Development of Light, Strong, and Water-Resistant PVA Composite Aerogels
Abstract
:1. Introduction
2. Experimental Methods
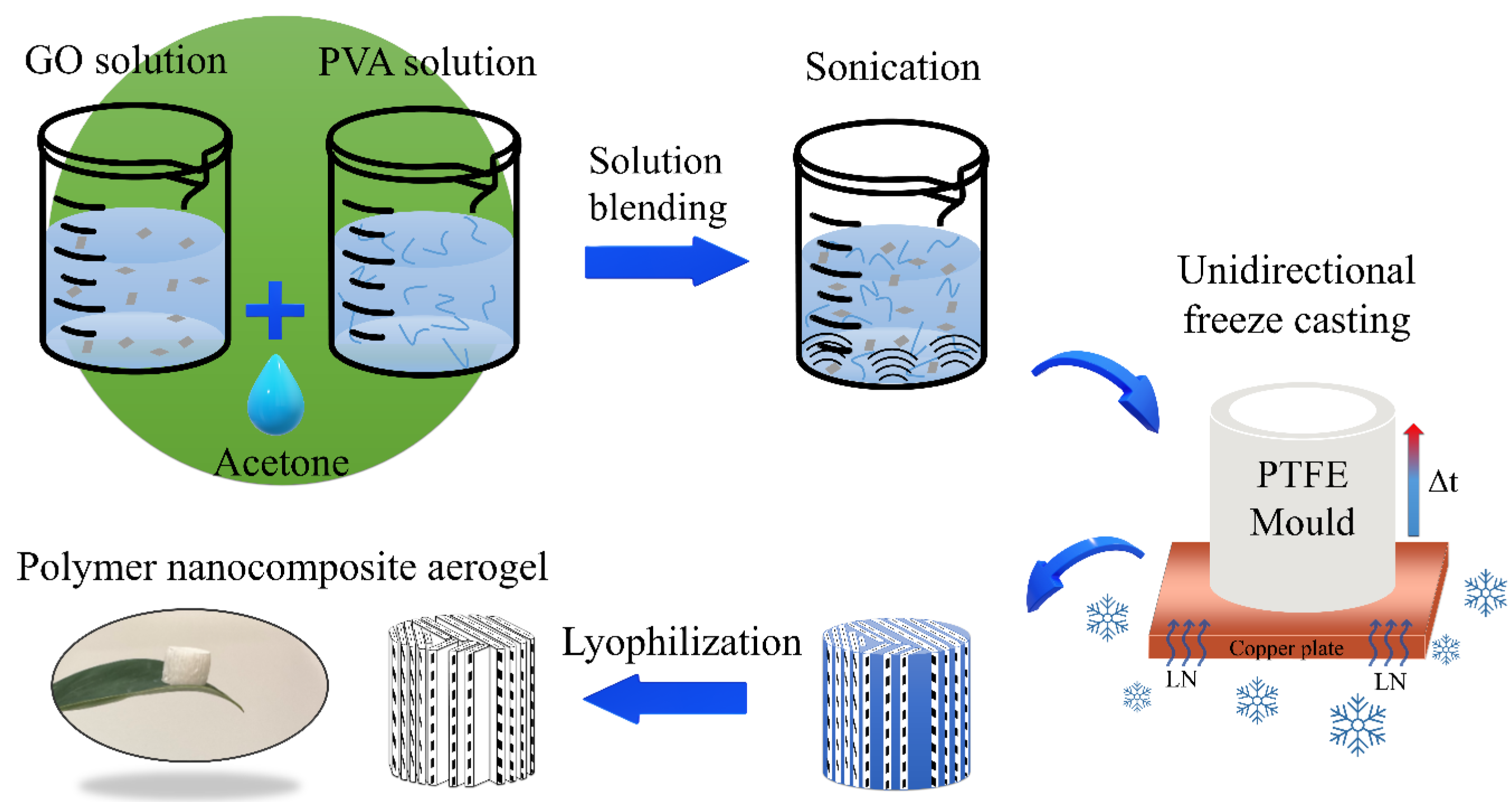
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
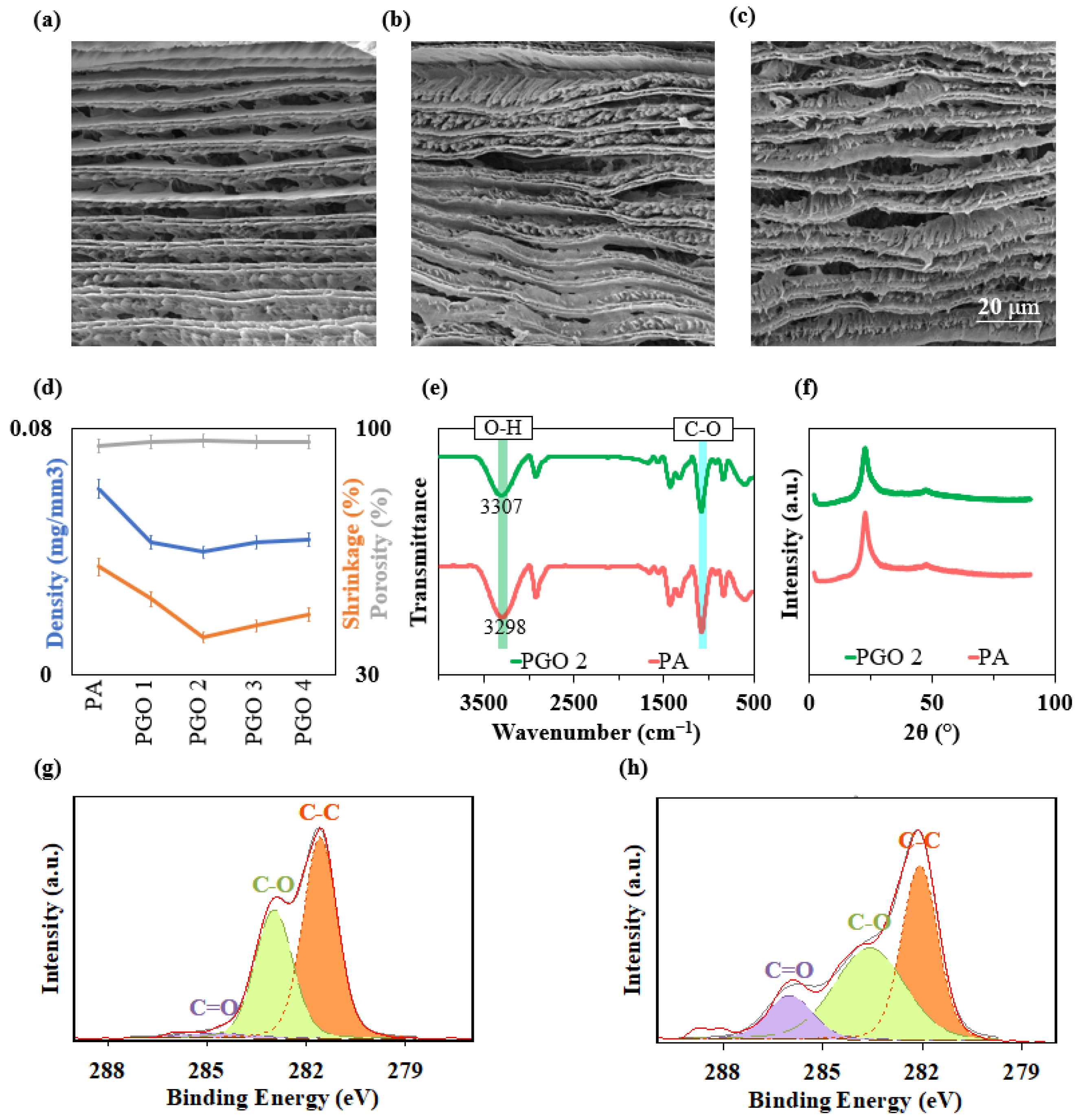
3.1. Characterisation of GO Nanosheets and Nanocomposite Aerogels
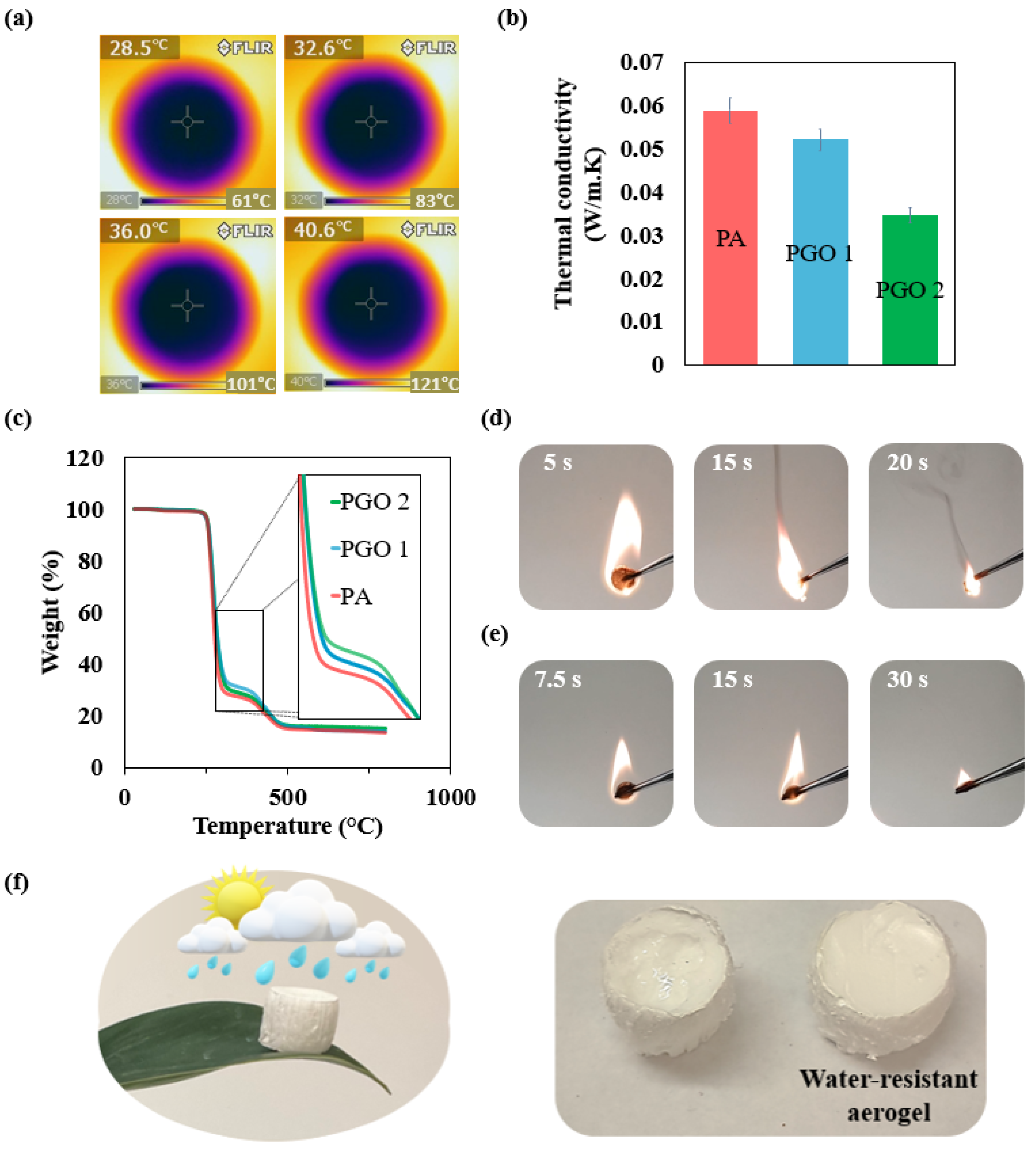
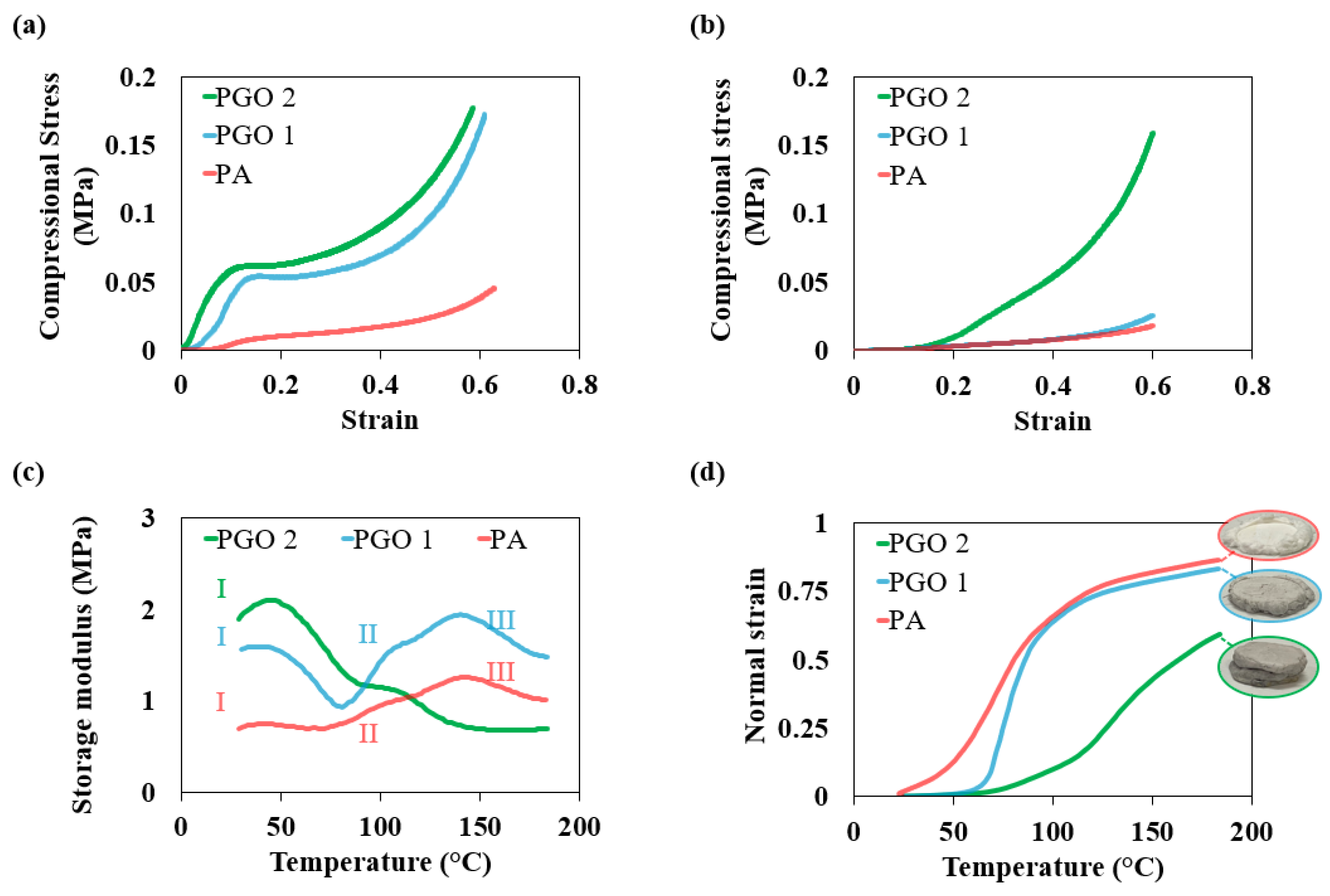
3.2. Thermal Properties and Environmental Stability of the Nanocomposite Aerogel
3.3. Mechanical Properties of the Nanocomposite Aerogels
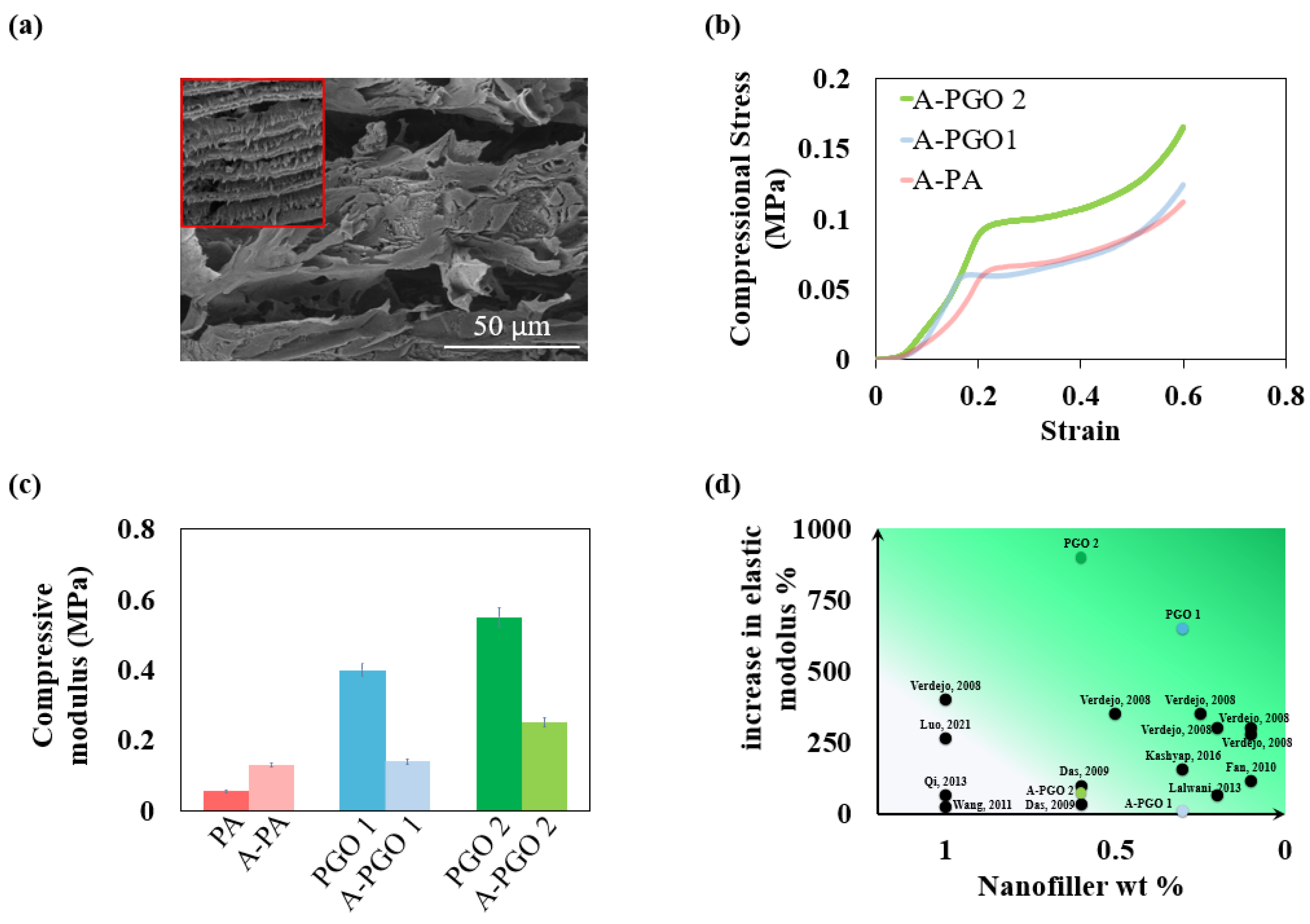
3.4. Effects of Acetone on Morphology and Mechanical Properties of Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhang, H.B.; Xie, X.; Yang, R.; Liu, Z.; Liu, Y.; Yu, Z.Z. Multifunctional, superelastic, and lightweight MXene/polyimide aerogels. Small 2018, 14, 1802479. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Pachfule, P.; Li, S.; Ye, M.Y.; Schmidt, J.; Thomas, A. Ultralight covalent organic framework/graphene aerogels with hierarchical porosity. Nat. Commun. 2020, 11, 4712. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Shi, L.A.; Wang, Y.C.; Zhao, H.Y.; Yao, H.B.; Zhu, Y.B.; Zhang, Y.; Zhu, H.W.; Wu, H.A.; Yu, S.H. Joule-heated graphene-wrapped sponge enables fast clean-up of viscous crude-oil spill. Nat. Nanotechnol. 2017, 12, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Q.; Hao, M.; Hu, Y.; Lin, Z.; Peng, L.; Wang, T.; Ren, X.; Wang, C.; Zhao, Z.; et al. Double-negative-index ceramic aerogels for thermal superinsulation. Science 2019, 363, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J. Superexpansive gels. Nature 1995, 374, 409–410. [Google Scholar] [CrossRef]
- Cai, B.; Sayevich, V.; Gaponik, N.; Eychmüller, A. Emerging Hierarchical Aerogels: Self-Assembly of Metal and Semiconductor Nanocrystals. Adv. Mater. 2018, 30, 1707518. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Anderson, M.L.; Stroud, R.M.; Merzbacher, C.I.; Rolison, D.R. Silica sol as a nanoglue: Flexible synthesis of composite aerogels. Science 1999, 284, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X. Binary crystallized supramolecular aerogels derived from host–guest inclusion complexes. ACS Nano 2015, 9, 11389–11397. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured assembly of nanocarbons: Fullerenes, nanotubes, and graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef]
