, W.; Chen, J.; Wu, Q.; Zhang, Y.; Huang, H.; et al. Accelerating Hydrogen Evolution in Ru-Doped FeCoP Nanoarrays with Lattice Distortion toward Highly Efficient Overall Water Splitting. Catal. Sci. Technol. 2020, 10, 8314–8324. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; He, H.; Zhang, P. Novel Electrocatalyst of Nickel Sulfide Boron Coating for Hydrogen Evolution Reaction in Alkaline Solution. Appl. Surf. Sci. 2019, 480, 689–696. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-Doped Nitrogen-Deficient Carbon Nitride-Based Z-Scheme Heterostructures for Photocatalytic Overall Water Splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Habib, M.A.; Mandavkar, R.; Burse, S.; Lin, S.; Kulkarni, R.; Patil, C.S.; Jeong, J.-H.; Lee, J. Design of Boron-Based Ternary W3CoB3 Electrocatalyst for the Improved HER and OER Performances. Mater. Today Energy 2022, 26, 101021. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Influence of Precursor Concentration and Deposition Temperature on the Photoactivity of Hematite Electrodes for Water Splitting. Mater. Today Commun. 2020, 25, 101459. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Zhang, W.; Guo, S. Electronic-Structure Tuning of Water-Splitting Nanocatalysts. Trends Chem. 2019, 1, 259–271. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, Y.; Wang, G.; Yan, K.; Zhao, J. Effect of NH4F Concentration and Controlled-Charge Consumption on the Photocatalytic Hydrogen Generation of TiO2 Nanotube Arrays. Electrochim. Acta 2015, 155, 312–320. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.-K.; Rama Raju, G.S.; Huh, Y.-S.; Han, Y.-K. Fluorine Engineered Self-Supported Ultrathin 2D Nickel Hydroxide Nanosheets as Highly Robust and Stable Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reactions. Small 2022, 18, 2103326. [Google Scholar] [CrossRef]
- Oliveira, F.G.S.; Santos, L.P.M.; da Silva, R.B.; Correa, M.A.; Bohn, F.; Correia, A.N.; Vieira, L.; Vasconcelos, I.F.; de Lima-Neto, P. FexNi(1−x) Coatings Electrodeposited from Choline Chloride-Urea Mixture: Magnetic and Electrocatalytic Properties for Water Electrolysis. Mater. Chem. Phys. 2022, 279, 125738. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Mu, Z.; Wang, Y.; Ali, U.; Jing, S.; Xing, S. Urea-Assisted Enhanced Electrocatalytic Activity of MoS2-Ni3S2for Overall Water Splitting. Inorg. Chem. Front. 2020, 7, 3588–3597. [Google Scholar] [CrossRef]
- Xu, T.; Yang, L.; Li, J.; Usoltseva, N.; An, V.; Jin, X.; Zhang, C.; Zhang, X.; Liu, B. NH4F-Induced Morphology Control of CoP Nanostructures to Enhance the Hydrogen Evolution Reaction. Inorg. Chem. 2021, 60, 10781–10790. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y. Designing Transition-Metal-Boride-Based Electrocatalysts for Applications in Electrochemical Water Splitting. Nanoscale 2020, 12, 9327–9351. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.-T.; Yuan, Z.-Y. Design Strategies of Phosphorus-Containing Catalysts for Photocatalytic, Photoelectrochemical and Electrocatalytic Water Splitting. Green Chem. 2022, 24, 713–747. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Zuo, P.; Kang, L.; Yin, G. A Novel Spherical Boron Phosphide as a High-Efficiency Overall Water Splitting Catalyst: A Density Functional Theory Study. Catal. Lett. 2020, 150, 544–554. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Liu, Y.; Liu, Z.; Sun, W.; Lin, K.A.; Li, T.; Luo, S. Ni2P Nanocrystals Embedded Ni-MOF Nanosheets Supported on Nickel Foam as Bifunctional Electrocatalyst for Urea Electrolysis. Sci. Rep. 2021, 11, 21414. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, F.; Faid, A.Y.; Sunde, S.; Pollet, B.G. Sonoactivated Polycrystalline Ni Electrodes for Alkaline Oxygen Evolution Reaction. Ultrason. Sonochem. 2022, 86, 106013. [Google Scholar] [CrossRef]
