Preparation and Gas-Sensitive Properties of SnO2@Bi2O3 Core-Shell Heterojunction Structure
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of SnO2
2.2. Synthesis of SnO2@Bi2O3
2.3. Material Characterization
2.4. Sensor Fabrication and Gas Sensing
3. Results and Discussion
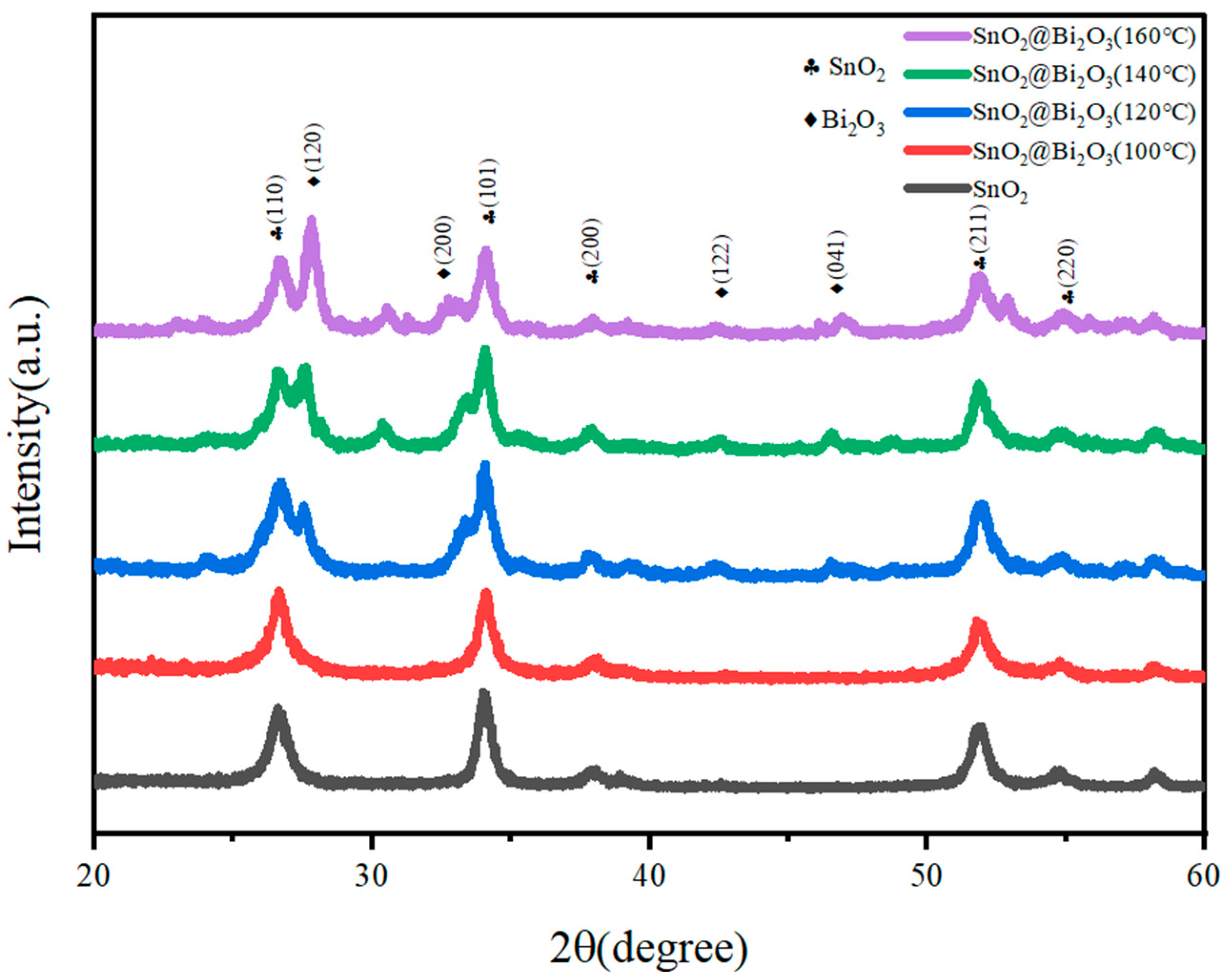
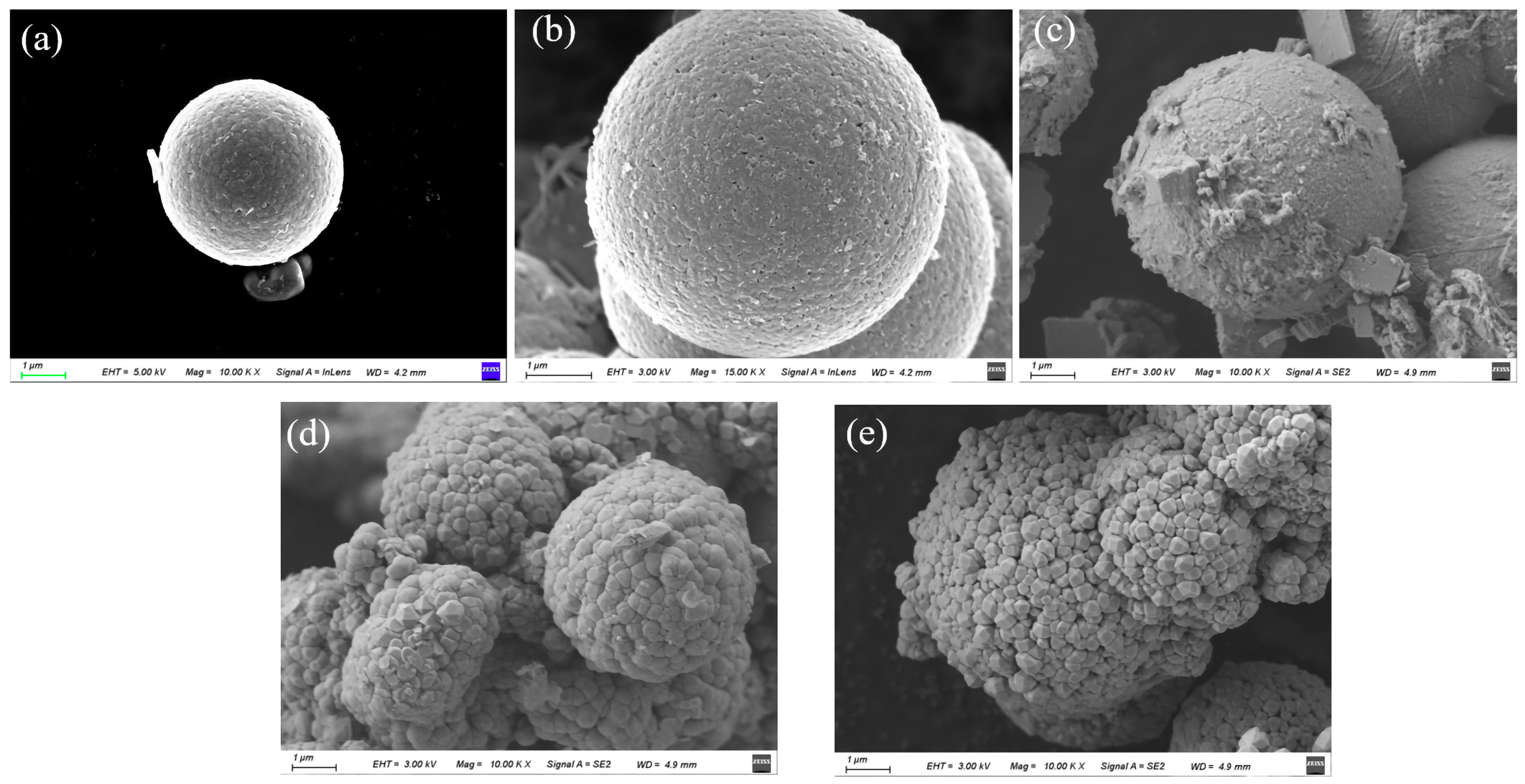
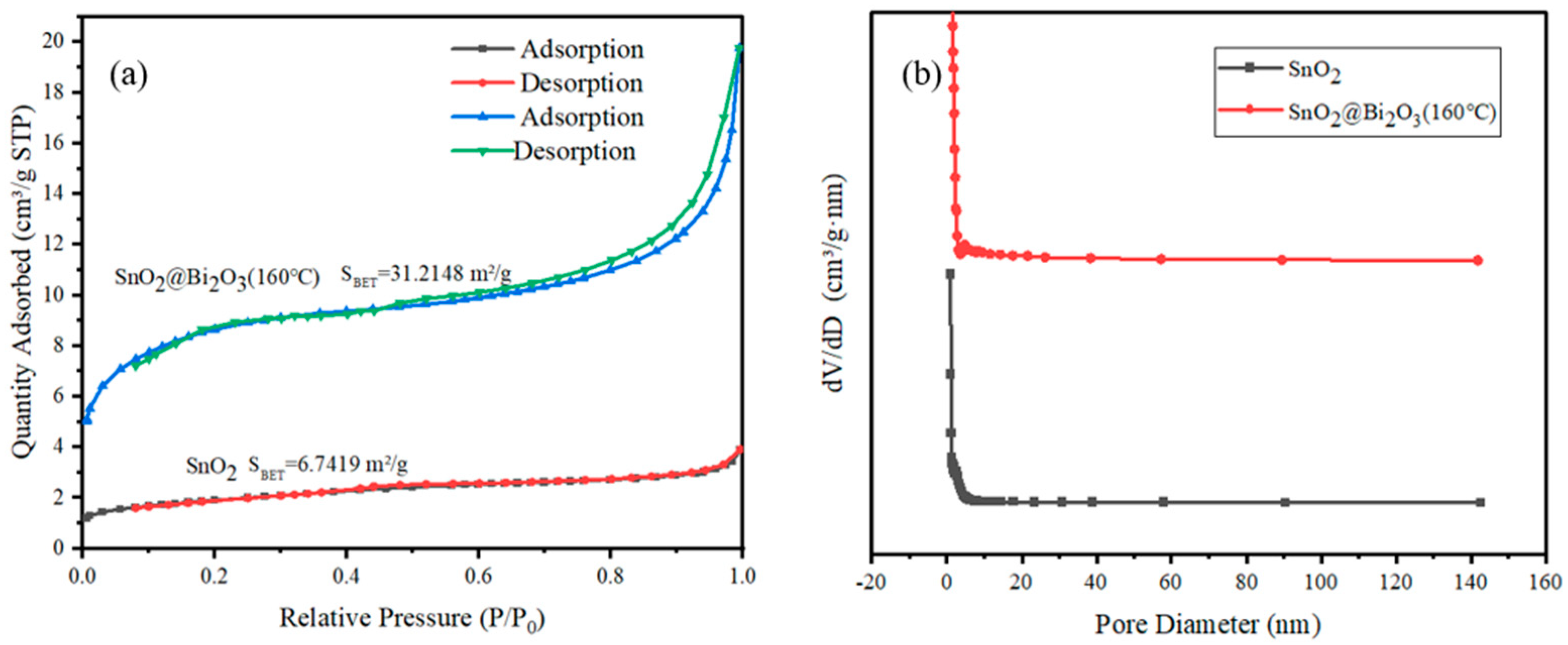
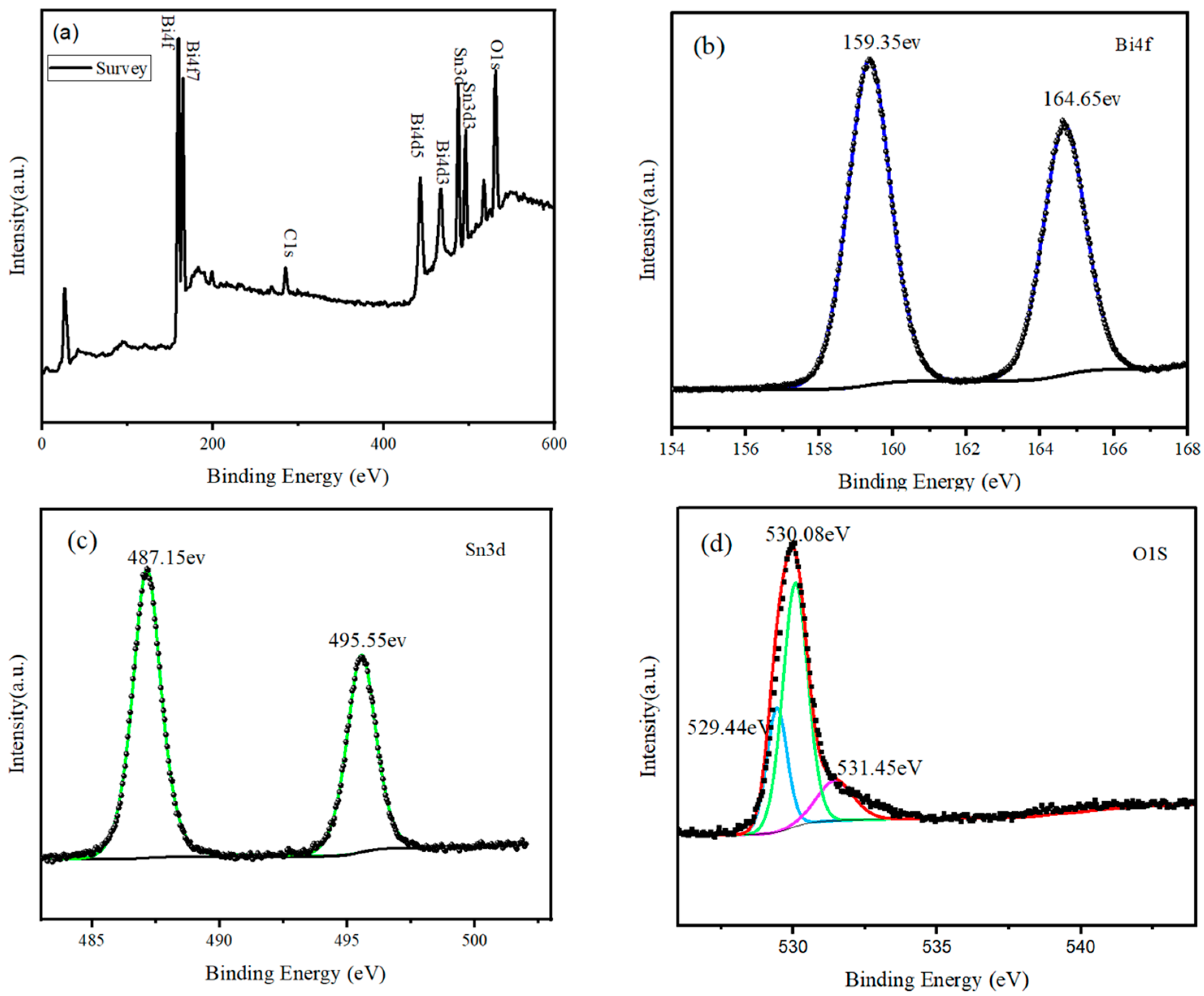
3.1. Characterization of the SnO2@Bi2O3
3.2. Gas-Sensing Performances
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A 2022, 341, 113578. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Chougule, S.S.; Bak, N.-H.; Nam, D.-J.; Jung, N.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. High performance langasite based SAW NO2 gas sensor using 2D g-C3N4@TiO2 hybrid nanocomposite. J. Hazard. Mater. 2022, 427, 128174. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators B 2022, 359, 131612. [Google Scholar] [CrossRef]
- Yu, H.M.; Li, J.Z.; Luo, W.B.; Li, Z.Y.; Tian, Y.W.; Yang, Z.D.; Gao, X.W.; Liu, H. Hetero-structure La2O3-modified SnO2–Sn3O4 from tin anode slime for highly sensitive and ppb-Level formaldehyde detection. Appl. Surf. Sci. 2020, 513, 145825. [Google Scholar] [CrossRef]
