A MOF-Templated Double-Shelled Co3O4/NiCo2O4 Nanocomposite for Electrochemical Detection of Alfuzosin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
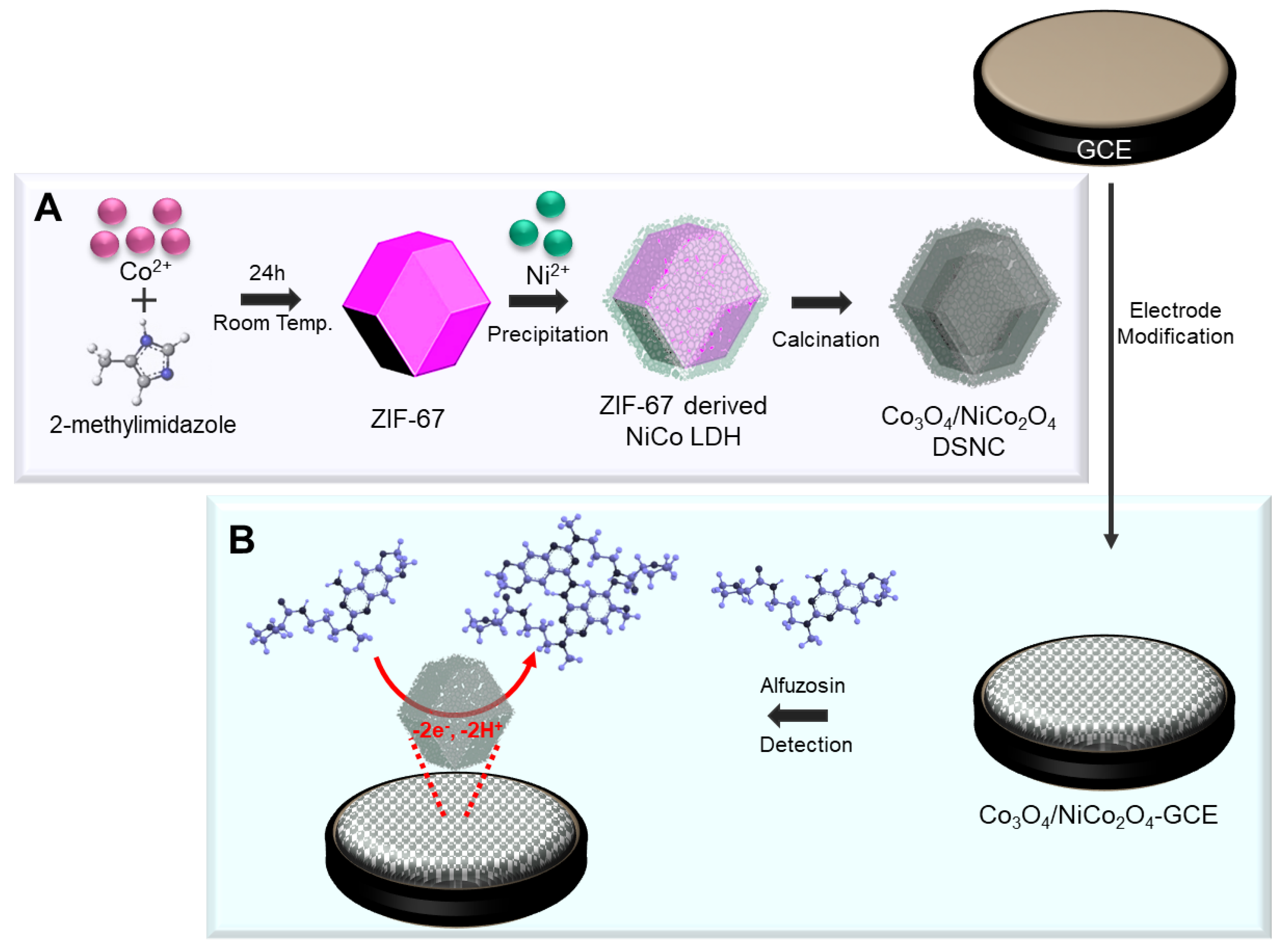
2.2. Synthesis of ZIF-67
2.3. Synthesis of Co3O4/NiCo2O4
2.4. Fabrication of the Co3O4/NiCo2O4-Modified Electrode
2.5. Instrumentation
3. Results and Discussion
3.1. Physical Characterization of the Co3O4/NiCo2O4 Nanocomposite
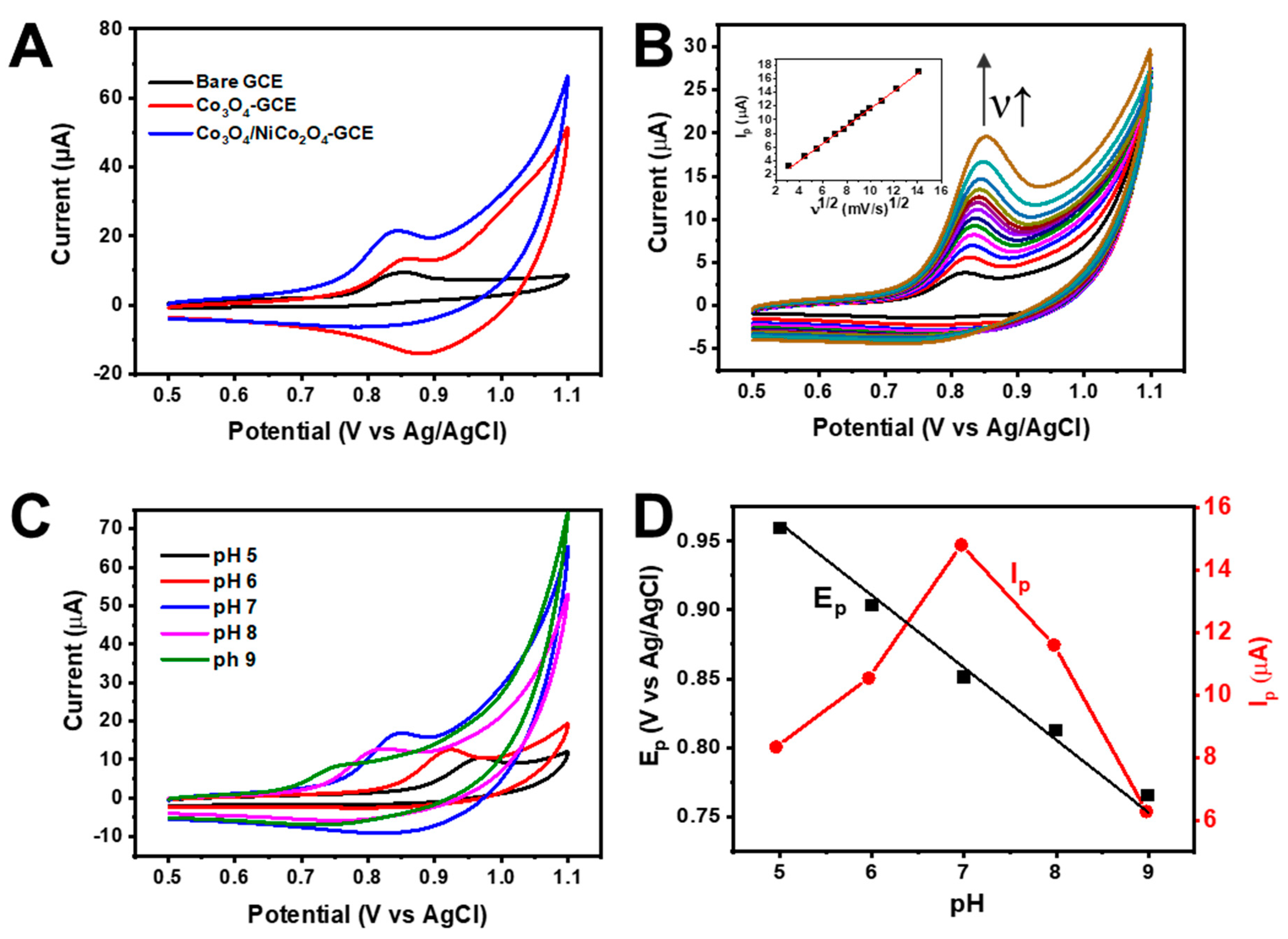
3.2. Electrochemical Characterization of Co3O4/NiCo2O4-GCE
3.3. Electrochemical Activity of Co3O4/NiCo2O4-GCE toward AFZ
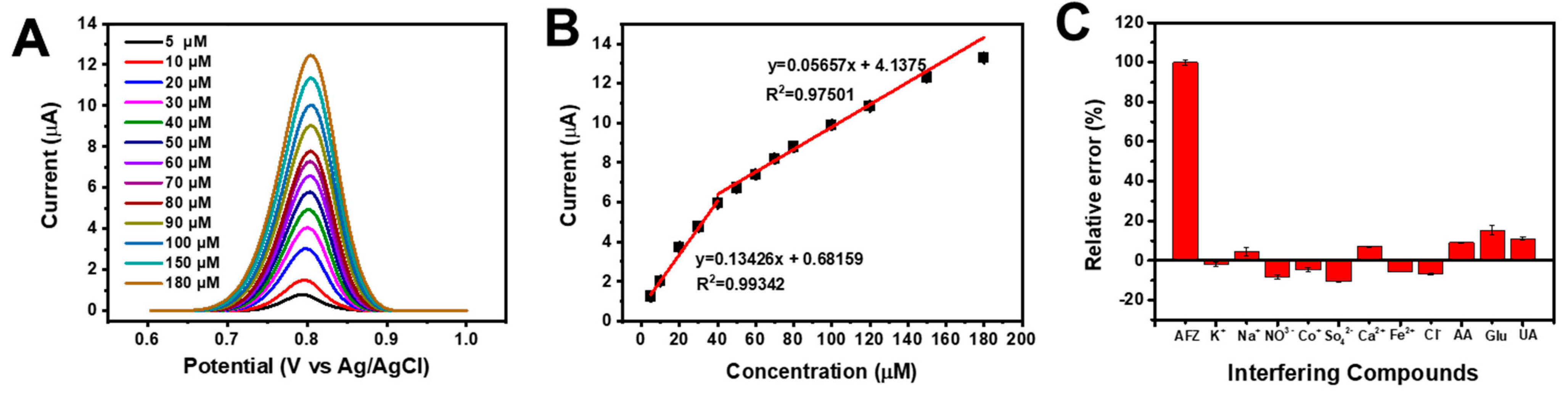
3.4. Electrochemical Sensing Performance
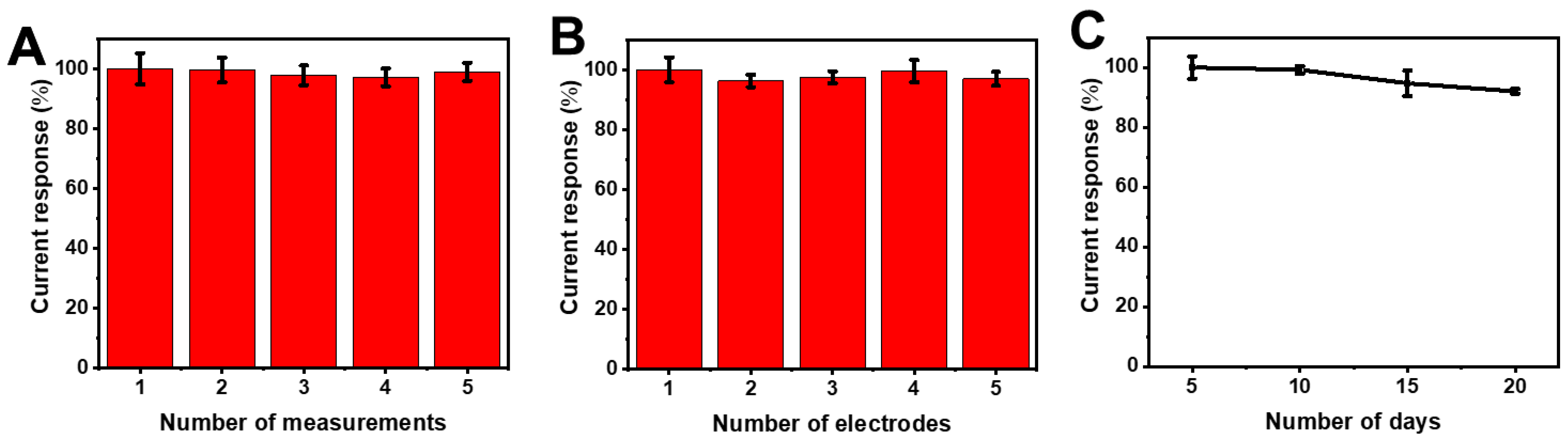
3.5. Effect of Repeatability, Reproducibility, and Stability
3.6. Detection of AFZ in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barry, M.J.; Roehrborn, C.G. Benign Prostatic Hyperplasia. BMJ 2001, 323, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign Prostatic Hyperplasia. Nat. Rev. Dis. Primer 2016, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Larson, J.A.; Andriole, G.L. Management of Benign Prostatic Hyperplasia. Annu. Rev. Med. 2016, 67, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; ALTESS Study Group. Alfuzosin 10 Mg Once Daily Prevents Overall Clinical Progression of Benign Prostatic Hyperplasia but Not Acute Urinary Retention: Results of a 2-year Placebo-controlled Study. BJU Int. 2006, 97, 734–741. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.; Wilt, T.J. Alfuzosin for Treatment of Lower Urinary Tract Symptoms Compatible with Benign Prostatic Hyperplasia: A Systematic Review of Efficacy and Adverse Effects. Urology 2005, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Mehik, A.; Alas, P.; Nickel, J.C.; Sarpola, A.; Helström, P.J. Alfuzosin Treatment for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled, Pilot Study. Urology 2003, 62, 425–429. [Google Scholar] [CrossRef] [PubMed]
- De la Rosette, J.; Karthaus, H.F.M.; van Kerrebroeck, P.E.; de Boo, T.; Debruyne, F.M.J. Research in’Prostatitis Syndromes’: The Use of Alfuzosin (a New α (1)-Receptor-Blocking Agent) in Patients Mainly Presenting with Micturition Complaints of an Irritative Nature and Confirmed Urodynamic Abnormalities. Eur. Urol. 1992, 22, 222–227. [Google Scholar] [CrossRef]