- Lin, S.; Habib, M.A.; Mandavkar, R.; Kulkarni, R.; Burse, S.; Chung, Y.-U.; Liu, C.; Wang, Z.; Lin, S.; Jeong, J.-H.; et al. Higher Water-Splitting Performance of Boron-Based Porous CoMnB Electrocatalyst over the Benchmarks at High Current in 1 m KOH and Real Sea Water. Adv. Sustain. Syst. 2022, 6, 2200213. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, R.; Dong, C.; Liu, H.; Yang, J.; Du, X. Polycrystalline CoO–Co9S8 Heterostructure Nanoneedle Arrays as Bifunctional Catalysts for Efficient Overall Water Splitting. ChemElectroChem 2022, 9, e202101566. [Google Scholar] [CrossRef]
- Guo, C.; Shi, Y.; Lu, S.; Yu, Y.; Zhang, B. Amorphous Nanomaterials in Electrocatalytic Water Splitting. Chin. J. Catal. 2021, 42, 1287–1296. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.; Zhang, B.; Yin, S.; Huang, Y.; Kraft, M.; Xu, R. Nickel Nanoparticles Encapsulated in Few-Layer Nitrogen-Doped Graphene Derived from Metal–Organic Frameworks as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef]
- Makimizu, Y.; Yoo, J.; Poornajar, M.; Nguyen, N.T.; Ahn, H.-J.; Hwang, I.; Kment, S.; Schmuki, P. Effects of Low Oxygen Annealing on the Photoelectrochemical Water Splitting Properties of α-Fe2O3. J. Mater. Chem. A 2020, 8, 1315–1325. [Google Scholar] [CrossRef]
- Mandavkar, R.; Habib, M.A.; Lin, S.; Kulkarni, R.; Burse, S.; Jeong, J.-H.; Lee, J. Electron Enriched Ternary NiMoB Electrocatalyst for Improved Overall Water Splitting: Better Performance as Compared to the Pt/C || RuO2 at High Current Density. Appl. Mater. Today 2022, 29, 101579. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ferrus-Suspedra, D.; Koper, M.T.M. The Importance of Nickel Oxyhydroxide Deprotonation on Its Activity towards Electrochemical Water Oxidation. Chem. Sci. 2016, 7, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, B.; Wu, Y.; Ruan, Q.; Liu, L.; Su, J.; Tang, Y.; Liu, R.; Chu, P.K. Experimental and Theoretical Investigation of Reconstruction and Active Phases on Honeycombed Ni3N-Co3N/C in Water Splitting. Appl. Catal. B Environ. 2021, 297, 120461. [Google Scholar] [CrossRef]
- Guo, M.; Meng, H.; Jin, J.; Mi, J. Amine-Assisted Synthesis of the Ni3Fe Alloy Encapsulated in Nitrogen-Doped Carbon for High-Performance Water Splitting. J. Mater. Chem. A 2023, 11, 6452–6464. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Guo, T.; Chen, L.; Li, Y.; Shen, K. Controllable Synthesis of Ultrathin Defect-Rich LDH Nanoarrays Coupled with MOF-Derived Co-NC Microarrays for Efficient Overall Water Splitting. Small 2022, 18, 2107739. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Li, C.-F.; Zhao, J.-W.; Xie, L.-J.; Wu, J.-Q.; Li, G.-R. Fe Doping and Oxygen Vacancy Modulated Fe-Ni5P4/NiFeOH Nanosheets as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Appl. Catal. B Environ. 2021, 291, 119987. [Google Scholar] [CrossRef]
- Teng, W.; Huo, M.; Sun, Z.; Yang, W.; Zheng, X.; Ding, C.; Zhang, S. FeCoNi Sulfides Derived from in Situ Sulfurization of Precursor Oxides as Oxygen Evolution Reaction Catalyst. Front. Chem. 2020, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yan, P.; Sun, B.; Elshekh, H.; Yan, B. An Excellent Soft Magnetic Fe/Fe3O4-FeSiAl Composite with High Permeability and Low Core Loss. Results Phys. 2019, 14, 102498. [Google Scholar] [CrossRef]
