Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms
Abstract
1. Introduction
2. Mechanisms of Hydrogen Evolution and Catalytic Performance
2.1. Thermochemical Mechanisms
2.2. Electrochemical Hydrogen Evolution Reaction
2.3. Photocatalytic Mechanisms
2.4. Key Performance Indicators (Activity, Stability, Selectivity)
3. Traditional Catalysts for Hydrogen Production
3.1. Noble Metal Catalysts
3.2. Transition Metal Catalysts
4. Advanced Materials for Hydrogen Production Catalysts
4.1. Nanostructured Catalysts
4.1.1. Carbon-Based Nanomaterials
4.1.2. Metal Oxide Nanoparticles
4.2. Single-Atom Catalysts
4.3. Bimetallic and Multi-Metallic Catalysts
4.4. Metal–Organic Frameworks (MOFs)
4.5. Practical Devices and AI-Supported Methods
5. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yüksel Alpaydın, C.; Gülbay, S.K.; Ozgur Colpan, C. A review on the catalysts used for hydrogen production from ammonia borane. Int. J. Hydrogen Energy 2020, 45, 3414–3434. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of advanced transition metal-based catalyst for hydrogen production via electrolysis of water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Faur Ghenciu, A. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Trincado, M.; Banerjee, D.; Grützmacher, H. Molecular catalysts for hydrogen production from alcohols. Energy Environ. Sci. 2014, 7, 2464–2503. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A review of recent advances in water-gas shift catalysis for hydrogen production. Emergent Mater. 2020, 3, 881–917. [Google Scholar] [CrossRef]
- Alinejad, Z.; Parham, N.; Tawalbeh, M.; Al-Othman, A.; Almomani, F. Progress in green hydrogen production and innovative materials for fuel cells: A pathway towards sustainable energy solutions. Int. J. Hydrogen Energy 2024, in press. [CrossRef]
- Martínez de León, C.; Molina, P.; Ríos, C.; Brey, J.J. Green hydrogen production’s impact on sustainable development goals. Int. J. Hydrogen Energy 2025, in press. [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Su, Z.; Guan, J.; Liu, Y.; Shi, D.; Wu, Q.; Chen, K.; Zhang, Y.; Li, H. Research progress of ruthenium-based catalysts for hydrogen production from ammonia decomposition. Int. J. Hydrogen Energy 2024, 51, 1019–1043. [Google Scholar] [CrossRef]
- AlZaabi, A.; AlMarzooqi, F.; Choi, D. Ammonia electro-catalysis for hydrogen production: Mechanisms, materials, and scalability. Int. J. Hydrogen Energy 2024, 94, 23–52. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A review of hydrogen production processes by photocatalytic water splitting—From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Xu, F.; Ren, J.; Ma, J.; Wang, Y.; Zhang, K.; Cao, Z.; Sun, Q.; Wu, S.; Li, G.; Bai, S. A review of hydrogen production kinetics from the hydrolysis of NaBH4 solution catalyzed by Co-based catalysts. Int. J. Hydrogen Energy 2024, 50, 827–844. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sannegowda, L.K.; Singh, V. Developments in electrocatalysts for electrocatalytic hydrogen evolution reaction with reference to bio-inspired phthalocyanines. Int. J. Hydrogen Energy 2023, 48, 16569–16592. [Google Scholar] [CrossRef]
- Pedram, S.; Batool, M.; Yapp, K.; Bonville, L.; Jankovic, J. A Review on Bioinspired Proton Exchange Membrane Fuel Cell: Design and Materials. Adv. Energy Sustain. Res. 2021, 2, 2000092. [Google Scholar] [CrossRef]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Tayebee, R.; Sajjadifar, S.; Lotfi, S.; Maleki, B.; Hemmati, S. Bio-inspired synthesis of palladium nanoparticles fabricated magnetic Fe3O4 nanocomposite over Fritillaria imperialis flower extract as an efficient recyclable catalyst for the reduction of nitroarenes. Sci. Rep. 2021, 11, 4515. [Google Scholar] [CrossRef]
