Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Physical Chemical Characterizations
2.3. Electrochemical Measurements
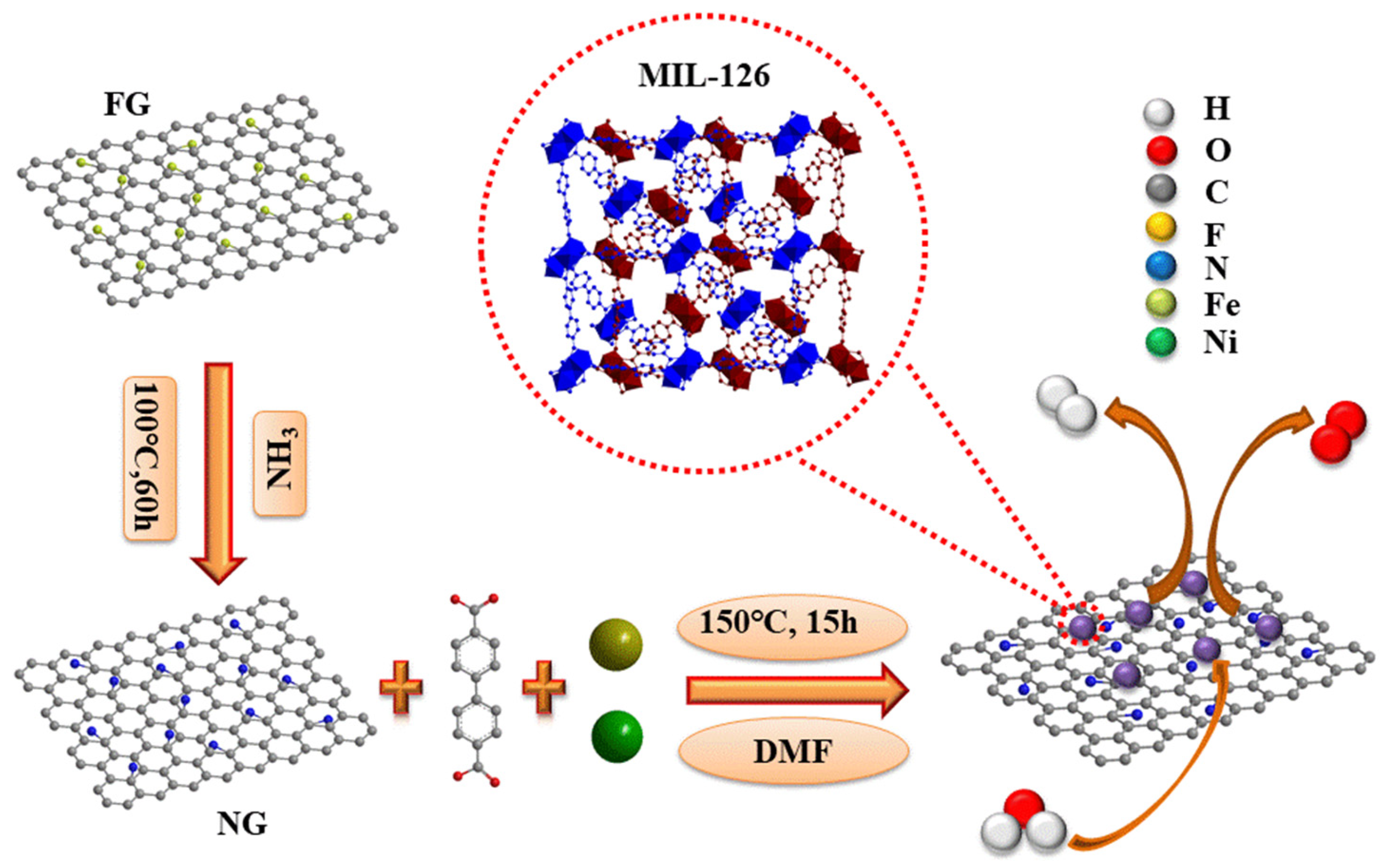
2.4. Synthesis of NG
2.5. Synthesis of Bimetallic (Fe,Ni)-MIL-126
2.6. Synthesis of MIL-NG-n Hybrid
2.7. Synthesis of Bimetallic (Fe,Ni)-NG
2.8. Synthesis of (Fe,Ni)-MIL-126-NG-mix
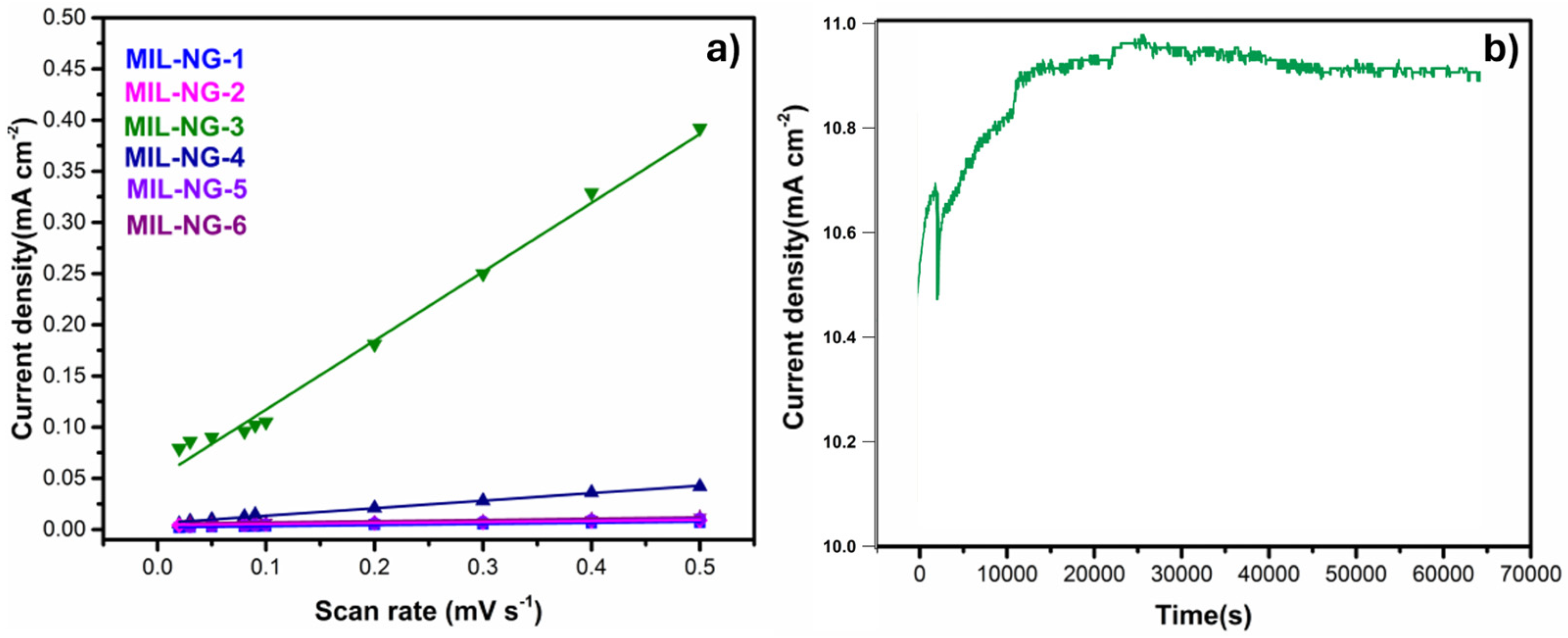
3. Results and Discussion
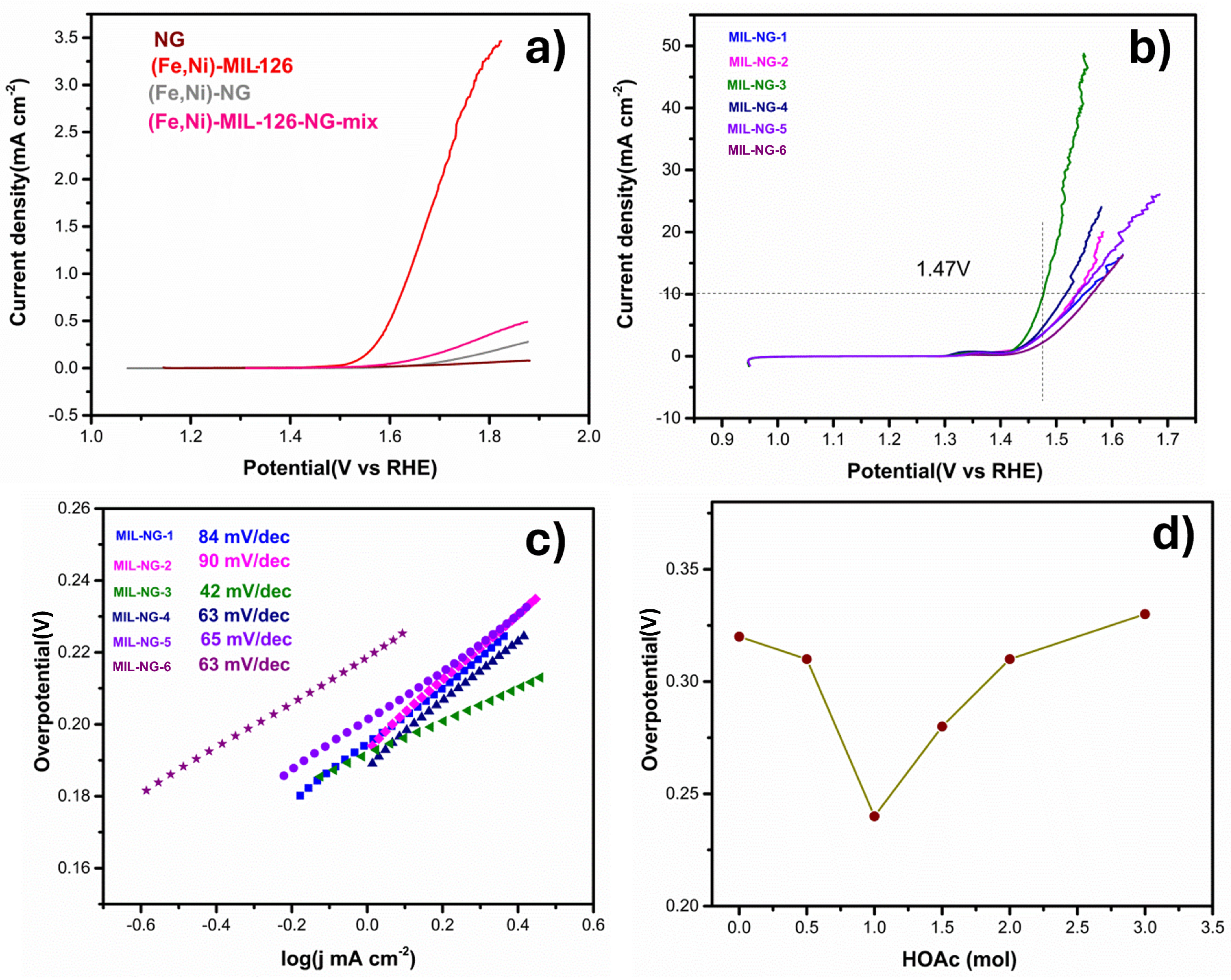
| Sample | Tafel Slope/mV dec−1 | Overpotential at 10 mA cm−2/mV | Cdl/mF cm−2 |
|---|---|---|---|
| MIL-NG-1 | 84 | 320 | 0.01 |
| MIL-NG-2 | 90 | 310 | 0.009 |
| MIL-NG-3 | 42 | 240 | 0.67 |
| MIL-NG-4 | 63 | 280 | 0.07 |
| MIL-NG-5 | 65 | 310 | 0.012 |
| MIL-NG-6 | 63 | 330 | 0.015 |
| Components | EOER (V) at 10 mA cm−2 | Tafel mV·dec−1 | Refs. |
|---|---|---|---|
| MIL-NG-3 | 1.47 | 42 | / |
| MIL-126(FeNi)-700 | 2.0 | [61] | |
| Fe1Ni2-BDC | 1.49 | 35 | [26] |
| Fe/Ni/Co(Mn)-MIL-53/NF | 1.45 | 53.5 | [35] |
| Fe2+-NiFe LDH | 1.479 | 40.4 | [62] |
| NiFeV LDHs | 1.422 | 42 | [63] |
| NiFe LDHs Nanosheets | 1.459 | 62.9 | [64] |
| flame-engraved NiFe-LDH | 1.48 | 69 | [65] |
| Fe−Ni−P/rGO-400 | 1.47 | 63 | [66] |
| Ni2P@C/GO (NiBTC) | 1.515 | 44 | [67] |
| NGO/Ni7S6 (Ni-MOF-74) | 1.61 | 45 | [68] |
| Ni-NiO/N-rGO | 1.47 | 43 | [28] |
| Ni-MOF-600 | 1.6 | 66 | [69] |
| CoP/rGO-400 (ZIF 67) | 1.57 | 66 | [70] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Lunardon, M.; Kosmala, T.; Durante, C.; Agnoli, S.; Granozzi, G. Atom-by-Atom Identification of Catalytic Active Sites in Operando Conditions by Quantitative Noise Detection. Joule 2022, 6, 617–635. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Kosmala, T.; Baby, A.; Lunardon, M.; Perilli, D.; Liu, H.; Durante, C.; Di Valentin, C.; Agnoli, S.; Granozzi, G. Operando Visualization of the Hydrogen Evolution Reaction with Atomic-Scale Precision at Different Metal–Graphene Interfaces. Nat. Catal. 2021, 4, 850–859. [Google Scholar] [CrossRef]