- Li, X.; Xiao, L.; Zhou, L.; Xu, Q.; Weng, J.; Xu, J.; Liu, B. Adaptive Bifunctional Electrocatalyst of Amorphous CoFe Oxide @ 2D Black Phosphorus for Overall Water Splitting. Angew. Chem. 2020, 132, 21292–21299. [Google Scholar] [CrossRef]
- Hao, W.; Yao, D.; Xu, Q.; Wang, R.; Zhang, C.; Guo, Y.; Sun, R.; Huang, M.; Chen, Z. Highly Efficient Overall-Water Splitting Enabled via Grafting Boron-Inserted Fe-Ni Solid Solution Nanosheets onto Unconventional Skeleton. Appl. Catal. B Environ. 2021, 292, 120188. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, P.; Yue, Y.; Wang, M.; Fu, W.; Si, S.; Wei, L.; Zhao, X.; Hu, G.; Xin, H.L. One-Pot Synthesis of B/P-Codoped Co-Mo Dual-Nanowafer Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 20024–20033. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The Pitfalls of Using Potentiodynamic Polarization Curves for Tafel Analysis in Electrocatalytic Water Splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Peng, Y.; Mak, C.H.; Kai, J.-J.; Du, M.; Ji, L.; Yuan, M.; Zou, X.; Shen, H.-H.; Santoso, S.P.; Colmenares, J.C.; et al. Recent Progress on Post-Synthetic Treatments of Photoelectrodes for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 26628–26649. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning Synergy between Nickel and Iron in Ruddlesden–Popper Perovskites through Controllable Crystal Dimensionalities towards Enhanced Oxygen-evolving Activity and Stability. Carbon Energy 2024, e465. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water Splitting Performance of Metal and Non-Metal-Doped Transition Metal Oxide Electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, S.; Ma, Y.; Qiu, Y.; An, Y.; Cheng, L. Interfacial Engineering of Cobalt Nitrides and Mesoporous Nitrogen-Doped Carbon: Toward Efficient Overall Water-Splitting Activity with Enhanced Charge-Transfer Efficiency. ACS Energy Lett. 2020, 5, 692–700. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Shalom, M.; Ressnig, D.; Yang, X.; Clavel, G.; Fellinger, T.P.; Antonietti, M. Nickel Nitride as an Efficient Electrocatalyst for Water Splitting. J. Mater. Chem. A 2015, 3, 8171–8177. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-Based Phosphides as Electrocatalysts for the Hydrogen Evolution Reaction: Recent Advances and Future Prospects. J. Mater. Chem. A 2020, 8, 19729–19745. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2020, 30, 1906481. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kunwar, S.; Pandit, S.; Lee, J. CoP2Nanoparticles Deposited on Nanometer-Thick Pt-Coated Fluorine-Doped Tin Oxide Substrates as Electrocatalysts for Simultaneous Hydrogen Evolution and Oxygen Evolution. ACS Appl. Nano Mater. 2020, 3, 6507–6515. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S. Do the Evaluation Parameters Reflect Intrinsic Activity of Electrocatalysts in Electrochemical Water Splitting? ACS Energy Lett. 2019, 4, 1260–1264. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Z.; Jiao, L. Development Strategies in Transition Metal Borides for Electrochemical Water Splitting. Energy Environ. Mater. 2022, 5, 470–485. [Google Scholar] [CrossRef]
- Bahadur, A.; Hussain, W.; Iqbal, S.; Ullah, F.; Shoaib, M.; Liu, G.; Feng, K. A Morphology Controlled Surface Sulfurized CoMn2O4 Microspike Electrocatalyst for Water Splitting with Excellent OER Rate for Binder-Free Electrocatalytic Oxygen Evolution. J. Mater. Chem. A 2021, 9, 12255–12264. [Google Scholar] [CrossRef]