- Dharmarajan, N.P.; Vidyasagar, D.; Yang, J.-H.; Talapaneni, S.N.; Lee, J.; Ramadass, K.; Singh, G.; Fawaz, M.; Kumar, P.; Vinu, A. Bio-Inspired Supramolecular Self-Assembled Carbon Nitride Nanostructures for Photocatalytic Water Splitting. Adv. Mater. 2024, 36, 2306895. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Hu, X.; Zhang, X. Photoredox catalysis over semiconductors for light-driven hydrogen peroxide production. Green Chem. 2021, 23, 1466–1494. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef]
- Alamiery, A. Advancements in materials for hydrogen production: A review of cutting-edge technologies. ChemPhysMater 2024, 3, 64–73. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Bai, S.; Jia, A.; Song, J.; Cao, S.; Wang, N.; Liu, X. Metal-support interactions in heterogeneous catalytic hydrogen production of formic acid. Chem. Eng. J. 2023, 474, 145612. [Google Scholar] [CrossRef]
- Zhou, Z.; Jia, Y.; Wang, Q.; Jiang, Z.; Xiao, J.; Guo, L. Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review. Sustainability 2023, 15, 4556. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Aklilu, E.G.; Bounahmidi, T. Machine learning applications in catalytic hydrogenation of carbon dioxide to methanol: A comprehensive review. Int. J. Hydrogen Energy 2024, 61, 578–602. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Wu, Y.; Chen, C.; Hong, Y.; Yue, Y.; Zhang, J.; Hou, B. Molecular Dynamics and Machine Learning in Catalysts. Catalysts 2021, 11, 1129. [Google Scholar] [CrossRef]
- Ojelade, O.A. CO2 Hydrogenation to Gasoline and Aromatics: Mechanistic and Predictive Insights from DFT, DRIFTS and Machine Learning. ChemPlusChem 2023, 88, e202300301. [Google Scholar] [CrossRef]
- Mou, T.; Pillai, H.S.; Wang, S.; Wan, M.; Han, X.; Schweitzer, N.M.; Che, F.; Xin, H. Bridging the complexity gap in computational heterogeneous catalysis with machine learning. Nat. Catal. 2023, 6, 122–136. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Kuang, Y.; Yang, F.; Feng, L. Advancements in Ruthenium (Ru)-Based Heterostructure Catalysts: Overcoming Bottlenecks in Catalysis for Hydrogen Evolution Reaction. Adv. Energy Mater. 2024, 14, 2402043. [Google Scholar] [CrossRef]
- Li, Y.; Xin, T.; Cao, Z.; Zheng, W.; He, P.; Yoon Suk Lee, L. Optimized Transition Metal Phosphides for Direct Seawater Electrolysis: Current Trends. ChemSusChem 2024, 17, e202301926. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, T.; Xia, M.; Wei, Q.-H.; Xie, Z. Core–shell Mo2C@NC/Mo2C hollow microspheres as highly efficient electrocatalysts for the hydrogen evolution reaction. Dalton Trans. 2023, 52, 6267–6272. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; Xiao, X.; Zhang, F.; Song, S.; Chen, S.; Ren, Z. Recent Advances in Self-Supported Layered Double Hydroxides for Oxygen Evolution Reaction. Research 2020, 2020, 3976278. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Peng, X.; Lyu, S.; Li, Z.; Yang, B.; Zhang, Q.; He, Q.; Lei, L.; Hou, Y. Advancements in MXene-based nanohybrids for electrochemical water splitting. Chin. Chem. Lett. 2024, 110392. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Xin, J.; Zhang, X.; Cui, G.; Zhang, X.; Bao, J.; Tang, B.; Xie, Y. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction. Nano Res. 2017, 10, 1178–1188. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Cai, Y.; Zhuang, Y.; Wang, L. The Advanced Progress of MoS2 and WS2 for Multi-Catalytic Hydrogen Evolution Reaction Systems. Catalysts 2023, 13, 1148. [Google Scholar] [CrossRef]
- Sun, H.; Lee, S.-Y.; Park, S.-J. Bimetallic CuPd alloy nanoparticles decorated ZnO nanosheets with enhanced photocatalytic degradation of methyl orange dye. J. Colloid Interface Sci. 2023, 629, 87–96. [Google Scholar] [CrossRef]
