Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase
Abstract
:1. Introduction
2. Materials and Methods
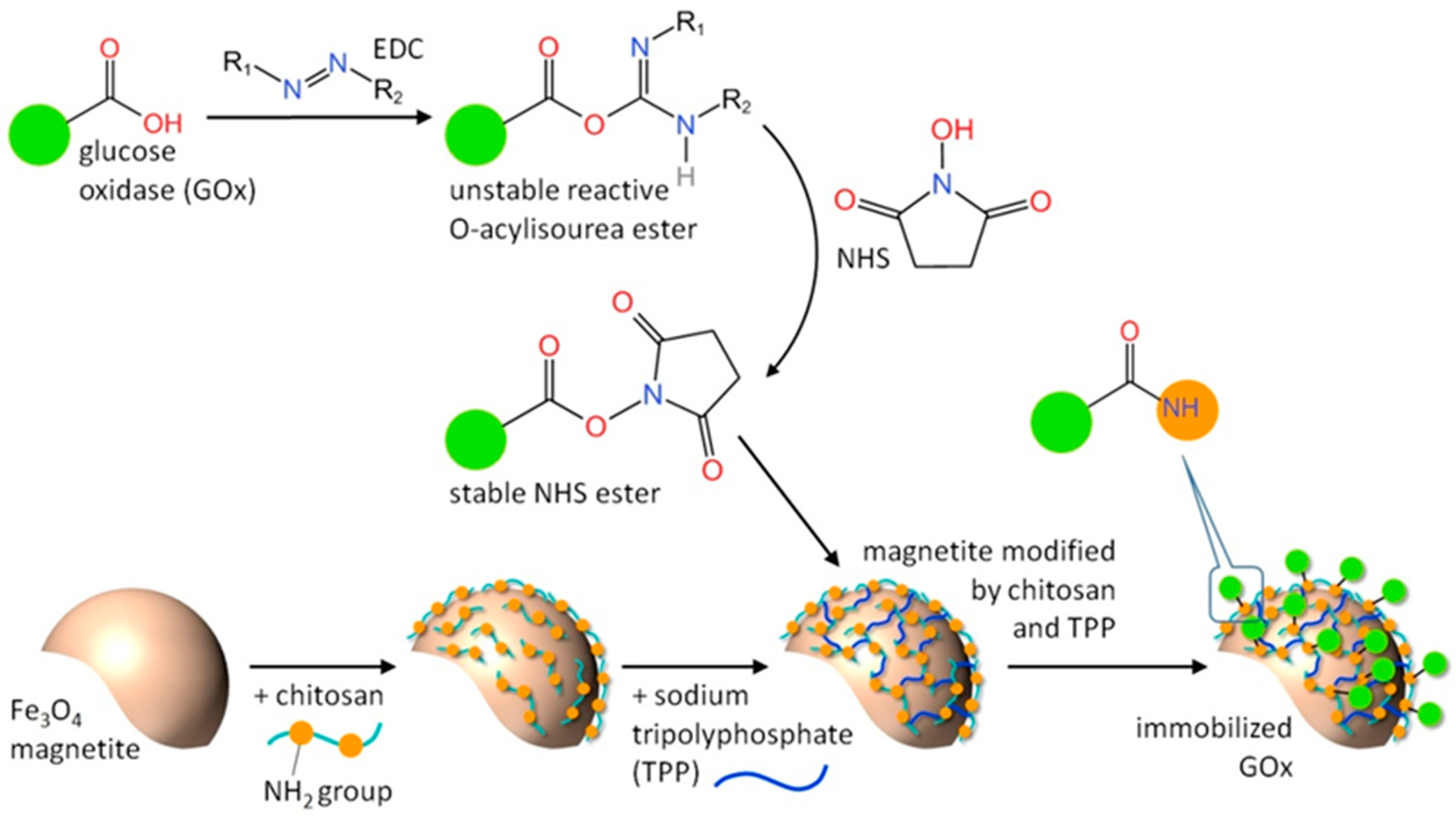
2.1. Magnetic Biocatalyst Synthesis
2.2. The Catalytic Reaction
3. Results and Discussion
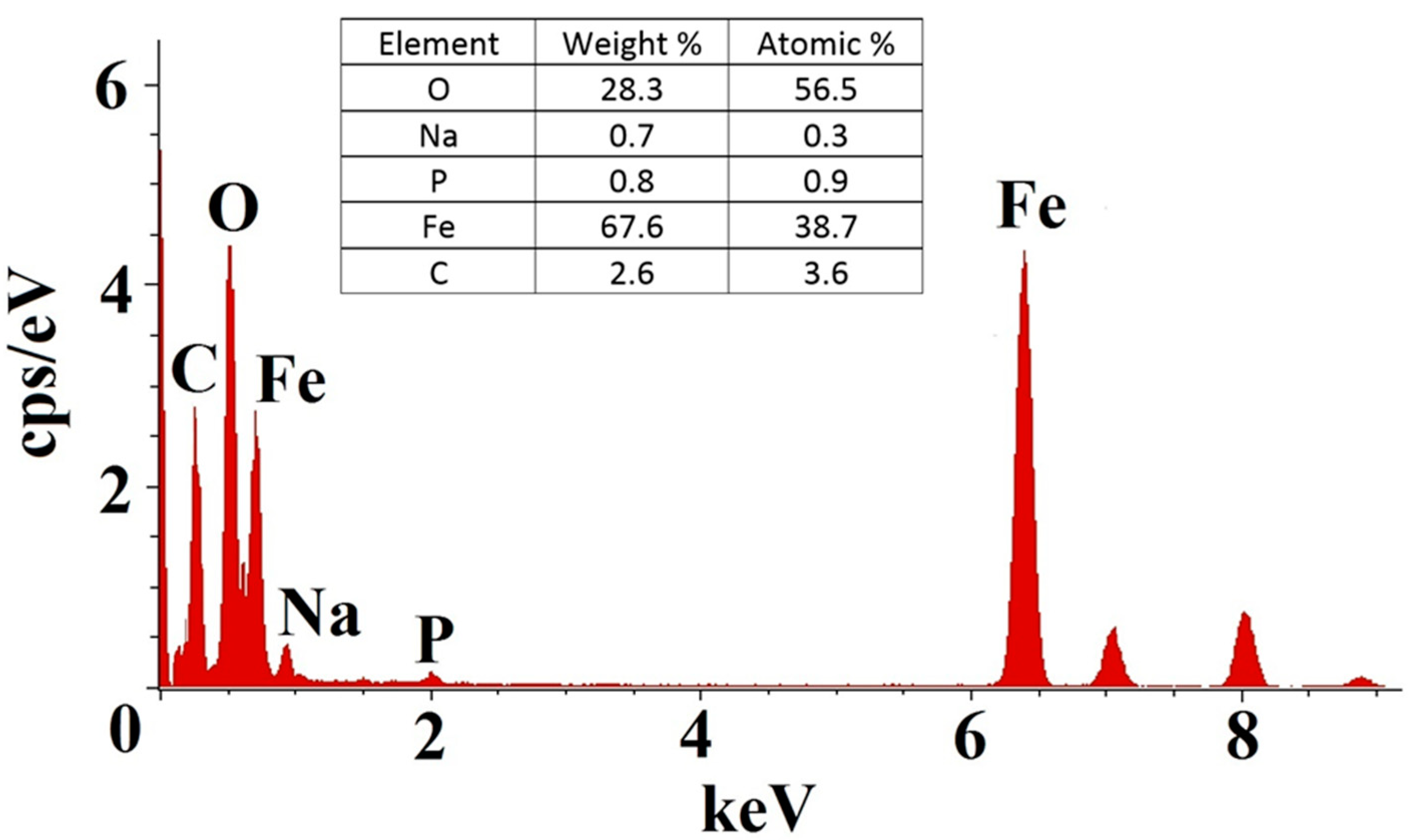
3.1. Characterization of MNAs
3.2. Biocatalyst Catalytic Activity
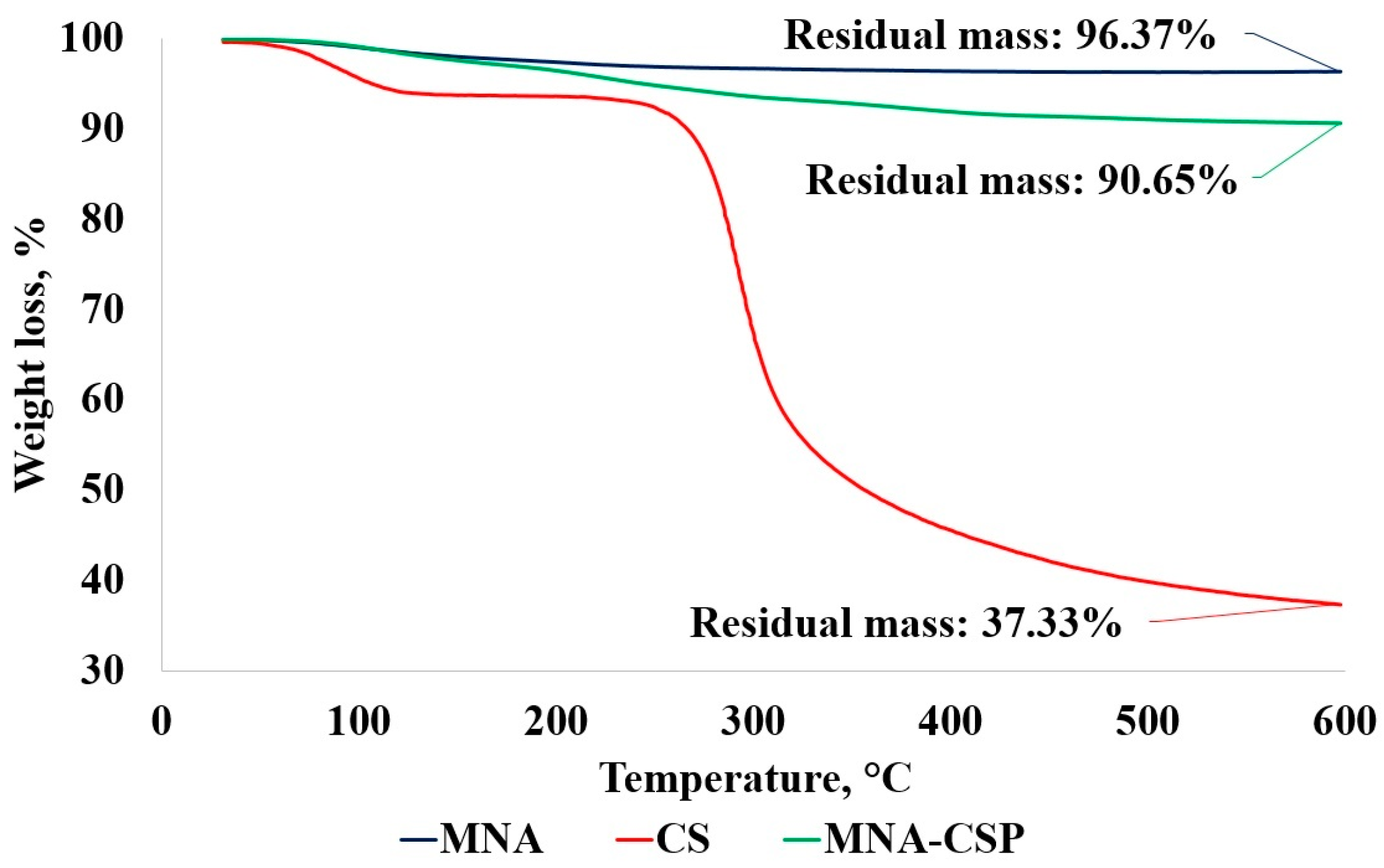
3.3. Characterization of MNA-CSP-3
3.4. Analysis of GOx Activity
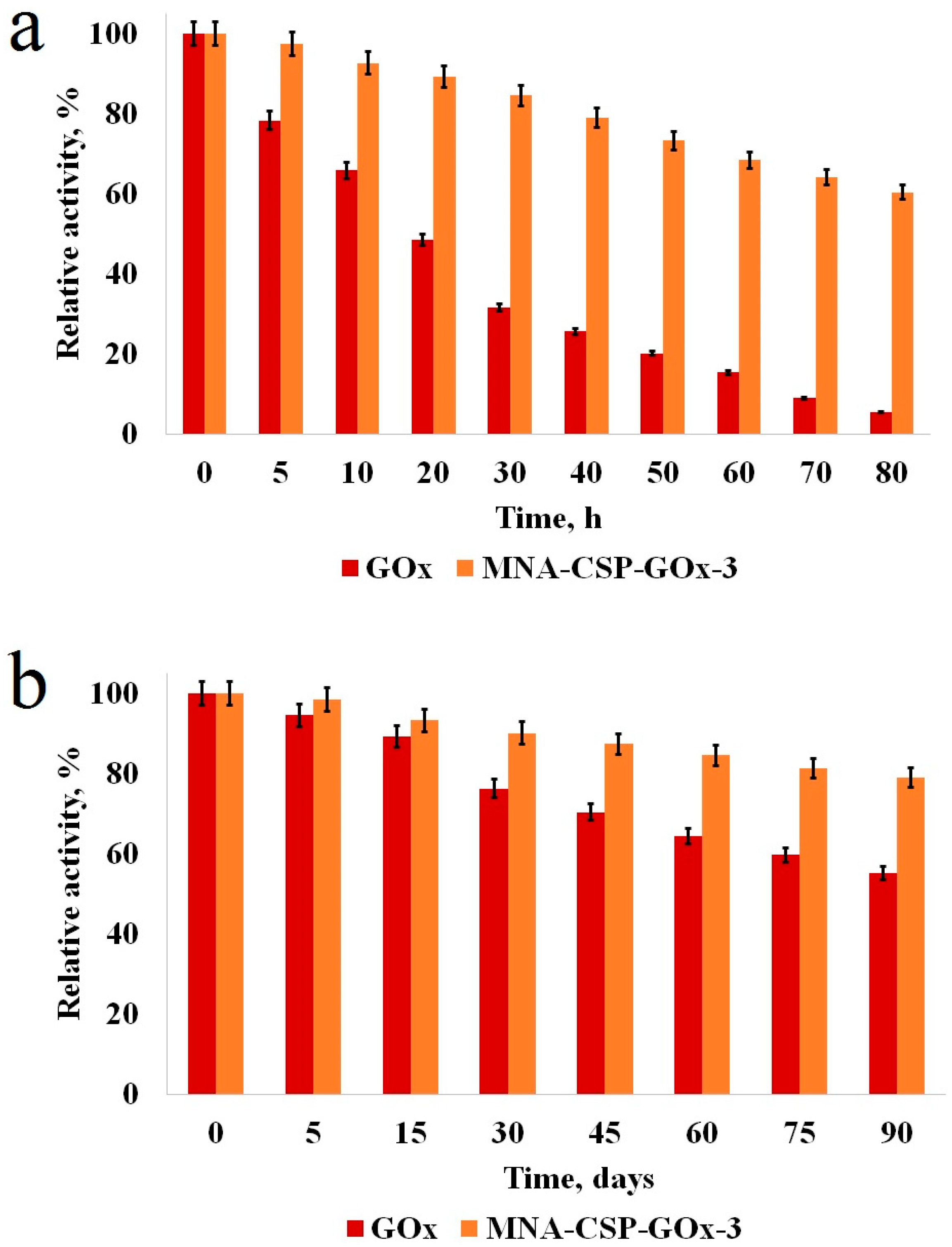
3.5. Repeated Use and Stability of the Biocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Jing, Z.; Ma, R.; Wan, X.; Liu, J.; Huang, W. A critical review of enzymes immobilized on chitosan composites: Characterization and applications. Bioprocess Biosyst. Eng. 2023, 46, 1539–1567. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Qamar, S.A.; Carballares, D.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Proteases immobilized on nanomaterials for biocatalytic, environmental and biomedical applications: Advantages and drawbacks. Biotechnol. Adv. 2024, 70, 108304. [Google Scholar] [CrossRef] [PubMed]
- Bisht, M.; Thayallath, S.K.; Bharadwaj, P.; Franklin, G.; Mondal, D. Biomass-derived functional materials as carriers for enzymes: Towards sustainable and robust biocatalysts. Green Chem. 2023, 25, 4591–4624. [Google Scholar] [CrossRef]
- Caparco, A.A.; Dautel, D.R.; Champion, J.A. Protein Mediated Enzyme Immobilization. Small 2022, 18, 2106425. [Google Scholar] [CrossRef] [PubMed]
- Dik, G.; Bakar, B.; Ulu, A.; Ates, B. Propelling of Enzyme Activity by Using Different Triggering Strategies: Applications and Perspectives. Ind. Eng. Chem. Res. 2023, 62, 14111–14129. [Google Scholar] [CrossRef]
- Gan, J.; Iqbal, H.M.N.; Show, P.L.; Rahdar, A.; Bilal, M. Upgrading recalcitrant lignocellulosic biomass hydrolysis by immobilized cellulolytic enzyme-based nanobiocatalytic systems: A review. Biomass Convers. Biorefin. 2024, 14, 4485–4509. [Google Scholar] [CrossRef]
- Lu, J.; Nie, M.; Li, Y.; Zhu, H.; Shi, G. Design of composite nanosupports and applications thereof in enzyme immobilization: A review. Colloids Surf. B 2022, 217, 112602. [Google Scholar] [CrossRef]
- Nikoshvili, L.Z.; Tikhonov, B.B.; Ivanov, P.E.; Stadolnikova, P.Y.; Sulman, M.G.; Matveeva, V.G. Recent Progress in Chitosan-Containing Composite Materials for Sustainable Approaches to Adsorption and Catalysis. Catalysts 2023, 13, 367. [Google Scholar] [CrossRef]