- Lunardon, M.; Cattelan, M.; Agnoli, S.; Granozzi, G. Toward Sustainable and Effective HER Electrocatalysts: Strategies for the Basal Plane Site Activation of Transition Metal Dichalcogenides. Curr. Opin. Electrochem. 2022, 34, 101025. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeon, K.-J.; Show, P.L.; Lee, I.H.; Jung, S.-C.; Choi, Y.J.; Rhee, G.H.; Lin, K.-Y.A.; Park, Y.-K. Mini Review on H2 Production from Electrochemical Water Splitting According to Special Nanostructured Morphology of Electrocatalysts. Fuel 2022, 308, 122048. [Google Scholar] [CrossRef]
- Lv, X.-W.; Tian, W.-W.; Yuan, Z.-Y. Recent Advances in High-Efficiency Electrocatalytic Water Splitting Systems. Electrochem. Energy Rev. 2023, 6, 23. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2016, 117, 3717–3797. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured Nonprecious Metal Catalysts for Oxygen Reduction Reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Lunardon, M.; Kosmala, T.; Ghorbani-Asl, M.; Krasheninnikov, A.V.; Kolekar, S.; Durante, C.; Batzill, M.; Agnoli, S.; Granozzi, G. Catalytic Activity of Defect-Engineered Transition Metal Dichalcogenides Mapped with Atomic-Scale Precision by Electrochemical Scanning Tunneling Microscopy. ACS Energy Lett. 2023, 8, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Hess, F. Corrosion Mechanism and Stabilization Strategies for RuO2 and IrO2 Catalysts in the Electrochemical Oxygen Evolution Reaction. Curr. Opin. Electrochem. 2023, 41, 101349. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Ge, J.; Liu, C.; Xing, W. Discontinuously Covered IrO2–RuO2@Ru Electrocatalysts for the Oxygen Evolution Reaction: How High Activity and Long-Term Durability can Be Simultaneously Realized in the Synergistic and Hybrid Nano-Structure. J. Mater. Chem. A 2017, 5, 17221–17229. [Google Scholar] [CrossRef]
