Abstract
Adatoms as co-catalysts may play a key role in photocatalysis, yet control of their exact configuration remains challenging. Specifically, there is converging evidence that ultra-small structures may be optimal as co-catalysts; however, a comprehensive distinction between single atoms (SAs), sub-nanoclusters (SNCs), and quantum-sized small particles (QSSPs) has yet to be established. Herein, we present a critical review addressing these distinctions, along with challenges related to the controlled synthesis of SAs, SNCs, and QSSPs; their detection methods; and their functional benefits in photocatalysis. Our discussion focuses on perovskite oxides (e.g., such as ABO3, where A and B are cations) and metal oxides (MxOy, where M is a metal) decorated with adatoms, which demonstrate superior photocatalytic performance compared to their unmodified counterparts. Finally, we highlight cases of misinterpretation between SA, SNC, and QSSP configurations emerging from limitations in high-resolution detection techniques and synthesis methods.
1. Introduction
The advancement in photocatalytic technologies plays a pivotal role in addressing global energy and environmental challenges, particularly in the domains of sustainable energy conversion and pollutant degradation [1,2,3,4]. Depending on each catalytic process, different types of photocatalysts can be selected, such as metal oxides and perovskites, which exhibit the desired structural and physicochemical properties. However, these systems appear to face challenges that restrict their overall photocatalytic performance, such as limited light absorption, rapid charge recombination, or unfavorable surface properties that might restrict the transfer of electrons to the final acceptor, e.g., H+ in the case of H2 production or reduced forms of HCO3−/CO2 toward CO, HCOOH, and CH4. In this context, it is well-anticipated that diligent engineering can be applied to the lattice and/or the surface of a semiconductor, including the incorporation of cocatalysts, which can facilitate photoinduced charge separation and improve reaction kinetics [5,6]. The most common co-catalysts are noble metals such as Pt, Pd, Ag, and Au [7]. Alternatively, recent research has provided encouraging results that non-noble metals, i.e., Cu, Ni, Co, or Sn, can be efficient co-catalysts [8,9,10,11,12]. Furthermore, there is mounting evidence that Single atoms dispersed on the surfaces of semiconducting supports can boost their catalytic activity and selectivity [13], i.e., acting as singe-atom (SA) co-catalysts.
1.1. Single Atom Catalysts (SACs) and Single Atom Co-Catalysts (SACCs)
The terminology “single atom catalyst-SAC” was first introduced in 2011 by Zhang et al. [14] based on the discovery that anchoring isolated Pt adatoms as “single-atoms” on FeOx significantly improved the system’s catalytic performance in CO oxidation [14]. The use of single atoms in photocatalysis was exemplified by Xing et al. [15], who utilized various noble metal single atoms (Pt, Pd, Rh, and Ru) as co-catalysts anchored on a TiO2 substrate for photocatalytic H2 production. These single metal atoms were stably dispersed on TiO2 and demonstrated superior photocatalytic hydrogen evolution performance compared to the corresponding metallic nanoparticles or clusters [15]. Since then, the field of single atom catalysis for photocatalytic applications has gained substantial attention due to its unique ability to maximize atomic efficiency and enhance catalytic activity, selectivity, and stability [13,16]. Single atoms can demonstrate more efficient utilization per atom vs. their nanoparticle counterparts. The importance of highly dispersed single atoms of a co-catalyst on a catalyst’s surface lies in the low coordination environment of atoms, and, more interestingly, the tuning of electronic states and efficient charge transfer between the catalyst and co-catalyst, boosted by strong metal–support interaction (SMSI) phenomena [17]. Operationally, it is useful to distinguish the roles of these adatoms as single atom catalysts (SACs) vs. single atom co-catalysts (SACCs) as follows: single atom catalysts refer to cases where an adatom is the catalyst itself. Typically, in such cases, the support plays an auxiliary role. On the other hand, a single atom co-catalysts refer to cases where the support is the catalyst, e.g., such as in the case of photocatalysis, and the adatom plays the role of co-catalyst.
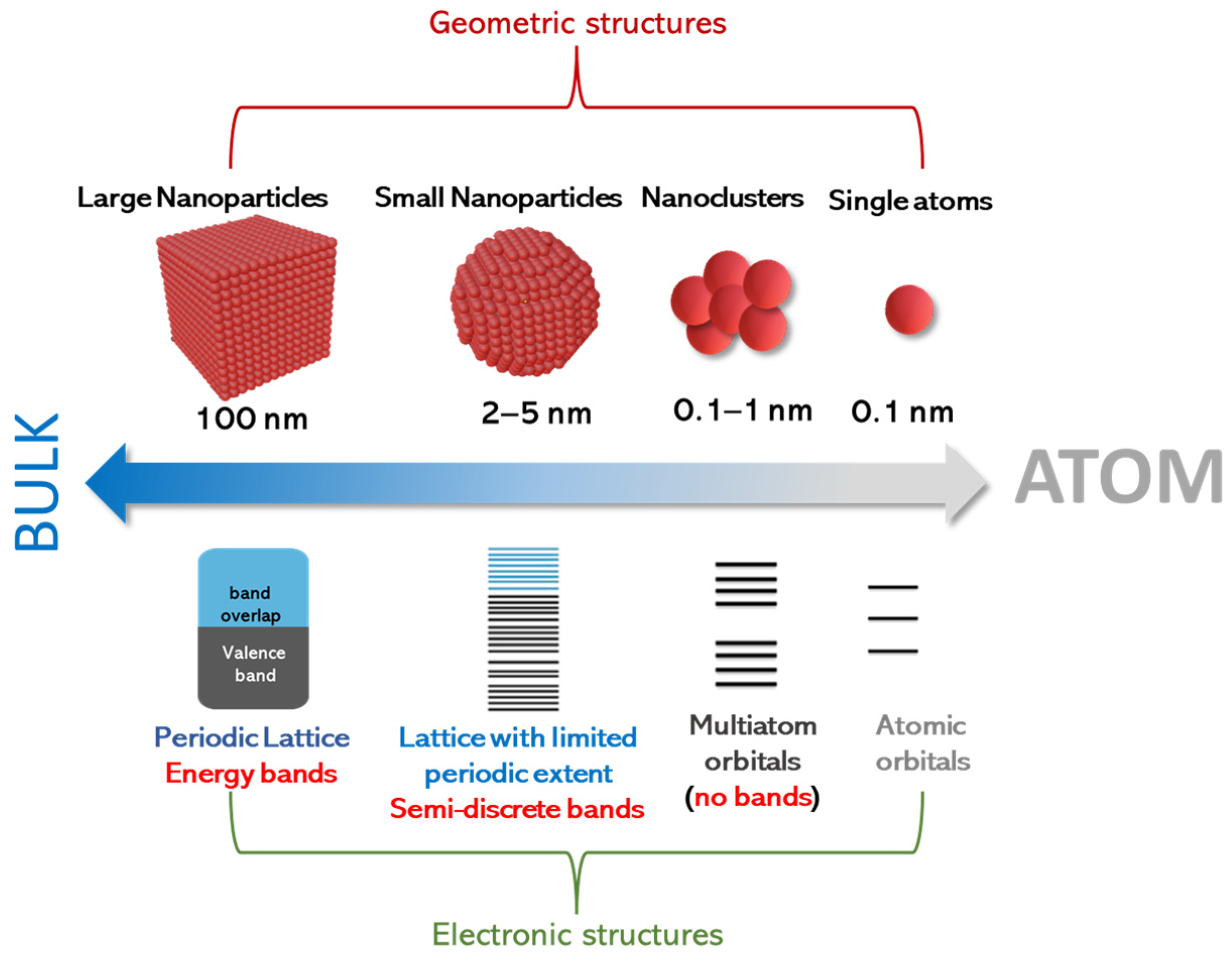
Despite this operational distinction, the physical configuration, and, often, the chemistry and synthesis, of SACs and SACCs may follow similar rules. As shown in Figure 1, in the case of adatoms that are only metal atoms (i.e., not oxides), precise control of their aggregation state is essential for regulating their electronic properties [18,19,20,21]. It is important to notice that, in contrast to previous similar schemes in the literature on the effect of size on electron levels [22,23,24,25,26], in Figure 1, we emphasize the cases of nanoclusters, small nanoparticles, and larger nanoparticles as pertinent configurations that conceptually should be anticipated to be between single atoms and bulk materials. Also, it should be noted that the term “large nanoparticles” herein is used to signify that for nanosizes near 100 nm, the electronic properties, e.g., density of states, do not exhibit quantum-sized effects [27]. Thus, we use the term “large nanoparticles” to highlight that, even if these structures are in the “nanoscale”, their properties might tend to resemble those of bulk particles. On a rigorous basis, all these trends can be well documented by theoretical quantum computing tools. However, herein, we present only a conceptual analysis, based on the fundamental concepts of nanoscale solid-state physics [28].
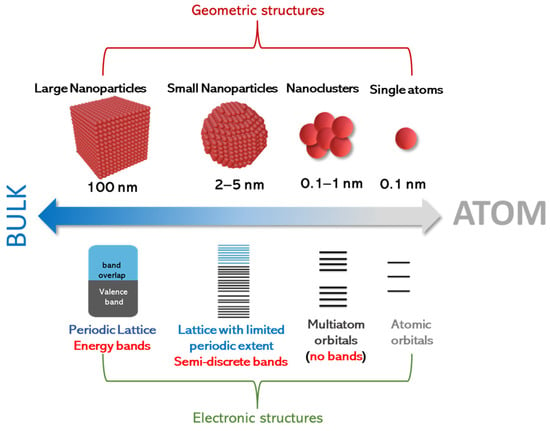
Figure 1.
Geometric and electronic structure of single atoms, clusters, small nanoparticles, and larger nanoparticles.
In this context, Figure 1 exemplifies that small nanoclusters do form multiple energy levels that do not exist in single atoms. However, in such small nanoclusters, we do not have the formation of energy bands. This is because small nanoclusters do not favor the stabilization of a periodic lattice; thus, they cannot stabilize Bloch wave functions that would allow for the hybridization of atomic orbitals toward bands [27]. To obtain a quantitative estimate, geometry-wise, a quantum-sized small particle (QSSPs) of 2 nm that clearly exhibits quantum-size effects would have a number of atoms (NQSSP) in the order of ~103 (assuming a typical atomic size of 1–3 Å). This implies that we should consider entities of size < 1 nm as small nanoclusters, with their number of atoms NSN being below NQSSP (i.e., NSN < ). This operational distinction helps us classify the energy configuration trends, i.e., the transition from discrete energy multiplicities (not-bands) in small nanoclusters to semi-discrete-bands in quantum-sized small particles to the full-periodic density of states in large nanoparticles with a well-formed periodic lattice.
When these limited-size entities are interfaced with a photocatalyst, interesting phenomena may arise that ultimately allow for the tuning of electronic states of the co-catalyst with the photocatalyst and efficient charge transfer between the catalyst and co-catalyst caused by strong metal–support interaction (SMSI) [29,30,31,32].
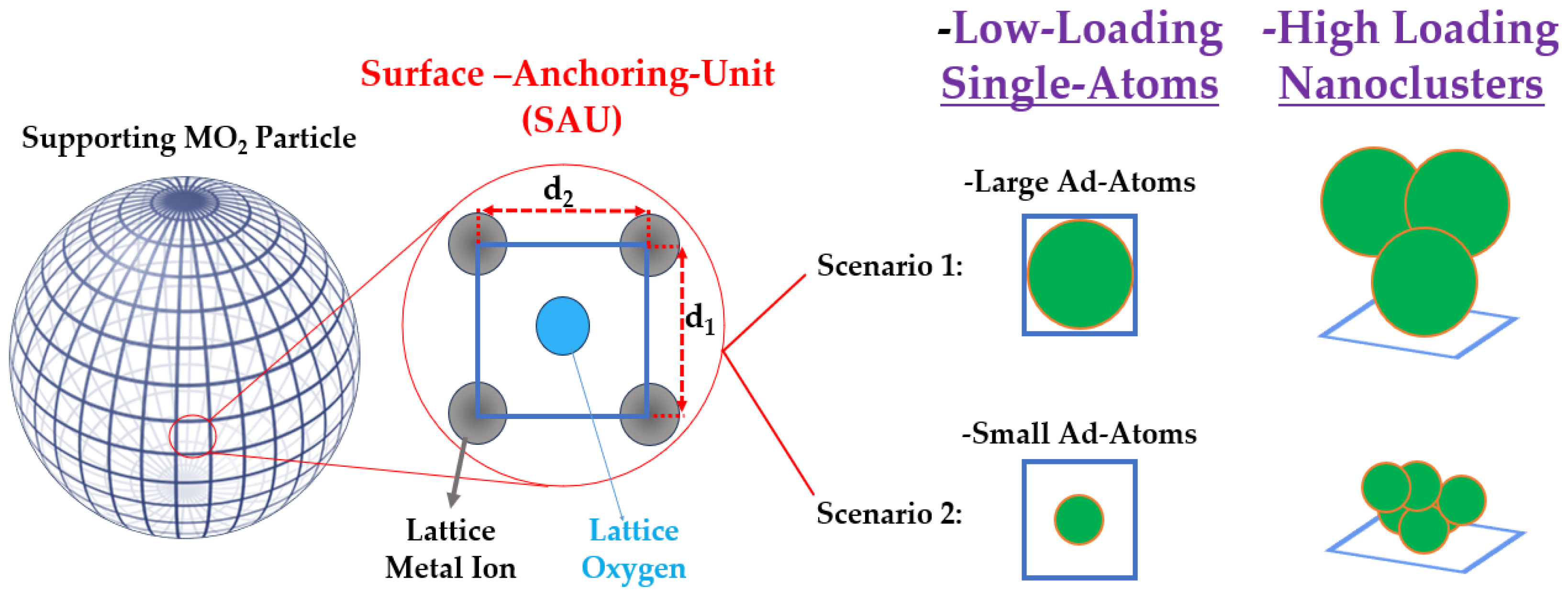
Determining the optimal loading on a supporting particle is inherently challenging, partly because there can be a significant difference between the nominal amount of ad atoms, i.e., used in the precursors during the anchoring process, and the actual amount that remains anchored. As shown schematically in Figure 2, the geometrical characteristics of the semiconductor’s surface and the size of the ad atoms allow us to anticipate some limits of single atoms versus nanocluster deposition.
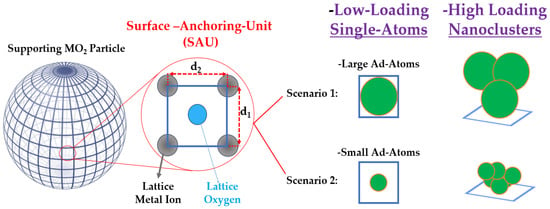
Figure 2.
Mode of ad atom anchoring per surface anchoring unit (SAU) on the support. Assuming that anchoring of ad atoms occurs on the O atom for each SAU, then at low loadings, 1 ad atom or less is anchored per SAU. This corresponds to single atom co-catalyst configuration. At higher loadings > 1 ad atom/per SAU will result in nanocluster formation, where the agglomeration degree is influenced by the SAU and the interactions between eh ad atoms. When the interactions between the ad atoms predominantly stabilize larger agglomerates, then small nanoparticles start to be favored.
To illustrate this, Figure 2 depicts an ideal spherical particle whose surface is divided into “surface-anchoring-unit” (SAU). In a typical metal oxide, ad atoms preferentially anchor to the oxygen (O) atoms of the lattice. On a simple MO2 oxide surface, four metal atoms (M) frame each O atom, defining a SAU that spans an elementary surface of 0.1 to 1 nm2, i.e., based on typical atomic diameters of 1–3 Å and M–O bond lengths of 1–2 Å. In this configuration, if one SAU binds one ad atom, that constitutes the single atom scenario; if the number of ad atoms per SAU exceeds one, clusters will inevitably form. In this context, the size of the ad atom itself (see Table 1) will dictate how many atoms can be accommodated at a minimum in a small nanocluster per SAU.

Table 1.
Parameters of atoms commonly used as ad atoms in photocatalysis.
Table 1.
Parameters of atoms commonly used as ad atoms in photocatalysis.
| Ad Atom | Co | Ni | Cu | Sn | Bi | Pd | Ag | Pt | Au |
|---|---|---|---|---|---|---|---|---|---|
| Most common Oxidation State while on support | 2+ | 2+ | 2+ | 2+ | 3+ | 0 | 0 | 0 | 0 |
| Atomic Diameter (Å) * | 1.09 | 1.10 | 1.14 | 1.44 | 1.92 | 4.04 | 3.44 | 1.2 | 2.74 |
| Atomic Weight ** | 58.93 | 58.69 | 63.55 | 195.08 | 106.42 | 107.87 | 118.71 | 196.97 | 208.98 |
* Data taken from [33]; ** data taken from [34].
So far, although great progress has been achieved, the precise control of ad atom deposition in photocatalytic systems still faces many challenges, and diligent optimization is required in order to reach their maximum performance. A maximized co-catalyst loading may be targeted (increased surface density) while avoiding single atom agglomeration into sub-nanoclusters or even small nanoparticles [35,36,37]. Interestingly, recent works [38,39] in photocatalytic hydrogen production show that a low ad atom loading can be sufficient for enhanced reaction rate in cases where the semiconductors possess efficient electronic properties. For example, Qin et al. [39] studied the effect of Pt co-catalysts on TiO2 semiconductors using different loadings of Pt leading from low to high SACC density. Their data revealed that the optimal Pt loading, i.e., for maximum H2 production, is 2 × 105 Pt atoms per μm2 of TiO2 surface. A further increase in SACC density did not enhance the reaction rate [39]. Schmuki and Wu, in their review [40], clearly emphasized this aspect, discussing that semiconductors with well-defined structures and favorable electronic properties, e.g., materials with high carrier transport and surface harvesting lengths, can significantly reduce the need for a high loading density of Pt atoms. Conversely, using highly defective semiconductors or semi-insulating materials necessitates a greater co-catalyst loading and/or results in underperforming systems [40].