- Qiao, S.; Jin, H.; Zuo, A.; Chen, Y. Integration of Enzyme and Covalent Organic Frameworks: From Rational Design to Applications. Acc. Chem. Res. 2024, 57, 93–105. [Google Scholar] [CrossRef]
- Saddique, Z.; Imran, M.; Javaid, A.; Rizvi, N.B.; Akhtar, M.N.; Iqbal, H.M.N.; Bilal, M. Enzyme-Linked Metal Organic Frameworks for Biocatalytic Degradation of Antibiotics. Catal. Lett. 2024, 154, 81–93. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, S.; Princy; Arya, S.K.; Kaur, A. Enzyme immobilization: Implementation of nanoparticles and an insight into polystyrene as the contemporary immobilization matrix. Process Biochem. 2022, 120, 22–34. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Khoo, K.S.; Soto-Moscoso, M. Enzyme Immobilized Nanomaterials: An Electrochemical Bio-Sensing and Biocatalytic Degradation Properties Toward Organic Pollutants. Top. Catal. 2023, 66, 691–706. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Sekar, K.; Jalil, A.A.; Soto-Moscoso, M. Role of nanotechnology for the conversion of lignocellulosic biomass into biopotent energy: A biorefinery approach for waste to value-added products. Fuel 2022, 322, 124236. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S. Advances in the application of immobilized enzyme for the remediation of hazardous pollutant: A review. Chemosphere 2022, 299, 134390. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Shanahan, J.; Rizaldo, S.; Kissel, D.S.; Stone, K.L. Co-immobilization of an enzyme system on a metal-organic framework to produce a more effective biocatalyst. Catalysts 2020, 10, 499. [Google Scholar] [CrossRef]
- Goldhahn, C.; Taut, J.A.; Schubert, M.; Burgert, I.; Chanana, M. Enzyme immobilization inside the porous wood structure: A natural scaffold for continuous-flow biocatalysis. RSC Adv. 2020, 10, 20608–20619. [Google Scholar] [CrossRef] [PubMed]
- Haskell, A.K.; Sulman, A.M.; Golikova, E.P.; Stein, B.D.; Pink, M.; Morgan, D.G.; Lakina, N.V.; Karpenkov, A.Y.; Tkachenko, O.P.; Sulman, E.M.; et al. Glucose Oxidase Immobilized on Magnetic Zirconia: Controlling Catalytic Performance and Stability. ACS Omega 2020, 5, 12329–12338. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jung, G.A.; Glover, D.J.; Clark, D.S. Enhanced enzyme activity through scaffolding on customizable self-assembling protein filaments. Small 2019, 15, 1805558. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, L.; Sun, T.; Yin, Y.; Wang, Q. Enzyme Immobilization on a Delignified Bamboo Scaffold as a Green Hierarchical Bioreactor. ACS Sustain. Chem. Eng. 2022, 10, 6244–6254. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, Q.; Hu, M.; Li, S.; Jiang, Y. Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: A kinetic and thermodynamic study of structure-activity. Catal. Sci. Technol. 2021, 11, 2446–2455. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Li, H.; Alhalhooly, L.; Li, Y.; Chen, B.; Choi, Y.; Yang, Z. Size-Tunable Metal-Organic Framework-Coated Magnetic Nanoparticles for Enzyme Encapsulation and Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 41794–41801. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Chen, Y.; Dai, W.; He, X.; Li, B.; Tian, S. Immobilized short-chain dehydrogenase/reductase on Fe3O4 particles acts as a magnetically recoverable biocatalyst component in patulin bio-detoxification system. J. Hazard. Mater. 2023, 448, 130986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Fang, X.; Lv, J.; Yang, Y.; Zeng, Y.; Liu, Y.; Wei, W.; Huang, G.; Zhang, B.; Wu, C. Site-Specific Modification of Single Domain Antibodies by Enzyme-Immobilized Magnetic Beads. Bioconjugate Chem. 2023, 34, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, X.; Wang, Y.; Zhai, Q.; Hu, M.; Li, S.; Jiang, Y. Amino modified magnetic halloysite nanotube supporting chloroperoxidase immobilization: Enhanced stability, reusability, and efficient degradation of pesticide residue in wastewater. Bioprocess Biosyst. Eng. 2021, 44, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Rehm, T.H.; Bogdan, A.; Hofmann, C.; Loeb, P.; Shifrina, Z.B.; Morgan, D.G.; Bronstein, L.M. Proof of Concept: Magnetic Fixation of Dendron-Functionalized Iron Oxide Nanoparticles Containing Palladium Nanoparticles for Continuous-Flow Suzuki Coupling Reactions. ACS Appl. Mater. Interfaces 2015, 7, 27254–27261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, X.; Liao, H.; Liang, Z.; Li, F.; Tian, J.; Ling, D. Artificially Engineered Cubic Iron Oxide Nanoparticle as a High-Performance Magnetic Particle Imaging Tracer for Stem Cell Tracking. ACS Nano 2020, 14, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism Beyond Magnetosomes. IEEE