- Siroosbakht, S.; Rezakhaniha, S.; Namdari, F.; Rezakhaniha, B. Is There Relationship between Serum Uric Acid Levels and Lower Urinary Tract Symptoms, Prostate Volume, and PSA in Men without Cancer? A Prospective Population-Based Study. Andrologia 2021, 53, e14200. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.L.; Sutherland, F.C.W.; Van Essen, G.H.; Hundt, H.K.L.; Swart, K.J.; Hundt, A.F. Selective, Sensitive and Rapid Liquid Chromatography–Tandem Mass Spectrometry Method for the Determination of Alfuzosin in Human Plasma. J. Chromatogr. B 2003, 788, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ashour, S.; Chehna, M.F.; Bayram, R. Spectrophotometric Determination of Alfuzosin HCl in Pharmaceutical Formulations with Some Sulphonephthalein Dyes. Int. J. Biomed. Sci. 2006, 2, 273. [Google Scholar] [CrossRef]
- Karasakal, A.; Ulu, S.T. Sensitive Spectrofluorimetric Determination of Alfuzosin in Pharmaceutical Preparations and Human Urine Using Dansyl Chloride. J. Anal. Chem. 2015, 70, 708–711. [Google Scholar] [CrossRef]
- Belal, F.; Walash, M.; Fathy, M.; Zayed, S.; Borg, H. Sensitive Analysis of Five Alpha Blockers in Dosage Forms and Human Plasma by Field Amplified Sample Injection Combined with Micellar Electrokinetic Chromatography. Microchem. J. 2019, 146, 1173–1180. [Google Scholar] [CrossRef]
- Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts 2022, 12, 306. [Google Scholar] [CrossRef]
- Lopes, L.C.; Santos, A.; Bueno, P.R. An Outlook on Electrochemical Approaches for Molecular Diagnostics Assays and Discussions on the Limitations of Miniaturized Technologies for Point-of-Care Devices. Sens. Actuators Rep. 2022, 4, 100087. [Google Scholar] [CrossRef]