- Xue, Y.; Dai, P.; Zhou, M.; Wang, X.; Pakdel, A.; Zhang, C.; Weng, Q.; Takei, T.; Fu, X.; Popov, Z.I.; et al. Multifunctional superelastic foam-like boron nitride nanotubular cellular-network architectures. ACS Nano 2017, 11, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, M.; Gong, W.; Du, R.; Eychmüller, A.; Li, T.; Lv, W.; Zhang, X. Boron nitride aerogels with super-flexibility ranging from liquid nitrogen temperature to 1000 °C. Adv. Funct. Mater. 2019, 29, 1900188. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, H.; Niu, M.; Fan, X.; Ma, M.; Shi, Z.; Guo, S.W. Ultralight, recoverable, and high-temperature-resistant SiC nanowire aerogel. ACS Nano 2018, 12, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Rivera Virtudazo, R.V.; Tingaut, P.; Koebel, M.M. Multiscale assembly of superinsulating silica aerogels within silylated nanocellulosic scaffolds: Improved mechanical properties promoted by nanoscale chemical compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Wang, T.; Long, M.C.; Zhao, H.B.; Liu, B.W.; Shi, H.G.; An, W.L.; Li, S.L.; Xu, S.M.; Wang, Y.Z. An ultralow-temperature superelastic polymer aerogel with high strength as a great thermal insulator under extreme conditions. J. Mater. Chem. A 2020, 8, 18698–18706. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Xu, X.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.; Wang, H.; Song, P. Bioinspired, highly adhesive, nanostructured polymeric coatings for superhydrophobic fire-extinguishing thermal insulation foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G.; Zhou, M.; Zhang, T.; Ji, G. Polyimide-based foams: Fabrication and multifunctional applications. ACS Appl. Mater. Interfaces 2020, 12, 48246–48258. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jiao, F.; Khan, Z.U.; Edberg, J.; Fabiano, S.; Crispin, X. Thermoelectric polymer aerogels for pressure–temperature sensing applications. Adv. Funct. Mater. 2017, 27, 1703549. [Google Scholar] [CrossRef]