- Mohanty, R.; Mansingh, S.; Parida, K.; Parida, K. Boosting sluggish photocatalytic hydrogen evolution through piezo-stimulated polarization: A critical review. Mater. Horiz. 2022, 9, 1332–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, W.; Zhang, C.; Huang, D.; Lai, C.; Cheng, M.; Qin, L.; Yang, Y.; Zhou, C.; Li, B.; et al. Sustainable hydrogen production by molybdenum carbide-based efficient photocatalysts: From properties to mechanism. Adv. Colloid Interface Sci. 2020, 279, 102144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, Y.; Yin, X.; Shen, L.; Zhu, K.; Huang, X.; Cao, D.; Yao, J.; Wang, G.; Yan, Q. Defect-rich MnxOy complex Fe–Ni sulfide heterogeneous electrocatalyst for a highly efficient hydrogen evolution reaction. J. Power Sources 2022, 540, 231664. [Google Scholar] [CrossRef]
- Tian, L.; Liu, H.; Yi, X.; Wang, X.; Pang, L.; Li, J. Synergistic effects of Mo2C/CeO2 nanoparticles anchored on nitrogen-doped carbon as an efficiently electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 23831–23841. [Google Scholar] [CrossRef]
- Xu, T.; Yang, L.; Li, J.; Usoltseva, N.; An, V.; Jin, X.; Zhang, C.; Zhang, X.; Liu, B. NH4F-Induced Morphology Control of CoP Nanostructures to Enhance the Hydrogen Evolution Reaction. Inorg. Chem. 2021, 60, 10781–10790. [Google Scholar] [CrossRef]
- Wu, P.F.; Yang, Y.Q.; Xi, H.Y.; Si, Y.; Chu, Y.H.; Su, X.Z.; Yan, W.S.; You, T.T.; Gao, Y.K.; Wang, Y.; et al. Operando Spectroscopy Observation of Mo Clusters-Ti3C2T Catalyst/Support Interface’s Dynamic Evolution in Hydrogen Evolution Reaction. Small 2024, 20, 2306716. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. Electrocatalytic and photocatalytic sustainable conversion of carbon dioxide to value-added chemicals: State-of-the-art progress, challenges, and future directions. J. Environ. Chem. Eng. 2022, 10, 108219. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Z.; Yi, H.; Cao, Y.; Du, L.; Hu, Y.; Kong, F.; Kramer Campen, R.; Gao, Y.; Du, C.; et al. Polyvinylpyrrolidone-Coordinated Single-Site Platinum Catalyst Exhibits High Activity for Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 15902–15907. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, X.; Jiang, H.; Li, S.; Li, C.; Li, S.; Ma, R.; Wang, J. Surface Engineering of Cr-Doped Cobalt Molybdate toward High-Performance Hydrogen Evolution. ACS Appl. Mater. Interfaces 2022, 14, 18607–18615. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Yu, T.; Huang, S.; Zhai, Z.; Wen, H.; Yin, S. Amorphous-crystalline heterostructure: Efficient catalyst for biomass oxidation coupled with hydrogen evolution. J. Colloid Interface Sci. 2024, 655, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, H.; Chen, Q.; Chen, H.; He, G. ZIF-67 derived Mo2N/Mo2C heterostructure as high-efficiency electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2022, 922, 166216. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Luo, J.; Chen, L.; He, K.; Ma, D.; Lv, S.; Xing, D. Carbon-based materials as highly efficient catalysts for the hydrogen evolution reaction in microbial electrolysis cells: Mechanisms, methods, and perspectives. Chem. Eng. J. 2023, 471, 144670. [Google Scholar] [CrossRef]
- Gao, L.; Zhong, X.; Chen, J.; Zhang, Y.; Liu, J.; Zhang, B. Optimizing the electronic structure of Fe-doped Co3O4 supported Ru catalyst via metal-support interaction boosting oxygen evolution reaction and hydrogen evolution reaction. Chin. Chem. Lett. 2023, 34, 108085. [Google Scholar] [CrossRef]
- Choi, J.; Nguyen, Q.T.; Park, S.; Ghule, B.G.; Park, J.H.; Park, J.R.; Nakate, U.T.; Jang, J.-H.; Kim, D.-W.; Park, S. Interfacially engineered palladium nanoparticle-decorated nickel oxide nanostructured electrocatalysts for high-performance hydrogen evolution reaction. Chem. Eng. J. 2024, 497, 154407. [Google Scholar] [CrossRef]