- Li, X.-P.; Huang, C.; Han, W.-K.; Ouyang, T.; Liu, Z.-Q. Transition Metal-Based Electrocatalysts for Overall Water Splitting. Chin. Chem. Lett. 2021, 32, 2597–2616. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, e2201099. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, 4, e202301534. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Ho, J.C.; Yang, J.; Wang, X. Recent Advances in Layered Double Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2019, 7, 5069–5089. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Zhu, S.; Feng, L.; Xing, W. Ni-Based Layered Double Hydroxide Catalysts for Oxygen Evolution Reaction. Mater. Today Phys. 2021, 16, 100292. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Luo, S.; Zhao, Z.; Wang, X.; Luo, Y.; Wang, Z.; Jin, J.; Ma, J. Lateral-Size-Mediated Efficient Oxygen Evolution Reaction: Insights into the Atomically Thin Quantum Dot Structure of NiFe2O4. ACS Catal. 2017, 7, 5557–5567. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Li, W. Electrocatalytic Oxygen Evolution over Supported Small Amorphous Ni–Fe Nanoparticles in Alkaline Electrolyte. Langmuir 2014, 30, 7893–7901. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, X.; Guo, K.; Dong, L.; Miao, X. Alkaline Media Regulated NiFe-LDH-Based Nickel–Iron Phosphides toward Robust Overall Water Splitting. Catalysts 2023, 13, 198. [Google Scholar] [CrossRef]
- Salvò, D.; Mosconi, D.; Neyman, A.; Bar-Sadan, M.; Calvillo, L.; Granozzi, G.; Cattelan, M.; Agnoli, S. Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials 2023, 13, 683. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional Hydrogen Storage Achieved by Screening Nearly Half a Million Metal-Organic Frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. How to Effectively Utilize MOFs for Electrocatalysis. ACS Energy Lett. 2019, 4, 1443–1445. [Google Scholar] [CrossRef]
- Tang, P.; Paganelli, S.; Carraro, F.; Blanco, M.; Riccò, R.; Marega, C.; Badocco, D.; Pastore, P.; Doonan, C.J.; Agnoli, S. Postsynthetic Metalated MOFs as Atomically Dispersed Catalysts for Hydroformylation Reactions. ACS Appl. Mater. Interfaces 2020, 12, 54798–54805. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-Metal Oxides/Graphene Nanocomposites as Multifunctional Electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating Metal–Organic Frameworks for Catalyzing Acidic Oxygen Evolution for Proton Exchange Membrane Water Electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Chevreau, H.; Devic, T.; Horcajada, P.; Maurin, G.; Férey, G.; Popov, D.; Riekel, C.; Wuttke, S.; Lavalley, J.-C.; et al. How Interpenetration Ensures Rigidity and Permanent Porosity in a Highly Flexible Hybrid Solid. Chem. Mater. 2012, 24, 2486–2492. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials Institute Lavoisier (MIL) Based Materials for Photocatalytic Applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Taffa, D.H.; Balkenhohl, D.; Amiri, M.; Wark, M. Minireview: Ni–Fe and Ni–Co Metal–Organic Frameworks for Electrocatalytic Water-Splitting Reactions. Small Struct. 2022, 4, 263. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal–Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 584. [Google Scholar] [CrossRef]
- Li, F.; Shao, Q.; Huang, X.; Lang, J. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2017, 57, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhang, W.; Jin, Z.; An, W.; Gao, Y.; Zhang, X.; Liu, J. Electrocatalytically Active MOF/Graphite Oxide Hybrid for Electrosynthesis of Dimethyl Carbonate. Electrochim. Acta 2014, 144, 1–6. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H. Synthesis of Iron(iii)-Based Metal–Organic Framework/Graphene Oxide Composites with Increased Photocatalytic Performance for Dye Degradation. RSC Adv. 2014, 4, 40435–40438. [Google Scholar] [CrossRef]
- Lalitha, A.; Shin, J.E.; Bonakala, S.; Oh, J.Y.; Park, H.B.; Maurin, G. Unraveling the Enhancement of the Interfacial Compatibility between Metal–Organic Framework and Functionalized Graphene Oxide. J. Phys. Chem. C 2019, 123, 4984–4993. [Google Scholar] [CrossRef]
- Favaro, M.; Agnoli, S.; Cattelan, M.; Moretto, A.; Durante, C.; Leonardi, S.; Kunze-Liebhäuser, J.; Schneider, O.; Gennaro, A.; Granozzi, G. Shaping Graphene Oxide by Electrochemistry: From Foams to Self-Assembled Molecular Materials. Carbon 2014, 77, 405–415. [Google Scholar] [CrossRef]