1.2. Perovskites in Photocatalysis
Originally, the term “perovskite” was used to describe crystal structures that follow the chemical formula ABX3, where A and B are usually metal cations (with A being larger than B), and X is a non-metal anion [41,42]. The structure formed is a cubic unit cell with the A cation occupying the corners, the X anion occupying the face-centered positions, and the B cation occupying the middle of the cube [42]. This configuration is highly symmetric, with different patterns of periodicity depending on the lattice point of reference. Over the years, the group of perovskites has been expanded to fit all compounds that form the perovskite structure without strictly obeying the ABX3 chemical formula [43]. The wide category of ABX3 used in photocatalysis includes perovskite-oxides like ABO3. This gave rise to new perovskite genres like high-entropy perovskites, e.g., La(Mn0.2Co0.2Fe0.2Ni0.2Cr0.2)O3 [44] and A-site deficient perovskites, e.g., La0.6Sr0.2Ti0.85Ni0.15O3−−δ [45]. In addition, non-oxide perovskites have been developed, e.g., halide perovskites. CH(NH2)2PbBr3−xIx [46,47].
Perovskite-oxides, ABO3, are usually semiconductors [48]. Strontium titanate (SrTiO3), for example [26,49], a widely used perovskite-oxide photocatalyst [50], forms a valence band predominantly comprised of oxygen 2p orbitals hybridized with states from the A-site (Sr) and B-site (Ti) cations, while the conduction band is mainly composed of the transition metal B-site cation’s 3d t2g and eg orbitals [51]. Titanium is in a Ti4+ oxidation state (3d0 configuration), which results in a fully occupied valence band and a completely empty conduction band [52]. Consequently, the Fermi level sits at the top of the valence band. However, the energy band structure discussed above concerns an ideal perovskite structure without defects. If defects were to be considered, they would add mid-gap states, which would allow some electrons to jump to the conduction band [53]. Therefore, the Fermi level would shift toward the middle of the band gap, charge separation would become more efficient, and the semi-conductor properties of the material would further improve [54,55,56]. In general, most perovskite-oxides, like ABO3, exhibit an electronic structure where the valence band is predominantly formed by oxygen 2p orbitals and the conduction band mainly consists of transition metal d orbitals [57]. As a result, the band gap is highly tunable through compositional variations and defect engineering, making perovskites favorable for catalytic applications [58].
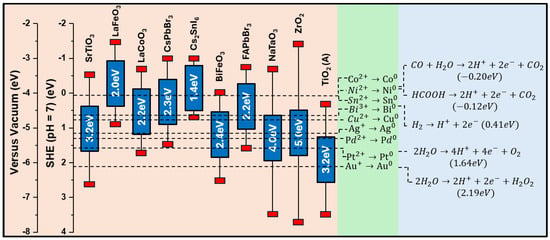
Figure 3.
Band structure of several perovskite photocatalysts, including ZrO2 and TiO2 as reference single metal oxides, are displayed on the right side of the figure. The redox potentials of selected atoms, commonly used as ad atoms/co-catalysts, are also shown in the middle of the figure. Some fundamental catalytic reactions, followed by photocatalysis, are displayed on the right side of figure. Redox potential values were obtained from [59,60,61,62,63,64,65,66]. Notice that all redox values in (eV) in the Y-axis are [T = 25 °C] vs. the standard hydrogen electrode at pH = 7, i.e., all Y values are offset by +0.59 mV pH vs. the value at pH = 0.
Figure 3.
Band structure of several perovskite photocatalysts, including ZrO2 and TiO2 as reference single metal oxides, are displayed on the right side of the figure. The redox potentials of selected atoms, commonly used as ad atoms/co-catalysts, are also shown in the middle of the figure. Some fundamental catalytic reactions, followed by photocatalysis, are displayed on the right side of figure. Redox potential values were obtained from [59,60,61,62,63,64,65,66]. Notice that all redox values in (eV) in the Y-axis are [T = 25 °C] vs. the standard hydrogen electrode at pH = 7, i.e., all Y values are offset by +0.59 mV pH vs. the value at pH = 0.
As a result, perovskites may possess favorable light-absorption properties [67], high extinction coefficients, and extended charge-carrier diffusion lengths, allowing them to efficiently capture light and transport photoinduced charge carriers over relatively long distances to surface sites for redox reactions [67]. Furthermore, in this context, perovskites, characterized by their high thermal stability and optimal crystal structure, have been shown to significantly improve the stability of precious metals, making them excellent candidates as supports for developing single atom catalysts [68,69].
Aspects of Energetics of Photocatalysts and Single Atom Co-catalysts: Photocatalytic reactions can be considered to consist of reduction and oxidation. In this context, it is important to view the electronic properties of perovskites as well as their decorating ad atoms. In Figure 3, we summarize the energy states (at pH = 7) of some prominent perovskite and oxide materials, along with the redox energies of several single atoms that are commonly used as ad atoms/co-catalysts in conjunction with the metal oxide perovskites. In addition, in Figure 3, some fundamental redox reactions (pH = 7), typically created via photocatalysis, are presented.
Some combinations of these materials decorated with single atoms have been studied to develop a comprehension of the underlying energetics. Two Y-axes are present in the graph: one represents the redox energy scale versus the standard hydrogen electrode (SHE) at pH = 7, and the other is versus the vacuum energy scale. Both scales serve a different analysis purpose but are of equal importance. The redox energy scale was created as a reference for all catalytic procedures that occur in aquatic environments. This scale offers the flexibility of altering the pH of the aquatic solution, i.e., the Nernst equation [70] implies that for every unit increase in pH, one must add 59 mV per electron to the potentials at scale. In Figure 3, a SHE pH = 7 scale was used, and the 7 points of increased pH caused a +0.413 eV shift to all values. Thus, the hydrogen oxidation reaction is +0.41 eV at pH = 7. While reading values of the redox energy scale, one must bear in mind that the more positive an energy level is, the more oxidizing the environment it creates, while the more negative a redox energy level gets, the more reducing its environment becomes. The vacuum scale is referenced to the commonly accepted value of energy required to excite an electron to the point it goes far away from any interaction (to the vacuum). This energy level is, by definition, located at 0 eV on the vacuum scale, and it corresponds to −4.44 eV in the redox energy scale vs. SHE at pH = 0 scale, i.e., −4.03 eV at pH = 7.
Regarding the positioning of the ad atoms/co-catalysts in Figure 3, more positive redox energy values indicate atoms requiring a strong oxidizing potential to be stabilized in their ionic form. Otherwise stated, atoms located at the bottom of the redox energy scale are more easily stabilized than their M0 metal state. Thus, atoms of Ag and Pt on oxide surfaces tend to be stabilized in their reduced M0 metal state. This is determined by the local redox potential, which is created locally at the interface of the ad atom and the metal oxide photocatalyst. Although Pd and Ag are noble metals, they are more prone to oxidation than Au or Pt. This has been elucidated recently for the case of Pd on TiO2, where we discovered multiple Pd oxidation states [71], i.e., Pd3+, Pd2+, and Pd1+, formed depending on the layer thickness of Pd on TiO2 [71]. Using electron paramagnetic resonance as high-resolution spectroscopy, we demonstrated that ultrafine, single atom Pd deposition formed Pd0/Pd1+, while thicker nanoclusters also contained Pd2+/Pd3+ states [71]. These Pd-ad atoms have been shown to be highly active in H2 photocatalysis and catalytic H2 production from HCOOH [72]. On the other end, atoms with redox energy at more negative values in Figure 3 require very negative potentials to be reduced, and thus they tend to remain in their oxidized states. Consequently, when deposited on the photocatalyst surface, they will, in principle, be found in their cationic state.
These trends can be altered by the redox action of the photoinduced holes and electrons, which possess the redox energies of the corresponding VB-top and CB-bottom of their parent semiconductor (see Figure 3). Thus, semiconductors with highly reducing CB (e.g., NaTaO3, SrTiO3, etc.) should generate electrons in their CB that are capable of reducing Ni2+ to Ni0, Co2+ to Co0, and Cu2+ to Cu0. On the other hand, TiO2 with less-reducing CB cannot reduce Ni2+ to Ni0 or Co2+ to Co0, but it can reduce Cu2+ to Cu0. Meanwhile, all the semiconductors in Figure 3 should be able to donate electrons to Au, Pt, Pd, and Ag.
In the same context, the reduction of CO2 to HCOOH is only favorable for the high-CB semiconductors and less so for TiO2 and BiFeO3. It is important to take into account that interfacial energetics can change these considerations drastically, i.e., the energy redox states of CO2/HCO3/HCOOH may differ at the interface with a semiconductor, and therefore, the efficiency of CO2/HCO3 photocatalytic reduction toward HCOOH can be significantly influenced.
As mentioned above, perovskites possess flexible structural and electronic properties divergent from their parental metal oxide, which makes them desirable for various photocatalytic applications. For example, as shown in the redox energy diagram (Figure 3), the TiO2 (Anatase) semiconductor possesses a mildly reducing CB edge, with a band gap of 3.2 eV. After perovskitization, in which TiO2 transforms into ABO3-type SrTiO3, the conduction band is shifted toward more negative redox energies. However, due to their special chemistry, perovskite synthesis is quite demanding, as it requires the simultaneous incorporation of two cations, A and B, into the ABO3 lattice with the precise stoichiometric ratio of 1:1:3 [73,74]. Consequently, the control of atomic dispersion of single atoms or sub-nanoclusters on their surface is more complex compared to metal oxide substrates.
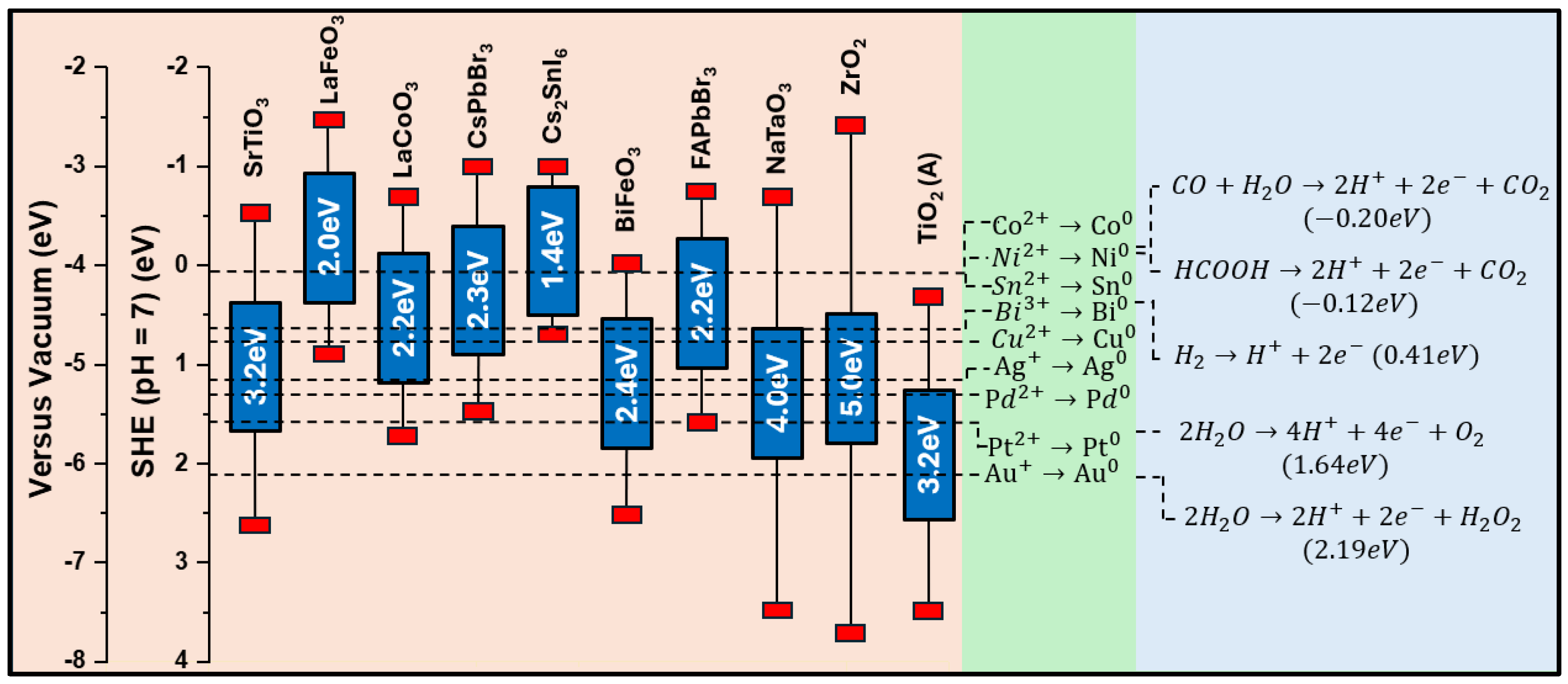
In the present review, we discuss the main key challenges in achieving efficient interfacing of a photocatalyst with single atoms, sub-nanoclusters, or quantum-sized small particles. We discuss three different aspects (as shown in Figure 4):
- The synthesis methods. Due to the demanding nature of the controlled formation of SAs vs. NCs vs. QSSPs and their anchoring on the catalytic particle, synthesis poses standalone challenges. This may result in a mixture of SAs and NCs or NCs and QSSPs. We discuss cases where these issues are not clear or there are misassignments of SAs to NCs or NCs to QSSP and vice versa.
- The detection–analysis methods. The de facto low-atom concentration required to form SAs, NCs, or QSSPs excludes their detection by traditional material characterization methods, e.g., X-ray diffraction, and requires specific spectroscopic microscopy techniques, often a combination of them. We discuss examples where a specific method provides the most convincing data on the distinction between SAs, NCs, and QSSPs.
- The performance characteristics. The gain in performance determines whether SA, NCs, or QSSPs are preferable for a specific photocatalytic system. We discuss examples where a clear benefit is achieved by a certain configuration and discuss possible trends and generalizations, if allowed by the data.
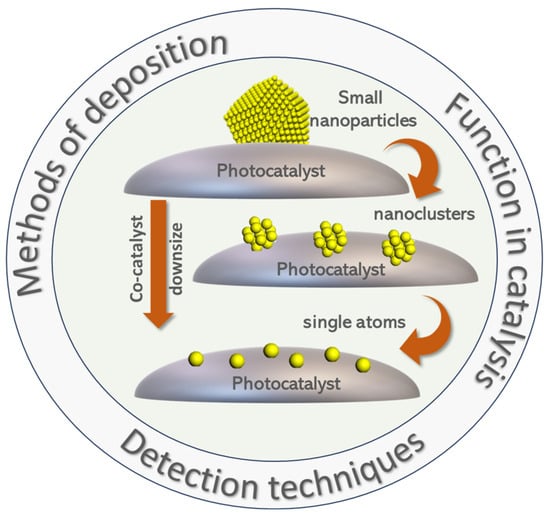
Figure 4.
The main challenges concerning SAs/NCs/QSSPs as co-(photo)catalysts: methods of deposition on the substrate, characterization techniques used for the detection of the aggregation state of the co-catalyst, and their function in various photocatalytic applications.
Figure 4.
The main challenges concerning SAs/NCs/QSSPs as co-(photo)catalysts: methods of deposition on the substrate, characterization techniques used for the detection of the aggregation state of the co-catalyst, and their function in various photocatalytic applications.
For coherence, this review focuses on metal atom co-catalysts interfaced with metal oxides as photocatalysts. Specifically, we focus on metal-oxide perovskite photocatalysts, e.g., of the type ABO3, since they show high-premise and higher photocatalytic efficiency vs. the corresponding single-metal oxides [48]. For completeness, we include selected non-oxide perovskites as pertinent examples to discuss the synthesis or the detection method for the co-catalyst and their role.
2. Synthesis Methods
The fundamental concept of a single atom co-catalyst depends on achieving this highly dispersed state of isolated single metal atoms anchored on suitable supports. This mechanism is not trivial, as metal atoms possess high surface free energy and therefore tend to aggregate into clusters or even nanoparticles during synthesis or subsequent treatments. In order to navigate toward successful single atom anchoring, synthetic strategies must adopt strong metal–support interactions and often involve carefully selected modifications of the support. For instance, lattice or surface defects in TiO2 and vacant sites have been reported as prominent options to stabilize single Pt atoms on the support’s surface [75,76,77,78]. Defects may prevent atom aggregation via the promotion of the formation of strong bonds between single atoms [79]. For instance, a DFT study on the surface of Fe3O4 demonstrated that oxygen vacancies favor the anchoring of single, noble metal atoms [76]. Awide range of synthetic approaches have been employed to prepare SACCs, encompassing both wet and dry chemistry categories, each with its own advantages and drawbacks that influence factors such as uniform distribution and overall catalyst stability. Metal loading is another critical parameter influenced by the synthesis method. Currently, most practices resort to low metal loading (0–2 wt.%), to minimize aggregation. Thus, further advancements in synthesis techniques and support engineering will be necessary. Alternatively, flame spray pyrolysis [73] is an industrial-scale approach to achieve precise control over the size and structure of the catalyst and co-catalyst. Furthermore, due to the high temperatures involved in the synthesis, strong metal–support interactions occur between the co-catalyst and the support [80].