- Xu, L.; Ge, M.Y.; Zhang, F.; Huang, H.J.; Sun, Y.; He, D.N. Nanostructured of SnO2/NiO composite as a highly selective formaldehyde gas sensor. J. Mater. Res. 2020, 35, 3079–3090. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An Efficient Bicomponent TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with a Side-by-Side Dual Spinneret Method. Nano Lett. 2007, 7, 1081–1085. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.; Bandaranayake, P.K.M.; Kumara, G.R.A.; Konno, A. Enhanced Efficiency of a Dye-Sensitized Solar Cell Made from MgO-Coated Nanocrystalline SnO2. Jpn. J. Appl. Phys. 2001, 40, 732. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, L.; Xie, T.; Wang, D.; Lin, Y. Study on the Gas-Sensitive Properties for Formaldehyde Based on SnO2-ZnO Heterostructure in UV Excitation. Sens. Actuators B Chem. 2018, 254, 863–871. [Google Scholar] [CrossRef]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.J.; Tse, M.S.; Tan, O.K. Low-Temperature Growth of SnO2 Nanorod Arrays and Tunable n-p-n Sensing Response of a ZnO/SnO2 Heterojunction for Exclusive Hydrogen Sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.L.; Zhang, Z.T. Visible Light Assisted Room-Temperature NO2 Gas Sensor Based on Hollow SnO2@SnS2 Nanostructures. Sens. Actuators B Chem. 2020, 324, 128754. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Xu, Q.; Gong, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. High Triethylamine-Sensing Properties of NiO/SnO2 Hollow Sphere P–N Heterojunction Sensors. Sens. Actuators B Chem. 2015, 215, 39–44. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and Gas Sensing Mechanism of n-SnO2-p-Co3O4 Composite Nanofibers. Sens. Actuators B Chem. 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Zhang, B.W.; Fu, W.Y.; Li, H.Y.; Fu, X.L.; Wang, Y.; Bala, H.; Sun, G.; Wang, X.D.; Wang, Y.; Cao, J.L.; et al. Actinomorphic ZnO/SnO2 Core-Shell Nanorods: Two-Step Synthesis and Enhanced Ethanol Sensing Properties. Mater. Lett. 2015, 160, 227–230. [Google Scholar] [CrossRef]
- Chen, K.; Chen, S.; Pi, M.; Zhang, D. SnO2 Nanoparticles/TiO2 Nanofibers Heterostructures: In Situ Fabrication and Enhanced Gas Sensing Performance. Solid State Electron. 2019, 157, 42–47. [Google Scholar] [CrossRef]
- Sardar, K.; Fang, T.; Yang, T.W. A novel route involving an in situ chemical reduction process to the one-dimensional nanostructures of bismuth trioxide. J. Am. Ceram. Soc. 2007, 90, 4033–4035. [Google Scholar] [CrossRef]
- Bonyani, M.; Lee, J.K.; Sun, G.-J.; Lee, S.; Ko, T.; Lee, C. Benzene sensing properties and sensing mechanism of Pd-decorated Bi2O3-core/ZnO-shell nanorods. Thin Solid Films 2017, 636, 257–266. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Dastan, D.; Zhang, Z.; Liu, Z.; Yue, C.; Yang, Z.; Mu, Y.; Wang, X.; Chen, X.; et al. Ultrasensitive n-butanol gas sensor based on Bi2O3-In2O3 heterostructure. J. Alloys Compd. 2024, 1003, 175585. [Google Scholar] [CrossRef]