- Gautam, J.; Lee, S.-Y.; Park, S.-J. Copper-doped zinc cobalt sulfide nanosheets as advanced bifunctional electrocatalysts for sustainable hydrogen production via electrochemical water splitting. Adv. Compos. Hybrid Mater. 2024, 7, 155. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-W.; Lee, S.-Y.; Park, S.-J. Facile production of highly porous graphitic nanosheets for enhanced hydrogen storage. Chem. Eng. J. 2024, 486, 149988. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Q.; Huang, C.; Zhang, M.; Yan, Y.; Liu, T.; Zhou, W. Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction. Appl. Sci. 2023, 13, 2177. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, R.; Xu, X.; He, C.; Zhou, M.; Zeng, Z.; Ma, T.; Wang, M.; Li, S. Next Generation Noble Metal-Engineered Catalysts: From Structure Evolution to Structure-Reactivity Correlation in Water Splitting. Adv. Funct. Mater. 2024, 34, 2308813. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts 2020, 10, 788. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Li, F.; Feng, X.; Zhang, P.; Gao, L. Recent Progress in Non-Precious Metal Single Atomic Catalysts for Solar and Non-Solar Driven Hydrogen Evolution Reaction. Adv. Sustain. Syst. 2020, 4, 2000151. [Google Scholar] [CrossRef]
- Wang, P.; Yi, X.; Lu, M.; Huang, P.; Wang, Y.; Gao, D.; Wei, Y.; Xie, X.; Zhu, J. An amphiphilic nitrogen-doped graphene prepared by ammonia cold plasma and its supported platinum nanocatalyst for aqueous-phase hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2023, 663, 119286. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, Z.; Fang, S.; Jin, H.; Zhu, C.; Zhao, S.; Zhuang, G.; Chen, Q.; Zhu, Y. Fcc/hcp PtNi heterostructured alloy nanocrystals with ultrathin Pt shell for enhanced catalytic performance towards hydrogen evolution reaction. Nano Res. 2024, 17, 9822–9829. [Google Scholar] [CrossRef]
- Yuan, H.; Hong, M.; Huang, X.; Qiu, W.; Dong, F.; Zhou, Y.; Chen, Y.; Gao, J.; Yang, S. Graphene Chainmail Shelled Dilute Ni─Cu Alloy for Selective and Robust Aqueous Phase Catalytic Hydrogenation. Adv. Sci. 2024, 11, 2304349. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wang, X.; Ge, J. Recent progress of noble metal-based single-atom electrocatalysts for acidic oxygen evolution reaction. Curr. Opin. Electrochem. 2023, 42, 101379. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, D.; Deng, T.; He, G.; Chen, A.; Sun, X.; Yang, Y.; Miao, P. Research Progress of Oxygen Evolution Reaction Catalysts for Electrochemical Water Splitting. ChemSusChem 2021, 14, 5359–5383. [Google Scholar] [CrossRef]
- Kazemi, A.; Manteghi, F.; Tehrani, Z. Metal Electrocatalysts for Hydrogen Production in Water Splitting. ACS Omega 2024, 9, 7310–7335. [Google Scholar] [PubMed]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 14971–15005. [Google Scholar] [CrossRef]
- Parthiban, J.; Awasthi, M.K.; Kharde, T.A.; Kalita, K.; Singh, S.K. Recent progress in molecular transition metal catalysts for hydrogen production from methanol and formaldehyde. Dalton Trans. 2024, 53, 4363–4389. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-W.; Yao, Y.-F.; Xu, S.-Y.; Cao, X.-Y.; Ren, Y.-W.; Si, L.-P.; Liu, H.-Y. Electrocatalytic Hydrogen Evolution of Transition Metal (Fe, Co and Cu)–Corrole Complexes Bearing an Imidazole Group. Catalysts 2024, 14, 5. [Google Scholar] [CrossRef]
- Zhang, H.; Han, G.; Liu, Y.; Zhao, L.; Zhang, W.; Tahir Khalil, M.; Wei, H.; Wang, C.; Liu, T.; Guo, X.; et al. CoP/Co heterojunction on porous g-C3N4 nanosheets as a highly efficient catalyst for hydrogen generation. J. Colloid Interface Sci. 2024, 658, 22–31. [Google Scholar] [CrossRef]
- Qiao, L.; Xi, C.; Li, C.; Zhang, K.; Li, Q.; Han, J.; Ding, Y. Self-Limited Formation of Nanoporous Nickel Heterostructure Catalyst for Electrochemical Hydrogen Production. Adv. Funct. Mater. 2024, 34, 2402286. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Liu, X.; Zhao, W.; Liu, S.; Huang, X.; Zhao, Q. Tetra-Coordinated W2S3 for Efficient Dual-pH Hydrogen Production. Angew. Chem. Int. Ed. 2024, 63, e202316306. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. An introductory review on advanced multifunctional materials. Heliyon 2023, 9, e18060. [Google Scholar] [CrossRef]