- Friebel, D.; Bajdich, M.; Yeo, B.S.; Louie, M.W.; Miller, D.J.; Sanchez Casalongue, H.; Mbuga, F.; Weng, T.-C.; Nordlund, D.; Sokaras, D.; et al. On the Chemical State of Co Oxide Electrocatalysts during Alkaline Water Splitting. Phys. Chem. Chem. Phys. 2013, 15, 17460–17467. [Google Scholar] [CrossRef] [PubMed]
- Behl, W.K.; Toni, J.E. Anodic Oxidation of Cobalt in Potassium Hydroxide Electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 63–75. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. An Account of the Strategies to Enhance the Water Splitting Efficiency of Noble-Metal-Free Electrocatalysts. J. Energy Chem. 2021, 59, 160–190. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in Noble Metal (Ru, Rh, and Ir) Doping for Boosting Water Splitting Electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Menezes, P.W. Why Should Transition Metal Chalcogenides Be Investigated as Water Splitting Precatalysts Even Though They Transform into (Oxyhydr)Oxides? Curr. Opin. Electrochem. 2022, 34, 100991. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Lu, Q.; Xing, Z.; Yang, X. Atomic and Electronic Modulation of Self-Supported Nickel-Vanadium Layered Double Hydroxide to Accelerate Water Splitting Kinetics. Nat. Commun. 2019, 10, 3899. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Li, C.; Pham, B.T.; Zhang, D. Electrodeposition of Ni–Fe–Mn Ternary Nanosheets as Affordable and Efficient Electrocatalyst for Both Hydrogen and Oxygen Evolution Reactions. Int. J. Hydrogen Energy 2020, 45, 24670–24683. [Google Scholar] [CrossRef]
- Lu, W.; Liu, T.; Xie, L.; Tang, C.; Liu, D.; Hao, S.; Qu, F.; Du, G.; Ma, Y.; Asiri, A.M.; et al. In Situ Derived Co—B Nanoarray: A High-Efficiency and Durable 3D Bifunctional Electrocatalyst for Overall Alkaline Water Splitting. Small 2017, 13, 1700805. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, J.; Chen, Y.; Wei, M.; Liu, X.; Li, X.; Wu, Q.; Feng, B.; Zhang, Y.; Yang, L. Regulation of the Morphology and Electrochemical Properties of Ni0.85Se via Fe Doping for Overall Water Splitting and Supercapacitors. CrystEngComm 2022, 24, 1704–1718. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. Binder-Free Growth of Nickel-Doped Iron Sulfide on Nickel Foam via Electrochemical Deposition for Electrocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 18015–18026. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, G.; Song, W.; Zhong, W.; Wang, X.; Wang, M.; Sun, T.; Tang, Y. Heterogeneous Bimetallic Mo-NiPx/NiSy as a Highly Efficient Electrocatalyst for Robust Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2101532. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Von Lim, Y.; Wang, M.; Xu, T.; Zang, J.; Li, X.; Yang, H.Y. Defect-Engineered 3D Hierarchical NiMo3S4 Nanoflowers as Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 607, 1876–1887. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, e202301534. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Wei, W.; Zhang, M.; Jiang, Z.; Yan, Z.; Xie, J. Chrysanthemum-like FeS/Ni3S2 Heterostructure Nanoarray as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 608, 536–548. [Google Scholar] [CrossRef]
- Ren, G.; Hao, Q.; Mao, J.; Liang, L.; Liu, H.; Liu, C.; Zhang, J. Ultrafast Fabrication of Nickel Sulfide Film on Ni Foam for Efficient Overall Water Splitting. Nanoscale 2018, 10, 17347–17353. [Google Scholar] [CrossRef]