Trans. Nanobiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Demortiere, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Begin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Baghshahi, S.; Yousefi, F. A New Systematic Approach to the Morphology and Magnetic Properties of Spherical, Cubic, and Rod-like Magnetite Nanoparticles. J. Supercond. Novel Magn. 2021, 34, 1949–1954. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Y.; Hu, S.; Song, L.; Dong, J.; Chen, Z.; Gu, N. Influence of morphology and surface exchange reaction on magnetic properties of monodisperse magnetite nanoparticles. Colloids Surf. A 2012, 408, 114–121. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Nakamatsu, E.H.; Netto, L.E.S.; Novak, M.A.; Zuin, A.; Nakamura, M.; Araki, K.; Toma, H.E. Catalytic properties of thioredoxin immobilized on superparamagnetic nanoparticles. J. Inorg. Biochem. 2011, 105, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Pita, M.; Kramer, M.; Zhou, J.; Poghossian, A.; Schoning, M.J.; Fernandez, V.M.; Katz, E. Optoelectronic Properties of Nanostructured Ensembles Controlled by Biomolecular Logic Systems. ACS Nano 2008, 2, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.-Y.; Chen, Y.-J.; Ou, J.-J.; Ho, L. Preparation and characterization of Pseudomonas putida esterase immobilized on magnetic nanoparticles. Enzyme Microb. Technol. 2006, 39, 1089–1095. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Li, Y.; Chen, X.; Liu, Y. Enzymes Immobilized on Superparamagnetic Fe3O4@Clays Nanocomposites: Preparation, Characterization, and a New Strategy for the Regeneration of Supports. J. Phys. Chem. C 2011, 115, 6350–6359. [Google Scholar] [CrossRef]
- Garcia-Colmenares, J.M.; Reyes-Cuellar, J.C. Immobilization of bromelain on cobalt-iron magnetic nanoparticles (CoFe2O4) for casein hydrolysis. Rev. Colomb. Quim. 2020, 49, 3–10. [Google Scholar] [CrossRef]
- Gracida, J.; Arredondo-Ochoa, T.; Garcia-Almendarez, B.E.; Escamilla-Garcia, M.; Shirai, K.; Regalado, C.; Amaro-Reyes, A. Improved Thermal and Reusability Properties of Xylanase by Genipin Cross-Linking to Magnetic Chitosan Particles. Appl. Biochem. Biotechnol. 2019, 188, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Styevko, G.; Madaras, E.; Haktanirlar, G.; Tran, A.T.M.; Bujna, E.; Dam, M.S.; Nguyen, Q.D. Immobilization of β-galactosidase on chitosan-coated magnetic nanoparticles and its application for synthesis of lactulose-based galactooligosaccharides. Process Biochem. 2019, 84, 30–38. [Google Scholar] [CrossRef]
