Amyloid Fibrils and Their Applications: Current Status and Latest Developments
Abstract
:1. Amyloid Proteins
2. Amyloid Fibrils
2.1. Existence in Nature
2.2. Artificial Synthesis
2.2.1. Induced Synthesis
2.2.2. Programmable Controllable Backup
2.2.3. Electrostatic Spinning
2.2.4. Others
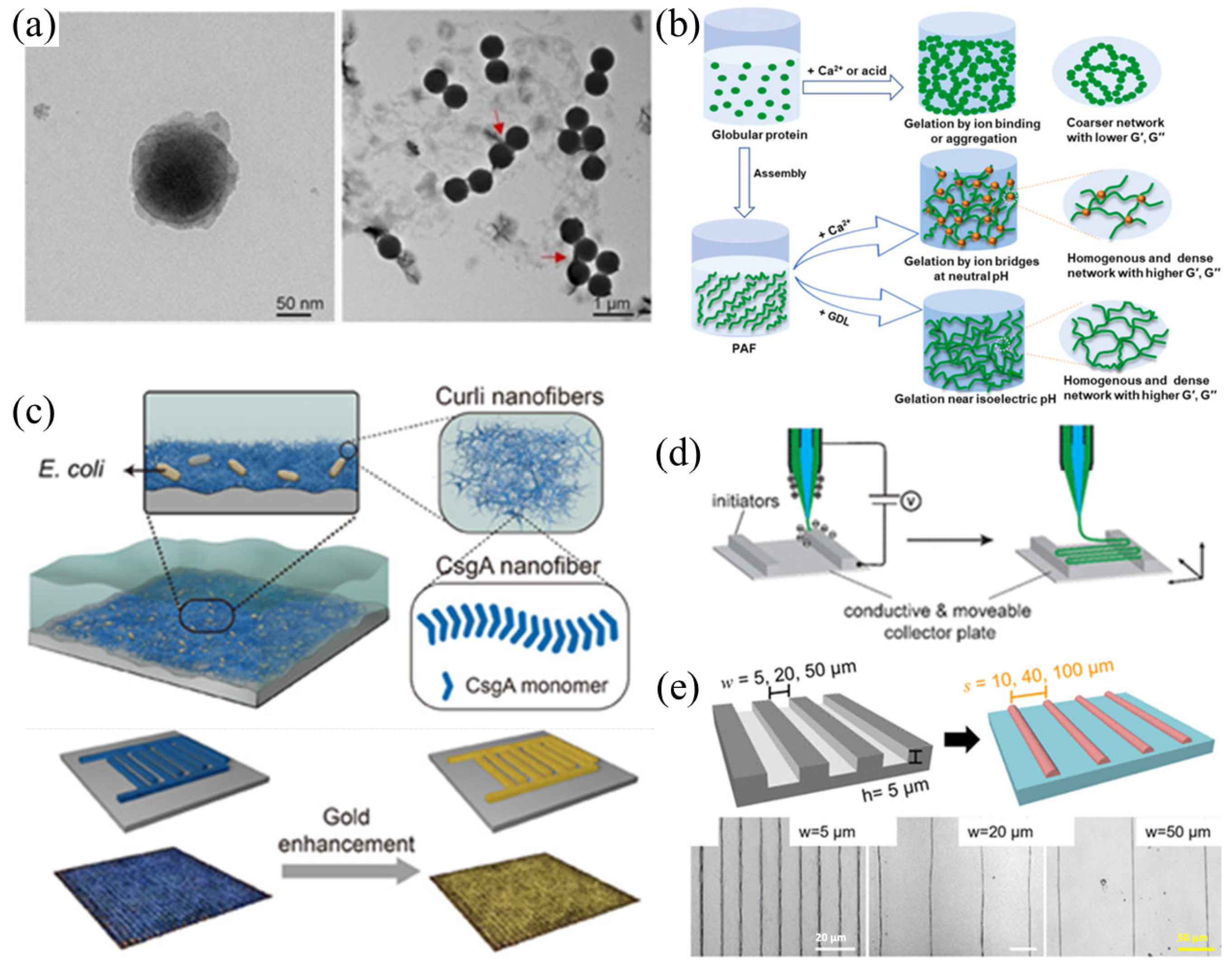
3. Research Progress on Functionalization of Amyloid-like Nanofibers
3.1. One-Dimensional
3.2. Two-Dimensional
3.3. Three-Dimensional
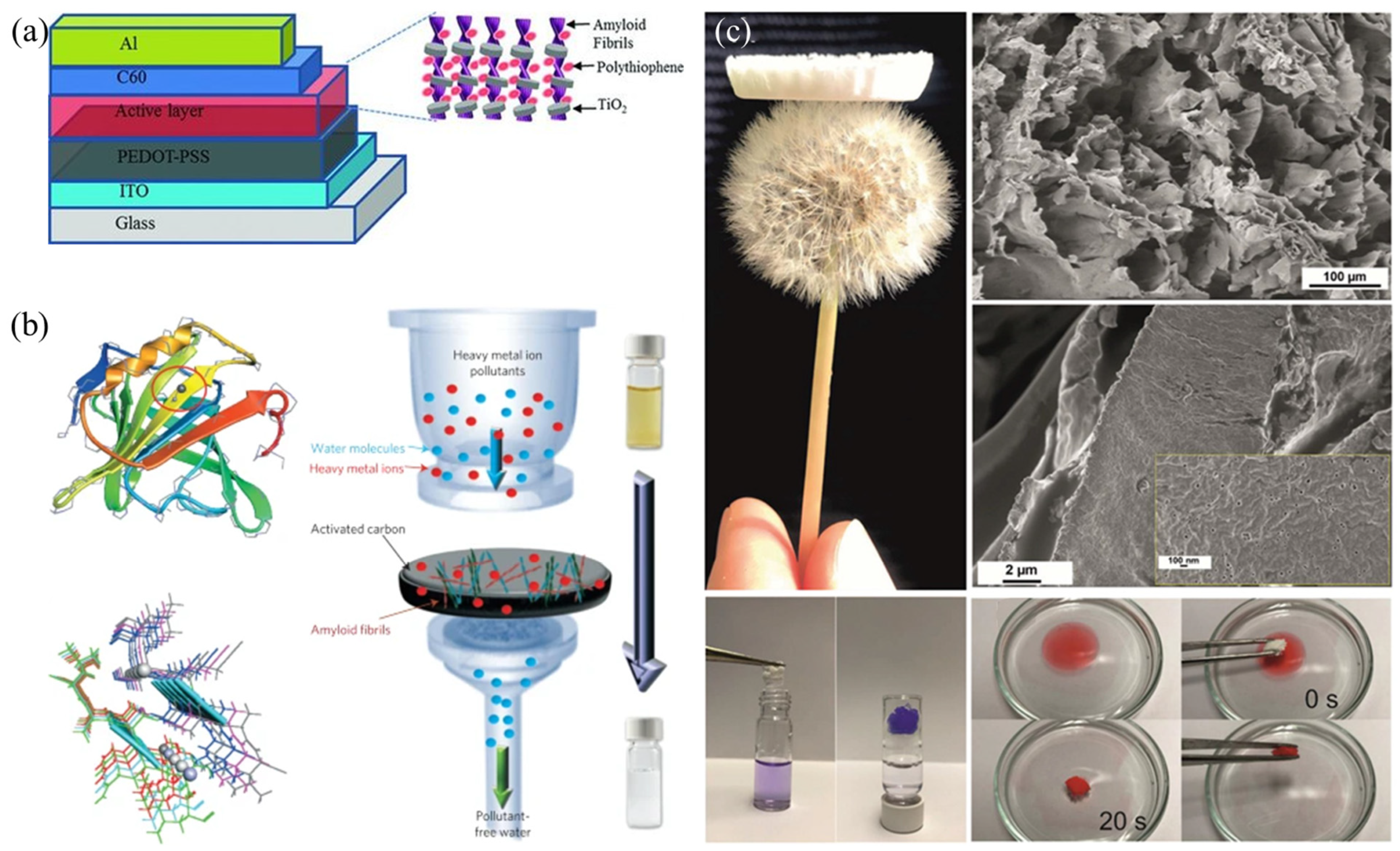
4. Application Based on Amyloid Nanofibrils
4.1. Water Treatment
4.2. Biomedicine
4.3. Biocatalyst
4.4. Structural Control
4.5. Pressure Sensors
4.6. Others
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| EISA | enzyme-instructed self-assembly |
| ZIF | zeolitic imidazolate frameworks |
| PEI | polyethyleneimine |
References
- Knowles, T.P.J.; Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Hartley, D.; Petre, B.M.; Walz, T.; Lansbury, P.T., Jr. Neurodegenerative disease-amyloid pores from pathogenic mutations. Nature 2002, 418, 291. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, S.; Jin, C.; Ren, F.; Wang, J. Wheat gluten amyloid fibrils: Conditions, mechanism, characterization, application, and future perspectives. Int. J. Biol. Macromol. 2023, 253, 126435. [Google Scholar] [CrossRef]
- Kutzli, I.; Zhou, J.; Li, T.; Baier, S.K.; Mezzenga, R. Formation and characterization of plant-based amyloid fibrils from hemp seed protein. Food Hydrocoll. 2023, 137, 108307. [Google Scholar] [CrossRef]
- Song, Y.; Li, T.; Zhang, X.; Wang, L. Investigating the effects of ion strength on amyloid fibril formation of rice proteins. Food Biosci. 2023, 51, 102068. [Google Scholar]
- Zhou, Q.; Lv, S.; Wang, W.; Zhu, S.; Xu, J.; Zheng, M.; Liu, Y.; Zhou, Y.; Sui, X.; Xiao, Y. Remodeling mechanism of gel network structure of soy protein isolate amyloid fibrils mediated by cellulose nanocrystals. Carbohydr. Polym. 2024, 332, 121919. [Google Scholar]
- Wei, Z.; Dai, S.; Huang, J.; Hu, X.; Ge, C.; Zhang, X.; Yang, K.; Shao, P.; Sun, P.; Xiang, N. Soy Protein Amyloid fibril scaffold for cultivated meat application. ACS Appl. Mater. Interfaces 2023, 15, 15108–15119. [Google Scholar]