- Yang, J.; Yang, W.; Chen, W.; Tao, X. An elegant coupling: Freeze-casting and versatile polymer composites. Prog. Polym. Sci. 2020, 109, 101289. [Google Scholar] [CrossRef]
- Sun, H.; Schiraldi, D.A.; Chen, D.; Wang, D.; Sánchez-Soto, M. Tough polymer aerogels incorporating a conformal inorganic coating for low flammability and durable hydrophobicity. ACS Appl. Mater. Interfaces 2016, 8, 13051–13057. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K.Y.; Venkatesan, H.; Kim, E.; Adegun, M.H.; Lee, J.H.; Shen, X.; Kim, J.K. Superinsulating BNNS/PVA composite aerogels with high solar reflectance for energy-efficient buildings. Nano-Micro Lett. 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Shen, X.; Yang, J.; Lin, K.T.; Venkatesan, H.; Kim, E.; Zhang, H.; Lee, J.H.; Yu, J.; Yang, J.; et al. Scalable anisotropic cooling aerogels by additive freeze-casting. Nat. Commun. 2022, 13, 5553. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef] [PubMed]
- Raidongia, K.; Tan, A.T.; Huang, J. Graphene oxide: Some new insights into an old material. In Carbon Nanotubes and Graphene; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–374. [Google Scholar]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Wang, B.; Huang, M.; Wang, B.; Jiang, Y.; Ruoff, R.S. Freeze-casting produces a graphene oxide aerogel with a radial and centrosymmetric structure. ACS Nano 2018, 12, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Ahankari, S.; Paliwal, P.; Subhedar, A.; Kargarzadeh, H. Recent developments in nanocellulose-based aerogels in thermal applications: A review. ACS Nano 2021, 15, 3849–3874. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, X.; Yang, W.; Kim, J.K. Templating strategies for 3D-structured thermally conductive composites: Recent advances and thermal energy applications. Prog. Mater. Sci. 2022, 133, 101054. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, T.; He, S.; Xiao, H.