- Singh, C.; Mukhopadhyay, S.; Hod, I. Metal–organic framework derived nanomaterials for electrocatalysis: Recent developments for CO2 and N2 reduction. Nano Converg. 2021, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Sarkar, D.; Awasthi, K.; Menezes, P.W. Functional role of single-atom catalysts in electrocatalytic hydrogen evolution: Current developments and future challenges. Coord. Chem. Rev. 2022, 452, 214289. [Google Scholar] [CrossRef]
- Bao, X.; Wang, T.; Yang, Y. Recent progress in bimetallic carbide-based electrocatalysts for water splitting. Mater. Chem. Front. 2024, 8, 627–651. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.H.; Jeoung, S.; Moon, H.R. Transformation of Metal–Organic Frameworks/Coordination Polymers into Functional Nanostructured Materials: Experimental Approaches Based on Mechanistic Insights. Acc. Chem. Res. 2017, 50, 2684–2692. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Al-Azzawi, W.K. Nanomaterials: Paving the way for the hydrogen energy frontier. Discov. Nano 2024, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.A.; Sattar, R.; Anum, A.; Al-Hussain, S.A.; Ahmad, S.; Irfan, A.; Zaki, M.E.A. An Insight into Carbon Nanomaterial-Based Photocatalytic Water Splitting for Green Hydrogen Production. Catalysts 2023, 13, 66. [Google Scholar] [CrossRef]
- Yin, H.; Pan, R.; Zou, M.; Ge, X.; Shi, C.; Yuan, J.; Huang, C.; Xie, H. Recent Advances in Carbon-Based Single-Atom Catalysts for Electrochemical Oxygen Reduction to Hydrogen Peroxide in Acidic Media. Nanomaterials 2024, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Kadja, G.T.M.; Mualliful Ilmi, M.; Mardiana, S.; Khalil, M.; Sagita, F.; Culsum, N.T.U.; Fajar, A.T.N. Recent advances of carbon nanotubes as electrocatalyst for in-situ hydrogen production and CO2 conversion to fuels. Results Chem. 2023, 6, 101037. [Google Scholar] [CrossRef]
- Hameed, S.; Ahmed, K.; Ghouri, Z.K. Carbon-based electrocatalysts for hydrogen evolution reaction. Energy Convers. Manag. X 2025, 100892. [Google Scholar] [CrossRef]
- He, H.; Liu, S.; Liu, Y.; Zhou, L.; Wen, H.; Shen, R.; Zhang, H.; Guo, X.; Jiang, J.; Li, B. Review and perspectives on carbon-based electrocatalysts for the production of H2O2via two-electron oxygen reduction. Green Chem. 2023, 25, 9501–9542. [Google Scholar] [CrossRef]
- Danish, M.S.S. Exploring metal oxides for the hydrogen evolution reaction (HER) in the field of nanotechnology. RSC Sustain. 2023, 1, 2180–2196. [Google Scholar] [CrossRef]
- Awogbemi, O.; Ojo, A.A.; Adeleye, S.A. Advancements in the application of metal oxide nanocatalysts for sustainable biodiesel production. Discov. Appl. Sci. 2024, 6, 250. [Google Scholar] [CrossRef]
- de Medeiros, F.G.; Lopes, F.W.; Rego de Vasconcelos, B. Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts 2022, 12, 363. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.-J.; Thorat, H.N.; Bodkhe, G.A.; Ramanavicius, A.; Ramanavicius, S.; Shirsat, M.D.; Ha, T.-J. Advanced energy materials: Current trends and challenges in electro- and photo-catalysts for H2O splitting. J. Ind. Eng. Chem. 2023, 119, 90–111. [Google Scholar] [CrossRef]
- Lin, S.; Sun, Z.; Wang, Z.; Zhou, Q.; Wang, A.; Wu, Z.; Qian, J.; He, M.-Y.; Chen, Q. Highly dispersed Ru immobilized on Ni-modified Al2O3 for selective hydrogenation of bisphenol A to hydrogenated bisphenol A. New J. Chem. 2024. [Google Scholar] [CrossRef]
- Gahlawat, A.; Sindhu, M.; Sharma, A.; Singh Maan, K.; Pratap Singh, P.; Aepuru, R.; Kumar, D. Synthesis and characterization of Bismuth titanate perovskite materials for hydrogen production. Mater. Lett. 2023, 342, 134316. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from Cation-Nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Ren, B.; Cao, J.; Zhang, H.; Han, C.; Xu, W. Recent progress in the development of single-atom electrocatalysts for highly efficient hydrogen evolution reactions. Mater. Chem. Front. 2023, 7, 3209–3231. [Google Scholar] [CrossRef]