- Camara-Almiron, J.; Navarro, Y.; Díaz-Martínez, L.; Magno-Pérez-Bryan, M.C.; Molina-Santiago, C.; Pearson, J.R.; de Vicente, A.; Pérez-García, A.; Romero, D. Dual functionality of the amyloid protein TasA in Bacillus physiology and fitness on the phylloplane. Nat. Commun. 2020, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Belousov, M.V.; Kosolapova, A.O.; Fayoud, H.; Sulatsky, M.I.; Sulatskaya, A.I.; Romanenko, M.N.; Bobylev, A.G.; Antonets, K.S.; Nizhnikov, A.A. OmpC and OmpF outer membrane proteins of Escherichia coli and Salmonella enterica form Bona Fide amyloids. Int. J. Mol. Sci. 2023, 24, 15522. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Hurbain, I.; Geerts, W.J.C.; Boudier, T.; Marco, S.; Verkleij, A.J.; Marks, M.S.; Raposo, G. Electron tomography of early melanosomes: Implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc. Natl. Acad. Sci. USA 2008, 105, 19726–19731. [Google Scholar] [CrossRef]
- Abidi, S.M.S.; Sharma, C.; Randhawa, S.; Shukla, A.K.; Acharya, A. A review on nanotechnological perspective of “the amyloid cascade hypothesis” for neurodegenerative diseases. Int. J. Biol. Macromol. 2023, 253, 126821. [Google Scholar] [CrossRef]
- Salahuddin, P.; Fatima, M.T.; Uversky, V.N.; Khan, R.H.; Islam, Z.; Furkan, M. The role of amyloids in Alzheimer’s and Parkinson’s diseases. Int. J. Biol. Macromol. 2021, 190, 44–55. [Google Scholar] [CrossRef]
- Hur, S.; Methivier, C.; Wilson, A.; Salmain, M.; Boujday, S.; Miserez, A. Biomineralization in barnacle base plate in association with adhesive cement protein. ACS Appl. Bio Mater. 2023, 6, 3423–3432. [Google Scholar] [CrossRef]
- Mykolenko, S.; Soon, W.L.; Mezzenga, R. Production and characterization of amaranth amyloid fibrils from food protein waste. Food Hydrocoll. 2024, 149, 109604. [Google Scholar] [CrossRef]
- Hasecke, F.; Niyangoda, C.; Borjas, G.; Pan, J.; Matthews, G.; Muschol, M.; Hoyer, W. Protofibril-fibril interactions inhibit amyloid fibril assembly by obstructing secondary nucleation. Angew. Chem. Int. Ed. 2021, 60, 3016–3021. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; Rossell, M.D.; Dachraoui, W.; Thompson, D.; Mayer, M. In situ observation of chemically induced protein denaturation at solvated interfaces. ACS Appl. Mater. Interfaces 2023, 15, 48015–48026. [Google Scholar] [CrossRef] [PubMed]
- Adamcik, J.; Mezzenga, R. Amyloid polymorphism in the protein folding and aggregation energy landscape. Angew. Chem. Int. Ed. 2018, 57, 8370–8382. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Yang, F.; Yu, H.; Xie, Y.; Yao, W. Submicron-size polystyrene modulates amyloid fibril formation: From the perspective of protein corona. Colloids Surf. B 2022, 218, 112736. [Google Scholar] [CrossRef] [PubMed]