- Meng, X.; Kang, S.; Zhao, Z.; Jin, G.; Shao, Z.; Wu, L. Core-Shell Bi2O3/CeO2 heterojunction for enhanced formaldehyde gas sensor. Ceram. Int. 2024. [Google Scholar] [CrossRef]
- Devi, G.S.; Manorama, S.V.; Rao, V.J. SnO2/Bi2O3: A Suitable System for Selective Carbon Monoxide Detection. J. Electrochem. Soc. 1998, 145, 1039–1044. [Google Scholar] [CrossRef]
- Montenegro, A.; Ponce, M.; Castro, M.S.; Rodríguez-Paez, J.E. SnO2–Bi2O3 and SnO2–Sb2O3 Gas Sensors Obtained by Soft Chemical Method. J. Eur. Ceram. Soc. 2007, 27, 4143–4146. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO₂ Sensor Based on Bi2O3 Branched SnO2 Nanowires. Sens. Actuators B Chem. 2018, 274, 356–369. [Google Scholar] [CrossRef]
- Yang, H.; Suematsu, K.; Mashiba, F.H.; Watanabe, K.; Shimanoe, K. Oxygenated VOC Detection Using SnO2 Nanoparticles with Uniformly Dispersed Bi2O3. Nanomaterials 2024, 14, 2032. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; Han, N.; Liao, D.; Bai, S.; Yang, X. rGO decorated W doped BiVO4 novel material for sensing detection of trimethylamine. Sens. Actuators B 2019, 298, 126749. [Google Scholar] [CrossRef]
- Yu, C.F.; Wang, K.; Yang, P.Y.; Yang, S.N.; Lu, C.; Song, Y.Z.; Dong, S.Y.; Sun, J.Y.; Sun, J.H. One-pot facile synthesis of Bi2S3/SnS2/Bi2O3 ternary heterojunction as advanced double Z-scheme photocatalytic system for efficient dye removal under sunlight irradiation. Appl. Surf. Sci. 2017, 420, 233–242. [Google Scholar] [CrossRef]