- Hu, Q.; Wan, J.; Wang, H.; Cao, X.; Li, S.; Liang, Y.; Luo, Y.; Wang, W.; Niu, L. Boronate-Affinity Cross-Linking-Based Ratiometric Electrochemical Detection of Glycoconjugates. Anal. Chem. 2022, 94, 9481–9486. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Z.; Liu, C.; Zhang, Y. FeMoO 4 Nanospheres-Based Nanozymatic Colorimetry for Rapid and Sensitive Pyrophosphate Detection. J. Mater. Chem. B 2022, 10, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, S.; Gautam, R.K.; Singh, A.K.; Tiwari, I. Nanoscale Materials-Based Hybrid Frameworks Modified Electrochemical Biosensors for Early Cancer Diagnostics: An Overview of Current Trends and Challenges. Microchem. J. 2022, 172, 106980. [Google Scholar] [CrossRef]
- Singh, S.; Gill, A.A.; Nlooto, M.; Karpoormath, R. Prostate Cancer Biomarkers Detection Using Nanoparticles Based Electrochemical Biosensors. Biosens. Bioelectron. 2019, 137, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Della Pelle, F.; Del Carlo, M.; Escarpa, A.; Compagnone, D. Nanomaterial-Based Electrochemical Sensing Strategies for Cell Lines Oxidative Stress Evaluation and for Bio-Compounds Detection in Food. In Proceedings of the 2nd European Biosensor Symposium 2019, Florence, Italy, 18–21 February 2019. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Channon, R.B. Electrochemical Sensors. In Bioengineering Innovative Solutions for Cancer; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–71. [Google Scholar]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, P.; Meyerhoff, M.E. Electrochemistry and Chemical Sensors. In Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 151. [Google Scholar]
- Lee, Y.-Y.; Sriram, B.; Wang, S.-F.; Kogularasu, S.; Chang-Chien, G.-P. A Comprehensive Review on Emerging Role of Rare Earth Oxides in Electrochemical Biosensors. Microchem. J. 2023, 193, 109140. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Hu, H.; Zhou, X.; You, F.; Yao, C.; Liu, F.J.; Yu, P.; Wu, D.; Yao, J. Advanced Metal–Organic Frameworks-Based Catalysts in Electrochemical Sensors. Front. Chem. 2022, 10, 881172. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wu, W.; Li, M.; Li, Z.; Pan, L.; Ahamad, T.; Alshehri, S.M.; Bando, Y.; Yamauchi, Y.; Xu, X. Metal-Organic Framework-Based Nanoarchitectonics: A Promising Material Platform for Electrochemical Detection of Organophosphorus Pesticides. Coord. Chem. Rev. 2024, 501, 215534. [Google Scholar] [CrossRef]