- Kaumbekova, S.; Torkmahalleh, M.A.; Shah, D. Impact of ultrafine particles and secondary inorganic ions on early onset and progression of amyloid aggregation: Insights from molecular simulations. Environ. Pollut. 2021, 284, 117147. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, X.; Xu, Z.; Zhang, Y.; Valle, J.; Fan, S.; Zuo, X.; Lasa, I.; Fang, X. Structural mechanism for modulation of functional amyloid and biofilm formation by Staphylococcal Bap protein switch. EMBO J. 2021, 40, e107500. [Google Scholar] [CrossRef]
- Yue, J.; Yao, X.; Gou, Q.; Li, D.; Liu, N.; Yang, D.; Gao, Z.; Midgley, A.; Katsuyoshi, N.; Zhao, M. Recent advances of interfacial and rheological property based techno-functionality of food protein amyloid fibrils. Food Hydrocoll. 2022, 132, 107827. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Yu, J.; Wu, J.; Wang, F.; Liu, Y.; Li, X. Self-assembly mechanism of rice glutelin amyloid fibril aggregates obtained through experimental and molecular dynamics simulation analysis. Food Hydrocoll. 2023, 143, 108867. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Wang, X.; Cui, M.; Ge, P.; Zhang, J.; Qiu, F.; Zhong, C. Conformable self-assembling amyloid protein coatings with genetically programmable functionality. Sci. Adv. 2020, 6, eaba1425. [Google Scholar] [CrossRef]
- Duraj-Thatte, A.M.; Manjula-Basavanna, A.; Rutledge, J.; Xia, J.; Hassan, S.; Sourlis, A.; Rubio, A.G.; Lesha, A.; Zenkl, M.; Kan, A.; et al. Programmable microbial ink for 3D printing of living materials produced from genetically engineered protein nanofibers. Nat. Commun. 2021, 12, 6600. [Google Scholar] [CrossRef]
- Peng, X.; Hao, J.; Tao, W.; Guo, D.; Liang, T.; Hu, X.; Xu, H.; Fan, X.; Chen, C. Amyloid-like aggregates of short self-assembly peptide selectively induce melanoma cell apoptosis. J. Colloid Interface Sci. 2023, 640, 498–509. [Google Scholar] [CrossRef]
- Ji, D.; Lin, Y.; Guo, X.; Ramasubramanian, B.; Wang, R.; Radacsi, N.; Jose, R.; Qin, X.; Ramakrishna, S. Electrospinning of nanofibres. Nat. Rev. Methods Primers 2024, 4, 1. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, P.; Shi, Q.-F.; Ning, X.; Chen, Z.; Ramakrishna, S.; Zheng, J.; Long, Y.-Z. Advances in wet electrospinning: Rich morphology and promising applications. Adv. Fiber Mater. 2024. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Zhou, J.; Yao, Y.; Wu, X.; Bolisetty, S.; Diener, M.; Handschin, S.; Lu, C.; Mezzenga, R. Interfacial electrostatic self-assembly of amyloid fibrils into multifunctional protein films. Adv. Sci. 2023, 10, 2206867. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Ates, C.; Dincer, C. Stress monitoring with wearable technology and AI. Nat. Electron. 2024, 7, 98–99. [Google Scholar] [CrossRef]