- Bai, J.Z.; Shen, Y.B.; Zhao, S.K.; Li, A.; Kang, Z.K.; Cui, B.Y.; Wei, D.Z.; Yuan, Z.Y.; Meng, F.L. Room-Temperature NH3 Sensor Based on SnO2 Quantum Dots Functionalized SnS2 Nanosheets. Adv. Mater. Technol. 2023, 8, 2201671. [Google Scholar] [CrossRef]
- Kou, X.Y.; Meng, F.Q.; Chen, K.; Wang, T.S.; Sun, P.; Liu, F.M.; Yan, X.; Sun, Y.F.; Liu, F.M.; Shimanoe, K.; et al. High-performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B 2020, 320, 128292. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Li, M.; Zhang, S.; Bai, H.; Yang, L.; Zhang, S. One-Pot Hydrothermal Synthesis of SnO2/BiOBr Heterojunction Photocatalysts for the Efficient Degradation of Organic Pollutants Under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 28686–28694. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Gao, W. Shape and composition effects of PdPt bimetallic nanocrystals on hydrogen sensing properties of SnO2 based sensors. Sens. Actuators B 2023, 390, 133976. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, Z.; Yue, J.; Zakaria, Q.M.D.; Chen, C.; Jiang, X.; Yu, A. Crystal plane-dependent gas-sensing properties of zinc oxide nanostructures: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2014, 16, 11471–11480. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Basu, P.K.; Mondal, B.; Saha, H. A low power MEMS gas sensor based on nanocrystalline ZnO thin films for sensing methane. Microelectron. Reliab. 2008, 48, 1772–1779. [Google Scholar] [CrossRef]