- Mosconi, D.; Kosmala, T.; Lunardon, M.; Neyman, A.; Bar-Sadan, M.; Agnoli, S.; Granozzi, G. One-Pot Synthesis of MoS2(1−x)Se2x on N-Doped Reduced Graphene Oxide: Tailoring Chemical and Structural Properties for Photoenhanced Hydrogen Evolution Reaction. Nanoscale Adv. 2020, 2, 4830–4840. [Google Scholar] [CrossRef]
- Favaro, M.; Carraro, F.; Cattelan, M.; Colazzo, L.; Durante, C.; Sambi, M.; Gennaro, A.; Agnoli, S.; Granozzi, G. Multiple Doping of Graphene Oxide Foams and Quantum Dots: New Switchable Systems for Oxygen Reduction and Water Remediation. J. Mater. Chem. A 2015, 3, 14334–14347. [Google Scholar] [CrossRef]
- Carraro, F.; Cattelan, M.; Favaro, M.; Calvillo, L. Aerosol Synthesis of N and N-S Doped and Crumpled Graphene Nanostructures. Nanomaterials 2018, 8, 406. [Google Scholar] [CrossRef]
- Lunardon, M.; Ran, J.; Mosconi, D.; Marega, C.; Wang, Z.; Xia, H.; Agnoli, S.; Granozzi, G. Hybrid Transition Metal Dichalcogenide/Graphene Microspheres for Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 2376. [Google Scholar] [CrossRef]
- Lyu, S.; Guo, C.; Wang, J.; Li, Z.; Yang, B.; Lei, L.; Wang, L.; Xiao, J.; Zhang, T.; Hou, Y. Exceptional Catalytic Activity of Oxygen Evolution Reaction via Two-Dimensional Graphene Multilayer Confined Metal-Organic Frameworks. Nat. Commun. 2022, 13, 6171. [Google Scholar] [CrossRef] [PubMed]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef] [PubMed]
- Pasarakonda, S.L.; Ponnada, S.; Gorle, D.B.; Chandra Bose, R.S.; Palariya, A.; Kiai, M.S.; Gandham, H.B.; Kathiresan, M.; Sharma, R.K.; Nowduri, A. On the Role of Graphene Oxide in Bifunctional Ni/MOF/RGO Composites in Electrochemical Nitrate Detection and Oxygen Evolution Reaction. New J. Chem. 2023, 47, 725–736. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Yu, Q.; Luo, Y.; Zhang, Z.; Zhang, C.; Qiu, L.; Liu, B. Controllable Structure Reconstruction of Nickel–Iron Compounds toward Highly Efficient Oxygen Evolution. Nanoscale 2020, 12, 10751–10759. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A Review Unveiling the Critical Roles of Fe in Enhancing OER Activity of Ni and Co Based Catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous Nanorods Fabricated by Coordination Modulation and Oriented Attachment Growth. Angew. Chem. Int. Ed. 2009, 48, 4739–4743. [Google Scholar] [CrossRef] [PubMed]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Bagherzadeh, E.; Zebarjad, S.M.; Hosseini, H.R.M. Morphology Modification of the Iron Fumarate MIL-88A Metal–Organic Framework Using Formic Acid and Acetic Acid as Modulators. Eur. J. Inorg. Chem. 2018, 2018, 1909–1915. [Google Scholar] [CrossRef]
- Bara, D.; Wilson, C.; Mörtel, M.; Khusniyarov, M.M.; Ling, S.; Slater, B.; Sproules, S.; Forgan, R.S. Kinetic Control of Interpenetration in Fe–Biphenyl-4,4′-Dicarboxylate Metal–Organic Frameworks by Coordination and Oxidation Modulation. J. Am. Chem. Soc. 2019, 141, 8346–8357. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent Progress in Metal–Organic Framework/Graphene-Derived Materials for Energy Storage and Conversion: Design, Preparation, and Application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel Hydroxides and Related Materials: A Review of Their Structures, Synthesis and Properties. Proc. Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Dai, H. A Mini Review of NiFe-Based Materials as Highly Active Oxygen Evolution Reaction Electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Tang, Z. Insight into Structural Evolution, Active Sites, and Stability of Heterogeneous Electrocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202110186. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Cai, B.; Huang, Z.; Garcia-Esparza, A.T.; Sokaras, D.; Zhang, Y.; Giordano, L.; Akkiraju, K.; Zhu, Y.G.; et al. Tunable Metal Hydroxide–Organic Frameworks for Catalysing Oxygen Evolution. Nat. Mater. 2022, 21, 673–680. [Google Scholar] [CrossRef]