- Choudhary, N.; Nabeela, K.; Mate, N.; Mobin, S.M. Recent advances in CO2 hydrogenation to methane using single-atom catalysts. RSC Sustain. 2024, 2, 1179–1201. [Google Scholar] [CrossRef]
- Li, S.; Xin, Z.; Luo, Y.; Pan, J.; Liao, G.; Li, Q.; Sun, Y.; Feng, Z.; Tan, R. Recent advances in the development of single atom catalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 82, 1081–1100. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Yu, H.; Yi, K.; Zhang, W.; Yuan, X.; Huang, J.; Deng, Y.; Zeng, G. Single-Atom Catalysts for Hydrogen Generation: Rational Design, Recent Advances, and Perspectives. Adv. Energy Mater. 2022, 12, 2200875. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Z.; Chen, W.; Shen, Q.; Yuan, D.; Zhang, C.; Zhu, Y.; Liang, H.-W.; Wang, Y.-G.; Song, W.; et al. Fully Exposed Iridium Clusters Enable Efficient Hydrogenation of N-Heteroarenes. ACS Catal. 2023, 13, 12153–12162. [Google Scholar] [CrossRef]
- Wei, T.; Zhou, J.; An, X. Recent advances in single-atom catalysts (SACs) for photocatalytic applications. Mater. Rep. Energy 2024, 4, 100285. [Google Scholar] [CrossRef]
- Kumar, N.; Lee, S.-Y.; Park, S.-J. Advancements in hydrogen storage technologies: A comprehensive review of materials, methods, and economic policy. Nano Today 2024, 56, 102302. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Deng, J.; Jing, L.; Wang, Z.; Wei, L.; Wei, Z.; Hou, Z.; Tao, J.; Dai, H. Bimetallic nanoparticles: Advances in fundamental investigations and catalytic applications. Environ. Sci. Adv. 2024, 4, 33–56. [Google Scholar] [CrossRef]
- Solanki, B.S.; Lim, H.; Yoon, S.J.; Ham, H.C.; Park, H.S.; Lee, H.E.; Lee, S.H. Recent advancement of non-noble metal catalysts for hydrogen production by NH3 decomposition. Renew. Sustain. Energy Rev. 2025, 207, 114974. [Google Scholar] [CrossRef]
- Wei, C.; Ding, H.; Zhang, Z.; Lin, F.; Xu, Y.; Pan, W. Research progress of bimetallic catalysts for CO2 hydrogenation to methane. Int. J. Hydrogen Energy 2024, 58, 872–891. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, K.; Sun, N.; Zhuang, Y.; Wang, L.; Yan, D. Bimetallic Single-Atom Catalysts for Electrocatalytic and Photocatalytic Hydrogen Production. Catalysts 2023, 13, 1409. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Kotob, E.; Abdulhakam, A.; Taialla, O.A.; Awad, M.M.; Hussain, I.; Alhooshani, K.R.; Ganiyu, S.A. Recent Advances in Bimetallic Catalysts for Methane Steam Reforming in Hydrogen Production: Current Trends, Challenges, and Future Prospects. Chem.-Asian J. 2024, 19, e202300641. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Srivastava, V.K.; Osman, A.I.; Rooney, D.W.; Fakeeha, A.H.; Abasaeed, A.E.; Alotibi, M.F.; Kumar, R. Iron-promoted zirconia-alumina supported Ni catalyst for highly efficient and cost-effective hydrogen production via dry reforming of methane. J. Environ. Sci. 2025, 148, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Anjum, M.S.; Ali, A.; Mehmood, Y.; Ahmad, M.; Alwadai, N.; Iqbal, M.; Akyürekli, S.; Hassan, N.; Shoukat, R. Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction. Sustainability 2024, 16, 1953. [Google Scholar] [CrossRef]
- Jadhav, G.S.; Mehta, A.K.; Tripathi, A.; Ghangrekar, M.M. Multi-metal ferrite as a promising catalyst for oxygen reduction reaction in microbial fuel cell. Environ. Sci. Pollut. Res. 2024, 31, 54402–54416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhong, J.; Gao, W.; Wang, Y. Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction. Coatings 2024, 14, 817. [Google Scholar] [CrossRef]
