Preparation of Electrochemical Sensors Based on Graphene/Ionic Liquids and the Quantitative Detection and Toxicity Evaluation of Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
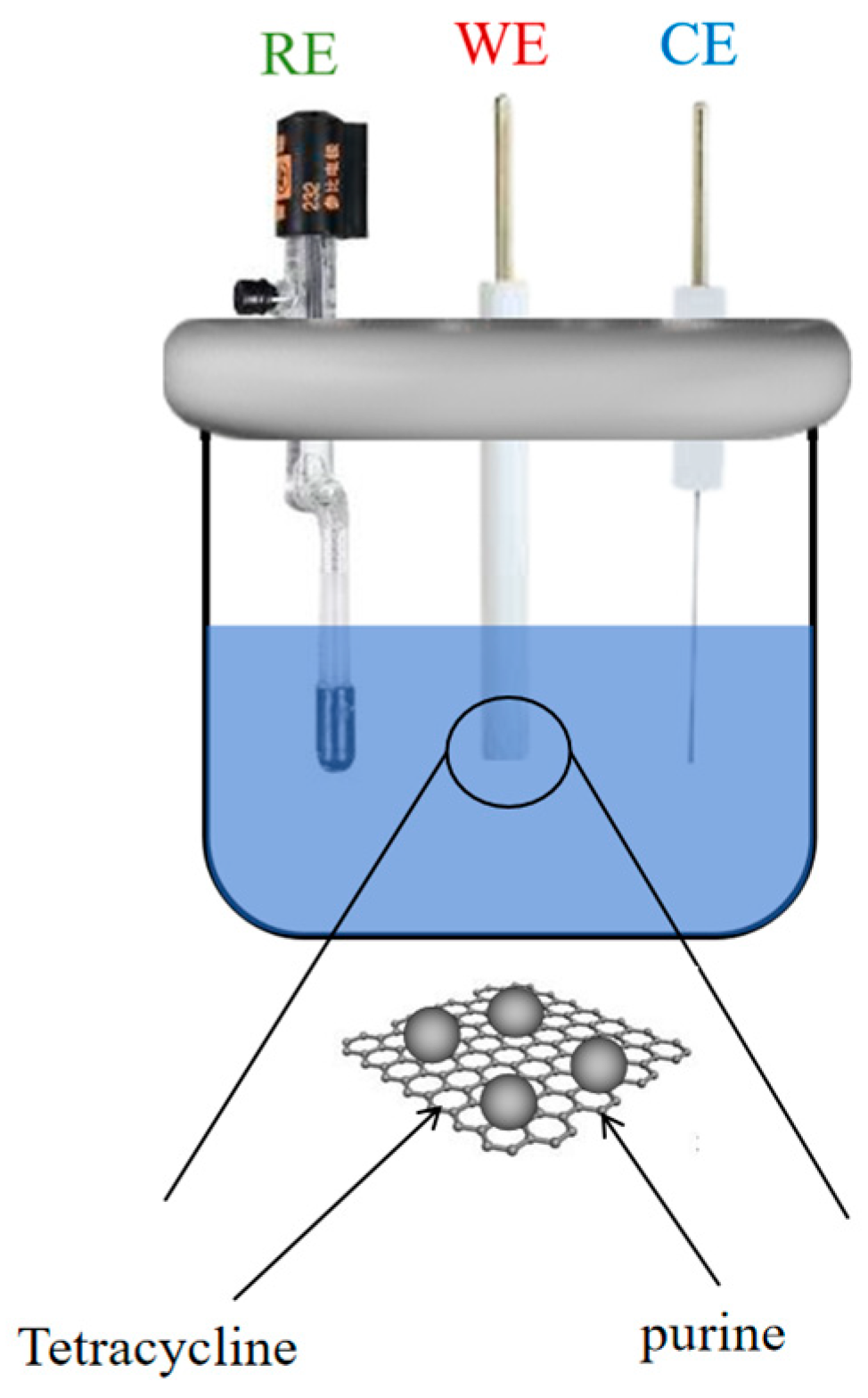
2.2. Apparatus
2.3. Preparation of the Nanocomposite-Modified Electrode
2.4. Cytotoxicity Assay