- Lionet, Z.; Nishijima, S.; Kim, T.-H.; Horiuchi, Y.; Lee, S.W.; Matsuoka, M.; Persidis, A. Bimetallic MOF-Templated Synthesis of Alloy Nanoparticle-Embedded Porous Carbons for Oxygen Evolution and Reduction Reactions. Nature 1999, 17, 229. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, D.; Wang, M.; Bak, S.; Wu, Y.; Wu, Z.; Tian, Y.; Xiong, X.; Li, Y.; Liu, W.; et al. Introducing Fe2+ into Nickel–Iron Layered Double Hydroxide: Local Structure Modulated Water Oxidation Activity. Angew. Chem. 2018, 130, 9536–9540. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 3341. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning Surface Electronic Configuration of NiFe LDHs Nanosheets by Introducing Cation Vacancies (Fe or Ni) as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, X.; Cai, Z.; Han, N.; Jia, Y.; Xie, Q.; Duan, X.; Xie, T.; Zheng, X.; Sun, X.; et al. Flame-Engraved Nickel–Iron Layered Double Hydroxide Nanosheets for Boosting Oxygen Evolution Reactivity. Small Methods 2018, 2, 83. [Google Scholar] [CrossRef]
- Fang, X.; Jiao, L.; Zhang, R.; Jiang, H.-L. Porphyrinic Metal–Organic Framework-Templated Fe–Ni–P/Reduced Graphene Oxide for Efficient Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 23852–23858. [Google Scholar] [CrossRef]
- Wang, M.; Lin, M.; Li, J.; Huang, L.; Zhuang, Z.; Lin, C.; Zhou, L.; Mai, L. Metal–Organic Framework Derived Carbon-Confined Ni2P Nanocrystals Supported on Graphene for an Efficient Oxygen Evolution Reaction. Chem. Commun. 2017, 53, 8372–8375. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Masa, J.; Tomanec, O.; Peeters, D.; Ranc, V.; Schneemann, A.; Zboril, R.; Schuhmann, W.; Fischer, R.A. Nanoporous Nitrogen-Doped Graphene Oxide/Nickel Sulfide Composite Sheets Derived from a Metal-Organic Framework as an Efficient Electrocatalyst for Hydrogen and Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 451. [Google Scholar] [CrossRef]