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl. Surf. Sci. 2019, 497, 143775. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Li, D.S.; Wang, S.J.; Zhou, Y.; Jiang, L. Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding. Nanotechnol. Rev. 2022, 11, 1722–1732. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, X.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.; Mendez, S.; Yang, R.; Qu, L.; Yu, G. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.; Wang, J.; Zhu, Y.; Jiang, L. Bioinspired hierarchical alumina–graphene oxide–poly (vinyl alcohol) artificial nacre with optimized strength and toughness. ACS Appl. Mater. Interfaces 2015, 7, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yao, X.; Deng, S.; Zhou, T.; Fu, Q. Water-induced shape memory effect of graphene oxide reinforced polyvinyl alcohol nanocomposites. J. Mater. Chem. A 2014, 2, 2240–2249. [Google Scholar] [CrossRef]
- Islam, M.R.; Mollik, S.I. Enhanced electrochemical performance of flexible and eco-friendly starch/graphene oxide nanocomposite. Heliyon 2020, 6, e05292. [Google Scholar] [CrossRef]
- Hurayra–Lizu, K.A.; Bari, M.W.; Gulshan, F.; Islam, M.R. GO based PVA nanocomposites: Tailoring of optical and structural properties of PVA with low percentage of GO nanofillers. Heliyon 2021, 7, e06983. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qin, Z.; Cui, Y.; Yang, C.; Deng, C.; Wang, Y.; Kang, J.S.; Xia, H.; Hu, Y. Ultralight and flexible monolithic polymer aerogel with extraordinary thermal insulation by a facile ambient process. Adv. Mater. Interfaces 2019, 6, 1900314. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Hassani, F.O.; Laachachi, A.; El Bouari, A. Effect of surface modification on morphological, mechanical and thermal conductivity of hemp fiber: Characterization of the interface of hemp–Polyurethane composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Elfordy, S.; Lucas, F.; Tancret, F.; Scudeller, Y.; Goudet, L. Mechanical and thermal properties of lime and hemp concrete (“hempcrete”) manufactured by a projection process. Constr. Build. Mater. 2008, 22, 2116–2123. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions–Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Sanchez-Calderon, I.; Merillas, B.; Bernardo, V.; Rodríguez-Pérez, M.Á. Methodology for measuring the thermal conductivity of insulating samples with small dimensions by heat flow meter technique. J. Therm. Anal. Calorim. 2022, 147, 12523–12533. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, J.; Cheng, X.; Gong, L.; Li, Y.; Zhang, H. Porous thermal insulation materials derived from fly ash using a foaming and slip casting method. Energy Build. 2014, 81, 262–267. [Google Scholar] [CrossRef]
- Hanif, A.; Diao, S.; Lu, Z.; Fan, T.; Li, Z. Green lightweight cementitious composite incorporating aerogels and fly ash cenospheres–Mechanical and thermal insulating properties. Constr. Build. Mater. 2016, 116, 422–430. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Zhang, P.; Song, L.; Wang, X.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Zeolitic imidazolate framework-8/polyvinyl alcohol hybrid aerogels with excellent flame retardancy. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105720. [Google Scholar] [CrossRef]
- Glicksman, L.R. Heat transfer in foams. In Low Density Cellular Plastics: Physical Basis of Behaviour; Springer: Dordrecht, The Netherlands, 1994; pp. 104–152. [Google Scholar]
- Zhan, H.J.; Wu, K.J.; Hu, Y.L.; Liu, J.W.; Li, H.; Guo, X.; Xu, J.; Yang, Y.; Yu, Z.L.; Gao, H.L.; et al. Biomimetic carbon tube aerogel enables super-elasticity and thermal insulation. Chem 2019, 5, 1871–1882. [Google Scholar] [CrossRef]
- Apostolopoulou-Kalkavoura, V.; Munier, P.; Bergström, L. Thermally insulating nanocellulose-based materials. Adv. Mater. 2021, 33, 2001839. [Google Scholar] [CrossRef]
- Adegun, M.H.; Chan, K.Y.; Yang, J.; Venkatesan, H.; Kim, E.; Zhang, H.; Shen, X.; Yang, J.; Kim, J.K. Anisotropic thermally superinsulating boron nitride composite aerogel for building thermal management. Compos. Part A Appl. Sci. Manuf. 2023, 169, 107522. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yu, M.Y.; Liu, J.; Li, X.; Min, P.; Yu, Z.Z. Efficient preconstruction of three-dimensional graphene networks for thermally conductive polymer composites. Nano-Micro Lett. 2022, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Pernot, G.; Stoffel, M.; Savic, I.; Pezzoli, F.; Chen, P.; Savelli, G.; Jacquot, A.; Schumann, J.; Denker, U.; Mönch, I.; et al. Precise control of thermal conductivity at the nanoscale through individual phonon-scattering barriers. Nat. Mater. 2010, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Graczykowski, B.; El Sachat, A.; Reparaz, J.S.; Sledzinska, M.; Wagner, M.R.; Chávez-Angel, E.; Wu, Y.; Volz, S.; Wu, Y.; Alzina, F.; et al. Thermal conductivity and air-mediated losses in periodic porous silicon membranes at high temperatures. Nat. Commun. 2017, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, N.M.; Kim, J.; Lee, J.; Kim, J.K.; Jeon, S. Highly conductive and fracture-resistant epoxy composite based on non-oxidized graphene flake aerogel. ACS Appl. Mater. Interfaces 2018, 10, 37507–37516. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.R.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Cherednichenko, K.; Kopitsyn, D.; Smirnov, E.; Nikolaev, N.; Fakhrullin, R. Fireproof Nanocomposite Polyurethane Foams: A Review. Polymers 2023, 15, 2314. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Mannix, A.J.; Kiraly, B.; Hersam, M.C.; Guisinger, N.P. Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 2017, 1, 0014. [Google Scholar] [CrossRef]
- Abdolazizi, A.; Chen, S.; Golberg, D.; Yan, C. Anisotropy of two-dimensional materials under tension: A molecular dynamic study across graphene, hexagonal boron nitride and molybdenum disulphide. FlatChem 2022, 36, 100442. [Google Scholar] [CrossRef]
- Unalan, I.U.; Wan, C.; Figiel, Ł.; Olsson, R.T.; Trabattoni, S.; Farris, S. Exceptional oxygen barrier performance of pullulan nanocomposites with ultra-low loading of graphene oxide. Nanotechnology 2015, 26, 275703. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Trigueiro, J.P.; Woellner, C.F.; Pedrotti, J.J.; Miquita, D.R.; Silva, W.M.; Lopes, M.C.; Fechine, G.J.; Luciano, M.A.; Silva, G.G.; et al. Higher thermal conductivity and mechanical enhancements in hybrid 2D polymer nanocomposites. Polym. Test. 2020, 87, 106510. [Google Scholar] [CrossRef]