- Song, M.-K.; Park, S.; Alamgir, F.M.; Cho, J.; Liu, M. Nanostructured Electrodes for Lithium-Ion and Lithium-Air Batteries: The Latest Developments, Challenges, and Perspectives. Mater. Sci. Eng. R Rep. 2011, 72, 203–252. [Google Scholar] [CrossRef]
- Prasad, G.V.; Vinothkumar, V.; Jang, S.J.; Kim, T.H. An Ultra-Sensitive Electrochemical Sensor Based on MOF-Derived ZnO/Co 3 O 4 Decorated on Graphene for Low-Level Monitoring of the α 1-AR Antagonist Alfuzosin in Tablets and Human Samples. Inorg. Chem. Front. 2023, 10, 4829–4844. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The Concept of Mixed Organic Ligands in Metal–Organic Frameworks: Design, Tuning and Functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating Metal–Organic Frameworks for Natural Gas Storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.H. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef]
- Farrusseng, D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Qiu, S.; Xue, M.; Zhu, G. Metal–Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF Nanosheet Array: A High-Performance Electrochemical Sensor for Non-Enzymatic Glucose Detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal Organic Frameworks for Electrochemical Sensor Applications: A Review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; Martins, P.R.; Rocha, D.P.; Matias, T.A.; Juliao, M.S.; Munoz, R.A.; Angnes, L. Recent Trends and Perspectives in Electrochemical Sensors Based on MOF-Derived Materials. J. Mater. Chem. C 2021, 9, 8718–8745. [Google Scholar] [CrossRef]
- Lin, R.-B.; Zhang, Z.; Chen, B. Achieving High Performance Metal–Organic Framework Materials through Pore Engineering. Acc. Chem. Res. 2021, 54, 3362–3376. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. Ultrahigh Porosity in Mesoporous MOFs: Promises and Limitations. Chem. Commun. 2014, 50, 7089–7098. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, K.J.; Cheon, J.Y.; Lee, J.H.; Joo, S.H.; Moon, H.R. Nanoporous Metal Oxides with Tunable and Nanocrystalline Frameworks via Conversion of Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 8940–8946. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Liu, X.; Elshahawy, A.M.; Zhang, H.; Wu, H.; Pennycook, S.J.; Wang, J. Metal–Organic Framework Derived Hollow CoS 2 Nanotube Arrays: An Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Nanoscale Horiz. 2017, 2, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yin, H.; Du, L.; He, L.; Zhao, K.; Chang, L.; Yin, G.; Zhao, H.; Liu, S.; Tang, Z. Carbonized Nanoscale Metal–Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction. ACS Nano 2014, 8, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, C.; Wang, J.; Huang, R.; Hao, X.; Li, K. Research Progress on Nanoporous Carbons Produced by the Carbonization of Metal Organic Frameworks. New Carbon Mater. 2021, 36, 322–335. [Google Scholar] [CrossRef]
- Park, J.; Shen, X.; Wang, G. Solvothermal Synthesis and Gas-Sensing Performance of Co3O4 Hollow Nanospheres. Sens. Actuators B Chem. 2009, 136, 494–498. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The Advances of Co3O4 as Gas Sensing Materials: A Review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Zheng, X.; Yao, S.; Yang, X.; Zhai, T. Core-Shell Structured Co3O4@ NiCo2O4 Electrodes Grown on Flexible Carbon Fibers with Superior Electrochemical Properties. Nano Energy 2017, 31, 410–417. [Google Scholar] [CrossRef]
- Wang, Y.; Che, P.; Du, X.; Zhang, X. Three-Dimensional Co3O4@NiCo2O4 Nanoarrays with Different Morphologies as Electrocatalysts for Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2020, 45, 28598–28606. [Google Scholar] [CrossRef]
- Xue, B.; Li, K.; Feng, L.; Lu, J.; Zhang, L. Graphene Wrapped Porous Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Electrocatalytic Performance for Glucose Sensor. Electrochimica Acta 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Sudha, V.; Senthil Kumar, S.M.; Thangamuthu, R. NiCo2O4 Nanorod: Synthesis and Electrochemical Sensing of Carcinogenic Hydrazine. Inorg. Chem. Commun. 2020, 116, 107927. [Google Scholar] [CrossRef]
- Hasseba, A.A.; Mohameda, G.G.; Elasherya, S.E. Potentiometric Determination of Alfuzosin Hydrochloride in Pharmaceutical Preparations and Biological Fluids Using Modified Carbon Paste Electrodes. J. Basic Environ. Sci. 2019, 6, 318–323. [Google Scholar]