2.1. Wet Chemical Synthesis Methods
Wet chemical synthesis methods for single atom co-catalysts (SACCs) typically involve dispersing metal precursors within a liquid medium and subsequently anchoring individual metal atoms onto a support under carefully controlled conditions [31]. Such approaches (see Table 2), which include wetness impregnation, adsorption, and strong electrostatic adsorption, can produce highly dispersed single atoms by promoting strong metal–support interactions and employing tailored reaction environments [31,75]. However, since metal atoms inherently tend to aggregate into clusters or nanoparticles, controlling reaction kinetics is critical. Key parameters such as pH, precursor concentration, and reaction temperature must be closely monitored [76]. Various techniques, including incipient wetness impregnation and strong electrostatic adsorption, can enhance spatial confinement and maintain high dispersion [75]. Although these methods often produce efficient catalysts, long-term stability may still pose a challenge [75]. To address this, post-treatment methods such as controlled reduction and pyrolysis have been employed [81].
2.2. Dry Chemistry Synthesis Methods
Dry chemistry methods are prominent strategies [31] for synthesizing single atom catalysts (SACCs) by achieving confined atomic dispersion on support. The dry chemistry mechanisms typically rely on controlled physical and chemical processes to anchor single metal atoms without involving solution-phase reactions. The most widespread examples of these methods are Atomic Layer Deposition (ALD) and pyrolysis [82,83,84,85]. ALD is characterized by the sequential exposure of support to metal precursors, enabling the precise deposition of single atoms through controlled ligand exchange reactions and surface binding [84]. This method provides high uniformity, atomic precision, and tunability of active site density, making it a valuable approach for producing stable SACCs [83]. On the other hand, pyrolysis methods [81] involve the thermal decomposition of metal–organic precursors under controlled atmospheres. Dry chemistry methods are notable for their ability to generate high-purity SACCs with exceptional structural stability, though challenges such as scalability and high energy demands remain to be addressed.

Table 2.
Literature summary of the synthesis methods used for single atom deposition on various substrates.
Table 2.
Literature summary of the synthesis methods used for single atom deposition on various substrates.
|
Nanostructures/ Substrates | Co-Catalyst | Synthesis Method | Classification of Synthesis Method | Ref. |
|---|---|---|---|---|
| LaFeO3 | Au | Deposition Precipitation | Wet chemistry | [68] |
| CsPbBr3 | Pt | Photo-deposition | Wet chemistry | [86] |
| SrTiO3 | Pt | Wet Impregnation | Wet chemistry | [87] |
| Cs2SnI6 | Pt | Wet Impregnation | Wet chemistry | [88] |
| CsPbBr3 | Pt | Photoreduction | Wet chemistry | [89] |
| FAPbBr3−xIx (FA = CH(NH2)2) | Pt | Co-precipitation | Wet chemistry | [90] |
| NaTaO3 | Ni | Flame Spray Pyrolysis | Dry chemistry | [74] |
| LaCoO3 | Pt | Solution-Mediated Adsorption | Wet chemistry | [91] |
| SrTiO3 | Ni | E-Beam Evaporation | Dry chemistry | [92] |
| BiFeO3 | Co | Immersion | Wet chemistry | [93] |
| SrTiO3 | Pd | Photo-deposition | Wet chemistry | [94] |
| SrTiO3 | Au | Sputtering | Dry chemistry | [95] |
| TiO2 | Cu | MOF synthesis | Wet chemistry | [96] |
| TiO2 | Cu | Wet Impregnation | Wet chemistry | [97] |
| TiO2 | Pd | Flame Spray Pyrolysis | Dry chemistry | [71] |
| ZrO2 | Ni | Solvothermal | Wet chemistry | [98] |
| ZrO2 | Pt | Wet-Impregnation | Wet chemistry | [99] |
3. Characterization Techniques for Detection of SACCs/NCs/QSSPs
One of the most challenging aspects of creating SACCs or NCs is to prove the atom anchoring on the support’s surface [100,101,102]. This objective pushes current characterization techniques and analyzation methods to their limits and it usually requires a combination of them in order to produce the desirable results [103] (see Figure 5). However, even with many techniques and methods in hand, it might still be difficult to determine the outcome of the single atom anchoring [102]. This is because not all single atoms have the same fate once they get on the surface of the support [75]. Some atoms might develop a strong metal–support interaction and remain as single atoms [104], but others might aggregate into clusters or nanoparticles or even infiltrate the support’s lattice [94]. These are all possible outcomes, and statistically speaking, they can all occur at once [103]. Nevertheless, each case becomes observable only when it accumulates a major percentage over the others. Bearing in mind that, in most studies, the SACCs’ loading percentage usually does not exceed 2.0 wt.%, one must be very aware of local phenomena while examining an SACC sample.
3.1. Indirect Characterization of SACCs/NCs
The characterization techniques used for SACCs/NCs can be divided into two major categories (see Figure 5): techniques that provide direct and indirect proof of single atoms [102,103]. The first group is comprised of procedures that can interact directly with single atoms [95,105] and provide data accordingly, and the second group can only exclude undesired outcomes [101]. For instance, aberration-corrected high-angle annular dark field scanning transmission electron microscopy (AC HAADF-STEM) can picture single atoms on the support’s surface if the Z contrast is favorable [95], but an X-ray diffraction (XRD) pattern can only exclude the formation of nanoparticles. This information is not very helpful as it only excludes one possible outcome, but it is necessary, nevertheless. Another technique that provides indirect data is Energy Dispersive Spectrometry (EDS) [96,99,103]. Although detecting the presence of single atoms is beyond its limits, EDS can either confirm or exclude the formation of clusters (and nanoparticles) by detecting areas with high aggregation (hot spots). In addition, it can provide information on the uniform and dispersed atom distribution on the support’s surface, which is favorable for single atom anchoring. However, EDS provides localized data, and it can be misleading. To overcome this uncertainty, another technique could be utilized that gives information on the total loading on the support. For instance, Inductively Coupled Plasma (ICP) spectrometry can provide the total loading percentage (per weight) of the desired element [96,106]. This percentage is usually around 2 wt.%, but it can vary depending on the supporting material. If a system is known to support up to 1.5 wt.% of single metal atoms and the ICP gives a loading percentage of 5 wt.%, it should raise suspicions about cluster formation. Lastly, Raman Spectroscopy can be utilized to study the vibrational modes of the support material [107]. Shifts in peak positions, intensities, and line shapes can be interpreted as local structure changes caused by the bonding of atoms on the surface. The shifts might be subtle, but if systematic in the Raman spectrum, they can provide supporting evidence [99].
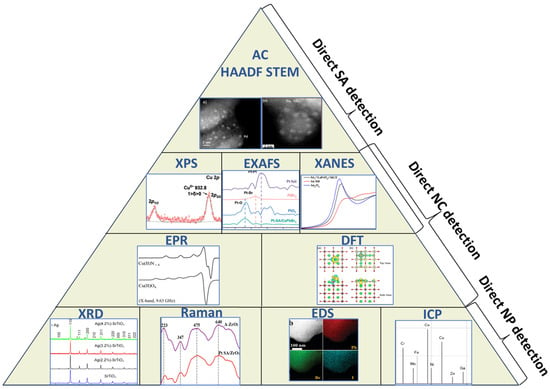
Figure 5.
Characterization techniques used for the detection of single atoms, nanoclusters, and small nanoparticles. AC HAADF-STEM: reproduced with permission from ref. [94]. Copyright {2024} Wiley-VCH. XPS: Reprinted (adapted) with permission from [97]. Copyright {2024}, American Chemical Society. EXAFS: Reprinted from [89] with permission from Elsevier. XANES: Reprinted from [68] with permission from Elsevier. EPR: Reprinted from [108] with permission from Elsevier. DFT: Reproduced from [109] with permission from the Royal Society of Chemistry. XRD: Reproduced from Ref. [110] with permission from the Royal Society of Chemistry. Raman: Reprinted from [99] with permission from Elsevier. EDS: Reproduced from Ref. [90] with permission from the Royal Society of Chemistry. ICP: Reproduced with permission from ref. [111]. Copyright {2016} Wiley-VCH.
Figure 5.
Characterization techniques used for the detection of single atoms, nanoclusters, and small nanoparticles. AC HAADF-STEM: reproduced with permission from ref. [94]. Copyright {2024} Wiley-VCH. XPS: Reprinted (adapted) with permission from [97]. Copyright {2024}, American Chemical Society. EXAFS: Reprinted from [89] with permission from Elsevier. XANES: Reprinted from [68] with permission from Elsevier. EPR: Reprinted from [108] with permission from Elsevier. DFT: Reproduced from [109] with permission from the Royal Society of Chemistry. XRD: Reproduced from Ref. [110] with permission from the Royal Society of Chemistry. Raman: Reprinted from [99] with permission from Elsevier. EDS: Reproduced from Ref. [90] with permission from the Royal Society of Chemistry. ICP: Reproduced with permission from ref. [111]. Copyright {2016} Wiley-VCH.
3.2. Direct Characterization of SACCs
AC HAADF-STEM: Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC HAADF-STEM) operates by focusing a finely tuned, aberration-corrected electron probe onto a thin, electron-transparent sample and collecting scattered electrons at high angles using an annular dark-field detector [68,96]. The electron scattering in the sample is proportional to the square of the atomic number (Z) of the scattering atom [103]. Heavier elements scatter electrons more and therefore appear brighter in AC HAADF-STEM imaging than lighter elements (see Figure 5). For ideal imaging, heavy elements (such as Pt and Au) should be anchored on a support comprising lighter elements. The bigger the difference in the atomic number, the greater the contrast. For instance, a study by J. Jones et al. reported Pt deposition over TiO2 or CeO2. Pt has an atomic number of 78, Ti 22, O 8, and Ce 58. While imaging Pt single atoms on TiO2 anatase, Pt appears 13 times brighter than the Ti atom (corresponding to the thickness variation of 3–4 nm along the (001) orientation) [112]. When the substrate was changed to CeO2, the contrast was way lower (corresponding to the thickness variation of ~0.5nm in the support) [113]. Consequently, AC HAADF-STEM in [112] could directly detect single atoms if the contrast with its support is adequate. An additional observation was that the sample examined must be sufficiently thin (e.g., <50 nm), and the area of interest must be at the edge of the support in order to have sufficient electron scattering [112].
Other complexities arise from surface morphology and thickness variations, as these can produce local bright signals unrelated to metal species [100]. To address such issues, the relative contrast between the metal atoms and the support must be carefully evaluated, sometimes through image simulations, complementary spectroscopic analysis (e.g., EDS or EELS), and comparison with known reference samples [103]. As such, AC HAADF-STEM, when combined with careful data interpretation, can provide compelling evidence for the presence of isolated metal atoms and their dispersion states on various supports, thereby aiding in the identification and characterization of SACCs.
SEM is also pertinent in SA research. Although SEM cannot directly visualize single atoms, given that the resolution is generally insufficient for sub-nanometer features, it can be employed to confirm the absence of nanoparticles above its resolution limit (~ nm in modern Field Emission-SEM instruments). Thus, if another technique (e.g., XPS) confirms Pt is present in the sample, but Pt particles are not visible by SEM, this indirectly supports the presence of sub-nanoclusters or single atoms. Furthermore, the combination of FE-SEM and XPS can efficiently scan large surfaces to compare nanoparticle and single atom contributions, facilitating rapid parameter screening during catalyst development [40].
X-ray Absorption Spectroscopy (XAS): X-ray Absorption Spectroscopy is an umbrella term for techniques that probe the local electronic and structural environment of an element within a material [101]. The main mechanism begins with the tunable X-ray radiation of the sample. As the energy of the X-rays is scanned across an absorption edge of interest, a portion of them will be absorbed and promote core electrons to unoccupied states (the near-edge region) or into the continuum (extended region) [114]. The first depends much on the density of unoccupied states and can therefore reveal changes in the oxidation state of the atom. The latter results in the emission of photoelectrons that will be scattered by neighboring atoms and interfere with each other either constructively or destructively [114]. This will create a fingerprint of the local coordination environment. Consequently, the absorption spectrum can be broken down into X-ray Absorption Near Edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS) [114].
XANES examines the absorption spectrum near the absorption edge of an element, providing insights into the oxidation state, electronic configuration, and coordination environment of the absorbing atom [114]. Single atom catalysts and small nanoclusters often display unique near-edge features, such as shifts in the edge energy or changes in the intensity of the “white line”, that differ from clusters and bulk materials [100]. The shift occurs because isolated atoms have distinctive arrangements for the valence electrons and better interaction with the support [102]. For instance, the “white line” intensity can reflect the number of unoccupied d-states, which differs when a metal atom gets anchored as an isolated atom because of the altered oxidation states [94]. By comparing the spectral fingerprints with well-studied references, one can find evidence of distinct electronic environments associated with single atom dispersion [86].
EXAFS analyzes oscillations in the absorption spectrum that occur tens to hundreds of eV above the absorption edge [114]. These oscillations arise from interference patterns of the photoelectron wave scattered by neighboring atoms, providing details on local atomic arrangements such as bond lengths, coordination numbers, and the identity of the elements surrounding the absorbing atom [114]. Each scattering path between atoms gives a distinct peak in the EXAFS spectrum and can be used to identify the existence of single atoms. For highly dispersed metal oxide species, metal-oxygen contributions will be primarily seen in the spectra because oxygen atoms (as well as other elements from the support) will typically be the nearest neighbors of the absorbing atom, and they comprise the “first shell” [114]. The next-nearest neighbors comprise the “second shell”, and it should include the same contributions as the first shell if single atoms are the case. If strong metal–metal contributions arise from either shell, it indicates that metal atoms are too close (suggesting below 5 Å), which is the effective range of the EXAFS spectra) and are probably forming clusters.
Although extensively applied, XAS methods have several notable drawbacks apart from the high cost mentioned in the following table. Firstly, the high-intensity synchrotron beams essential for detection can inflict “beam damage”, particularly if samples contain minimal amounts of the element in question or if measurements must be taken under in situ/operando conditions [115,116,117]. Prolonged exposure may artificially modify oxidation states or induce clustering, thereby complicating accurate data interpretation. Secondly, EXAFS does not directly yield a single atom structural model. Instead, interpreting its data requires fitting to proposed structural frameworks, making outcomes heavily dependent on the chosen assumptions. Lastly, EXAFS is inherently an averaging method, so all atoms of a given element contribute to the final signal. If a sample contains multiple local configurations, distinctions crucial to single atom behavior might be masked [114,116]. Therefore, corroborating EXAFS findings with additional analytical techniques, alongside control experiments to account for beam effects, is strongly recommended for reliable characterization.
X-ray Photoelectron Spectroscopy: XPS is a surface-sensitive analytical technique that measures the binding energies of core-level electrons by exciting them to the point they escape the atom and the material [118]. As a result, it provides insight into the chemical states and electronic environments of elements present at or near a material’s surface. By detecting subtle shifts in binding energy and changes in peak intensity or shape, XPS can distinguish between different oxidation states, coordination environments, and the influence of support interactions on atomic species [89]. When applied to the study of single atom catalysts, these principles are particularly useful. Isolated atoms anchored on support often exhibit unique electronic signatures compared to atoms in clusters or bulk materials [99]. The altered electron density and modified chemical bonding at the scale lead to characteristic binding energy shifts and distinct spectral features in XPS data [99]. Through careful analysis of these changes and with comparison to known reference states, XPS can assist in confirming the presence of single atom sites.
Despite its usefulness, XPS only probes a shallow depth (~1 nm), so it may struggle with very low loadings of SA. Additionally, beam-induced transformations can occur, as mentioned in Table 3, such as the reduction of Pt (IV) to Pt (II), sometimes in a matter of minutes [119,120]. Careful experimental design—like minimizing exposure times or using filters—is crucial to mitigate damage. Furthermore, accurate spectral fitting is essential; improper calibration, handling of spin–orbit coupling, and peak deconvolution can lead to incorrect conclusions. Consequently, while XPS is a key tool for identifying single atom sites, caution must be exercised to ensure reliable data.

Table 3.
Some advantages/limitations of main characterization techniques used to identify and distinguish single atoms and sub-nanoclusters.
Electron Paramagnetic Resonance: EPR spectroscopy is a technique applicable to paramagnetic species, as mentioned in Table 3, that utilizes the Zeeman effect on unpaired electrons and measures their microwave absorbance as they transit between energy states [121]. More specifically, as the magnetic field is scanned in a selected range, unpaired electron spins in the sample absorb energy at specific resonant frequencies, producing characteristic EPR signals [121]. These signals are influenced by factors such as the electronic environment, the nature of neighboring atoms, and the symmetry of the local coordination site. For single atom catalysts, if the individual metal atoms possess unpaired electrons (as is often the case for certain transition metals like Cu) [122], their EPR spectra may exhibit distinct signatures that differ substantially from those of clusters or bulk phases [96], which tend to have more complex and broadened EPR responses. By comparing the EPR parameters such as g-values, hyperfine splittings, and line shapes with known references and simulated data, one can conclude the presence of isolated atoms [121]. Thus, EPR can be a valuable tool for confirming single atom confinement of paramagnetic species [123].