- Massie, C.; Stewart, G.; McGregor, G.; Gilchrist, J.R. Design of a portable optical sensor for methane gas detection. Sens. Actuators B Chem. 2006, 113, 830–836. [Google Scholar] [CrossRef]
- Han, Z.J.; Qi, Y.; Yang, Z.Y.; Han, H.C.; Jiang, Y.Y.; Du, W.J.; Zhang, X.; Zhang, J.Z.; Dai, Z.F.; Wu, L.L.; et al. Recent advances and perspectives on constructing metal oxide semiconductor gas sensing materials for efficient formaldehyde detection. J. Mater. Chem. C 2020, 8, 13169–13188. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. Repeatability of indium oxide gas sensors for detecting methane at low temperature. Mater. Sci. Semicond. Process. 2016, 56, 260–264. [Google Scholar] [CrossRef]
- Lee, D.-D.; Chung, W.-Y.; Sohn, B.-K. High sensitivity and selectivity methane gas sensors doped with Rh as a catalyst. Sens. Actuators B Chem. 1993, 13, 252–255. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166–167, 156–164. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hamad, D. Low-temperature hydrogen sensor based on sputtered tin dioxide nanostructures through slow deposition rate. Appl. Surf. Sci. 2022, 598, 153857. [Google Scholar] [CrossRef]