- Podrepsek, G.H.; Knez, Z.; Leitgeb, M. Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef]
- Salehi, M.E.; Asoodeh, A. Immobilization of endoglucanase isolated from symbiotic bacterium Bacillus safensis CF99 on magnetic nanoparticles. Chem. Pap. 2022, 76, 6523–6536. [Google Scholar] [CrossRef]
- Lu, F.; Ding, G.; Ma, X.; Huang, B.; You, L. Chitosan-gelatin/cetyltrimethylammonium bromide magnetic polymer composites as reusable high performance adsorbent for AR 18 removal. J. Mol. Liq. 2023, 391, 123303. [Google Scholar] [CrossRef]
- Mostaraddi, S.; Pazhang, M.; Ebadi-Nahari, M.; Najavand, S. The Relationship Between the CrossLinker on Chitosan-Coated Magnetic Nanoparticles and the Properties of Immobilized Papain. Mol. Biotechnol. 2023, 65, 1809–1823. [Google Scholar] [CrossRef]
- Santillan, F.; Mejia, I.M.M.; Goicoechea, H.C. Comparative study of uncoated and tetraethylorthosilicate-coated magnetic chitosan beads in the adsorption of two textile dyes. Int. J. Environ. Sci. Technol. 2023, 20, 11821–11836. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Yunus, M.H.M.; Fauzi, M.B.; Tabata, Y.; Hiraoka, Y.; Phang, S.J.; Chia, M.R.; Buyong, M.R.; Yazid, M.D. Gelatin-chitosan-cellulose nanocrystals as an acellular scaffold for wound healing application: Fabrication, characterisation and cytocompatibility towards primary human skin cells. Cellulose 2023, 30, 5071–5092. [Google Scholar] [CrossRef]
- Choi, H.S.; Jo, Y.K.; Ahn, G.-N.; Joo, K.I.; Kim, D.-P.; Cha, H.J. Magnetically Guidable Proteinaceous Adhesive Microbots for Targeted Locoregional Therapeutics Delivery in a Highly Dynamic Environment of Esophagus. Adv. Funct. Mater. 2021, 31, 2104602. [Google Scholar] [CrossRef]
- Chandra, D.; Molla, T.H.M.; Bashar, A.M.; Islam, S.M.; Ahsan, S.M. Chitosan-based nano-sorbents: Synthesis, surface modification, characterization and application in Cd (II), Co (II), Cu (II) and Pb (II) ions removal from wastewater. Sci. Rep. 2023, 13, 6050. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Deng, C.; Wang, Y.; Adisa, I.O.; Zhou, J.; White, J.C. Chitosan and Zinc Oxide Nanoparticle-Enhanced Tripolyphosphate Modulate Phosphorus Leaching in Soil. ACS Agric. Sci. Technol. 2023, 3, 487–498. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Lee, Y.-Y.; Qiu, C.; Zeng, X.; Wang, Y. Pickering emulsions stabilized by chitosan-flaxseed gum-hyaluronic acid nanoparticles for controlled topical release of ferulic acid. Int. J. Biol. Macromol. 2024, 255, 128086. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zou, P.P.; Huang, J.; Cai, J. Co-immobilization of glucose oxidase and catalase in porous magnetic chitosan microspheres for production of sodium gluconate. Int. J. Chem. React. Eng. 2022, 20, 989–1001. [Google Scholar] [CrossRef]
- Yin, D.; Wu, H.; Liu, C.; Zhang, J.; Zhou, T.; Wu, J.; Wan, Y. Fabrication of composition-graded collagen/chitosan-polylactide scaffolds with gradient architecture and properties. React. Funct. Polym. 2014, 83, 98–106. [Google Scholar] [CrossRef]
- Zhang, X.L.; Niu, H.Y.; Zhang, S.X.; Cai, Y.Q. Preparation of a chitosan-coated C18-functionalized magnetite nanoparticle sorbent for extraction of phthalate ester compounds from environmental water samples. Anal. Bioanal. Chem. 2010, 397, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Q.; Yan, Y.R.; Zhang, W.; Luo, H.Y.; Yao, B.; Huang, H.Q.; Tu, T. Review of glucose oxidase as a feed additive: Production, engineering, applications, growth-promoting mechanisms, and outlook. Crit. Rev. Biotechnol. 2023, 43, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.N.; Wang, Y.G.; Li, X.; Wan, M.L.; Zhang, G.; Leng, F.F.; Zhang, H.B. Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. Int. J. Mol. Sci. 2022, 23, 9841. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, N.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production ofd-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.Y.; Wang, C.; Chen, W. Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mera, I.; Espinosa-Pesqueira, M.E.; Pérez-Hernández, R.; Arenas-Alatorre, J. Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Mater. Lett. 2007, 61, 4447–4451. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res.—Part A 2007, 80, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, P.A.; Madarász, J.; Kuzmann, E.; Vértes, A.; Molnár, G.; Bousseksou, A.; Sharma, V.K.; Homonnay, Z. Thermal stability of the FeIIIEDTA complex in its monomeric form. Thermochim. Acta 2008, 479, 53–58. [Google Scholar] [CrossRef]