- Hadad, G.M.; Emara, S.; Mahmoud, W.M. Development and Validation of a Stability-Indicating RP-HPLC Method for the Determination of Paracetamol with Dantrolene or/and Cetirizine and Pseudoephedrine in Two Pharmaceutical Dosage Forms. Talanta 2009, 79, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Attia, K.A.; El-Abasawi, N.M.; Abdelazim, A.H. Colorimetric Estimation of Alfuzosin Hydrochloride in Pharmaceutical Preparation Based on Computational Studies. Anal. Methods 2016, 8, 1798–1805. [Google Scholar] [CrossRef]
- Baezzat, M.R.; Banavand, F.; Fasihi, F. Electrooxidation Study and Highly Sensitive Voltammetric Determination of Alfuzosin Employing Multi-Walled Carbon Nanotubes and the Ionic Liquid 1-Hexylpyridinium Hexafluorophosphate Nanocomposite Sensor. J. Mol. Liq. 2017, 233, 391–397. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal Synthesis of Zeolitic Imidazolate Framework-67 (ZIF-67) Nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Hou, W.; Huang, Y.; Liu, X. Highly Efficient and Recyclable ZIF-67 Catalyst for the Degradation of Tetracycline. Catal. Lett. 2020, 150, 3017–3022. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A Universal Chemical-Induced Tensile Strain Tuning Strategy to Boost Oxygen-Evolving Electrocatalysis on Perovskite Oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Kim: Analysis of the NiCo2O4 Spinel Surface with Auger and X-ray Photoelectron Spectroscopy. Available online: https://scholar.google.com/scholar_lookup?title=Analysis%20of%20the%20NiCo2O4%20spinel%20surface%20with%20Auger%20and%20X-ray%20photoelectron%20spectroscopy&publication_year=2000&author=J.G.%20Kim&author=D.L.%20Pugmire&author=D.%20Battaglia&author=M.A.%20Langell (accessed on 14 April 2024).
- Marco: Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. Available online: https://scholar.google.com/scholar_lookup?title=Characterization%20of%20the%20nickel%20cobaltite%2C%20NiCo2O4%2C%20prepared%20by%20several%20methods%3A%20an%20XRD%2C%20XANES%2C%20EXAFS%2C%20and%20XPS%20study&publication_year=2000&author=J.F.%20Marco&author=J.R.%20Gancedo&author=M.%20Gracia&author=J.L.%20Gautier&author=E.%20R%C3%ADos&author=F.J.%20Berry (accessed on 14 April 2024).