- Hecker, L.; Wang, W.; Mela, I.; Fathi, S.; Poudel, C.; Soavi, G.; Huang, Y.Y.S.; Kaminski, C.F. Guided assembly and patterning of intrinsically fluorescent amyloid fibers with long-range order. Nano Lett. 2021, 21, 938–945. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Li, K.; Kang, A.; Zhang, S.; Feng, T.; Zhang, H.; Zhong, C. Functional amyloid-chitin hybrid ink coupled with flexible fabrication approaches for diverse macro and micro-structures. Mater. Today Bio 2022, 13, 100179. [Google Scholar] [CrossRef]
- Wu, C.; Bagnani, M.; Jin, T.; Yuan, Y.; Mezzenga, R. Cholesteric tactoids with tunable helical pitch assembled by lysozyme amyloid fibrils. Small 2024, 20, 2305839. [Google Scholar] [CrossRef]
- Nystrom, G.; Arcari, M.; Mezzenga, R. Confinement-induced liquid crystalline transitions in amyloid fibril cholesteric tactoids. Nat. Nanotechnol. 2018, 13, 330–336. [Google Scholar] [CrossRef]
- Ishibe, T.; Kaneko, T.; Uematsu, Y.; Sato-Akaba, H.; Komura, M.; Iyoda, T.; Nakamura, Y. Tunable thermal switch via order-order transition in liquid crystalline block copolymer. Nano Lett. 2022, 22, 6105–6111. [Google Scholar] [CrossRef]
- Li, Y.; Suen, J.J.Y.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D.; et al. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef]
- Khadem, S.A.; Bagnani, M.; Mezzenga, R.; Rey, A.D. Relaxation dynamics in bio-colloidal cholesteric liquid crystals confined to cylindrical geometry. Nat. Commun. 2020, 11, 4616. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Bagnani, M.; Yun, H.S.; Han, M.J.; Mezzenga, R.; Yoon, D.K. Hierarchically fabricated amyloid fibers via evaporation-induced self-assembly. ACS Nano 2021, 15, 20261–20266. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Adamcik, J.; Heier, J.; Mezzenga, R. Amyloid directed synthesis of titanium dioxide nanowires and their applications in hybrid photovoltaic devices. Adv. Funct. Mater. 2012, 22, 3424–3428. [Google Scholar] [CrossRef]
- Huang, S.; Qian, C.; Liu, X.; Zhang, L.; Meng, F.; Yan, Z.; Zhou, Y.; Du, J.; Ding, B.; Shi, J.; et al. A review on flexible solar cells. Sci. China Mater. 2024, 67, 2717–2736. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, Q.; Tan, Y.; Wang, F.; Salih, K.A.; Zheng, N.; Hamza, M.F.; Ning, S.; Wei, Y.; Yin, X. Synthesis of amyloid fibrils-PEI hybrid membrane for efficient removal of 99mTc from medical wastewater. Sep. Purif. Technol. 2025, 359, 130617. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Karbasi, M.; Sanchez-Ferrer, A.; Adamcik, J.; Askari, G.; Madadlou, A.; Mezzenga, R. Covalent β-lactoglobulin-maltodextrin amyloid fibril conjugate prepared by the Maillard reaction. Food Chem. 2021, 342, 128388. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, X.; Luan, C.; Wen, N.; Shi, C.; Lin, X.; Zhao, C.; Zhang, Y.; Luo, L.; Zhang, L.; et al. Fabrication of high-preformance emulsifier from conjugating maltodextrin onto myofibrillar protein peptide with microwave-ultrasound synergy. Ultrason. Sonochem. 2024, 104, 106818. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef]
- Song, J.; Sun, C.; Xiang, Y.; Xie, Y.; Mata, A.; Fang, Y. Fabrication of composite structures of lysozyme fibril-zein using antisolvent precipitation: Effects of blending and pH adjustment sequences. J. Agric. Food Chem. 2020, 68, 11802–11809. [Google Scholar] [CrossRef]
- Choi, J.; Seo, S.; Lee, S.; Ko, H.; Luo, Y.; Han, Y.; Shin, J.H.; Cho, H.; Lee, H. Low-temperature layer-by-layer growth of semiconducting few-layer γ-graphyne to exploit robust biocompatibility. ACS Appl. Mater. Interfaces 2023, 15, 41708–41719. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid fibrils aerogel for sustainable removal of organic contaminants from water. Adv. Mater. 2020, 32, 1907932. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Boschi, E.; Donat, F.; Mezzenga, R. Gold recovery from e-waste by food-waste amyloid aerogels. Adv. Mater. 2024, 36, 2310642. [Google Scholar] [CrossRef]
- Peydayesh, M.; Boschi, E.; Bagnani, M.; Tay, D.; Donat, F.; Almohammadi, H.; Li, M.; Usuelli, M.; Shiroka, T.; Mezzenga, R. Hybrid amyloid-chitin nanofibrils for magnetic and catalytic aerogels. ACS Nano 2024, 18, 6690–6701. [Google Scholar] [CrossRef]
- Usuelli, M.; Germerdonk, T.; Cao, Y.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nyström, G.; Mezzenga, R. Polysaccharide-reinforced amyloid fibril hydrogels and aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef]
- Kummer, N.; Giacomin, C.E.; Fischer, P.; Campioni, S.; Nyström, G. Amyloid fibril-nanocellulose interactions and self-assembly. J. Colloid Interface Sci. 2023, 641, 338–347. [Google Scholar] [CrossRef]
- Ha, Y.; Kwon, Y.; Nam, E.-J.; Park, H.; Paik, S.R. Short communication disulfide-mediated elongation of amyloid fibrils of a-synuclein for use in producing self-healing hydrogel and dye-absorbing aerogel. Acta Biomater. 2022, 145, 52–61. [Google Scholar] [CrossRef]
- Jia, X.; Peydayesh, M.; Huang, Q.; Mezzenga, R. Amyloid fibril templated MOF aerogels for water purification. Small 2022, 18, 2105502. [Google Scholar] [CrossRef]