- Ai, L.; Tian, T.; Jiang, J. Ultrathin Graphene Layers Encapsulating Nickel Nanoparticles Derived Metal–Organic Frameworks for Highly Efficient Electrocatalytic Hydrogen and Oxygen Evolution Reactions. ACS Sustain. Chem. Eng. 2017, 5, 4771–4777. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Chemical Science Metal-Organic Framework-Based CoP/Reduced Graphene Oxide: High-Performance Bifunctional Electrocatalyst for Overall Water Splitting Metal-Organic Framework-Based CoP/Reduced Graphene Oxide: High-Performance Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Sci. 2016, 7, 1615–2442. [Google Scholar] [CrossRef]
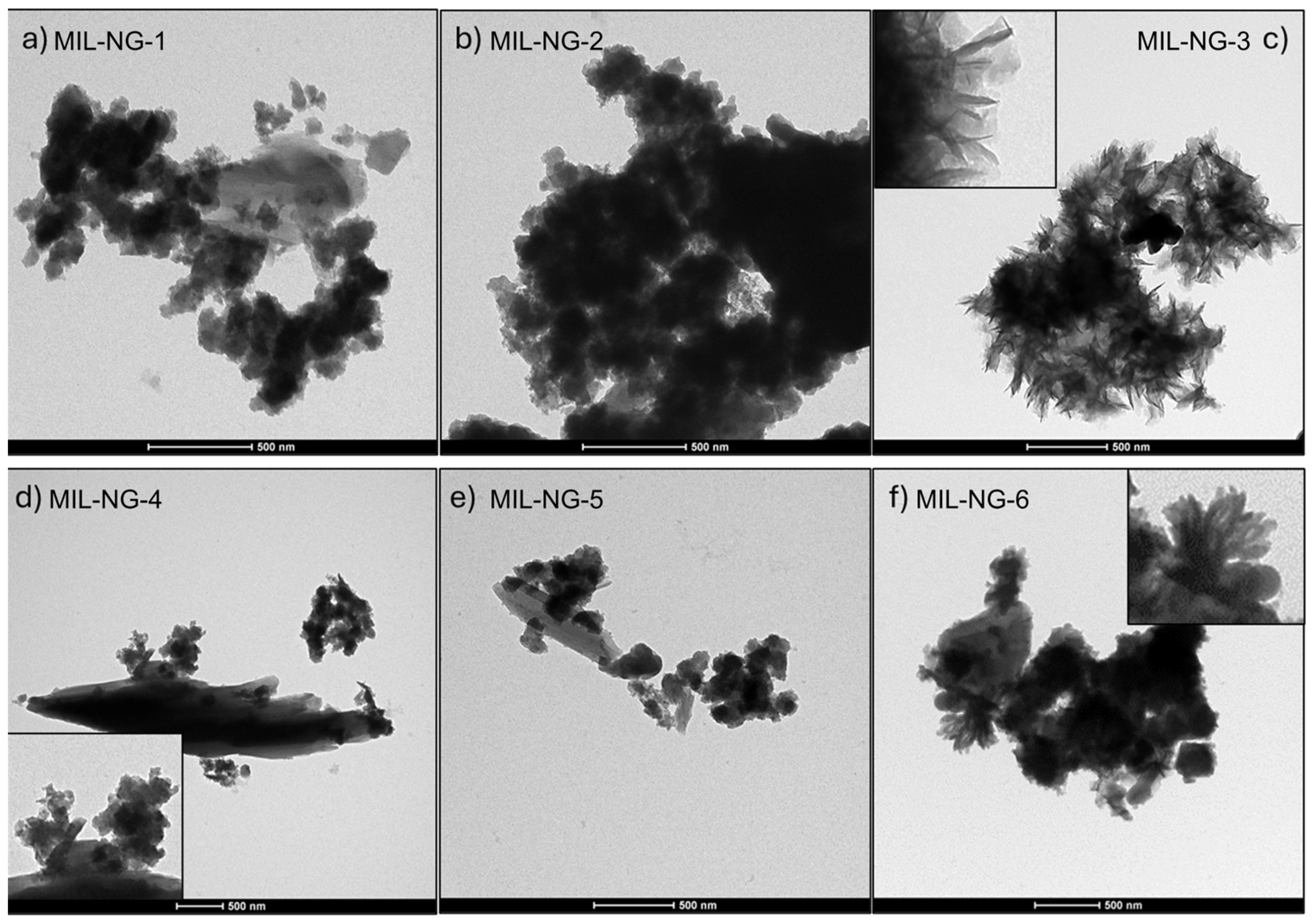

| MIL-NG-1 | MIL-NG-2 | MIL-NG-3 | MIL-NG-4 | MIL-NG-5 | MIL-NG-6 | |
|---|---|---|---|---|---|---|
| HOAc (mmol) | / | 0.5 | 1 | 1.5 | 2 | 3 |
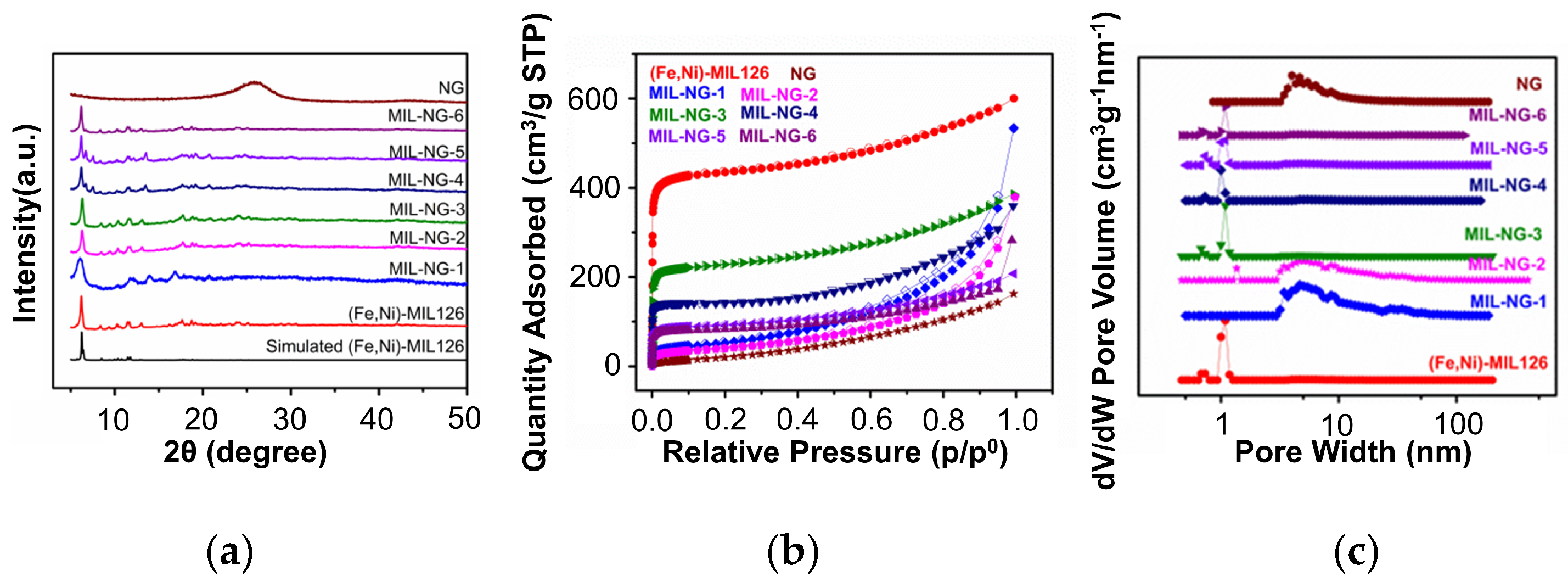
| Sample | Type of Porosity | Specific Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|---|
| NG | IV type and hysteresis loop, mesoporous | 92 | 5 |
| (Fe,Ni)-MIL-126 | I type, microporous | 1720 | 1 |
| MIL-NG-1 | IV type and hysteresis loop, mesoporous | 148 | 5 |
| MIL-NG-2 | IV type and hysteresis loop, mesoporous | 196 | 5 |
| MIL-NG-3 | I type, microporous | 884 | 1 |
| MIL-NG-4 | I type, microporous | 595 | 1 |
| MIL-NG-5 | I type, microporous | 352 | 1 |
| MIL-NG-6 | I type, microporous | 285 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Di Vizio, B.; Yang, J.; Patil, B.; Cattelan, M.; Agnoli, S. Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanomaterials 2024, 14, 751. https://doi.org/10.3390/nano14090751
Tang P, Di Vizio B, Yang J, Patil B, Cattelan M, Agnoli S. Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanomaterials. 2024; 14(9):751. https://doi.org/10.3390/nano14090751
Chicago/Turabian StyleTang, Panjuan, Biagio Di Vizio, Jijin Yang, Bhushan Patil, Mattia Cattelan, and Stefano Agnoli. 2024. "Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction" Nanomaterials 14, no. 9: 751. https://doi.org/10.3390/nano14090751
APA StyleTang, P., Di Vizio, B., Yang, J., Patil, B., Cattelan, M., & Agnoli, S. (2024). Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanomaterials, 14(9), 751. https://doi.org/10.3390/nano14090751