- Miaudet, P.; Derre, A.; Maugey, M.; Zakri, C.; Piccione, P.M.; Inoubli, R.; Poulin, P. Shape and temperature memory of nanocomposites with broadened glass transition. Science 2007, 318, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Jin, P.; Zhang, T.; Zhao, Y.; Ai, Y.; Xiu, H.; Zhang, Q.; Fu, Q. Poly(vinyl alcohol)/MXene biomimetic aerogels with tunable mechanical properties and electromagnetic interference shielding performance controlled by pore structure. Polymer 2021, 230, 124101. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Tai, Z.X.; Sun, D.F.; Chen, J.T.; Ma, H.B.; Yan, X.B.; Liu, B.; Xue, Q.J. Fabrication and characterization of poly (vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J. Appl. Polym. Sci. 2013, 127, 1885–1894. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Fang, J.; Xu, H.; Yin, J. Graphene oxide/polybenzimidazole composites fabricated by a solvent-exchange method. Carbon 2011, 49, 1199–1207. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef]
- Das, B.; Prasad, K.E.; Ramamurty, U.; Rao, C.N. Nano-indentation studies on polymer matrix composites reinforced by few-layer graphene. Nanotechnology 2009, 20, 125705. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.X.; Mikos, A.G.; Sitharaman, B. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Luo, X.; Wu, Y.; Guo, M.; Yang, X.; Xie, L.; Lai, J.; Li, Z.; Zhou, H. Multi-functional polyurethane composites with self-healing and shape memory properties enhanced by graphene oxide. J. Appl. Polym. Sci. 2021, 138, 50827. [Google Scholar] [CrossRef]
- Verdejo, R.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; De Saja, J.A.; Lopez-Manchado, M.A. Functionalized graphene sheet filled silicone foam nanocomposites. J. Mater. Chem. 2008, 18, 2221–2226. [Google Scholar] [CrossRef]
- Verdejo, R.; Saiz-Arroyo, C.; Carretero-Gonzalez, J.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Lopez-Manchado, M.A. Physical properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur. Polym. J. 2008, 44, 2790–2797. [Google Scholar] [CrossRef]