- Chen, M.-T.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Jiao, Y.; Zhang, L.; Wang, A.-J. Iron, Rhodium-Codoped Ni2P Nanosheets Arrays Supported on Nickel Foam as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 605, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Yuan, M.; Hao, H.; San, X.; Lv, Z.; Xu, L.; Wei, B. Operando Capturing of Surface Self-Reconstruction of Ni3S2/FeNi2S4 Hybrid Nanosheet Array for Overall Water Splitting. Chem. Eng. J. 2022, 427, 131944. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, M.; Yuan, J.; Luo, J.; Zhang, J.; Lu, Z.; Chen, D.; Fu, X.; Wang, L.; Liu, C. Oxygen Vacancies and Interface Engineering on Amorphous/Crystalline CrOx-Ni3N Heterostructures toward High-Durability and Kinetically Accelerated Water Splitting. Small 2022, 18, 2106554. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous Catalysts and Electrochemical Water Splitting: An Untold Story of Harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef]
- Browne, M.P.; Vasconcelos, J.M.; Coelho, J.; O’Brien, M.; Rovetta, A.A.; McCarthy, E.K.; Nolan, H.; Duesberg, G.S.; Nicolosi, V.; Colavita, P.E.; et al. Improving the Performance of Porous Nickel Foam for Water Oxidation Using Hydrothermally Prepared Ni and Fe Metal Oxides. Sustain. Energy Fuels 2017, 1, 207–216. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and Correctness in the Evaluation of Electrocatalytic Water Splitting: Revisiting Activity Parameters with a Critical Assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Feng, R.; Zhu, Q.; Chu, M.; Jia, S.; Zhai, J.; Wu, H.; Wu, P.; Han, B. Electrodeposited Cu–Pd Bimetallic Catalysts for the Selective Electroreduction of CO2 to Ethylene. Green Chem. 2020, 22, 7560–7565. [Google Scholar] [CrossRef]
- Han, A.; Chen, H.; Sun, Z.; Xu, J.; Du, P. High Catalytic Activity for Water Oxidation Based on Nanostructured Nickel Phosphide Precursors. Chem. Commun. 2015, 51, 11626–11629. [Google Scholar] [CrossRef]
- Wang, G.; Hua, C.; Chen, W.; Fan, H.; Feng, P.; Zhu, Y. Intriguing 3D Micro-Flower Structure of Co1.11Te2 Deposited on Te Nanosheets Showing an Efficient Bifunctional Electrocatalytic Property for Overall Water Splitting. Electrochim. Acta 2023, 447, 142133. [Google Scholar] [CrossRef]
- Sun, H.; Meng, J.; Jiao, L.; Cheng, F.; Chen, J. A Review of Transition-Metal Boride/Phosphide-Based Materials for Catalytic Hydrogen Generation from Hydrolysis of Boron-Hydrides. Inorg. Chem. Front. 2018, 5, 760–772. [Google Scholar] [CrossRef]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Wu, A.; Dong, X.; Guo, D.; Tian, C.; Fu, H. Interfacial Engineering of MoO2-FeP Heterojunction for Highly Efficient Hydrogen Evolution Coupled with Biomass Electrooxidation. Adv. Mater. 2020, 32, 2000455. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, X.; Zhang, D.; Wang, Y.; Wang, J.; Pi, M.; Zhou, H.; Li, J.; Chen, S. Porous Mn-Doped CoP3 Nanowires as a Janus Electrocatalyst for Efficient Overall Water Splitting in Alkaline Solution. J. Electrochem. Soc. 2018, 165, F1323. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.-Y.; Zhang, X.-L.; Li, P.; Sun, B.; Gao, X.; Yan, K.; Liu, H.; Duan, Y.; Gao, M.-R.; et al. Highly Disordered Cobalt Oxide Nanostructure Induced by Sulfur Incorporation for Efficient Overall Water Splitting. Nano Energy 2020, 71, 104652. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Qian, J.; Li, Y.; Shen, K. Intensified Kirkendall Effect Assisted Construction of Double-Shell Hollow Cu-Doped CoP Nanoparticles Anchored by Carbon Arrays for Water Splitting. Appl. Catal. B Environ. 2023, 325, 122295. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Han, C.; Lu, R.; Yang, S.; Song, X. Electrospun Hollow Cage-like α-Fe2O3 Microspheres: Synthesis, Formation Mechanism, and Morphology-Preserved Conversion to Fe Nanostructures. CrystEngComm 2014, 16, 10618–10623. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.S. Hybrid Microwave Annealing Synthesizes Highly Crystalline Nanostructures for (Photo)Electrocatalytic Water Splitting. Acc. Chem. Res. 2019, 52, 3132–3142. [Google Scholar] [CrossRef]
- Kim, T.; Roy, S.B.; Moon, S.; Yoo, S.; Choi, H.; Parale, V.G.; Kim, Y.; Lee, J.; Jun, S.C.; Kang, K. Highly Dispersed Pt Clusters on F-Doped Tin (IV) Oxide Aerogel Matrix: An Ultra-Robust Hybrid Catalyst for Enhanced Hydrogen Evolution. ACS Nano 2022, 16, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Gong, S.; Chen, Y.; Xie, R.; Wu, Q.; Tao, J.; Meng, F.; Zhao, P. A Novel Non-Enzymatic Electrochemical Biosensor Based on the Nanohybrid of Bimetallic PdCu Nanoparticles/Carbon Black for Highly Sensitive Detection of H2O2 Released from Living Cells. Sens. Actuators B Chem. 2019, 290, 249–257. [Google Scholar] [CrossRef]
- Sultan, S.; Ha, M.; Kim, D.Y.; Tiwari, J.N.; Myung, C.W.; Meena, A.; Shin, T.J.; Chae, K.H.; Kim, K.S. Superb Water Splitting Activity of the Electrocatalyst Fe3Co (PO4)4 Designed with Computation Aid. Nat. Commun. 2019, 10, 5195. [Google Scholar] [CrossRef]
- Yang, J.; An, Y.; Guo, K.; Ren, X.; Jiang, B. Nitrogen Doped FeCoNiS Nanoparticles on N, S-Co-Doped Vertical Graphene as Bifunctional Electrocatalyst for Water Splitting. Int. J. Hydrogen Energy 2023, 48, 4143–4157. [Google Scholar] [CrossRef]
- Surendran, S.; Jesudass, S.C.; Janani, G.; Kim, J.Y.; Lim, Y.; Park, J.; Han, M.-K.; Cho, I.S.; Sim, U. Sulphur Assisted Nitrogen-Rich CNF for Improving Electronic Interactions in Co-NiO Heterostructures toward Accelerated Overall Water Splitting. Adv. Mater. Technol. 2023, 8, 2200572. [Google Scholar] [CrossRef]
- Miao, L.; Sui, L.; Shen, X.; Yang, D.; Huang, H.; Kuang, Y. Realizing High Performance Bifunctional Energy Storage Devices and Electrocatalytic Water Splitting Catalysts through Regulated Interface Engineering of ZnCo2O4@Co3O4 Nanosheets. CrystEngComm 2023, 25, 4812–4821. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Deng, Y.-T.; Pao, C.-W.; Chen, J.-L.; Lee, J.-F.; Lai, K.-T.; Liaw, W.-F. The HER/OER Mechanistic Study of an FeCoNi-Based Electrocatalyst for Alkaline Water Splitting. J. Mater. Chem. A 2020, 8, 9939–9950. [Google Scholar] [CrossRef]
- Du, Y.; Li, Z.; Liu, H.; Qiao, S.; Chen, Y.; Zhu, Z.; Tang, Y.; Liu, C. Scalable Oxygen-Assisted-Fe2+ Etching Approach towards Amorphous/Crystalline Structure Fe-Ni2P Nanoarray for Efficient Water Splitting. J. Alloy. Compd. 2023, 936, 168073. [Google Scholar] [CrossRef]
- Yang, N.; Tian, S.; Feng, Y.; Hu, Z.; Liu, H.; Tian, X.; Xu, L.; Hu, C.; Yang, J. Introducing High-Valence Iridium Single Atoms into Bimetal Phosphides toward High-Efficiency Oxygen Evolution and Overall Water Splitting. Small 2023, 19, 2207253. [Google Scholar] [CrossRef]