- Liu, M.; Sun, R.Y.; Sima, Z.H.; Song, P.; Ding, Y.L.; Wang, Q. Au-decorated In2O3 nanospheres/exfoliated Ti3C2Tx MXene nanosheets for highly sensitive formaldehyde gas sensing at room temperature. Appl. Surf. Sci. 2022, 605, 154839. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.; Wang, G.; Shen, Y.; San, X.; Li, M.; Meng, F. Low-temperature formaldehyde gas sensors based on NiO-SnO2 heterojunction microflowers assembled by thin porous nanosheets. Sens. Actuators B Chem. 2018, 273, 418–428. [Google Scholar] [CrossRef]
- Chowdhuri, A.; Gupta, V.; Sreenivas, K.; Kumar, R.; Mozumdar, S.; Patanjali, P.K. Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl. Phys. Lett. 2004, 84, 1180–1182. [Google Scholar] [CrossRef]
- Xue, X.; Xing, L.; Chen, Y.; Shi, S.; Wang, Y.; Wang, T. Synthesis and H2S sensing properties of CuO−SnO2 core/shell pn-junction nanorods. J. Phys. Chem. C 2008, 112, 12157–12160. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Wang, Y.; Chen, Z. A Highly Sensitive and Room Temperature CNTs/SnO2/CuO Sensor for H2S Gas Sensing Applications. Nanoscale Res. Lett. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, S.; Du, K. Chemiresistive Gas Sensors Based on Hollow Heterojunction: A Review. Adv. Mater. Interfaces 2021, 8, 2002122. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors. Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
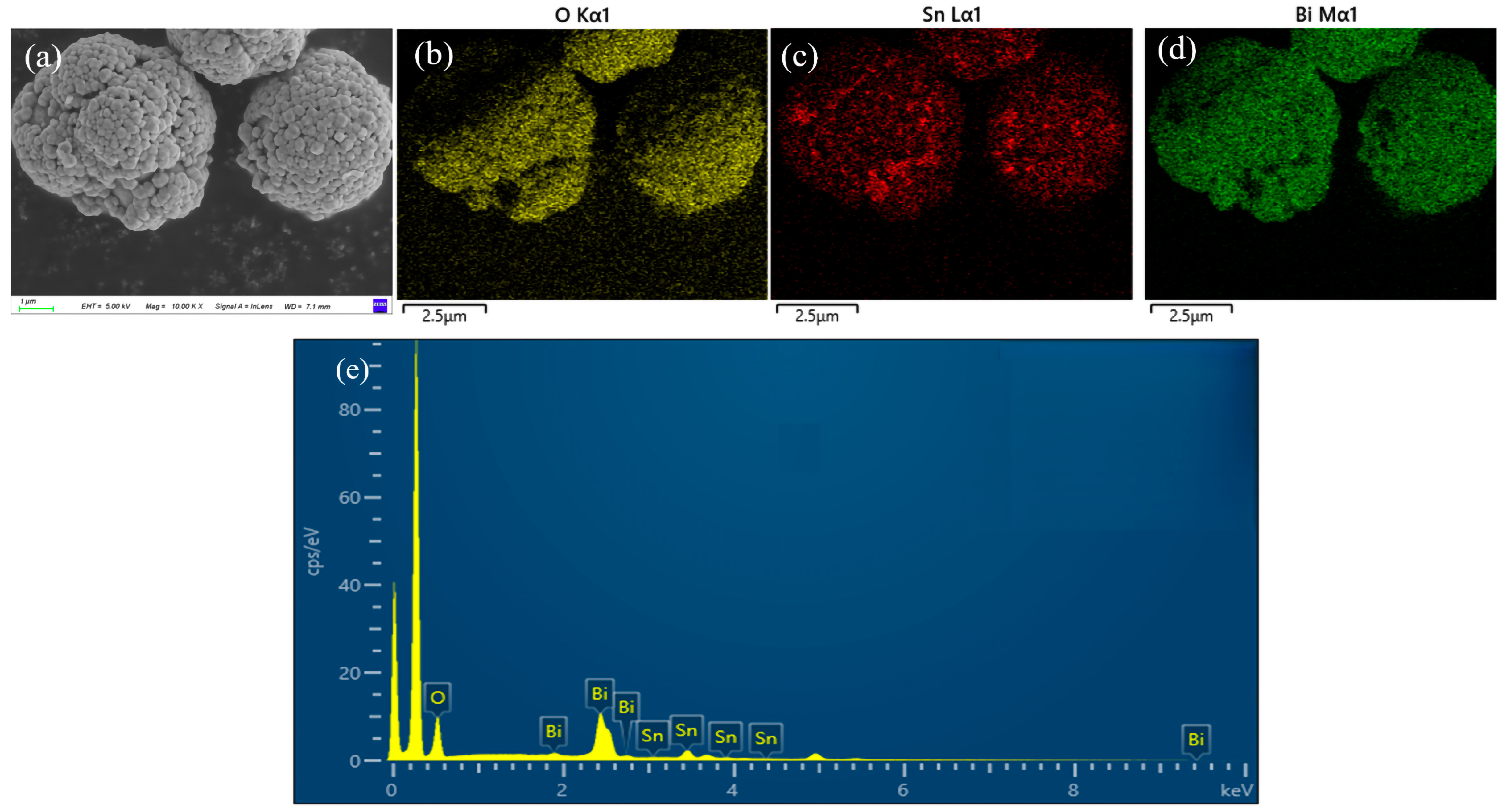




| Element | Atomic % |
|---|---|
| O | 79.3 |
| Sn | 6.1 |
| Bi | 14.6 |
| Total | 100 |
| Samples | SBET (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| SnO2 | 6.7419 | 2.8669 | 0.004116 |