- Fan, Y.; Lan, H.; Qi, Z.; Liu, R.; Hu, C. Removal of nickel and copper ions in strongly acidic conditions by in-situ formed amyloid fibrils. Chemosphere 2022, 297, 134241. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Yao, X.; Jiang, J.; Wang, M.; Yuan, S. Ultrasound-assisted preparation of Fe(OH)3@bacterial cellulose aerogel for efficient removal of organic contamination in water. Appl. Surf. Sci. 2023, 607, 154959. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suta, T.; Usuelli, M.; Handschin, S.; Canelli, G.; Bagnani, M.; Mezzenga, R. Sustainable removal of microplastics and natural organic matter from water by coagulation-flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021, 55, 8848–8858. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mo, S.; Du, K. Preparing macro-mesoporous membranes based on carbon-nanotube-supported “rattan-like” amyloid protein nanofibers for high-performance removal of Congo red. Ind. Eng. Chem. Res. 2024, 63, 18655–18666. [Google Scholar] [CrossRef]
- Bo, J.; Shi, B. Performances of residues from hydrolyzed corn-cobs for the adsorption of Congo red. Ind. Crops Prod. 2024, 220, 119311. [Google Scholar] [CrossRef]
- Zhang, Q.; Bolisetty, S.; Cao, Y.; Handschin, S.; Adamcik, J.; Peng, Q.; Mezzenga, R. Selective and efficient removal of fluoride from water: In situ engineered amyloid fibril/ZrO2 hybrid membranes. Angew. Chem. Int. Ed. 2019, 58, 6012–6016. [Google Scholar] [CrossRef]
- Yin, J.; Huang, G.; Xiao, H.; Chen, N.; An, C.; Chao, T.-C.; Feng, R.; Read, S. Bioinspired and dual-functional nanocellulose aerogels for water disinfection and heavy metal removal. Nano Today 2023, 51, 101918. [Google Scholar] [CrossRef]
- Favaro, M.T.P.; Lopez-Laguna, H.; Voltà-Durán, E.; Alba-Castellon, L.; Sánchez, J.M.; Casanova, I.; Unzueta, U.; Mangues, R.; Villaverde, A.; Vázquez, E. Lyophilization of biomimetic amyloids preserves their regulatable, endocrine-like functions for nanoparticle release. Appl. Mater. Today 2024, 39, 102348. [Google Scholar] [CrossRef]
- Wang, W.; Azizyan, R.A.; Garro, A.; Kajava, A.V.; Ventura, S. Multifunctional amyloid oligomeric nanoparticles for specific cell targeting and drug delivery. Biomacromolecules 2020, 21, 4302–4312. [Google Scholar] [CrossRef]