Carbon Monoxide Diffuse Reflective Infrared Fourier Transform Spectroscopy (CO-DRIFTS) uses CO as a probe molecule to identify and characterize metal or metal-oxide sites by tracking CO’s vibrational signals upon adsorption [124,125]. In practice, CO interacts with different surface sites—such as isolated atoms, nanoclusters, or bulk phases—yielding distinct IR peaks (e.g., linear and bridging) that reflect variations in bonding environments. This makes CO-DRIFTS especially valuable for detecting single atoms or nanoclusters anchored on supports, as linear CO often shows sharp, narrow peaks indicative of isolated sites. For instance, B. Han et al. utilized CO-FTIR to distinguish between single atoms and nanoparticles anchored on TiO2 [17]. Nonetheless, the method has certain drawbacks. For instance, surface sites that do not bind CO strongly may remain undetected, and overlapping signals from other metal oxides or structural vibrations can complicate data interpretation. Moreover, low metal loadings or highly heterogeneous samples may produce weak signals, affecting sensitivity [124]. Despite these limitations, with careful experimental design—such as selecting appropriate backgrounds and pretreatment protocols—CO-DRIFTS remains a robust tool for examining metal/metal oxide surfaces at the single atom or nanocluster level [126].
In addition to experimental detection methods, theoretical tools such as Density Functional Theory (DFT), a quantum mechanical modeling method, can be used to investigate the electronic structure of many-body systems, primarily atoms, molecules, and solids [127]. Reviewing the theoretical methods is out of the scope of the present review; however, the interested reader can consult pertinent review articles on DFT simulations on ad atoms on metal oxides [128,129,130]. When applied to single atom catalysts, DFT calculations can predict how isolated atoms interact with the support, estimate binding energies, forecast reaction pathways, and simulate spectroscopic signatures (for example, shifts in core-level energies or changes in vibrational frequencies). DFT simulation may also extend to catalytic performances [109]. If the simulated data align with the experimental results, they can serve as solid proof for single atoms’ existence [95].
4. Single Atoms/Sub-Nanoclusters and Quantum-Sized Small Particles of Co-Catalysts on Perovskite Oxide and Non-Perovskite Oxide Substrates
In this section, we discuss cases of SA/SNC and/or QSSPs deposited on perovskite oxide and non-perovskite oxide substratesreporting the synthetic routes and emphasizing the characterization techniques used to identify the exact configuration of the co-catalyst, in addition to their performance on specific photocatalytic applications.
4.1. Single Atoms/Sub-Nanoclusters and Quantum-Sized Small Particles of Co-Catalysts on Perovskite Oxide
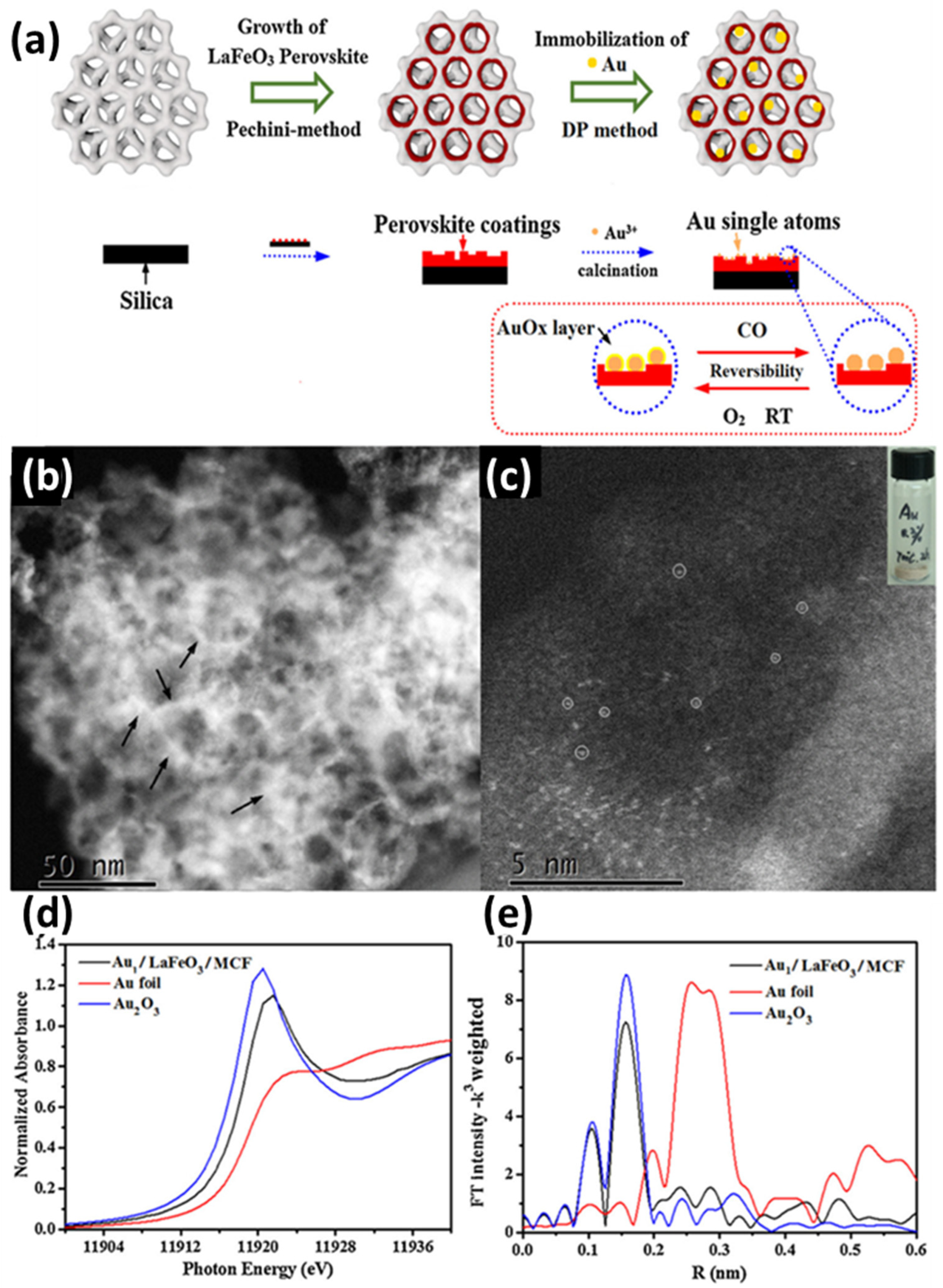
C. Tian et al. reported [68] the synthesis of a hierarchical LaFeO3 perovskite support decorated with stable single Au atoms. The synthesis of the substrate LaFeO3/MCF was based on the Pechini method [131], and the Au species was later incorporated through a deposition–precipitation process employing HAuCl4 in water, achieving an approximate loading of 0.3 wt.%. The final structure was obtained by calcinating the Au-loaded LaFeO3/MCF pre-catalysts in air at 700 °C for 2 h. To examine the dispersed state of the Au species, HAADF-STEM was utilized [68]. As anticipated, numerous randomly distributed atomic Au species were identified, marked by white circles (see Figure 6c). The atomically dispersed individual Au atoms were further verified via XAFS spectroscopy. Additionally, according to the XANES of Au1/LaFeO3/MCF, Au foil, and Au2O3, Au1/LaFeO3/MCF exhibits a strong peak around 11,921 eV (see Figure 6d [68]), which corresponds to the depletion of Au d orbitals from their metallic d10 configuration. This indicates that the Au species supported on the perovskite exhibit notable oxidic characteristics, similar to the Au3+ in Au2O3. DFT calculations were performed to confirm the presence of oxidized Au species on the LaFeO3 surface [68]. Overall, Au adsorption on LaFeO3 surfaces was found to be stronger than on TiO2, aligning with the observed greater resistance to sintering, proving that LaFeO3 perovskite is a better substrate to noble metals than TiO2 metal oxide [68]. The Au1/LaFeO3/MCF catalyst was evaluated by its catalytic activity toward CO oxidation. By leveraging the strong interaction between gold atoms and the LaFeO3 perovskite support, this system achieves exceptional stability and activity under reaction conditions. The synergistic combination of LaFeO3’s structural and electronic properties with the catalytic efficiency of gold single atoms enables highly selective and efficient photocatalytic performance, even under challenging conditions [68].
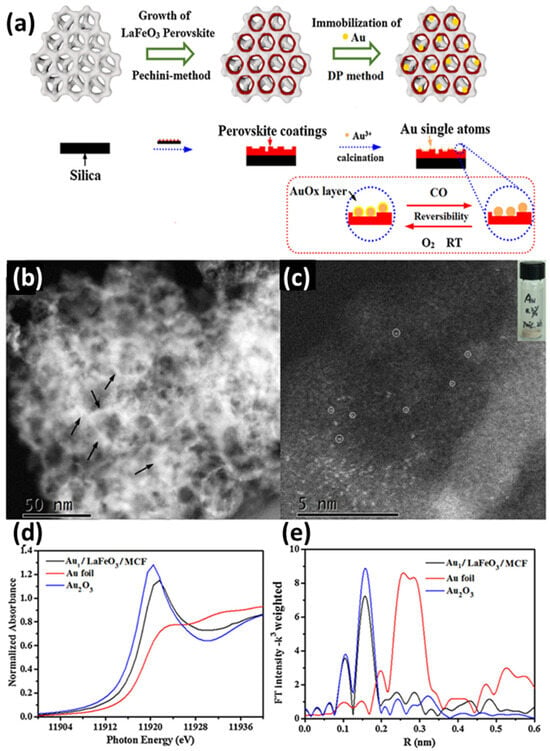
Figure 6.
(a) Schematic illustration of the Au1/LaFeO3/MCF preparation process; (b) STEM image (the black arrows highlight the region of the HAADF-STEM images) and (c) HAADF-STEM image of Au1/LaFeO3/MCF (the white circles marks the Au single atoms); (d) X-ray absorption near edge spectrum (XANES) of Au1/LaFeO3/MCF, Au foil, and Au2O3; (e) and Au LIII-edge EXAFS of Au1/LaFeO3/MCF, Au foil, and Au2O3. Reprinted from [68], with permission from Elsevier.
Figure 6.
(a) Schematic illustration of the Au1/LaFeO3/MCF preparation process; (b) STEM image (the black arrows highlight the region of the HAADF-STEM images) and (c) HAADF-STEM image of Au1/LaFeO3/MCF (the white circles marks the Au single atoms); (d) X-ray absorption near edge spectrum (XANES) of Au1/LaFeO3/MCF, Au foil, and Au2O3; (e) and Au LIII-edge EXAFS of Au1/LaFeO3/MCF, Au foil, and Au2O3. Reprinted from [68], with permission from Elsevier.
H. Shin and coworkers [91] reported the deposition of Pt single atoms on different supports, metallic, metal oxide, and perovskite nanosheets, reaching up to 3.94 wt.% Pt loading in the case of perovskite LaCoO3. The concept of the synthesis involved the use of N-doped graphene as a sacrificial template to locally constrain the single atoms [91]. The process includes adsorbing precursors of the desired support material onto the SA-stabilized template, followed by heat treatment to transfer the SAs onto the support material while removing the graphene layer [91]. By controlling the amount of the Pt during the synthesis, Pt single atoms (3.94 wt.% Pt) and Pt clusters (12.7 wt.% Pt) were formed on the LaCoO3 perovskite. HAADF-STEM analysis was performed on Pt-SAs/LaCoO3 and PtCl/LaCoO3 [91]. In the first case, Pt species were uniformly dispersed and atomically distributed across the perovskite’s surface, while in the latter, Pt clusters and small nanoparticles were observed. To further identify and compare single atoms and nanoclusters on LaCoO3, EXAFS analysis was utilized, confirming that in Pt-SAs/LaCoO3 [91] spectra, no Pt-Pt bond contribution is resolvable, while in PtCl/LaCoO3, the Pt-Pt bond appears, verifying the presence of clusters or small particles [91].
Djatoubai et al. [93] used Co single atoms to decorate the surface of BiFeO3 perovskite. The synthesis involved a facile hydrothermal method to form the perovskite and an immersion Wet chemistry method to deposit the Co species. The deposition of Co-SAs was successfully confirmed using aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC HAADF-STEM) and X-ray Absorption Near-Edge Structure (XANES) [93]. In the EDS spectrum, the homogeneous Co distribution on BiFeO3 was verified, while the high-resolution X-ray photoelectron spectroscopy confirmed the presence of Co2+ species. The photocatalytic system achieved a great O2 evolution response at solar water oxidation reactions, surpassing BiFeO3-based materials. The performance was attributed to the Co-SAs utilization, which enhanced charge-carrier separation and electron transport [93].
Y. Xing et al. [87] reported the synthesis of SrTiO3 perovskite decorated with either single atoms or large clusters and nanoparticles of Pt. The formation of Pt large clusters and nanoparticles on the substrate’s surface (Pt/SrTiO3) was conducted using an impregnation method, while the catalyst with the Pt single atoms was prepared by leaching the Pt/SrTiO3 catalyst (Pt/SrTiO3-L) [87]. The aggregation state of the Pt species was studied with High-Resolution STEM images (HR-STEM). In the case of Pt/SrTiO3 catalyst, Pt nanoparticles were observed and measured at 2.7 nm in size, while the image of Pt/SrTiO3-L catalyst single atomic Pt and small Pt clusters was detected [87], composed of 2–8 atoms. Based on the extended EXAFS results, unlike the standard Pt foil and PtO2 samples, no significant Pt–Pt bonds were detected in either of the catalysts [87]. In the Pt/SrTiO3-L catalyst, the peak at 1.58 Å, corresponding to Pt–O coordination, was sharper compared to that of Pt/SrTiO3. Additionally, the second peak observed at 2.8–3.2 Å, associated with PtO2 in the Pt/SrTiO3-L catalyst, deviates from the reference, indicating that the Pt species are predominantly sub-nanoscale and exist in a highly undercoordinated state [87]. The catalysts with different aggregation states of co-catalyst were evaluated via reverse water gas shift reactions, leading to the conclusion that the catalyst decorated with Pt single atoms/nanoclusters demonstrates higher CO2 conversion compared to the one with the large Pt clusters/nanoparticles [87]. The size effects of Pt in the RWGS reaction were studied using DRIFTs and DFT methods. Single Pt atoms/Sub-nanoclusters showed the highest activity and CO selectivity via the “–COOH route”, [87] whereas larger Pt particles promoted further CO hydrogenation. Over Pt/SrTiO3, the rate-determining step is the reaction between formate and H*, which can be hindered by the strong formate adsorption observed on large Pt clusters and nanoparticles [87].
Z. Wang et al. [92] studied the decoration of Ni atoms on SrTiO3. In their study, Ni atoms, as well as clusters, were reportedly deposited on the SrTiO3 (100) surface. The perovskite material was purchased readily in the form of single crystals (5 mm × 5 mm × 5 mm), doped with Nb 5 wt.% [92]. The Ni deposition was carried out through the e-beam evaporation method and produced two samples of different Ni coverage. The first sample had 0.01 Å of Ni coverage (an assumption based on the vapor deposition rate), and the second sample had 0.05 Å of Ni coverage on its surface [92]. Scanning transmission electron microscopy (STEM) experiments were carried out for these samples. For the first sample, the results revealed Sr adatoms, as well as two types of bright protuberances that were labeled as Ni atoms because of their adsorption site and their height of approximately 150 pm [92]. Three orientation models were examined using DFT calculations [92] and were proposed as the most energetically favorable over all other possible orientations. Regarding the second sample, Ni sites were observed at 300–400 pm height and were attributed to cluster formation. Valence-band photoelectron spectroscopy (VB-PES) experiments were later conducted and found new in-gap states on different energy levels for the two samples. While the techniques mentioned within this paragraph were used thoroughly, they cannot be considered adequate for precisely distinguishing the configuration of the ad atoms. As stated in previous sections, it is most possible that the results from an atom anchoring process follow a distribution that ranges from single atoms to nanoclusters and nanoparticles. STEM imaging can give a clear enough image but of local range, and more characterization techniques should be utilized to conclude this study [92].
A study on Pd-SrTiO3 by B. Yang et al. [94] does not report single atom deposition on the perovskite’s surface but rather single atom doping and decorating clusters [94] based on several pertinent characterization techniques (see Figure 7). Specifically in [94], two SrTiO3 samples were synthesized using a solvothermal method, and Pd introduction was created by means of an aqueous solution. There were several loading amounts ranging from 0.1 to 0.8 wt.%. The first sample was labeled as Pd-SrTiO3, and it would later be characterized as SrTiO3 with single atoms inside the lattice, and the second sample was labeled as Pd/SrTiO3, and it would later be characterized as Pd cluster-decorated SrTiO3. Regarding Pd-SrTiO3, AC HAADF-STEM (see Figure 7a) made a clear demonstration of highly dispersed Pd single atoms inside the perovskite lattice [94]. The existence of single atoms in the lattice environment was further confirmed by Pd K-edge EXAFS (see Figure 7b [94]). The same techniques were applied to the Pd/SrTiO3 sample and proved the existence of clusters through the observed Pd-Pd bonds [94]. The results were compared to Pd-foil for reference [94]. Several other techniques, including FTIR, Temperature-Programmed Desorption (TPD), EPR, and DFT, were used for the indirect identification of the single atoms and the clusters, as well as for the evaluation of the catalysts’ performance. The photocatalytic semi-hydrogenation of alkynes experiments demonstrated that the incorporation of Pd single atoms within the SrTiO3 lattice [94] (see Figure 7c), as opposed to being supported on its surface, effectively altered the adsorption behavior of phenylacetylene (PA) and styrene on the photocatalysts. Pd-SrTiO3, exhibiting a sufficiently strong interaction with PA but a relatively weak interaction with styrene, is expected to offer significant advantages in the photocatalytic semi-hydrogenation of PA, such as achieving high yield with excellent selectivity for styrene [94].