| Sample Name | Poly (Vinyl Alcohol) (PVA) (wt%) | Graphene Oxide (GO) (wt%) | Acetone (vol%) |
|---|---|---|---|
| PA | 100 | 0 | 0 |
| PGO 1 | 99.7 | 0.3 | 0 |
| PGO 2 | 99.4 | 0.6 | 0 |
| PGO 3 | 99.1 | 0.9 | 0 |
| PGO 4 | 98.8 | 1.2 | 0 |
| A-PA | 100 | 0 | 4 |
| A-PGO 1 | 99.7 | 0.3 | 4 |
| A-PGO 2 | 99.4 | 0.6 | 4 |
| Insulator | Thermal Conductivity (W/m·K) | Reference | |
|---|---|---|---|
| PGO 2 | 0.035 | Current study | |
| Commercial insulators | Hemp | 0.042 | [41] |
| Hempcrete | 0.179 | [42] | |
| Glass mineral wool | 0.03–0.04 | [22] | |
| Fiberglass | 0.033–0.044 | [22] | |
| Cork | 0.04–0.05 | [22] | |
| Cellulose insulation | 0.04–0.05 | [43] | |
| Foams | XPS | 0.037 | [44] |
| EPS | 0.036 | [44] | |
| PUR | 0.031 | [44] | |
| PE | 0.039 | [44] | |
| Coal fly ash composite foam | 0.051 | [45] | |
| PVA aerogels | PVA fibre | 0.320 | [46] |
| PVA/CNF (1:1.5) | 0.038 | [30] | |
| PVA/HAP aerogel (3:1) | 0.039 | [47] | |
| PVA/ZIF-8 aerogel (1:1) | 0.036 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdolazizi, A.; Wijesinghe, I.; Marriam, I.; Chathuranga, H.; Golberg, D.; Yan, C. Development of Light, Strong, and Water-Resistant PVA Composite Aerogels. Nanomaterials 2024, 14, 745. https://doi.org/10.3390/nano14090745
Abdolazizi A, Wijesinghe I, Marriam I, Chathuranga H, Golberg D, Yan C. Development of Light, Strong, and Water-Resistant PVA Composite Aerogels. Nanomaterials. 2024; 14(9):745. https://doi.org/10.3390/nano14090745
Chicago/Turabian StyleAbdolazizi, Amir, Ishara Wijesinghe, Ifra Marriam, Hiran Chathuranga, Dmitri Golberg, and Cheng Yan. 2024. "Development of Light, Strong, and Water-Resistant PVA Composite Aerogels" Nanomaterials 14, no. 9: 745. https://doi.org/10.3390/nano14090745
APA StyleAbdolazizi, A., Wijesinghe, I., Marriam, I., Chathuranga, H., Golberg, D., & Yan, C. (2024). Development of Light, Strong, and Water-Resistant PVA Composite Aerogels. Nanomaterials, 14(9), 745. https://doi.org/10.3390/nano14090745