2.5. Electrochemical Detection Method
2.6. Statistical Analysis
3. Results and Discussion
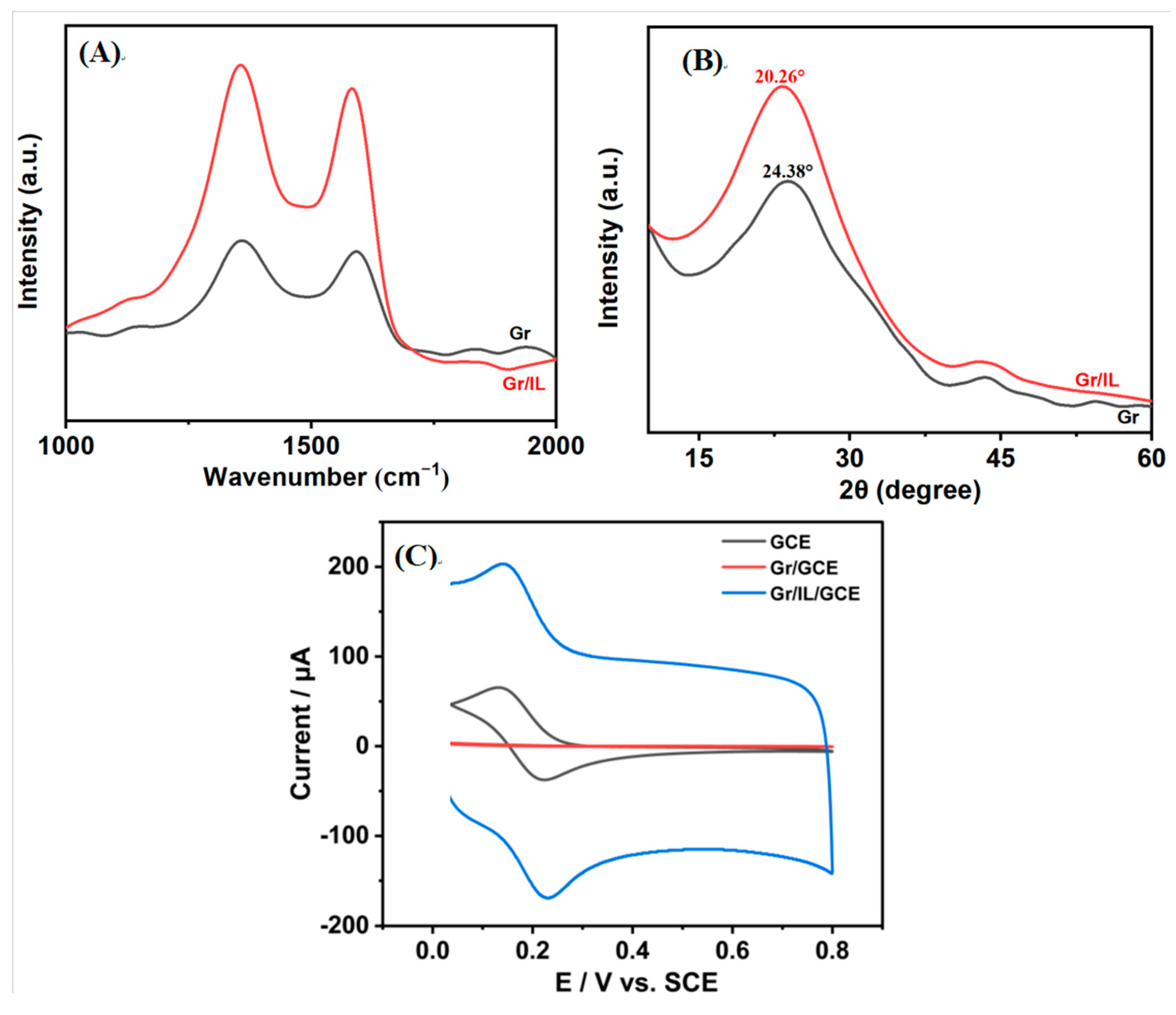