The use of SrTiO3 as support was also studied by J. Fend et al. [95] for decoration with Au single atoms. The team utilized commercially available SrTiO3 doped with 7 wt.% Nb and applied a thermal evaporation method for Au atom deposition. The sample preparation involved three steps: first, the deposition of Ti atoms to "reconstruct" the SrTiO3 surface, followed by the sputtering of Au+ ions, and, finally, calcination at high temperature to stabilize the structure [95]. The characterization part of this study only included scanning transmission electron microscopy equipped with Molecular Beam Epitaxy (MBE), Reflection High Energy Electron Diffraction (RHEED), and Low Energy Electron Diffraction (LEED) [95]. According to STEM images, bright and dark spots appear, while probably based on the Z factor previously discussed, the team attributed the bright spots to Au atoms [95]; however, these could also be small Au clusters since STAM cannot provide such resolution. They then compared the number of bright spots to the amount of Au loading to deduce that each bright spot contains only one Au atom.
Our group recently reported the synthesis of NaTaO3 perovskite oxide and simultaneously the deposition of ultrafine NiO nanoparticles using one-step flame spray pyrolysis technology [74]. By utilizing the Double Nozzle setup, two different nanocrystals were formed, leading to a tightly interfaced heterojunction. The deposited NiO nanoparticle’s size was calculated at an average size of 2 nm using TEM, XRD, and BET characterization techniques. Various Ni loadings were deposited on the NaTaO3 surface, and according to the H2 production evaluation, lower Ni content (0.5 wt.%) was optimal since, in the case of high Ni loadings, the coverage of NaTaO3 surface is higher, which leads to the inhibition of photoexcitation [74].
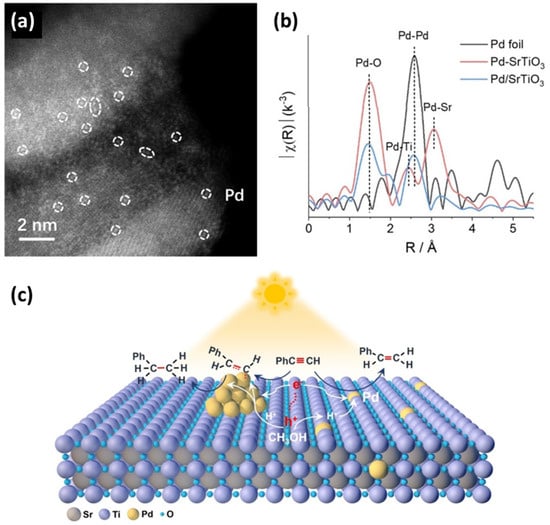
Figure 7.
(a) AC HAADF-STEM images of Pd-SrTiO3, with single atoms of Pd highlighted in white circles; (b) Pd K-edge EXAFS spectra of Pd foil, Pd-SrTiO3, and Pd/SrTiO3; (c) possible reaction mechanism of PA hydrogenation reaction over Pd-SrTiO3 and Pd/SrTiO3. Reproduced with permission from ref. [94]. Copyright {2024} Wiley-VCH.
Figure 7.
(a) AC HAADF-STEM images of Pd-SrTiO3, with single atoms of Pd highlighted in white circles; (b) Pd K-edge EXAFS spectra of Pd foil, Pd-SrTiO3, and Pd/SrTiO3; (c) possible reaction mechanism of PA hydrogenation reaction over Pd-SrTiO3 and Pd/SrTiO3. Reproduced with permission from ref. [94]. Copyright {2024} Wiley-VCH.
4.2. Single Atom/Sub-Nanoclusters and Quantum-Sized Small Particles of Co-Catalyst on Non-Perovskite Oxide
Hu et al. [89] reported that Pt single atoms can be deposited on the surface of CsPbBr3 nanocrystals using a photo-assisted method (see Figure 8a). This process involves partial oxidation of the surface, followed by anchoring Pt single atoms through the formation of Pt–O and Pt–Br bonds, resulting in a 1.04 wt.% loading amount of Pt. In the case of the non-oxidized surface, the formation of Pt nanoparticles is favorable since the metal–halide bind cannot efficiently stabilize the highly active Pt single atoms [89]. To verify the atomic deposition of single Pt atoms onto CsPbBr3 nanocrystals, energy-dispersive X-ray spectroscopy (EDS) (see Figure 8b) was performed, confirming that the Pt atoms were homogeneously dispersed on the perovskite’s surface [89]. Diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy was conducted using CO as a probe molecule to investigate the form of Pt in Pt-SAs/CsPbBr3. In the CO-DRIFTS spectrum of Pt-SAs/CsPbBr3 nanocrystals, a prominent peak appears around 2058 cm−1, corresponding to the linear adsorption of CO on Ptδ+ sites [89]. The absence of a CO bridge adsorption peak, typically associated with two Pt atoms, suggests that the Pt atoms remain isolated without forming Pt nanoparticles or experiencing significant agglomeration [89]. Additionally, according to the normalized XANES spectrum, the shape of Pt/CsPbBr3 differs from the other spectra, such as Pt foil, PtO2, and PtBr2, indicating a different electronic structure (see Figure 8d). The spectrum for the as-prepared 1.04% Pt-SAs/CsPbBr3 reveals two prominent features at approximately 1.7 Å and 2.2 Å, corresponding to Pt–O and Pt–Br scattering paths, respectively. Notably, no signal near 2.6 Å is detected, indicating the absence of Pt–Pt scattering paths [89], as observed in the reference Pt foil. These EXAFS findings confirm that Pt atoms are well-dispersed on the CsPbBr3 surface, forming Pt–O and Pt–Br bonds [89]. According to their photocatalytic performance in the semi-hydrogenation of propyne, Pt-SAs/CsPbBr3 displayed high quenching efficiency, which the researchers assigned to the formation of deep trap sites after Pt single atom deposition, resulting in fast transfer carrier from perovskite to Pt active sites during illumination [89].
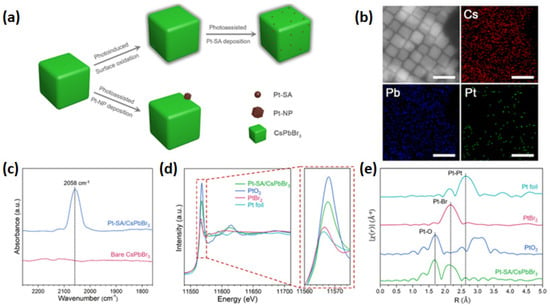
Figure 8.
(a) Schematic illustration of the formation mechanism of Pt-SAs/CsPbBr3 and Pt-NPs/CsPbBr3 and (b) HAADF-STEM image and EDS mapping of 1.04% Pt-SAs/CsPbBr3 nanocrystals. The scale bars are 20 nm. (c) DRIFT spectra of CO adsorption on bare CsPbBr3 nanocrystals and 1.04% Pt-SAs/CsPbBr3 nanocrystals. (d) Normalized XANES spectra at the Pt L3-edge of Pt-SAs/CsPbBr3, PtO2, PtBr2, and Pt foil. (e) Normalized k3-weighted Fourier transform spectra derived from EXAFS of 1.04% Pt-SAs/CsPbBr3, PtO2, PtBr2, and Pt foil. Reprinted (adapted) with permission from [89]. Copyright {2021} American Chemical Society.
Figure 8.
(a) Schematic illustration of the formation mechanism of Pt-SAs/CsPbBr3 and Pt-NPs/CsPbBr3 and (b) HAADF-STEM image and EDS mapping of 1.04% Pt-SAs/CsPbBr3 nanocrystals. The scale bars are 20 nm. (c) DRIFT spectra of CO adsorption on bare CsPbBr3 nanocrystals and 1.04% Pt-SAs/CsPbBr3 nanocrystals. (d) Normalized XANES spectra at the Pt L3-edge of Pt-SAs/CsPbBr3, PtO2, PtBr2, and Pt foil. (e) Normalized k3-weighted Fourier transform spectra derived from EXAFS of 1.04% Pt-SAs/CsPbBr3, PtO2, PtBr2, and Pt foil. Reprinted (adapted) with permission from [89]. Copyright {2021} American Chemical Society.
Another work on single Pt atom deposition on CsPbBr3 nanocrystals was provided by Y. Qin and coworkers [86]. The Pt/CsPbBr3 nanocrystals were prepared using the two-step wet chemistry method. First, the surface of the perovskite was partially oxidized via light irradiation in order to form Pb-O bonds. These active sites can effectively anchor and stabilize Pt single atoms through coordination with Br atoms. After Pt deposition, to avoid possible aggregates due to high temperature, a deuterium lamp was utilized at room temperature to stabilize the Pt single atoms on the perovskite substrate. The EXAFS profiles (see Figure 9d) [86] revealed that the Pt species were in an oxidized state, while from the EXAFS spectra in R space, it was found that except Pt-O and Pt-Br bonds, no other peaks assigned to Pt-Pt were observed (see Figure 9d). As concluded, Pt atoms are highly dispersed and isolated on CsPbBr3 perovskite. The Pt single atom/CsPbBr3 nanocrystals were evaluated to determine their electrocatalytic performance in the detection of ascorbic acid, reaching the detection limit of 0.0369 mM. Pt single atoms served as quantitative active sites for substrate adsorption and facilitated charge transfer by efficiently capturing electrons for electrochemical reactions [86].
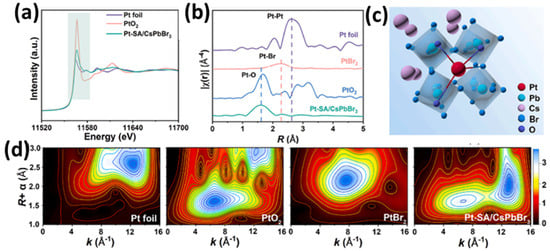
Figure 9.
(a) XANES spectra for Pt K-edge of Pt foil, PtO2, and Pt-SAs/CsPbBr3. (b) Fourier transform K-edge EXAFS spectra and (d) wavelet transforms of the k3-weighted EXAFS signals of Pt foil, PtO2, PtBr2, and Pt-SAs/CsPbBr3 and (c) schematic structural model of Pt-SAs/CsPbBr3. Reprinted from [86], with permission from Elsevier.
Figure 9.
(a) XANES spectra for Pt K-edge of Pt foil, PtO2, and Pt-SAs/CsPbBr3. (b) Fourier transform K-edge EXAFS spectra and (d) wavelet transforms of the k3-weighted EXAFS signals of Pt foil, PtO2, PtBr2, and Pt-SAs/CsPbBr3 and (c) schematic structural model of Pt-SAs/CsPbBr3. Reprinted from [86], with permission from Elsevier.
Based on a hydrothermal method followed by an impregnation method, P. Zhou et al. [88] reported strong anchoring of highly dispersed Pt single atoms on the surface of Cs2SnI6 perovskite. The conventional low-temperature and rapid crystallization methods, such as hydrothermal methods commonly used for synthesizing perovskites, inevitably result in the introduction of numerous defects into the perovskite structure [88]. As mentioned before, these defects on the substrate enhance the strong anchoring of the metal co-catalyst on the catalyst’s surface, resulting in strong metal–support interaction (SMSI). The loading of Pt single atoms on the substrate was calculated to be 0.12 wt.%. To confirm the formation of single Pt atoms on the perovskite’s surface, HAADF-STEM was employed [88]. According to the HAADF-STEM image and EDS mapping images presented, there is a uniform distribution of Pt single atoms on Cs2SnI6, while no Pt clusters or nanoparticles were observed [88]. Furthermore, the oxidation state and coordination environment of Pt single atoms were examined via XAFS. Based on the normalized Pt L3-edge XANES spectra for Pt-SAs/Cs2SnI6, PtI2, and Pt foil, it was found that the oxidation state of Pt in Pt-SAs/Cs2SnI6 exceeds +2, while the FT- EXAFS spectra [88] indicate that Pt atoms are anchored to the surface of PtSAs/Cs2SnI6 via a Pt–I bond. Pt-SAs/Cs2SnI6 was evaluated for photocatalytic hydrogen production in comparison to Cs2SnI6 decorated with Pt nanoparticles and pristine Cs2SnI6, resulting in an enhanced H2 evolution rate [88] for Pt-Cs2SnI6. Through a combination of charge-carrier dynamics studies and DFT calculations, we identified that the distinctive coordination structure and electronic properties of Pt–I3 species play a crucial role in the SMSI effect. This enhances photogenerated electron transfer from Cs2SnI6 to Pt single atoms while simultaneously lowering the Gibbs free energy and accelerating the kinetics of hydrogen production [88].
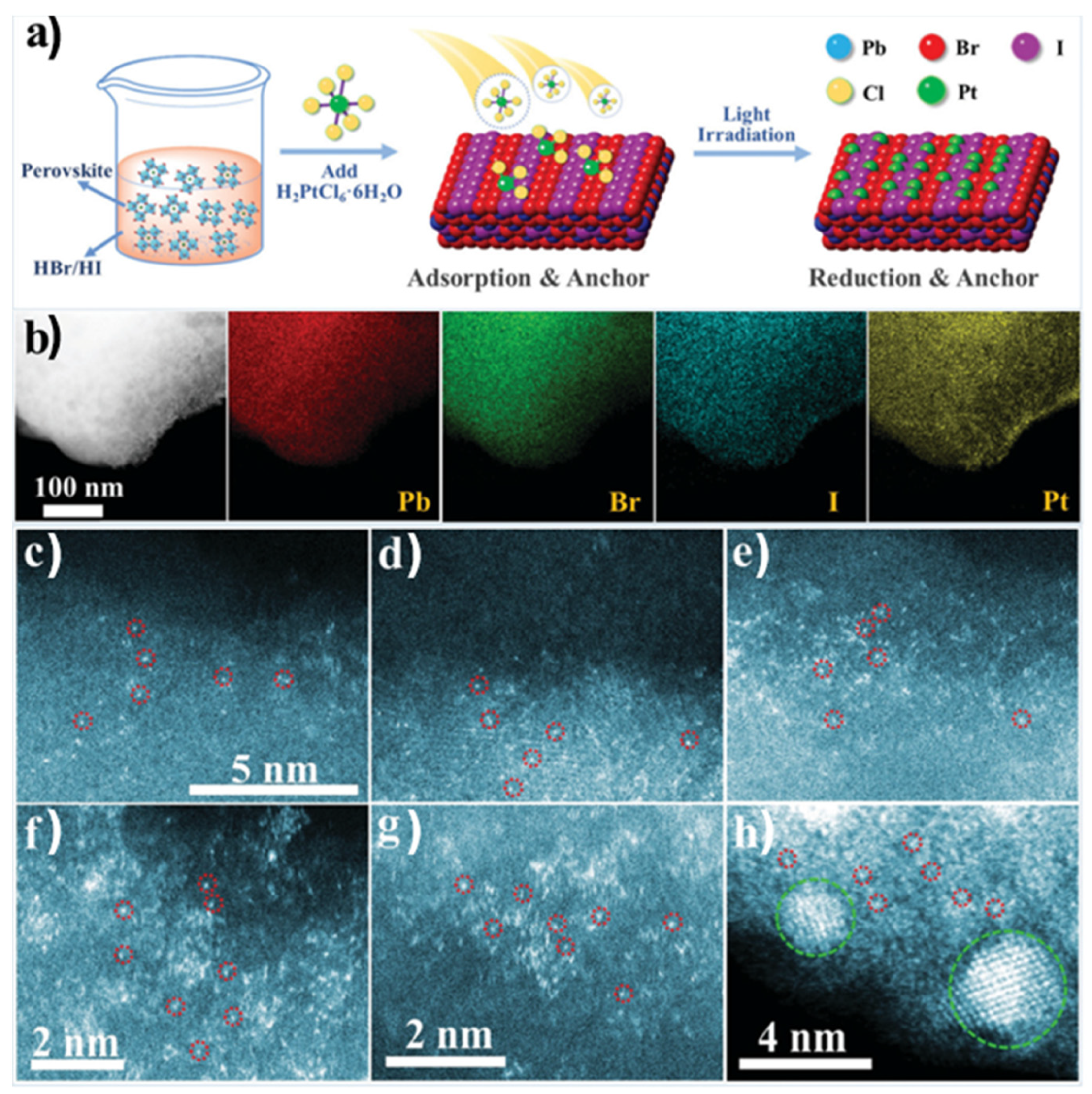
A different approach was followed by Y. Wu et al., employing an organometal halide perovskite as a substrate for single atoms Pt deposition [90] (see Figure 10a). The synthesis of FAPbBr3−xIx (FA = CH(NH2)2) particles involved a wet chemistry co-precipitation method, while for the Pt deposition on the surface of the perovskite, the Pt species were absorbed and reduced under light irradiation. Different Pt loadings were performed from 0.7 to 2.1 wt.% on the substate’s surface in order to explore the limits in which the Pt single atoms aggregate to nanoclusters and eventually to nanoparticles. The catalysts with different Pt loadings were evaluated by STEM-HAADF and EDS (see Figure 10b). The images of elemental mapping proved the existence of the Pt element on the perovskite and its uniform distribution. The HAADF images confirmed that as the Pt loading increases above 1.8 wt.%, Pt nanoclusters and Pt nanoparticles are formed. The researchers further used XPS and XAFS measurements [90], which revealed that the Pt species exist in distinct chemical environments, likely due to variations in their coordination with Br or I. Additionally, DFT was employed to develop the theoretical model, demonstrating that halide ions on the surface of the mixed-halide FAPbBr3−xIx perovskite are well-suited to serve as uniform anchoring sites for Pt atoms. The catalysts with different co-catalyst loadings were examined by photocatalytic hydrogen production experiments, demonstrating that the optimal Pt loading was 1.8 wt.%, which, based on the former analysis, corresponds to single Pt atoms dispersed on the perovskite surface [90].