- Yang, J.; Dear, A.J.; Yao, Q.-Q.; Liu, Z.; Dobson, C.M.; Knowles, T.P.J.; Wu, S.; Perrett, S. Amelioration of aggregate cytotoxicity by catalytic conversion of protein oligomers into amyloid fibrils. Nanoscale 2020, 12, 18663–18672. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, J.; Soon, W.L.; Kutzli, I.; Molière, A.; Diedrich, S.; Radiom, M.; Handschin, S.; Li, B.; Li, L.; et al. Food amyloid fibrils are safe nutrition ingredients based on in-vitro and in-vivo assessment. Nat. Commun. 2023, 14, 6806. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Shen, Z.-T.; Hou, T.; Zhao, Y.-H.; Huang, M.-S.; Li, J.; Chen, H.; Hu, P.-H.; Luo, Z.-J.; et al. Core-shell reactor partitioning enzyme and prodrug by ZIF-8 for NADPH-sensitive in situ prodrug activation. Angew. Chem. Int. Ed. 2023, 62, e202314025. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Zhang, A.; Arad, E.; Jelinek, R.; Warshel, A. Assessing the catalytic role of native glucagon amyloid fibrils. ACS Catal. 2024, 14, 4656–4664. [Google Scholar] [CrossRef] [PubMed]
- Rufo, C.M.; Moroz, Y.S.; Moroz, O.V.; Stöhr, J.; Smith, T.A.; Hu, X.; DeGrado, W.F.; Korendovych, I.V. Short peptides self-assemble to produce catalytic amyloids. Nature Chem. 2014, 6, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Diaz-Caballero, M.; Peccati, F.; Roldán-Martín, L.; Sodupe, M.; Ventura, S. Amyloid fibrils formed by short prion-inspired peptides are metalloenzymes. ACS Nano 2023, 17, 16968–16979. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Bolisetty, S.; Tian, T.; Handschin, S.; Lu, C.; Mezzenga, R. Amyloid fibril-templated high-performance conductive aerogels with sensing properties. Small 2020, 16, 2004932. [Google Scholar] [CrossRef]
- Fasciano, S.; Wheba, A.; Ddamulira, C.; Wang, S. Recent advances in scaffolding biomaterials for cultivated meat. Biomater. Adv. 2024, 162, 213897. [Google Scholar]
- Kamarainen, T.; Nakayama, Y.; Uchiyama, H.; Tozuka, Y.; Kadota, K. Amyloid nanofibril-assisted Spray drying of crumpled supraparticles. Small 2024, 20, 2309645. [Google Scholar]
- Groppe, P.; Reichstein, J.; Carl, S.; Collados, C.C.; Niebuur, B.; Zhang, K.; Zubiri, B.A.; Libuda, J.; Kraus, T.; Retzer, T.; et al. Catalyst supraparticles: Tuning the structure of spray-dried Pt/SiO2 supraparticles via salt-based colloidal manipulation to control their catalytic performance. Small 2024, 20, 2310813. [Google Scholar] [CrossRef]
- Ha, Y.; Paik, S.R. Robust and resilient piezoresistive sensor made of MWCNT-embedded aerogel prepared with elongated amyloid fibrils of α-synuclein. Sensor. Actuat. B-Chem. 2024, 418, 136351. [Google Scholar] [CrossRef]
- Chen, X.; Kim, D.-H.; Lu, N. Introduction: Wearable devices. Chem. Rev. 2024, 124, 6145–6147. [Google Scholar] [CrossRef]
- Xue, B.; Sheng, H.; Li, Y.; Li, L.; Di, W.; Xu, Z.; Ma, L.; Wang, X.; Jiang, H.; Qin, M.; et al. Stretchable and self-healable hydrogel artificial skin. Natl. Sci. Rev. 2022, 9, nwab147. [Google Scholar] [CrossRef] [PubMed]