- Zaman, N.; Noor, T.; Iqbal, N. Recent advances in the metal–organic framework-based electrocatalysts for the hydrogen evolution reaction in water splitting: A review. RSC Adv. 2021, 11, 21904–21925. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, Y.; Li, Y.; Weragoda, D.M.; Tian, G.; Zhang, W.; Zhang, Z.; Zhao, X.; Chen, B. Research progress on MOFs and their derivatives as promising and efficient electrode materials for electrocatalytic hydrogen production from water. RSC Adv. 2023, 13, 24393–24411. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Kim, Y.; Gopannagari, M.; Kumar, D.P.; Kim, T.K. Recent advances in metal–organic framework-based photocatalysts for hydrogen production. Sustain. Energy Fuels 2021, 5, 1597–1618. [Google Scholar] [CrossRef]
- Shi, W.; Jin, X.; Zhang, C.; Zhang, X.; Liu, X.; Gao, Y.; Ding, W.; Gao, H.; Li, A. Recent advancement in metal-organic frameworks for hydrogen storage: Mechanisms, influencing factors and enhancement strategies. Int. J. Hydrogen Energy 2024, 83, 432–449. [Google Scholar] [CrossRef]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand Challenges and Future Opportunities for Metal–Organic Frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Meshesha, M.M.; Chanda, D.; Yang, B.L. Efficient green hydrogen production through metal–organic framework-derived Ni and Co mediated iron selenide hexagonal nanorods and wireless coupled with photovoltaics for urea and alkaline water electrolysis. Appl. Catal. B Environ. Energy 2024, 344, 123635. [Google Scholar] [CrossRef]
- Shingole, M.; Banerjee, S.; Ruz, P.; Kolay, S.; Kumar, A.; Sudarsan, V. Catalytic Hydrogen Generation through Ammonia Borane Hydrolysis Using Metal–Organic Framework via O–H Bond Activation. Energy Fuels 2024, 38, 8968–8978. [Google Scholar] [CrossRef]
- Yao, Y.; Yuan, J.; Wang, Z.; Zhao, Y.; Xu, J.; Liu, M.; Cho, J.; Li, F. Efficient visible-light-driven photocatalytic degradation of indoor formaldehyde using an indium-based MOF/graphene oxide composite. J. Photochem. Photobiol. A Chem. 2025, 460, 116102. [Google Scholar] [CrossRef]
- Makhafola, M.D.; Balogun, S.A.; Modibane, K.D. A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction. Energies 2024, 17, 1646. [Google Scholar] [CrossRef]
- Tang, H.; Chen, W.; Wang, J.; Dugger, T.; Cruz, L.; Kisailus, D. Electrocatalytic N-Doped Graphitic Nanofiber—Metal/Metal Oxide Nanoparticle Composites. Small 2018, 14, 1703459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, J.; Cai, X.; Zhao, Y.; Pan, L.; Cao, Q.; Wang, D.; Du, Y. Ultrafine bimetallic phosphide nanoparticles embedded in carbon nanosheets: Two-dimensional metal–organic framework-derived non-noble electrocatalysts for the highly efficient oxygen evolution reaction. Nanoscale 2018, 10, 19774–19780. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, W.; Xu, M.; Wang, E.; Wei, Y.; Liu, W.; Gu, X.; Liu, L.; Chen, Q.; Zhai, P.; et al. Single atom catalysts in Van der Waals gaps. Nat. Commun. 2022, 13, 6863. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Concepción, P.; Corma, A. A new strategy to transform mono and bimetallic non-noble metal nanoparticles into highly active and chemoselective hydrogenation catalysts. J. Catal. 2017, 350, 218–225. [Google Scholar] [CrossRef]
- Ma, F.-X.; Xu, C.-Y.; Lyu, F.; Song, B.; Sun, S.-C.; Li, Y.Y.; Lu, J.; Zhen, L. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef]
- Guo, J.; Haghshenas, Y.; Jiao, Y.; Kumar, P.; Yakobson, B.I.; Roy, A.; Jiao, Y.; Regenauer-Lieb, K.; Nguyen, D.; Xia, Z. Rational Design of Earth-Abundant Catalysts toward Sustainability. Adv. Mater. 2024, 36, 2407102. [Google Scholar] [CrossRef]
- Taherdoost, H.; Madanchian, M. AI Advancements: Comparison of Innovative Techniques. AI 2024, 5, 38–54. [Google Scholar] [CrossRef]


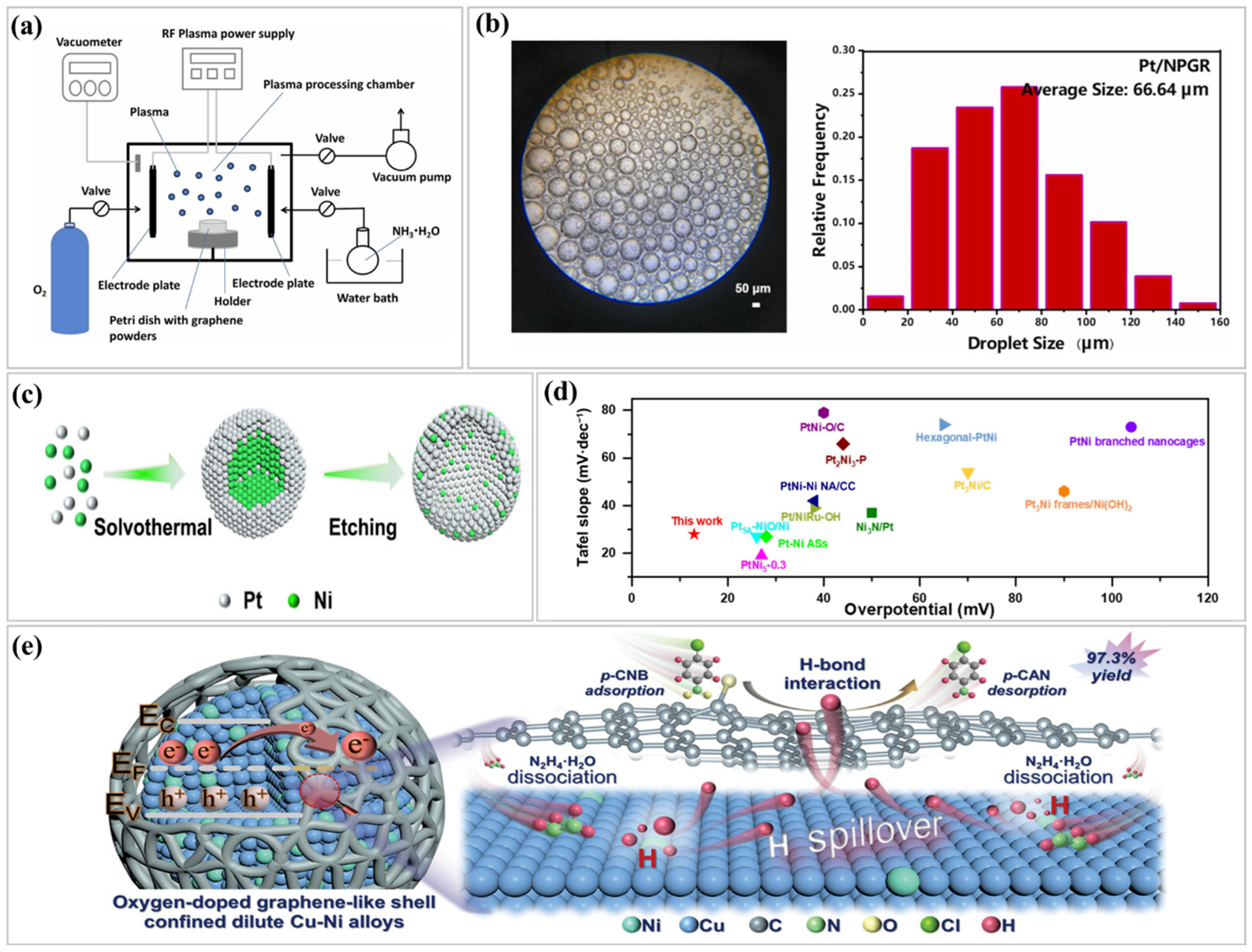
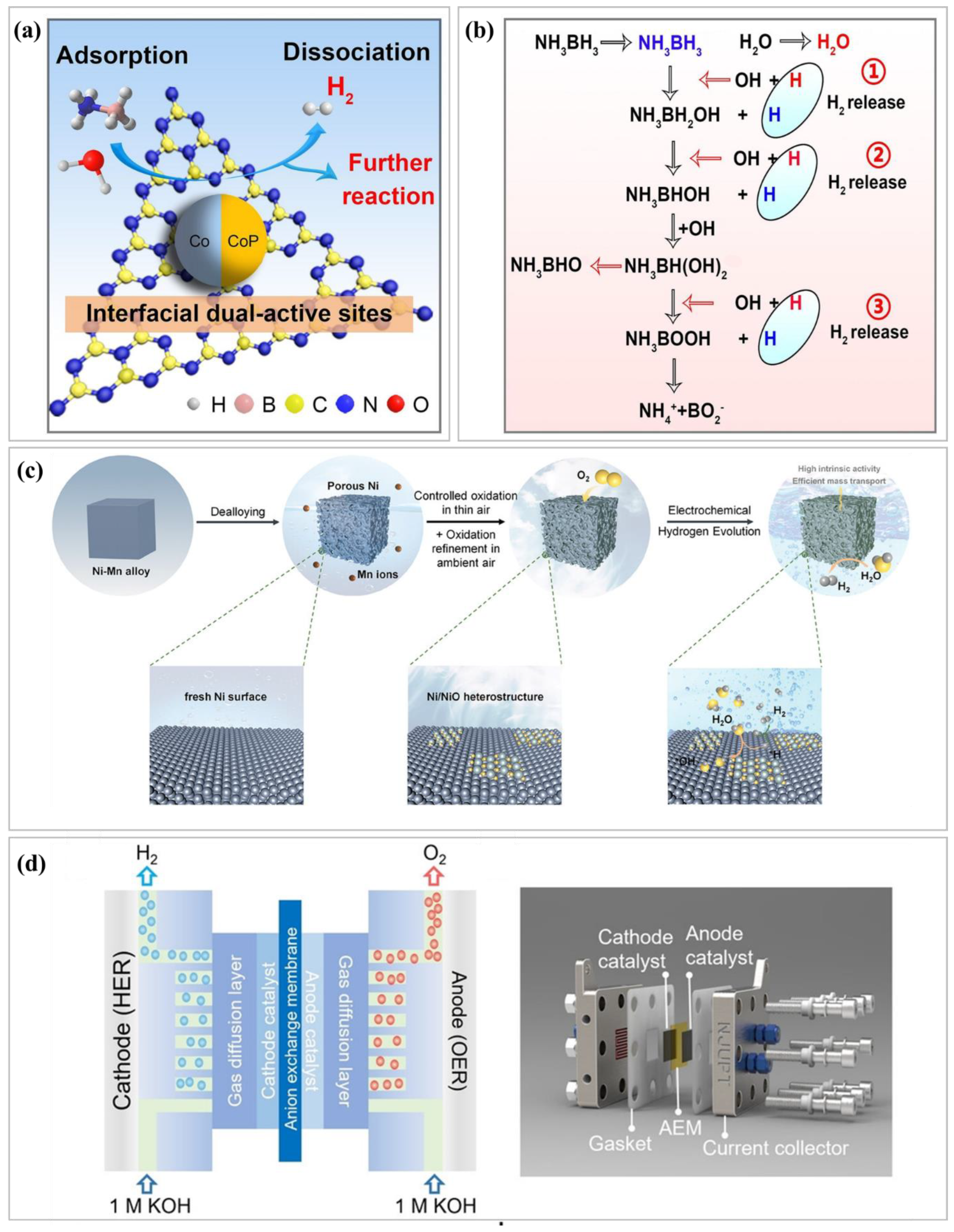


| Key Indicator | Specific Metrics | Enhancement Strategies | Ref. |
|---|---|---|---|
| Activity |
|
| [48] |
| Stability |
|
| [49] |
| Selectivity |
|
| [50] |
| Structural Design |
|
| [51] |
| Scalability |
|
| [52] |
| Electron Transfer |
|
| [53] |