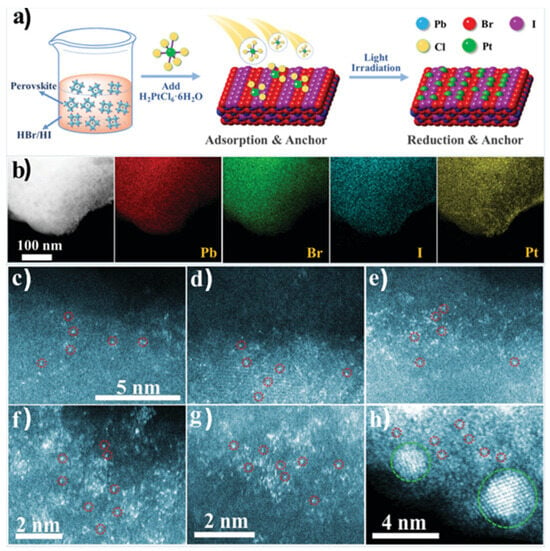
Figure 10.
Synthesis mechanism illustration and STEM-HAADF images of Pt/FAPbBr3−xIx. (a) Mechanism illustration of the synthesis process of Pt/FAPbBr3−xIx. (b) TEM images and corresponding EDS mapping images of a random area of Pt/FAPbBr3−xIx. STEM-HAADF images of Pt/FAPbBr3−xIx with different Pt loading amounts of (c), 0.7; (d), 1.2; (e), 1.5; (f), 1.8; (g,h), 2.1. (d,e) share the same scale bar with (c). Some of the Pt atoms are circled in red in (c–h), and Pt clusters are circled in green in (h). Reproduced from Ref. [90] with permission from the Royal Society of Chemistry.
Figure 10.
Synthesis mechanism illustration and STEM-HAADF images of Pt/FAPbBr3−xIx. (a) Mechanism illustration of the synthesis process of Pt/FAPbBr3−xIx. (b) TEM images and corresponding EDS mapping images of a random area of Pt/FAPbBr3−xIx. STEM-HAADF images of Pt/FAPbBr3−xIx with different Pt loading amounts of (c), 0.7; (d), 1.2; (e), 1.5; (f), 1.8; (g,h), 2.1. (d,e) share the same scale bar with (c). Some of the Pt atoms are circled in red in (c–h), and Pt clusters are circled in green in (h). Reproduced from Ref. [90] with permission from the Royal Society of Chemistry.
4.3. Single Atoms/Sub-Nanoclusters and Quantum-Sized Small Particles (QSSPs) of Co-Catalyst on Metal Oxides
For completeness, we include a few examples of popular metal oxides, such as TiO2 and ZrO2, to discuss and further highlight the controversies in the literature regarding the identification of the exact aggregation state of the co-catalyst on the substrate.
Y. Zhang et al. reported the dispersion of a high amount of Cu single atoms (~1.5 wt.%) on the surface of a TiO2 semiconductor. Utilizing a metal–organic framework (MOF), the metal ions were anchored into the MOF by a wet impregnation method, forming a metal–oxygen–titanium bond, followed by thermal annealing. HAADF-STEM was employed to verify that the Cu atoms, shown by the bright spots, were incorporated on the Ti vacancy sites in the intermediate MOF [96]. The line scan profiles, highlighted with three randomly selected blue lines, show that line 1 consists solely of Ti atoms, whereas lines 2 and 3 contain both Ti and Cu atoms, confirming the presence of Cu-O-Ti clusters [96]. The valence states of Cu in CuSA-TiO2 were examined by in situ Electron Paramagnetic Resonance (EPR) both before and after irradiation. Prior to irradiation, the EPR spectrum of CuSA-TiO2 exhibited a prominent Cu2+ signal, which diminished following 30 min of irradiation but subsequently intensified upon air exposure. This observation suggests that Cu+ species, which are EPR-silent, were generated during irradiation and later oxidized back to Cu2+ upon contact with air [96]. Although the concept of this work was based on the enhanced photocatalytic properties of Cu single atoms on TiO2, the EPR signal shape prior to the illumination reveals the coexistence of Cu sub-nanoclusters alongside Cu single atoms. CuSA/TiO2 with 1.5 wt.% Cu semiconductor showed excellent photocatalytic hydrogen production compared to the catalysts decorated with other Cu loadings (from 0.48 to 2.57 wt.%), while the overall enhanced performance of the system was attributed to the efficient electron transfer through the Cu2+–Cu+ process.
H. Zou and coworkers [97] developed a TiO2 semiconductor decorated with Cu sub-nanoclusters, achieving a metal mass loading of 1.8 wt.% (see Figure 11a). The TiO2 substrate was prepared via a hydrothermal method followed by calcination, while the Cu deposition occurred via a wet impregnation method. According to XRD patterns, no metallic diffraction peak of Cu or CuO NPs was observed [97], suggesting that Cu is in the aggregation state of nanoclusters. Furthermore, aberration-corrected STEM was employed, and Z-contrast HAADF images (see Figure 11b) demonstrated the presence of ultrafine Cu sub-nanoclusters (approximately 1 nm in diameter), distributed on the semiconductor’s surface [97]. The chemical states of Cu were investigated by XPS (see Figure 11c,d). The observed negative shifts in the Ti 2p and O 2p peaks upon introducing Cu, along with the positively charged valence states of the metals, indicate significant electron transfer from the metal co-catalysts to TiO2 [97].
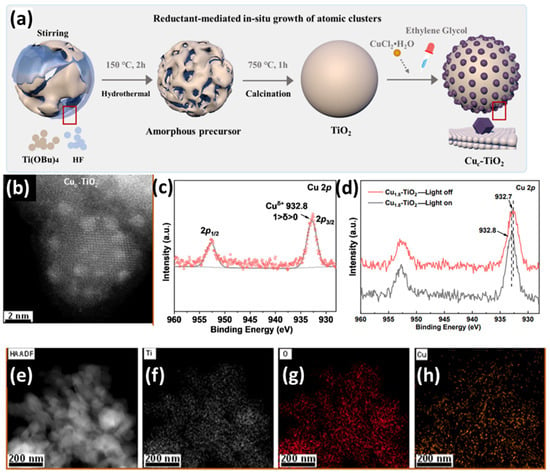
Figure 11.
(a) Schematic illustration of the specific synthesis process of Cuc-TiO2; (b) atomic resolution HAADF-STEM image of Cu1.8-TiO2, (c) high-resolution XPS analysis of Cu 2p, (d) in situ XPS Cu 2p spectra before and after irradiation, (e–h) HAADF-STEM image and EDX mapping of Cu1.8-TiO2. Reprinted (adapted) with permission from [97]. Copyright {2024} American Chemical Society.
Figure 11.
(a) Schematic illustration of the specific synthesis process of Cuc-TiO2; (b) atomic resolution HAADF-STEM image of Cu1.8-TiO2, (c) high-resolution XPS analysis of Cu 2p, (d) in situ XPS Cu 2p spectra before and after irradiation, (e–h) HAADF-STEM image and EDX mapping of Cu1.8-TiO2. Reprinted (adapted) with permission from [97]. Copyright {2024} American Chemical Society.
Moreover, the Cu 2p peaks confirm the successful integration of Cu clusters, emphasizing strong charge interactions with the substrate. The TiO2 decorated with Cu subnanoclusters exhibited remarkable hydrogen production, which indicates that the decoration of Cu clusters enhances both light absorption and the transport and separation of electron–hole pairs while also reducing photogenerated charge recombination. Furthermore, DFT calculations suggested that the surface-active, site-rich SNC-Cu exhibits favorable hydrogen adsorption and desorption energy barriers [97].
Our group recently reported a library of atomic Pd states formed on Pd-Nps, stabilized on TiO2 support, using flame spray pyrolysis (FSP) technology [71]. Our findings revealed that interfacial Pd atoms can stabilize in Pd1+ or Pd3+ oxidation states depending on the oxygen availability and local temperature at the Pd-TiO2 interface during FSP synthesis. Various post-FSP treatments were conducted, such as reduction with gas H2, treatment with NaHB4, and oxidation under O2. Moreover, these systems were evaluated under formic acid dehydrogenation catalytic conditions and via in situ photocatalytic EPR study [71]. Consequently, through EPR spectroscopy, we identified four distinct Pd states at the Pd/TiO2 interface, presented in Figure 12a: Pd0, Pd1+, {Pd2+–O2−}, and Pd3+. The Pd0 species were observed under oxygen-lean preparation conditions or in highly reducing environments, such as hydrogen treatment without H2O, while the formation of the less common Pd1+ and Pd3+ states was found to be directly linked to the abundance of oxygen [71]. Although Pd2+ species are not visible by EPR spectroscopy, a unique {Pd2+–OO−} state appeared under specific conditions, including H2/H2O treatment or during catalytic formic acid (HCOOH) dehydrogenation. The case of the O2-rich Pd/TiO2 sample shows a highly efficient transfer of photogenerated electrons to Pd-Nps, which prevents electron–hole recombination, thus enhancing photocatalytic performance [71] (Table 4).
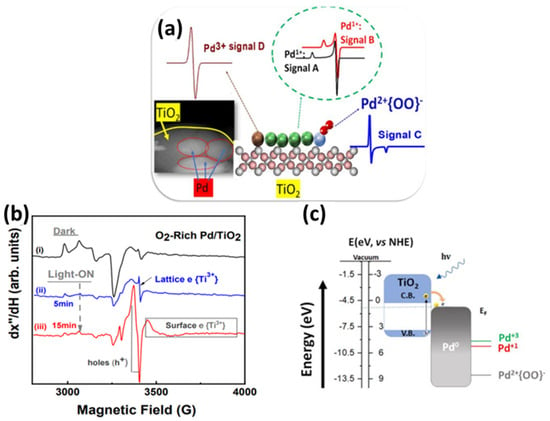
Figure 12.
(a) Schematic depiction of structural assignment of the detected Pdn+ EPR signals. The STEM image shows the Pd particles deposited on the TiO2 matrix. (b) 77K EPR spectra of O2-Rich Pd/TiO2 illuminated with a Xenon solar simulator at different illumination times. (c) Schematic photoinduced electron transfer mechanism under photocatalytic conditions. Reprinted (adapted) with permission from [71]. Copyright {2022} American Chemical Society.
Figure 12.
(a) Schematic depiction of structural assignment of the detected Pdn+ EPR signals. The STEM image shows the Pd particles deposited on the TiO2 matrix. (b) 77K EPR spectra of O2-Rich Pd/TiO2 illuminated with a Xenon solar simulator at different illumination times. (c) Schematic photoinduced electron transfer mechanism under photocatalytic conditions. Reprinted (adapted) with permission from [71]. Copyright {2022} American Chemical Society.

Table 4.
Literature summary of perovskites and metal-oxide substrates decorated with single atoms/sub-nanoclusters/quantum-sized small particles of various metals and their application in photocatalysis.
Table 4.
Literature summary of perovskites and metal-oxide substrates decorated with single atoms/sub-nanoclusters/quantum-sized small particles of various metals and their application in photocatalysis.
| Nanostructure | Co-Catalyst | Aggregation State of the Co-Catalyst | Method of Analysis | Catalytic Application | Ref. |
|---|---|---|---|---|---|
| Perovskites | |||||
| LaFeO3 | Au | SACs | HAADF-STEM, EDS, XAFS, DFT, EXAFS, XANES | CO oxidation | [68] |
| SrBO3 (B = 3d transition metals) | Pt | SACs | DFT | Methane activation | [109] |
| CsPbBr3 | Pt | SACs, NPs | HR-TEM, 3D-FP XANES, EXAFS | Photocatalytic semi-hydrogenation of propyne | [89] |
| SrTiO3 | Pt | SACs, SNCs, NPs | HAADF-STEM, EXAFS | Reverse water–gas shift reactions | [87] |
| Cs2SnI6 | Pt | SACs, NPs | HAADF-STEM, EDS, XAFS, XPS, EXAFS, EXANS, DFT | Photocatalytic hydrogen production | [88] |
| CsPbBr3 | Pt | SACs | HAADF-STEM, EDS, XPS, DRIFT, EXAFS, XANES | Electrochemical sensing of ascorbic acid | [86] |
| FAPbBr3−xIx (FA = CH(NH2)2) | Pt | SACs | HAADF-STEM, EDS, XPS, DFT, EXAFS, XANES | Photocatalytic hydrogen production | [90] |
| LaCoO3 | Pt | SACs, SNCs | HAADF-STEM, EDS, EXAFS, EPR, XANES, XPS | Chemiresistive sensing of acetone gas | [91] |
| NaTaO3 | Ni | NPs | TEM, XRD, BET | Photocatalytic hydrogen production | [73] |
| SrTiO3 | Ni | SACs, SNCs | STEM, PES, DFT | - | [92] |
| BiFeO3 | Co | SACs | AC HAADF-STEM, EDX, XPS, XANES, EXAFS | Photocatalytic oxygen evolution | [93] |
| SrTiO3 | Pd | SACs, SNCs | AC HAADF-STEM, XAS, XPS, XANES | Photocatalytic semihydrogenation of Alkynes | [94] |
| SrTiO3 | Au | SACs | STEM-MBE/RHEED/LEED | - | [95] |
| Metal Oxides | |||||
| TiO2 | Cu | SACs | HAADF-STEM, EDS, PL, UV-vis, XPS, FTIR, SPV, fs-TAS, EPR, ICP, DFT | Photocatalytic hydrogen production | [96] |
| TiO2 | Cu | SNCs | HAADF-STEM, EDS, XPS, ICP, UV-vis, PL, TRPL | Photocatalytic hydrogen production | [97] |
| TiO2 | Pd | SACs | STEM, XPS, EPR | - | [71] |
| ZrO2 | Ni | SACs, SNCs | AC HAADF-STEM, XANES, EXAFS, XPS, DFT | Photocatalytic CO2 Reduction to CO | [98] |
| ZrO2 | Pt | SACs | AC-TEM, EDS, ICP, XANES, EXAFS, XPS, Raman | Photocatalytic CO2 Reduction to CO | [99] |
Among metal oxides, zirconia ZrO2, a high conduction band semiconductor, is a promising candidate for photocatalytic procedures such as hydrogen production and CO2 reduction [132,133]. X. Xiong et al. [98] utilized a sol-gel approach to produce defect-rich zirconia with isolated Ni single atoms. The Ni loading ranges from 2 to 8 wt.%, depending on the weight ratio of Ni salts (Ni-SA-x/ZrO2). The researchers utilized HAADF-STEM microscopy to identify the aggregation state of Ni. Singe Ni atoms were present with dark spots in Z-contrast images for the Ni-SA-5/ZrO2 sample [98]. Additionally, the materials with different Ni loadings were evaluated using K-edge XANES and EXAFS. The Ni-Ni interactions of the first-shell Ni-Ni feature (2.1 Å) of metallic Ni foil and the second-shell Ni-Ni feature in NiO (2.5 Å), ref. [98] were absent in the Ni-SA-x/ZrO2 (x = 2, 5) photocatalysts, which indicates that Ni atoms were highly dispersed. On the contrary, in the sample with high Ni loading, the presence of the presence of Ni-Ni features in the R-space plots was observed [98], suggesting that Ni species aggregated into possible nanoclusters [98]. The photocatalysts, decorated with different Ni loadings, were evaluated in photocatalytic CO2 reduction to CO. The optimal photocatalyst for the highest CO yield was Ni-SA-5/ZrO2, which, according to the researchers, contained a large amount of single nickel atoms [98]. To support the experimental results, theoretical studies were performed, demonstrating that the atomically dispersed Ni sites reduce the energy barrier for CO2 to CO conversion through an adsorbed COOH intermediate while also inhibiting H2 desorption in the competing water-splitting reaction. As a result, the Ni single atom sites effectively promote both CO2 conversion and CO selectivity [98].
Another work employing zirconia substrate was reported by S. Dong et al. [99] in which single Pt atoms were anchored on amorphous ZrO2 nanowires. The amorphous support was prepared using a wet chemistry approach, followed by the incorporation of Pt SAs through an impregnated reduction process. Spherical aberration-corrected TEM was used to examine the aggregation state of the co-catalyst. Individual bright spots appeared on the image that were measured to be less than 1nm and assigned to the Pt single atoms. The EXAFS spectrum displayed a strong peak around 1.6 Å for Pt-SAs/ZrO2 [99], indicating Pt-O coordination, with no notable Pt-Pt bond signal between 2.5 and 3.0 Å [99], indicating that the Pt species on the ZrO2 were atomically dispersed. Photocatalytic CO2 reduction experiments were conducted, where Pt-SAs/ZrO2 exhibited the highest photocatalytic efficiency compared to crystal-ZrO2 and amorphous-ZrO2 nanowires. Overall, the researchers concluded that the amorphous ZrO2 nanowires contain numerous oxygen defects that help coordinate and stabilize the Pt single atoms [99]. These defects also promote the formation of Pt-O-Zr charge bridges, which improve the catalyst’s light absorption and the efficiency of photogenerated electron transport. As a result, these features enhance the effective use of photoelectrons, boost the formation of intermediate products, and facilitate CO desorption [99].