- Bagnani, M.; Ehrengruber, S.; Soon, W.L.; Peydayesh, M.; Miserez, A.; Mezzenga, R. Rapeseed cake valorization into bioplastics based on protein amyloid fibrils. Adv. Mater. Technol. 2023, 8, 2200932. [Google Scholar] [CrossRef]
- Grudniewska, A.; De Melo, E.M.; Chan, A.; Gniłka, R.; Boratyński, F.; Matharu, A.S. Enhanced protein extraction from oilseed cakes using glycerol-choline chloride-deep eutectic solvents: A biorefinery approach. ACS Sustain. Chem. Eng. 2018, 6, 15791–15800. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Y.; Weng, S.; Qi, W.; Su, H.; Li, T. Amyloid protein fibrils show enhanced ice recrystallization inhibition activity when serve as pickering emulsion stabilizer. Food Hydrocoll. 2023, 139, 108581. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Assembly of iron-bound ovotransferrin amyloid fibrils. Food Hydrocoll. 2019, 89, 579–589. [Google Scholar] [CrossRef]

| Source of Amyloid Proteins | Function | |
|---|---|---|
| Bacteria | Bacillus subtilis [11] | Improving the mechanical strength of biofilm and resisting chemical and biological degradation |
| Salmonella [12] | ||
| Escherichia coli [13] | ||
| Pseudomonas aeruginosa [14] | ||
| Staphylococcus aureus [14] | ||
| Animal | Premelanosome protein [15] | Forming amyloid fibrils and promoting melanin deposition |
| Parkinson’s disease [16,17] | Accumulation in the brain, triggering molecular cascades | |
| Alzheimer’s disease [16,17] | ||
| Acorn barnacles [18] | Regulating biomineralization and promoting the formation of fibrils | |
| Plant | Wheat gluten [6] | Unclear till now |
| Hemp seed [7] | ||
| Oryza sativa [8] | ||
| Soybean [10] | ||
| Amaranth seeds [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, H.; Qin, X. Amyloid Fibrils and Their Applications: Current Status and Latest Developments. Nanomaterials 2025, 15, 255. https://doi.org/10.3390/nano15040255
Liu B, Zhang H, Qin X. Amyloid Fibrils and Their Applications: Current Status and Latest Developments. Nanomaterials. 2025; 15(4):255. https://doi.org/10.3390/nano15040255
Chicago/Turabian StyleLiu, Bingxu, Hongnan Zhang, and Xiaohong Qin. 2025. "Amyloid Fibrils and Their Applications: Current Status and Latest Developments" Nanomaterials 15, no. 4: 255. https://doi.org/10.3390/nano15040255
APA StyleLiu, B., Zhang, H., & Qin, X. (2025). Amyloid Fibrils and Their Applications: Current Status and Latest Developments. Nanomaterials, 15(4), 255. https://doi.org/10.3390/nano15040255