- Amara, D.; Grinblat, J.; Margel, S. Solventless thermal decomposition of ferrocene as a new approach for one-step synthesis of magnetite nanocubes and nanospheres. J. Mater. Chem. 2012, 22, 2188–2195. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.R.; Duarte, L.G.R.; Fiocco, A.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Improving chitosan properties through ionic and chemical cross-linking and their impact on emulsified systems. Int. J. Food Sci. Technol. 2023, 58, 4324–4331. [Google Scholar] [CrossRef]
- Hua, Y.G.; Liu, S.L.; Xie, S.S.; Shi, L.J.; Li, J.C.; Luo, Q.F. Heterobifunctional Cross-Linker with Dinitroimidazole and N-Hydroxysuccinimide Ester Motifs for Protein Functionalization and Cysteine-Lysine Peptide Stapling. Org. Lett. 2023, 25, 8792–8796. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.T.; Mao, P.Z.; Chen, Z.L.; Tarn, C.; Dong, M.Q. Comparative Analysis of Chemical Cross-Linking Mass Spectrometry Data Indicates That Protein STY Residues Rarely React with N-Hydroxysuccinimide Ester Cross-Linkers. J. Proteome Res. 2023, 22, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of enzyme immobilization on magnetic microparticles using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) as a crosslinking agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef]
- Memon, A.H.; Ding, R.; Yuan, Q.; Liang, H.; Wei, Y. Coordination of GMP ligand with Cu to enhance the multiple enzymes stability and substrate specificity by co-immobilization process. Biochem. Eng. J. 2018, 136, 102–108. [Google Scholar] [CrossRef]
- Miron, J.; Gonzalez, M.P.; Vazquez, J.A.; Pastrana, L.; Murado, M.A. A mathematical model for glucose oxidase kinetics, including inhibitory, deactivant and diffusional effects, and their interactions. Enzyme Microb. Technol. 2004, 34, 513–522. [Google Scholar] [CrossRef]
- Sulman, A.M.; Haskell, A.K.; Tikhonov, B.B.; Grebennikova, O.V.; Sidorov, A.I.; Bronstein, L.M.; Matveeva, V.G. Larger pores dramatically enhance activity of an immobilized enzyme in mesoporous magnetic silica. Microporous Mesoporous Mater. 2022, 341, 112092. [Google Scholar] [CrossRef]
- Liao, L.; Meng, Y.; Wang, R.; Jia, B.; Li, P. Coupling and Regulation of Porous Carriers Using Plasma and Amination to Improve the Catalytic Performance of Glucose Oxidase and Catalase. Front. Bioeng. Biotechnol. 2019, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1479274.htm (accessed on 7 March 2024).