5. Concluding Remarks—Future Perspectives
Single atom catalysis provides remarkable efficiency and precise selectivity toward various photocatalytic applications, which is attributed to the maximized atomic utilization, distinctive active sites, and strong metal–support interaction (SMSI) between the co-catalyst and the semiconductor. Nevertheless, these systems face several challenges that limit their photocatalytic performance.
The synthesis of single atom co-catalysts (SACCs) poses significant challenges, as it requires control of the desirable metal loading while avoiding agglomeration on the semiconductor surface, uniformly dispersing single atoms or sub-nanoclusters, and establishing a robust interface to promote strong metal–support interactions. Perovskites possess promising photocatalytic properties, and furthermore, they appear to be stable and efficient substates to anchor and stabilize co-catalytic single atoms/sub-nanoclusters and/or quantum-sized small particles. However, due to their special chemistry, their synthesis, and the deposition of the co-catalyst on their surface, they become more complex compared to metal oxide substrates. Subsequently, in many cases, there is little control of the desirable aggregation state of the co-catalyst, and mostly, it is achieved via trial and error using different metal loadings. Although many synthesis processes have been utilized to produce these delicate materials, ranging from wet to dry methods, it is essential to establish more synthetic routes capable of controlling the atomic dispersion of single atoms or sub-nanoclusters, especially on complex substrates such as perovskites.
Furthermore, adequate characterization of the exact configuration of the co-catalyst has been an issue as it requires state-of-the-art machinery as well as stupendous analytical skills. In some cases, it leads to misunderstandings concerning the aggregation state of the co-catalyst on the catalyst’s surface. The most reliable set of techniques that have been established so far begins with HAADF-STEM, which provides a clear outlook on how the single atoms became anchored on the support’s surface. Then, X-ray absorption techniques are in place. XPS can illustrate the chemical states and electronic environments of the atoms involved. EXAFS gives data on bond lengths, coordination numbers, and the identity of the elements surrounding the absorbing atom. This information comprises the local atomic arrangement. XANES can reveal alterations in oxidation states, which further reveal the electronic environment of the atoms. EPR analysis may also assist in identifying single atoms if they possess unpaired electrons. Their sharp signals will serve as signatures for isolated atoms. Finally, comparing DFT simulations with experimental catalytic performance can offer further evidence of the presence and efficacy of isolated single atoms relative to established benchmarks, although reaching a definitive conclusion through this route is often more challenging than it may appear. Despite these techniques possibly being the best methods for identifying single atoms at present, they do not come without drawbacks and limitations. As previously discussed, it is most probable that the results of the anchoring process follow a distribution that ranges from single atoms to clusters and nanoparticles. STEM experiments can only provide local information that might not even represent the rest of the material. In addition, the STEM data are reliable only if the elements have a good enough Z factor to create adequate contrast. The techniques of the XAS umbrella often struggle with background signals and overlapping peaks, resulting in unreliable data. Similar problems apply to the XPS, where overlapping peaks may result in misinterpretation of oxidation states. EPR spectroscopy can provide detailed data and even quantitative analysis on single atoms and sub-nanoclusters, given (however) that the elements have unpaired electrons in order to be EPR-visible. In order for the subdivision of single atom co-catalysts to expand and the field of catalysis to achieve new heights, these techniques must evolve and combine to provide sufficient and reliable data on the results of the anchoring process.
In current literature, single atoms are preferred to in the majority of catalytic applications; however, a comprehensive study concerning the function of SA/SNC/QSSPs in a specific photocatalytic procedure is lacking. Different configurations of the co-catalyst lead to different electronic properties, from discrete energy multiplicities in small nanoclusters to semi-discrete bands in quantum-sized-small particles and full-periodic density of states in large nanoparticles. Thus, the optimal co-catalyst configuration should be selected and examined carefully to fit the requirements of each individual photocatalytic application.
Funding
This research was funded by the project “Advanced Nanostructured Materials for Sustainable Growth: Green Energy Production/Storage, Energy Saving and Environmental Remediation” (TAEDR-0535821), which is implemented under the action “Flagship actions in interdisciplinary scientific fields with a special focus on the productive fabric” (ID 16618), Greece 2.0—National Recovery and Resilience Fund and funded by European Union NextGenerationEU.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nosaka, Y.; Nosaka, A. Introduction to Photocatalysis: From Basic Science to Applications; Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, İ.H.; Karakaş, Z.K. Emerging trends and future prospects in photocatalysis-based environmental remediation and hydrogen production. In Photocatalysts for Energy and Environmental Sustainability; IOP Publishing: Bristol, UK, 2024; pp. 7–24. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.; Ahuja, S. Photocatalytic Technology: A review of environmental protection and renewable energy application for sustainable development. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tsang, S.C.E. Recent progress and strategies for enhancing photocatalytic water splitting. Mater. Today Sustain. 2020, 9, 100032. [Google Scholar] [CrossRef]
- Chu, S.; Wang, E.; Feng, F.; Zhang, C.; Jiang, J.; Zhang, Q.; Wang, F.; Bing, L.; Wang, G.; Han, D. A Review of Noble Metal Catalysts for Catalytic Removal of VOCs. Catalysts 2022, 12, 1543. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, X. Cocatalysts from types{,} preparation to applications in the field of photocatalysis. Nanoscale 2021, 13, 10649–10667. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, L.; Dong, L.; Sheng, X.; Zhang, W.; Liu, Z.; Shen, J.; Jiang, H.; Li, C. Cu Single-Atom Catalysts for High-Selectivity Electrocatalytic Acetylene Semihydrogenation. Angew. Chemie Int. Ed. 2023, 62, e202307848. [Google Scholar] [CrossRef] [PubMed]
- Millet, M.-M.; Algara-Siller, G.; Wrabetz, S.; Mazheika, A.; Girgsdies, F.; Teschner, D.; Seitz, F.; Tarasov, A.; Levchenko, S.V.; Schlögl, R.; et al. Ni Single Atom Catalysts for CO2 Activation. J. Am. Chem. Soc. 2019, 141, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Effective and Durable Co Single Atomic Cocatalysts for Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 42734–42741. [Google Scholar] [CrossRef]
- Li, M.; He, C.; Yang, X.; Liu, Z.; Li, J.; Wang, L.; Wu, S.; Zhang, J. Optimizing water dissociation dehydrogenation process via Sn single atom incorporation for boosting photocatalytic CO2 methanation. Chem Catal. 2023, 3, 100737. [Google Scholar] [CrossRef]
- Mitchell, S.; Pérez-Ramírez, J. Single atom catalysis: A decade of stunning progress and the promise for a bright future. Nat. Commun. 2020, 11, 4302. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Xing, J.; Chen, J.F.; Li, Y.H.; Yuan, W.T.; Zhou, Y.; Zheng, L.R.; Wang, H.F.; Hu, P.; Wang, Y.; Zhao, H.J.; et al. Stable Isolated Metal Atoms as Active Sites for Photocatalytic Hydrogen Evolution. Chem.—A Eur. J. 2014, 20, 2138–2144. [Google Scholar] [CrossRef]
- He, H.; Wang, H.; Liu, J.; Liu, X.; Li, W.; Wang, Y. Research Progress and Application of Single-Atom Catalysts: A Review. Molecules 2021, 26, 6501. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Guo, Y.; Huang, Y.; Xi, W.; Xu, J.; Luo, J.; Qi, H.; Ren, Y.; Liu, X.; Qiao, B.; et al. Strong Metal–Support Interactions between Pt Single Atoms and TiO2. Angew. Chemie Int. Ed. 2020, 59, 11824–11829. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, S.; Li, Q.; Wu, Z.; Shen, T.; Chu, J.; Song, Y.-F. Recent progress on atomic-scale modulation of single-atom-based catalysts for electrocatalytic CO2 reduction. Chem. Eng. J. 2023, 477, 146610. [Google Scholar] [CrossRef]
- Liu, L.; Chen, T.; Chen, Z. Understanding the Dynamic Aggregation in Single-Atom Catalysis. Adv. Sci. 2024, 11, 2308046. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Santiago, A.R.; Kong, Y.; Ahsan, M.A.; Luque, R.; Du, A.; Pan, H. Atomically Dispersed Heteronuclear Dual-Atom Catalysts: A New Rising Star in Atomic Catalysis. Small 2021, 18, 2106091. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, S. Advancements in Single Atom Catalysts for Electrocatalytic Nitrate Reduction Reaction. ChemCatChem 2024, 16, e202301641. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, J.; Jia, B.; Wang, X.; Jiang, S.; Ma, T. Isolating Single and Few Atoms for Enhanced Catalysis. Adv. Mater. 2022, 34, e2201796. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khan, M.; Hu, J.; Kibria, M. Single-atom catalysts for biomass-derived drop-in chemicals. In Advanced Catalysis for Drop-in Chemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–100. [Google Scholar] [CrossRef]
- Cai, J.; Javed, R.; Ye, D.; Zhao, H.; Zhang, J. Recent progress in noble metal nanocluster and single atom electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 22467–22487. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ma, X.; Liu, X.; Zhang, R.; Wang, Y. Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction. Nanoenergy Adv. 2023, 3, 48–72. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef] [PubMed]
- Takaaki, T.; Hiroyuki, H.; Martin, V.; Tomoyasu, T. Nanoscale Physics for Materials Science; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Li, Y.; Zhan, S. Towards single-atom photocatalysts for future carbon-neutral application. SmartMat 2022, 3, 417–446. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Li, D.; Wang, J.; Djitcheu, X.; He, D.; Zhang, Q. A minireview on the synthesis of single atom catalysts. RSC Adv. 2022, 12, 9373–9394. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ye, L.; Lo, T.W.B. Designing the electronic and geometric structures of single-atom and nanocluster catalysts. J. Mater. Chem. A 2021, 9, 18773–18784. [Google Scholar] [CrossRef]
- Atomic Radius in the Periodic Table of Elements. Available online: https://pubchem.ncbi.nlm.nih.gov/ptable/atomic-radius/ (accessed on 14 January 2025).
- Atomic Mass in the Periodic Table of Elements. Available online: https://pubchem.ncbi.nlm.nih.gov/ptable/atomic-mass/ (accessed on 14 January 2025).
- Sun, N.; Song, J.; Tao, Q.; Kan, E.; Kuai, L. High-loading single-atom Pt/TiO2 mesoporous catalysts for superior photocatalytic oxidation of benzyl alcohol. Microporous Mesoporous Mater. 2022, 337, 111949. [Google Scholar] [CrossRef]
- Luo, J.; Waterhouse, G.I.N.; Peng, L.; Chen, Q. Recent progress in high-loading single-atom catalysts and their applications. Ind. Chem. Mater. 2023, 1, 486–500. [Google Scholar] [CrossRef]
- Shi, R.; Tian, C.; Zhu, X.; Peng, C.-Y.; Mei, B.; He, L.; Du, X.-L.; Jiang, Z.; Chen, Y.; Dai, S. Achieving an exceptionally high loading of isolated cobalt single atoms on a porous carbon matrix for efficient visible-light-driven photocatalytic hydrogen production. Chem. Sci. 2019, 10, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Lazaar, N.; Wu, S.; Qin, S.; Hamrouni, A.; Bikash Sarma, B.; Doronkin, D.E.; Denisov, N.; Lachheb, H.; Schmuki, P. Single-Atom Catalysts on C3N4: Minimizing Single Atom Pt Loading for Maximized Photocatalytic Hydrogen Production Efficiency. Angew. Chemie Int. Ed. 2025, 64, e202416453. [Google Scholar] [CrossRef]
- Qin, S.; Will, J.; Kim, H.; Denisov, N.; Carl, S.; Spiecker, E.; Schmuki, P. Single Atoms in Photocatalysis: Low Loading Is Good Enough! ACS Energy Lett. 2023, 8, 1209–1214. [Google Scholar] [CrossRef]
- Wu, S.-M.; Schmuki, P. Single Atom Cocatalysts in Photocatalysis. Adv. Mater. 2024, 2414889. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2021, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Tilley, R.J.D. The ABX3 Perovskite Structure. In Perovskites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–41. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Wang, R.; He, B.; Wang, D.; Jia, C.; Cao, J.; Shi, L.; Pan, J.; Hai, G.; Li, C. Dual functional high entropy perovskite La(Mn0.2Cr0.2Fe0.2Co0.2Ni0.2)O3 interfacial transition layer modified Cu2Y2O5/CdIn2O4 transparent pn junction towards photovoltaic enhancement. Ceram. Int. 2024, 50, 52917–52926. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Li, W. Morphology effect of nano-hydroxyapatite as support for loading Ni in methane dry reforming. J. Fuel Chem. Technol. 2023, 51, 977–985. [Google Scholar] [CrossRef]
- Du, D.; He, H.; Zheng, R.; Zeng, L.; Wang, X.; Shu, C.; Zhang, C. Single-Atom Immobilization Boosting Oxygen Redox Kinetics of High-Entropy Perovskite Oxide Toward High-Performance Lithium-Oxygen Batteries. Adv. Energy Mater. 2024, 14, 2304238. [Google Scholar] [CrossRef]
- Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.; Vohs, J.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.T.; Zaman, M.; Aslam, M.Z.; Kousar, N.; Asim, M.; Rafique, M.; Siraj, K.; Tabish, A.N.; Usman, M.; et al. Photocatalysis and perovskite oxide-based materials: A remedy for a clean and sustainable future. RSC Adv. 2022, 12, 7009–7039. [Google Scholar] [CrossRef]
- Lamhani, M.; Chchiyai, Z.; Elomrani, A.; Manoun, B.; Hasnaoui, A. Enhanced Photocatalytic Water Splitting of SrTiO3 Perovskite through Cobalt Doping: Experimental and Theoretical DFT Understanding. Inorg. Chem. 2023, 62, 13405–13418. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Hisatomi, T.; Goto, Y.; Yoshida, M.; Higashi, T.; Katayama, M.; Takata, T.; Minegishi, T.; Nishiyama, H.; Yamada, T.; et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000 h of constant illumination. Chem. Sci. 2019, 10, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Dylla, M.; Kang, S.; Snyder, J. Effect of Two-Dimensional Crystal Orbitals on Fermi Surfaces and Electron Transport in Three-Dimensional Perovskite Oxides. Angew. Chemie Int. Ed. 2019, 58, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Shu, X.; Wan, D.; Wei, N.; Yu, X.; Breese, M.; Venkatesan, T.; Xue, J.; Liu, Y.; et al. From Titanium Sesquioxide to Titanium Dioxide: Oxidation-Induced Structural, Phase and Property Evolution. Chem. Mater. 2018, 30, 4383–4392. [Google Scholar] [CrossRef]
- Queisser, H.J.; Haller, E.E. Defects in Semiconductors: Some Fatal, Some Vital. Science 1998, 281, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Van Benthem, K.; Elsässer, C.; French, R.H. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef]
- Ikeue, K.; Yamamoto, Y.; Suzuki, M. Photocatalytic Activity for Hydrogen Evolution of Heteroatom-Doped SrTiO3 Prepared Using a Graphitic-Carbon Nitride Nanosheet. Ceramics 2020, 3, 22–30. [Google Scholar] [CrossRef]
- Park, N.-H.; Dang, F.; Wan, C.; Seo, W.-S.; Koumoto, K. Self-originating two-step synthesis of core–shell structured La-doped SrTiO3 nanocubes. J. Asian Ceram. Soc. 2013, 1, 35–40. [Google Scholar] [CrossRef]
- Eng, H.; Barnes, P.; Auer, B.; Woodward, P. Investigation of the Electronic Structure of d0 Transition Metal Oxides Belonging to the Perovskite Family. J. Solid State Chem. 2003, 175, 94–109. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, S.; Atri, S.; Tomar, R. A Brief Review on Key Role of Perovskite Oxides as Catalyst. ChemistrySelect 2021, 6, 12947–12959. [Google Scholar] [CrossRef]