- Yuan, C.; Li, J.; Hou, L.; Yang, L.; Shen, L.; Zhang, X. Facile Template-Free Synthesis of Ultralayered Mesoporous Nickel Cobaltite Nanowires towards High-Performance Electrochemical Capacitors. J. Mater. Chem. 2012, 22, 16084–16090. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- An, C.; Wang, Y.; Huang, Y.; Xu, Y.; Xu, C.; Jiao, L.; Yuan, H. Novel Three-Dimensional NiCo2O4 Hierarchitectures: Solvothermal Synthesis and Electrochemical Properties. CrystEngComm 2014, 16, 385–392. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning Synergy between Nickel and Iron in Ruddlesden–Popper Perovskites through Controllable Crystal Dimensionalities towards Enhanced Oxygen-Evolving Activity and Stability. Carbon Energy 2024, e465. [Google Scholar] [CrossRef]
- Budi, C.S.; Deka, J.R.; Hsu, W.-C.; Saikia, D.; Chen, K.-T.; Kao, H.-M.; Yang, Y.-C. Bimetallic Co/Zn Zeolitic Imidazolate Framework ZIF-67 Supported Cu Nanoparticles: An Excellent Catalyst for Reduction of Synthetic Dyes and Nitroarenes. J. Hazard. Mater. 2021, 407, 124392. [Google Scholar] [CrossRef] [PubMed]
- Mohtasham, H.; Rostami, M.; Gholipour, B.; Sorouri, A.M.; Ehrlich, H.; Ganjali, M.R.; Rostamnia, S.; Rahimi-Nasrabadi, M.; Salimi, A.; Luque, R. Nano-Architecture of MOF (ZIF-67)-Based Co3O4 NPs@N-Doped Porous Carbon Polyhedral Nanocomposites for Oxidative Degradation of Antibiotic Sulfamethoxazole from Wastewater. Chemosphere 2023, 310, 136625. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, J.; Zhang, Y.; Wang, D.; Zhang, L.; Guo, Y.; Ma, W.; Wang, J. Synthesis of Core–Shell ZIF-67@Co-MOF-74 Catalyst with Controllable Shell Thickness and Enhanced Photocatalytic Activity for Visible Light-Driven Water Oxidation. CrystEngComm 2018, 20, 7659–7665. [Google Scholar] [CrossRef]
- Venu Gopal, T.; Reddy, T.M.; Venkataprasad, G.; Shaikshavalli, P.; Gopal, P. Rapid and Sensitive Electrochemical Monitoring of Paracetamol and Its Simultaneous Resolution in Presence of Epinephrine and Tyrosine at GO/Poly(Val) Composite Modified Carbon Paste Electrode. Colloids Surf. Physicochem. Eng. Asp. 2018, 545, 117–126. [Google Scholar] [CrossRef]