- Farjadian, F.; Moradi, S.; Hosseini, M. Thin chitosan films containing super-paramagnetic nanoparticles with contrasting capability in magnetic resonance imaging. J. Mater. Sci. Mater. Med. 2017, 28, 47. [Google Scholar] [CrossRef] [PubMed]
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and application of magnetic chitosan in environmental remediation and other fields: A review. J. Appl. Polym. Sci. 2021, 138, 51241. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Cho, A.; Weon, S.; Lee, S.; Lee, J.; Han, J.W.; Kim, D.-P.; Choi, W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nat Commun 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.S.; Torres, J.A.; Silva, M.C.; Nogueira, F.G.E.; da Silva, A.C.; Ramalho, T.C. Soybean peroxidase immobilized on δ-FeOOH as new magnetically recyclable biocatalyst for removal of ferulic acid. Bioprocess Biosyst. Eng. 2018, 41, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Su, P.; Ma, R.A.; Yang, Y.; Yang, Y. Based on DNA Strand Displacement and Functionalized Magnetic Nanoparticles: A Promising Strategy for Enzyme Immobilization. Ind. Eng. Chem. Res. 2017, 56, 5127–5137. [Google Scholar] [CrossRef]
- Vishnu, D.; Neeraj, G.; Swaroopini, R.; Shobana, R.; Kumar, V.V.; Cabana, H. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ. Sci. Pollut. Res. 2017, 24, 17993–18009. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhu, G.X.; Wang, G.C.; Li, Y.L.; Tang, R.K. Robust glucose oxidase with a Fe3O4@C-silica nanohybrid structure. J. Mater. Chem. B 2016, 4, 4726–4731. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.X.; Zhou, H.M. Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules. Int. J. Mol. Sci. 2011, 12, 3042–3054. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Ariga, K.; Kunitake, T. Activity and stability of glucose oxidase in molecular films assembled alternately with polyions. J. Biosci. Bioeng. 1999, 87, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Penniston, J.T. Method for determination of hydrogen peroxide, with its aaplication illustrated by glucose assay. Clin. Chem. 1980, 26, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.C.; Perez Goncalves, G.M.; Pielak, G.J.; Gorensek-Benitez, A.H. Large cosolutes, small cosolutes, and dihydrofolate reductase activity. Protein Sci. 2017, 26, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Yang, K.-L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions. Enzyme Microb. Technol. 2017, 100, 52–59. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Li, D.; Jiang, Y. Co-immobilization of glucose oxidase and catalase in silica inverse opals for glucose removal from commercial isomaltooligosaccharide. Int. J. Biol. Macromol. 2018, 107, 2034–2043. [Google Scholar] [CrossRef]




| Sample | m(CS), g | m(TPP), g | m(EDC), g–m(NHS), g | m(GOx), g | R, % |
|---|---|---|---|---|---|
| MNA-CSP-GOx-1 | 0.050 | 0.050 | 0.100–0.040 | 0.010 | 55.5 |
| MNA-CSP-GOx-2 | 0.075 | 0.050 | 0.100–0.040 | 0.010 | 76.9 |
| MNA-CSP-GOx-3 | 0.100 | 0.050 | 0.100–0.040 | 0.010 | 100 |
| MNA-CSP-GOx-4 | 0.125 | 0.050 | 0.100–0.040 | 0.010 | 65.3 |
| MNA-CSP-GOx-5 | 0.150 | 0.050 | 0.100–0.040 | 0.010 | 60.5 |
| MNA-CSP-GOx-6 | 0.100 | 0.010 | 0.100–0.040 | 0.010 | 41.3 |
| MNA-CSP-GOx-7 | 0.100 | 0.025 | 0.100–0.040 | 0.010 | 58.4 |
| MNA-CSP-GOx-8 | 0.100 | 0.075 | 0.100–0.040 | 0.010 | 85.4 |
| MNA-CSP-GOx-9 | 0.100 | 0.100 | 0.100–0.040 | 0.010 | 65.1 |
| MNA-CSP-GOx-10 | 0.100 | 0.050 | 0.020–0.008 | 0.010 | 58.4 |
| MNA-CSP-GOx-11 | 0.100 | 0.050 | 0.050–0.020 | 0.010 | 74.9 |
| MNA-CSP-GOx-12 | 0.100 | 0.050 | 0.075–0.030 | 0.010 | 83.6 |
| MNA-CSP-GOx-13 | 0.100 | 0.050 | 0.125–0.050 | 0.010 | 92.5 |
| MNA-CSP-GOx-14 | 0.100 | 0.050 | 0.100–0.040 | 0.050 | 79.7 |
| MNA-CSP-GOx-15 | 0.100 | 0.050 | 0.100–0.040 | 0.075 | 81.3 |
| MNA-CSP-GOx-16 | 0.100 | 0.050 | 0.100–0.040 | 0.125 | 93.8 |
| MNA-CSP-GOx-17 | 0.100 | 0.050 | 0.100–0.040 | 0.150 | 94.7 |
| Kinetic Parameter | Free GOx | MNC-CSP-GOx-3 |
|---|---|---|
| Vmax, μM/s | 6.32 | 2.92 |
| Km, mM | 7.69 | 3.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonov, B.B.; Lisichkin, D.R.; Sulman, A.M.; Sidorov, A.I.; Bykov, A.V.; Lugovoy, Y.V.; Karpenkov, A.Y.; Bronstein, L.M.; Matveeva, V.G. Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase. Nanomaterials 2024, 14, 700. https://doi.org/10.3390/nano14080700
Tikhonov BB, Lisichkin DR, Sulman AM, Sidorov AI, Bykov AV, Lugovoy YV, Karpenkov AY, Bronstein LM, Matveeva VG. Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase. Nanomaterials. 2024; 14(8):700. https://doi.org/10.3390/nano14080700
Chicago/Turabian StyleTikhonov, Boris B., Daniil R. Lisichkin, Alexandrina M. Sulman, Alexander I. Sidorov, Alexey V. Bykov, Yury V. Lugovoy, Alexey Y. Karpenkov, Lyudmila M. Bronstein, and Valentina G. Matveeva. 2024. "Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase" Nanomaterials 14, no. 8: 700. https://doi.org/10.3390/nano14080700