- Kawawaki, T.; Mori, Y.; Wakamatsu, K.; Ozaki, S.; Kawachi, M.; Hossain, S.; Negishi, Y. Controlled colloidal metal nanoparticles and nanoclusters: Recent applications as cocatalysts for improving photocatalytic water-splitting activity. J. Mater. Chem. A 2020, 8, 16081–16113. Available online: https://api.semanticscholar.org/CorpusID:225383963 (accessed on 11 December 2024). [CrossRef]
- Pawar, G.S.; Tahir, A.A. Unbiased Spontaneous Solar Fuel Production using Stable LaFeO3 Photoelectrode. Sci. Rep. 2018, 8, 3501. [Google Scholar] [CrossRef]
- Sathiyamoorthy, K.; Silambarasan, A.; Navaneethan, M.; Harish, S. Boosting the performance of LaCoO3/MoS2 perovskite interface for sustainable decontaminants under visible light-driven photocatalysis. Chemosphere 2024, 348, 140575. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.-C.; Chan, C.-H. High brightness and low operating voltage CsPbBr3 perovskite LEDs by single-source vapor deposition. Sci. Rep. 2024, 14, 3351. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Liao, Y.; Pan, Z.; Rao, H.; Zhong, X. Cs2SnI6 nanocrystals enhancing hole extraction for efficient carbon-based CsPbI2Br perovskite solar cells. Chem. Eng. J. 2022, 440, 135710. [Google Scholar] [CrossRef]
- Man, S.; Leng, X.; Bai, J.; Kan, S.; Cui, Y.; Wang, J.; Xu, L. Enhancement of photoelectrochemical performance of BiFeO3 by Sm3+ doping. Ceram. Int. 2023, 49, 10255–10264. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Ding, Y.; Li, F.; Wang, L.; Cai, B.; Zhang, F.; Liu, T.; Boschloo, G.; Johansson, E.M.J.; et al. Monolithic FAPbBr3 photoanode for photoelectrochemical water oxidation with low onset-potential and enhanced stability. Nat. Commun. 2023, 14, 5486. [Google Scholar] [CrossRef]
- Gaggero, E.; Calza, P.; Cerrato, E.; Paganini, M.C. Cerium-, Europium- and Erbium-Modified ZnO and ZrO2 for Photocatalytic Water Treatment Applications: A Review. Catalysts 2021, 11, 1520. [Google Scholar] [CrossRef]
- Temerov, F.; Baghdadi, Y.; Rattner, E.; Eslava, S. A Review on Halide Perovskite-Based Photocatalysts: Key Factors and Challenges. ACS Appl. Energy Mater. 2022, 5, 14605–14637. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, H.; Zhu, X.; Lin, B.; Liu, X.; Chen, H.; Zhang, Y.; Mullins, D.R.; Abney, C.W.; Shakouri, M.; et al. A new trick for an old support: Stabilizing gold single atoms on LaFeO3 perovskite. Appl. Catal. B Environ. 2020, 261, 118178. [Google Scholar] [CrossRef]
- Onn, T.M.; Monai, M.; Dai, S.; Fonda, E.; Montini, T.; Pan, X.; Graham, G.W.; Fornasiero, P.; Gorte, R.J. Smart Pd Catalyst with Improved Thermal Stability Supported on High-Surface-Area LaFeO3 Prepared by Atomic Layer Deposition. J. Am. Chem. Soc. 2018, 140, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Rodes, A.; Herrero, E.; Aldaz, A. Understanding the Nernst Equation and Other Electrochemical Concepts: An Easy Experimental Approach for Students. J. Chem. Educ. 2012, 89, 936–939. [Google Scholar] [CrossRef]
- Stathi, P.; Belles, L.; Deligiannakis, Y. Multipotent Atomic Palladium Species Pd1+, Pd2+–O2–, and Pd3+ Formed at the Interface of Pd/TiO2 Nanoparticles: Electron Paramagnetic Resonance Study. J. Phys. Chem. C 2022, 126, 14125–14137. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Zindrou, A.; Belles, L.; Deligiannakis, Y. Atomic {Pdn+-X} States at Nanointerfaces: Implications in Energy-Related Catalysis. Energies 2023, 16, 913. [Google Scholar] [CrossRef]
- Psathas, P.; Zindrou, A.; Spyrou, A.V.; Deligiannakis, Y. Engineering of LiTaO3 Nanoparticles by Flame Spray Pyrolysis: Understanding In Situ Li-Incorporation into the Ta2O5 Lattice. Nanomaterials 2024, 14, 1257. [Google Scholar] [CrossRef]
- Psathas, P.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Highly Crystalline Nanosized NaTaO3/NiO Heterojunctions Engineered by Double-Nozzle Flame Spray Pyrolysis for Solar-to-H2 Conversion: Toward Industrial-Scale Synthesis. ACS Appl. Nano Mater. 2023, 6, 2658–2671. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-Atom Catalysts: From Design to Application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Tanaka, Y.; Ishikawa, R.; Toyoura, K.; Matsunaga, K.; Ikuhara, Y.; Shibata, N. Direct Imaging of Pt Single Atoms Adsorbed on TiO2 (110) Surfaces. Nano Lett. 2014, 14, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, S.; Mohajernia, S.; Osuagwu, B.; Zoppellaro, G.; Andryskova, P.; Tomanec, O.; Kment, S.; Zbořil, R.; Schmuki, P. On the Controlled Loading of Single Platinum Atoms as a Co-Catalyst on TiO2 Anatase for Optimized Photocatalytic H2 Generation. Adv. Mater. 2020, 32, 1908505. [Google Scholar] [CrossRef]
- Li, S.; Xin, Z.; Luo, Y.; Pan, J.; Liao, G.; Li, Q.; Sun, Y.; Feng, Z.; Tan, R. Recent advances in the development of single atom catalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 82, 1081–1100. [Google Scholar] [CrossRef]
- Wang, C.; Najimu, M.; Ko, B.; Sasmaz, E. Impact of Flame Conditions on the Pd-O Structure and Methane Oxidation Activity over Ceria Support. ChemCatChem 2024, 17, e202401449. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Cao, D.; Cheng, D. Carbon-based material-supported single-atom catalysts for energy conversion. iScience 2022, 25, 104367. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Chen, Z.; Akl, D.F.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Lu, J. Single-Atom Catalysts Designed and Prepared by the Atomic Layer Deposition Technique. ACS Catal. 2021, 11, 7018–7059. [Google Scholar] [CrossRef]
- Cheng, N.; Sun, X. Single atom catalyst by atomic layer deposition technique. Chinese J. Catal. 2017, 38, 1508–1514. [Google Scholar] [CrossRef]
- Butburee, T.; Ponchai, J.; Khemthong, P.; Mano, P.; Chakthranont, P.; Youngjan, S.; Phanthasri, J.; Namuangruk, S.; Faungnawakij, K.; Wang, X.; et al. General Pyrolysis for High-Loading Transition Metal Single Atoms on 2D-Nitro-Oxygeneous Carbon as Efficient ORR Electrocatalysts. ACS Appl. Mater. Interfaces 2024, 16, 10227–10237. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, H.; Luo, Y.; Zhang, J.; Zhou, K.; Leng, Y.; Zheng, J.; Chen, Z. Platinum single atom on CsPbBr3 nanocrystals as electrocatalyst boosts electrochemical sensing of ascorbic acid. Talanta 2024, 277, 126396. [Google Scholar] [CrossRef]
- Xing, Y.; Ouyang, M.; Zhang, L.; Yang, M.; Wu, X.; Ran, R.; Weng, D.; Kang, F.; Si, Z. Single Atomic Pt on SrTiO3 Catalyst in Reverse Water Gas Shift Reactions. Catalysts 2021, 11, 738. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, H.; Chao, Y.; Zhang, Q.; Zhang, W.; Lv, F.; Gu, L.; Zhao, Q.; Wang, N.; Wang, J.; et al. Single-atom Pt-I3 sites on all-inorganic Cs2SnI6 perovskite for efficient photocatalytic hydrogen production. Nat. Commun. 2021, 12, 4412. [Google Scholar] [CrossRef]
- Hu, H.; Guan, W.; Xu, Y.; Wang, X.; Wu, L.; Chen, M.; Zhong, Q.; Xu, Y.; Li, Y.; Sham, T.-K.; et al. Construction of Single-Atom Platinum Catalysts Enabled by CsPbBr3 Nanocrystals. ACS Nano 2021, 15, 13129–13139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Q.; Zhang, Q.; Lou, Z.; Liu, K.; Ma, Y.; Wang, Z.; Zheng, Z.; Cheng, H.; Liu, Y.; et al. An organometal halide perovskite supported Pt single-atom photocatalyst for H2 evolution. Energy Environ. Sci. 2022, 15, 1271–1281. [Google Scholar] [CrossRef]
- Shin, H.; Ko, J.; Park, C.; Kim, D.-H.; Ahn, J.; Jang, J.-S.; Kim, Y.H.; Cho, S.-H.; Baik, H.; Kim, I.-D. Sacrificial Template-Assisted Synthesis of Inorganic Nanosheets with High-Loading Single-Atom Catalysts: A General Approach. Adv. Funct. Mater. 2022, 32, 2110485. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, X.; Gerhold, S.; Mares, P.; Wagner, M.; Bliem, R.; Schulte, K.; Schmid, M.; Franchini, C.; Diebold, U. Stabilizing Single Ni Adatoms on a Two-Dimensional Porous Titania Overlayer at the SrTiO3(110) Surface. J. Phys. Chem. C 2014, 118, 19904–19909. [Google Scholar] [CrossRef] [PubMed]
- Djatoubai, E.; Khan, M.S.; Haq, S.U.; Heidari, G.; Dong, C.-L.; Nga, T.T.T.; Chen, J.-L.; Shen, S. Engineered Cobalt Single-Atoms@BiFeO3 Heteronanostructures for Highly Efficient Solar Water Oxidation. Small 2023, 19, 2206293. [Google Scholar] [CrossRef]
- Yang, B.; Liu, K.; Ma, Y.; Ma, J.-J.; Chen, Y.-Y.; Huang, M.; Yang, C.; Hou, Y.; Hung, S.-F.; Yu, J.C.; et al. Incorporation of Pd Single-Atom Sites in Perovskite with an Excellent Selectivity toward Photocatalytic Semihydrogenation of Alkynes. Angew. Chemie Int. Ed. 2024, 63, e202410394. [Google Scholar] [CrossRef]
- Feng, J.; Ding, S.; Guo, J. Stable Adsorption of Single Gold Atoms on the SrTiO3(111)-(9 × 9) Reconstructed Surface. J. Phys. Chem. C 2019, 123, 4866–4870. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 2022, 13, 58. [Google Scholar] [CrossRef]
- Zou, H.; Tong, Y.; Feng, Y.; Zhou, Y.; Xiao, J.; Xia, L.; Guha, A.; Polesso, B.; Zhang, B.; Liu, J.; et al. Cu Atomic Subnanoclusters on TiO2 for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2024, 7, 11680–11689. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Dong, S.; Liu, W.; Liu, S.; Li, F.; Hou, J.; Hao, R.; Bai, X.; Zhao, H.; Liu, J.; Guo, L. Single atomic Pt on amorphous ZrO2 nanowires for advanced photocatalytic CO2 reduction. Mater. Today Nano 2022, 17, 100157. [Google Scholar] [CrossRef]
- Shen, W. Atomically dispersed metal site in subnanometric clusters catalyze dynamically. Chem Catal. 2021, 1, 768–770. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Ma, Z.; Chen, J.; Tang, X. Fabrication{,} characterization{,} and stability of supported single-atom catalysts. Catal. Sci. Technol. 2017, 7, 4250–4258. [Google Scholar] [CrossRef]
- Liu, J.; Bunes, B.R.; Zang, L.; Wang, C. Supported single-atom catalysts: Synthesis, characterization, properties, and applications. Environ. Chem. Lett. 2018, 16, 477–505. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Identification of the active sites in supported subnanometric metal catalysts. Nat. Catal. 2021, 4, 453–456. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly Active and Stable Metal Single-Atom Catalysts Achieved by Strong Electronic Metal–Support Interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Loon, A.; Subramanian, A.; Gerhold, S.; McDermott, E.; Enterkin, J.A.; Hieckel, M.; Russell, B.C.; Green, R.J.; Moewes, A.; et al. Transition from Reconstruction toward Thin Film on the (110) Surface of Strontium Titanate. Nano Lett. 2016, 16, 2407–2412. [Google Scholar] [CrossRef]
- Van Acker, T.; Theiner, S.; Bolea-Fernandez, E.; Vanhaecke, F.; Koellensperger, G. Inductively coupled plasma mass spectrometry. Nat. Rev. Methods Prim. 2023, 3, 52. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Djitcheu, X.; He, D.; Liu, H.; Zhang, Q. Techniques for the characterization of single atom catalysts. RSC Adv. 2022, 12, 1216–1227. [Google Scholar] [CrossRef]
- Bennett, B.; Kowalski, J.M. Chapter Thirteen—EPR Methods for Biological Cu(II): L-Band CW and NARS. In Electron Paramagnetic Resonance Investigations of Biological Systems by Using Spin Labels, Spin Probes, and Intrinsic Metal Ions, Part A; Qin, P.Z., Warncke, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 563, pp. 341–361. [Google Scholar] [CrossRef]
- Wan, Q.; Fung, V.; Lin, S.; Wu, Z.; Jiang, D. Perovskite-supported Pt single atoms for methane activation. J. Mater. Chem. A 2020, 8, 4362–4368. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.J.; Dai, J.F. Enhanced photocatalytic activity of SrTiO3 particles by surface decoration with Ag nanoparticles for dye degradation. Phys. Scr. 2015, 90, 55801. [Google Scholar] [CrossRef]
- Vorberg, E.; Fleischer, H.; Junginger, S.; Stoll, N.; Thurow, K. Automated sample preparation for mercury analysis in wood materials. IET Sci. Meas. Technol. 2016, 10, 398–404. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.; Peterson, E.; Phạm, H.; Challa, S.; Qi, G.; Oh, S.; Wiebenga, M.; Hernandez, X.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Liu, L.; Meira, D.M.; Arenal, R.; Concepcion, P.; Puga, A.V.; Corma, A. Determination of the Evolution of Heterogeneous Single Metal Atoms and Nanoclusters under Reaction Conditions: Which Are the Working Catalytic Sites? ACS Catal. 2019, 9, 10626–10639. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]
- Zabilska, A.; Clark, A.H.; Ferri, D.; Nachtegaal, M.; Kröcher, O.; Safonova, O.V. Beware of beam damage under reaction conditions: X-ray induced photochemical reduction of supported VOx catalysts during in situ XAS experiments. Phys. Chem. Chem. Phys. 2022, 24, 21916–21926. [Google Scholar] [CrossRef]
- Tayal, A.; Coburn, D.S.; Abel, D.; Rakitin, M.; Ivashkevych, O.; Wlodek, J.; Wierzbicki, D.; Xu, W.; Nazaretski, E.; Stavitski, E.; et al. Five-analyzer Johann spectrometer for hard X-ray photon-in/photon-out spectroscopy at the Inner Shell Spectroscopy beamline at NSLS-II: Design, alignment and data acquisition. J. Synchrotron Radiat. 2024, 31, 1609–1621. [Google Scholar] [CrossRef]
- Jousseaume, T.; Colin, J.-F.; Chandesris, M.; Lyonnard, S.; Tardif, S. How Beam Damage Can Skew Synchrotron Operando Studies of Batteries. ACS Energy Lett. 2023, 8, 3323–3329. [Google Scholar] [CrossRef]
- Karak, N. Chapter 1—Fundamentals of Nanomaterials and Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar] [CrossRef]
- Karhu, H.; Kalantar, A.; Väyrynen, I.J.; Salmi, T.; Murzin, D.Y. XPS analysis of chlorine residues in supported Pt and Pd catalysts with low metal loading. Appl. Catal. A Gen. 2003, 247, 283–294. [Google Scholar] [CrossRef]
- Katrib, A.; Hemming, F.; Hilaire, L.; Wehrer, P.; Maire, G. XPS studies of supported tungsten carbide(s). J. Electron Spectros. Relat. Phenomena 1994, 68, 589–595. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef]
- Ju, M.; Kim, J.; Shin, J. EPR spectroscopy: A versatile tool for exploring transition metal complexes in organometallic and bioinorganic chemistry. Bull. Korean Chem. Soc. 2024, 45, 835–862. [Google Scholar] [CrossRef]
- Zollo, A.; Polliotto, V.; Livraghi, S.; Giamello, E. Self-Organisation of Copper Species at the Surface of Cu–TiO2 Systems During H2 Evolution Reaction: A Combined Investigation by EPR and Optical Spectroscopy. Appl. Magn. Reson. 2020, 51, 1497–1513. [Google Scholar] [CrossRef]
- Zhang, C.C.; Shi, J.; Hartlaub, S.; Palamara, J.P.; Petrovic, I.; Yilmaz, B. In-situ diffuse reflective infrared Fourier transform spectroscopy (DRIFTS) study on Ni passivation in FCC catalysts from boron-based technology. Catal. Commun. 2021, 150, 106273. [Google Scholar] [CrossRef]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon N. Y. 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Kottwitz, M.; Li, Y.; Wang, H.; Frenkel, A.I.; Nuzzo, R.G. Single Atom Catalysts: A Review of Characterization Methods. Chemistry–Methods 2021, 1, 278–294. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Hasnip, P.J.; Refson, K.; Probert, M.I.J.; Yates, J.R.; Clark, S.J.; Pickard, C.J. Density functional theory in the solid state. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130270. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Desmarais, J.K.; Casassa, S.; Civalleri, B.; Donà, L.; Bush, I.J.; Searle, B.; Maschio, L.; Edith-Daga, L.; Cossard, A.; et al. CRYSTAL23: A Program for Computational Solid State Physics and Chemistry. J. Chem. Theory Comput. 2023, 19, 6891–6932. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Pandey, S.K.; Tiwari, R. A computational method to estimate spin–orbital interaction strength in solid state systems. Comput. Mater. Sci. 2023, 221, 112090. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, X.; Abney, C.W.; Liu, X.; Foo, G.S.; Wu, Z.; Li, M.; Meyer, H.M.I.I.I.; Brown, S.; Mahurin, S.M.; et al. Toward the Design of a Hierarchical Perovskite Support: Ultra-Sintering-Resistant Gold Nanocatalysts for CO Oxidation. ACS Catal. 2017, 7, 3388–3393. [Google Scholar] [CrossRef]
- Zhang, H.; Itoi, T.; Konishi, T.; Izumi, Y. Dual Photocatalytic Roles of Light: Charge Separation at the Band Gap and Heat via Localized Surface Plasmon Resonance To Convert CO2 into CO over Silver–Zirconium Oxide. J. Am. Chem. Soc. 2019, 141, 6292–6301. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Mantzanis, A.; Zindrou, A.; Smykala, S.; Solakidou, M. Control of monomeric Vo’s versus Vo clusters in ZrO2−x for solar-light H2 production from H2O at high-yield (millimoles gr−1 h−1). Sci. Rep. 2022, 12, 15132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).