- Dai, Z.; Du, X.; Zhang, X. The Synthesis of Ni-Co-Fe-Se@NiCo-LDH Nanoarrays on Ni Foam as Efficient Overall Water Splitting Electrocatalyst. J. Alloy. Compd. 2023, 946, 169451. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Zhao, Q.; Abudula, A.; Ma, Y.; Yan, K.; Guan, G. Tuning Octahedron Sites in MnFe2O4 Spinel by Boron Doping for Highly Efficient Seawater Splitting. Appl. Catal. B Environ. 2023, 330, 122577. [Google Scholar] [CrossRef]
- Guo, F.; Li, W.; Liu, Y.; Chen, Q.; Zhong, Q. Heterogeneous Fe-Doped NiCoP–MoO3 Efficient Electrocatalysts for Overall Water Splitting. Langmuir 2023, 39, 1042–1050. [Google Scholar] [CrossRef]
- Lei, B.; Xu, D.; Wei, B.; Xie, T.; Xiao, C.; Jin, W.; Xu, L. In Situ Synthesis of α-Fe2O3/Fe3O4 Heterojunction Photoanode via Fast Flame Annealing for Enhanced Charge Separation and Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 4785–4795. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Zhou, B.; Xiao, W.; Wang, J.; Wang, X.; Xu, G.; Li, B.; Li, Z.; Wu, Z.; et al. Corrosive Engineering Assisted in Situ Construction of an Fe–Ni-Based Compound for Industrial Overall Water-Splitting under Large-Current Density in Alkaline Freshwater and Seawater Media. J. Mater. Chem. A 2023, 11, 1886–1893. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, Y.; Su, D.; Zhang, S.; Yang, J.; Yan, D.; Fang, S.; Wang, X. Engineering Cu1.96S/Co9S8 with Sulfur Vacancy and Heterostructure as an Efficient Bifunctional Electrocatalyst for Water Splitting. J. Mater. Sci. Technol. 2023, 154, 1–8. [Google Scholar] [CrossRef]
- He, B.; Wang, X.-C.; Xia, L.-X.; Guo, Y.-Q.; Tang, Y.-W.; Zhao, Y.; Hao, Q.-L.; Yu, T.; Liu, H.-K.; Su, Z. Metal-Organic Framework-Derived Fe-Doped Co1.11Te2 Embedded in Nitrogen-Doped Carbon Nanotube for Water Splitting. ChemSusChem 2020, 13, 5239–5247. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, A.-L.; Xiao, W.; Chao, D.; Zhang, X.; Tiep, N.H.; Chen, S.; Kang, J.; Wang, X.; Ding, J.; et al. In Situ Grown Epitaxial Heterojunction Exhibits High-Performance Electrocatalytic Water Splitting. Adv. Mater. 2018, 30, 1705516. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Yang, F.; Xing, L.; Wang, D.; Chen, X.; Gao, H.; Huang, X.; Lu, Y.; Wang, G. 3D Hydrangea Macrophylla-like Nickel–Vanadium Metal–Organic Frameworks Formed by Self-Assembly of Ultrathin 2D Nanosheets for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 48495–48510. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ouyang, T.; Zou, Y.; Li, N.; Liu, Z.-Q. Ultrathin NiCo2Px Nanosheets Strongly Coupled with CNTs as Efficient and Robust Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 7420–7427. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Wang, C.; Gao, B.; Li, J.; Zhu, M.; Wu, H.; Zhang, C.; Qin, Y. Photothermal–Magnetic Synergistic Effects in an Electrocatalyst for Efficient Water Splitting under Optical–Magnetic Fields. Adv. Mater. 2023, 35, 2303741. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-B.; Li, X.; Wei, W.-B.; Wu, X.-T.; Zhu, Q.-L. Nano-Engineering of Ru-Based Hierarchical Porous Nanoreactors for Highly Efficient PH-Universal Overall Water Splitting. Appl. Catal. B Environ. 2021, 294, 120230. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Gao, Y.; Zhang, J.; Huang, C.; Shi, Q.; Mu, S.; Xiao, Q.; Huo, S.; Xia, Z.; et al. D-Orbital Manipulated Ru Nanoclusters for High-Efficiency Overall Water Splitting at Industrial-Level Current Densities. Adv. Funct. Mater. 2023, 34, 2307917. [Google Scholar] [CrossRef]
- Nagappan, S.; Karmakar, A.; Madhu, R.; Dhandapani, H.N.; Singha Roy, S.; Kundu, S. Tuning the Active Sites and Optimizing the D-Spacing Value in CoFe-LDH by Ex Situ Intercalation of Guest Anions: An Innovative Electrocatalyst for Overall Water Splitting Reaction. Catal. Sci. Technol. 2023, 13, 6377–6391. [Google Scholar] [CrossRef]