- Prasad, G.V.; Jang, S.-J.; Sekhar, Y.C.; Reddy, T.M.; Sarma, L.S.; Kim, H.-B.; Kim, T.H. Fine-Tuning of Pd–CeO2/rGO Nanocomposite: A Facile Synergetic Strategy for Effective Electrochemical Detection of Dopamine in Pharmaceutical and Biological Samples. J. Electroanal. Chem. 2023, 941, 117544. [Google Scholar] [CrossRef]



| Method | Linear Range (µM) | LOD (µM) | Ref. | |
|---|---|---|---|---|
| Potentiometry using modified electrodes | Electrode I | 10–10,000 | 9.6 | [54] |
| Electrode II | 10–10,000 | 9.7 | ||
| RP-HPLC | - | 8.014 | [55] | |
| Colorimetry | 25–300 | 6.13 | [56] | |
| DPV | MWCNT/CLIE | 0.02–90 | 0.0041 | [57] |
| Amperometry | 10–100 | 2.5 | ||
| DPV using Co3O4/NiCo2O4-GCE | 5–180 | 1.37 | This work | |
| Samples | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tablet | 15 | 14.84 | 98.93 | 0.87 |
| 25 | 24.81 | 99.23 | 0.82 | |
| 35 | 34.09 | 97.41 | 1.14 | |
| Human Serum | 15 | 14.54 | 96.95 | 2.92 |
| 20 | 19.86 | 99.28 | 1.59 | |
| 25 | 24.90 | 99.60 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Amin; Prasad, G.V.; Jang, S.J.; Oh, J.-W.; Kim, T.H. A MOF-Templated Double-Shelled Co3O4/NiCo2O4 Nanocomposite for Electrochemical Detection of Alfuzosin. Nanomaterials 2024, 14, 757. https://doi.org/10.3390/nano14090757
Al-Amin, Prasad GV, Jang SJ, Oh J-W, Kim TH. A MOF-Templated Double-Shelled Co3O4/NiCo2O4 Nanocomposite for Electrochemical Detection of Alfuzosin. Nanomaterials. 2024; 14(9):757. https://doi.org/10.3390/nano14090757
Chicago/Turabian StyleAl-Amin, Gajapaneni Venkata Prasad, Seung Joo Jang, Jeong-Wook Oh, and Tae Hyun Kim. 2024. "A MOF-Templated Double-Shelled Co3O4/NiCo2O4 Nanocomposite for Electrochemical Detection of Alfuzosin" Nanomaterials 14, no. 9: 757. https://doi.org/10.3390/nano14090757
APA StyleAl-Amin, Prasad, G. V., Jang, S. J., Oh, J.-W., & Kim, T. H. (2024). A MOF-Templated Double-Shelled Co3O4/NiCo2O4 Nanocomposite for Electrochemical Detection of Alfuzosin. Nanomaterials, 14(9), 757. https://doi.org/10.3390/nano14090757