| Hydrogen Adsorption |
|
| [51] |
| Sustainability |
|
| [52] |
| Interfacial Engineering |
|
| [50] |
| Nanomaterials | Physical Properties | Catalytic Properties | Hydrogen Interaction Properties | Ref. |
|---|---|---|---|---|
| Carbon-based | -High surface area -Good thermal stability -Electrical conductivity | -Active sites on surface -High catalytic efficiency -Low overpotential | High hydrogen adsorption, catalytic efficiency in electrochemical reactions | [73] |
| Metal oxide | -High surface area -Thermal stability -Mechanical strength | -High TOF -Catalytic stability -Reduction in activation energy | Efficient hydrogen storage and catalytic activity in water splitting | [74] |
| Single-atom | -Supported on high-surface-area materials like porous carbons -Thermal stability | -Maximized TOF due to isolated active sites -Low overpotential | High hydrogen adsorption/desorption efficiency, excellent for water splitting | [75] |
| Bimetallic/multi-metallic | Enhanced mechanical and thermal strength through alloying | -Synergistic catalytic effects -Reduced activation energy -Stable performance | Enhanced hydrogen adsorption and storage, high catalytic turnover in hydrogen evolution reactions | [76] |
| MOFs | Large surface area, tunable pore size, thermal stability | -High catalytic stability -Efficient reduction of overpotentials -Tunable active sites | High hydrogen storage capacity, catalytic efficiency in water splitting, and other redox reactions | [77] |
| Catalyst Type | Nanomaterial Examples | Activity | Stability | Selectivity | Overpotential | Cost-Effectiveness | Surface Area and Active Sites | Reference |
|---|---|---|---|---|---|---|---|---|
| Carbon-based nanomaterials | Graphene, graphene oxide (GO), carbon nanotubes (CNTs), carbon dots (CDs) | High ORR performance, superior to Pt in alkaline solutions | Excellent in alkaline media | Comparable to Pt in methanol tolerance | Moderate (~300 mV at 10 mA/cm2) | Low-cost, non-precious materials | High surface area, large pore channels | [116] |
| Metal oxide nanoparticles | Co3O4, Fe2O3, MnO2, NiO | Superior for OER with 270 mV at 10 mA/cm2 | High durability in alkaline solutions | High due to structural optimization | Moderate (270 mV) | Cost-effective | Moderate surface area, enhanced by doping | [117] |
| Single-atom catalysts | Pt on SnS2, Co on N-doped graphene | Comparable to 10 wt% Pt/C in HER | Ultrahigh stability, long-term durability | High due to isolated active sites | Very low (~51 mV at 10 mA/cm2) | Moderate | Maximal utilization of atoms in intercalated structures | [118] |
| Bimetallic catalysts | Ni-Co, Fe-Co, Pt-Ni | ~5× improvement over Co@C monometallic | Superior under mild conditions | Excellent chemoselectivity | Low (~41.7 mV dec⁻1) | High for non-noble catalysts | Large active sites due to bimetallic synergy | [119] |
| MOFs | ZIF-8, UiO-66, MIL-101 | Highly active with ultralow overpotential | High stability, resistant to deactivation | Selective to desired products | Low (~286 mV at 10 mA/cm2) | Moderate | Exceptional surface area, diverse active sites | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Aepuru, R.; Lee, S.-Y.; Park, S.-J. Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms. Nanomaterials 2025, 15, 256. https://doi.org/10.3390/nano15040256
Kumar N, Aepuru R, Lee S-Y, Park S-J. Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms. Nanomaterials. 2025; 15(4):256. https://doi.org/10.3390/nano15040256
Chicago/Turabian StyleKumar, Niraj, Radhamanohar Aepuru, Seul-Yi Lee, and Soo-Jin Park. 2025. "Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms" Nanomaterials 15, no. 4: 256. https://doi.org/10.3390/nano15040256
APA StyleKumar, N., Aepuru, R., Lee, S.-Y., & Park, S.-J. (2025). Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms. Nanomaterials, 15(4), 256. https://doi.org/10.3390/nano15040256


_Stamatis.png)