| SnO2@Bi2O3 (160 °C) | 31.2148 | 2.9790 | 0.020202 |
| Ethanol Gas (100 ppm) | Sensor Response |
|---|---|
| SnO2 | 9 |
| SnO2@Bi2O3 (100 °C) | 9 |
| SnO2@Bi2O3 (120 °C) | 10.5 |
| SnO2@Bi2O3 (140 °C) | 13.6 |
| SnO2@Bi2O3 (160 °C) | 19.7 |
| Concentration of Ethanol (ppm) | Sensor Response |
|---|---|
| 1 | 1.21 |
| 1.1 | 1.21 |
| 1.2 | 1.21 |
| 1.3 | 1.21 |
| 1.4 | 1.21 |
| 1.5 | 1.562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gao, Y.; Lv, Y.; Yang, M.; Guo, H.; Li, N.; Bai, D.; Wang, A. Preparation and Gas-Sensitive Properties of SnO2@Bi2O3 Core-Shell Heterojunction Structure. Nanomaterials 2025, 15, 129. https://doi.org/10.3390/nano15020129
Liu J, Gao Y, Lv Y, Yang M, Guo H, Li N, Bai D, Wang A. Preparation and Gas-Sensitive Properties of SnO2@Bi2O3 Core-Shell Heterojunction Structure. Nanomaterials. 2025; 15(2):129. https://doi.org/10.3390/nano15020129
Chicago/Turabian StyleLiu, Jin, Yixin Gao, Yuanyuan Lv, Mengdi Yang, Haoru Guo, Neng Li, Danyang Bai, and Anyi Wang. 2025. "Preparation and Gas-Sensitive Properties of SnO2@Bi2O3 Core-Shell Heterojunction Structure" Nanomaterials 15, no. 2: 129. https://doi.org/10.3390/nano15020129
APA StyleLiu, J., Gao, Y., Lv, Y., Yang, M., Guo, H., Li, N., Bai, D., & Wang, A. (2025). Preparation and Gas-Sensitive Properties of SnO2@Bi2O3 Core-Shell Heterojunction Structure. Nanomaterials, 15(2), 129. https://doi.org/10.3390/nano15020129