| Electrochemical Properties | HER | OER |
|---|---|---|
| EIS values | 7.1 Ω | 13.3 Ω |
| Tafel slopes | 62 mV/dec | 199 mV/dec |
| Cdl values | 4.6 mF/cm2 | 14.6 mF/cm2 |
| ECSA | 28.75 cm2 | 91.25 cm2 |
| TOF values (at 500 and 800 mA/cm2) | 0.193 site−1s−1 | 0.101 site−1s−1 |
| Faradic efficiency (FE) | 90.51% | 90.16% |
| Electrocatalyst | Overpotentials [mV] | Year | References | ||
|---|---|---|---|---|---|
| @20 mA/cm2 | @50 mA/cm2 | @200 mA/cm2 | |||
| RuVNi | 15 | 26 | 48 | 2019 | [72] |
| CoFeBP | 20.1 | 46 | 145 | - | This work |
| NiMoB | 54 | 97 | 210 | 2022 | [40] |
| Ru/CoFeP | 61 | 82 | - | 2020 | [19] |
| NiFeMn | 80 | 121 | - | 2020 | [73] |
| CoB@CoO/Ti | 81 | 110 | 181 | 2017 | [74] |
| NiCoP | 134 | 165 | 204 | 2018 | [13] |
| Ni/Ni3N | 151 | 232 | 420 | 2015 | [59] |
| Fe-NiSe | 182 | 265 | - | 2022 | [75] |
| Fe-S | 193 | 235 | 324 | 2019 | [76] |
| Electrocatalyst | Overpotentials [mV] | Year | References | ||
|---|---|---|---|---|---|
| @20 mA/cm2 | @50 mA/cm2 | @200 mA/cm2 | |||
| Ni2P-CuP | 140 | 190 | - | 2021 | [3] |
| MoNiPx/NiSy | 143 | 156 | 221 | 2021 | [77] |
| NiMo3S4 | 162 | 252 | 617 | 2022 | [78] |
| P-Co3O4 | 208 | 295 | 330 | 2018 | [18] |
| RuVNi | 217 | 227 | 312 | 2019 | [72] |
| CoFeBP | 219 | 303 | 426 | - | This work |
| NiMoB | 248 | 267 | 500 | 2022 | [40] |
| NiFeMn | 260 | 291 | 352 | 2020 | [73] |
| NiCoP | 296 | 328 | 370 | 2018 | [13] |
| Mo2NiB2 | 311 | 342 | - | 2021 | [2] |
| NiSe2 | 320 | 521 | - | 2022 | [75] |
| Electrolytes | @200 mA/cm2 | @1000 mA/cm2 |
|---|---|---|
| 1 M KOH | 1.84 V | 2.66 V |
| 6 M KOH | 1.76 V | 2.54 V |
| 0.5 M H2SO4 | 2.13 V | - |
| 1 M PBS | 2.25 V | - |
| River water | - | - |
| River water + 1 M KOH | 3.87 V | - |
| Seawater | - | - |
| Seawater + 1 M KOH | 2.34 V | - |
| Electrocatalyst | Electrolyte | Cell Voltage (V) | Year | References | |
|---|---|---|---|---|---|
| @50 mA/cm2 | @200 mA/cm2 | ||||
| Fe-NiSe | 1 M KOH | 1.34 V | 1.61 V | 2022 | [75] |
| Ru/CoFeP | 1 M KOH | 1.46 V | - | 2020 | [19] |
| MoNiPx/NiSy | 1 M KOH | 1.55 V | 1.88 V | 2021 | [77] |
| FeS/Ni3S2 | 1 M KOH | 1.56 V | - | 2022 | [80] |
| NiFeMn | 1 M KOH | 1.57 V | 1.66 V | 2020 | [73] |
| CoFeBP | 1 M KOH | 1.60 V | 1.84 V | - | This work |
| NiMoB | 1 M KOH | 1.61 V | 1.96 V | 2022 | [40] |
| Ni3S2 | 1 M KOH | 1.63 V | - | 2018 | [81] |
| Fe,Rh-Ni2P | 1 M KOH | 1.64 V | 1.85 V | 2022 | [82] |
| Mo2NiB2 | 1 M KOH | 1.67 V | - | 2021 | [2] |
| NiFeS | 1 M KOH | 1.73 V | - | 2022 | [83] |
| P-Co3O4 | 1 M KOH | 1.79 V | - | 2018 | [18] |
| NiCoP | 1 M KOH | 1.82 V | - | 2018 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Habib, M.A.; Joni, M.H.; Dristy, S.A.; Mandavkar, R.; Jeong, J.-H.; Chung, Y.-U.; Lee, J. CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials 2024, 14, 698. https://doi.org/10.3390/nano14080698
Lin S, Habib MA, Joni MH, Dristy SA, Mandavkar R, Jeong J-H, Chung Y-U, Lee J. CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials. 2024; 14(8):698. https://doi.org/10.3390/nano14080698
Chicago/Turabian StyleLin, Shusen, Md Ahasan Habib, Mehedi Hasan Joni, Sumiya Akter Dristy, Rutuja Mandavkar, Jae-Hun Jeong, Young-Uk Chung, and Jihoon Lee. 2024. "CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts" Nanomaterials 14, no. 8: 698. https://doi.org/10.3390/nano14080698