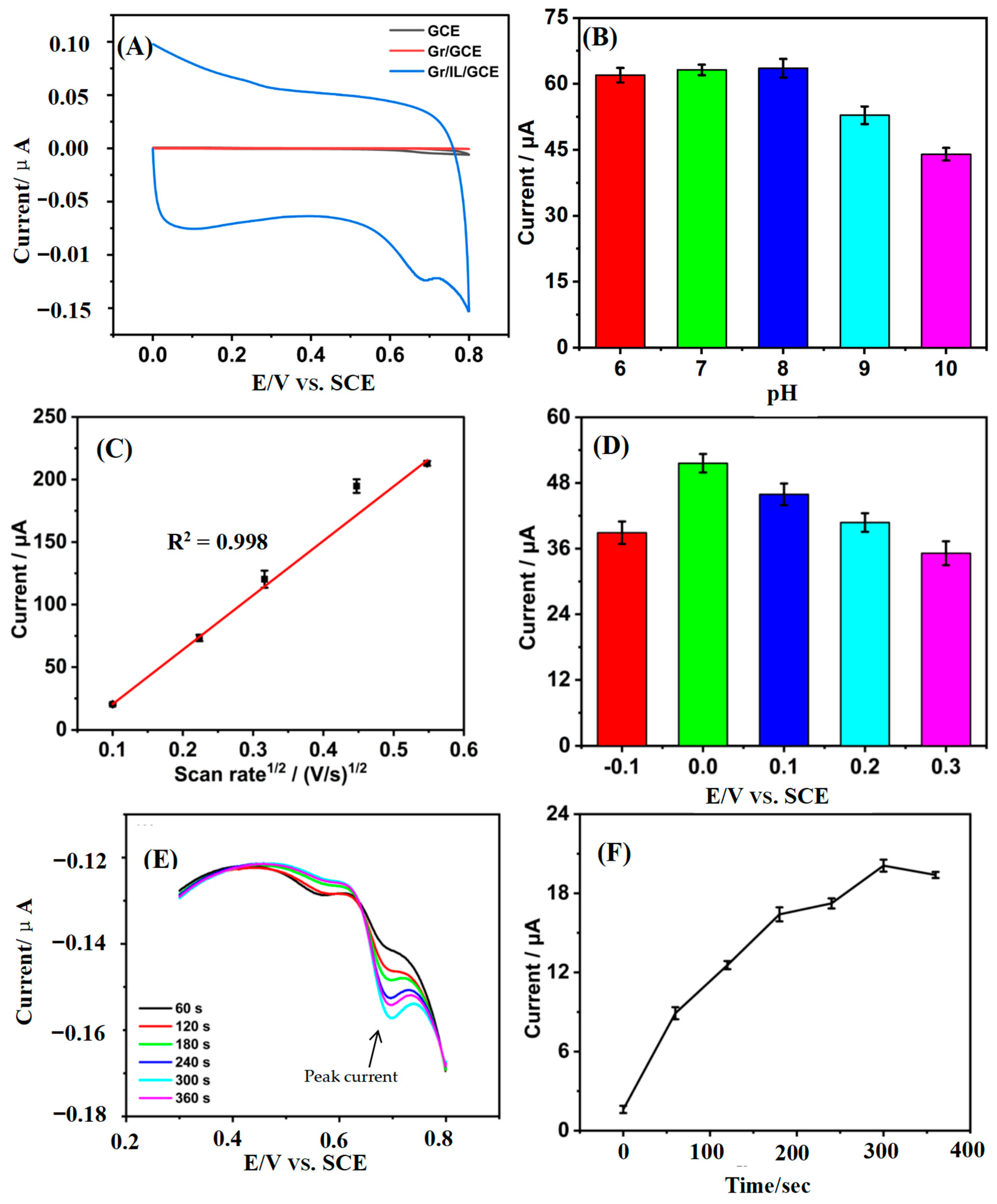
3.1. Characterization of the Gr/IL/GCE
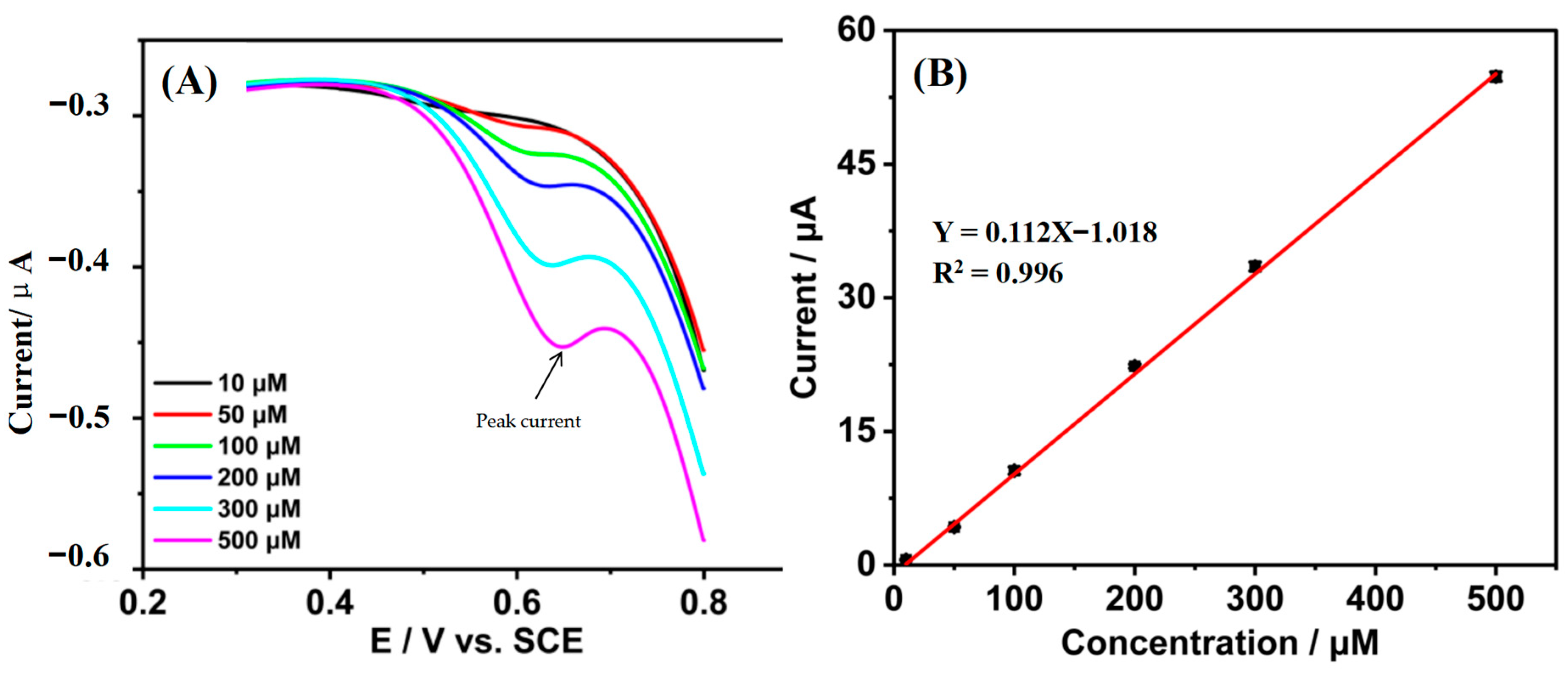
3.2. Quantification of Tetracycline by the Gr/IL/GCE
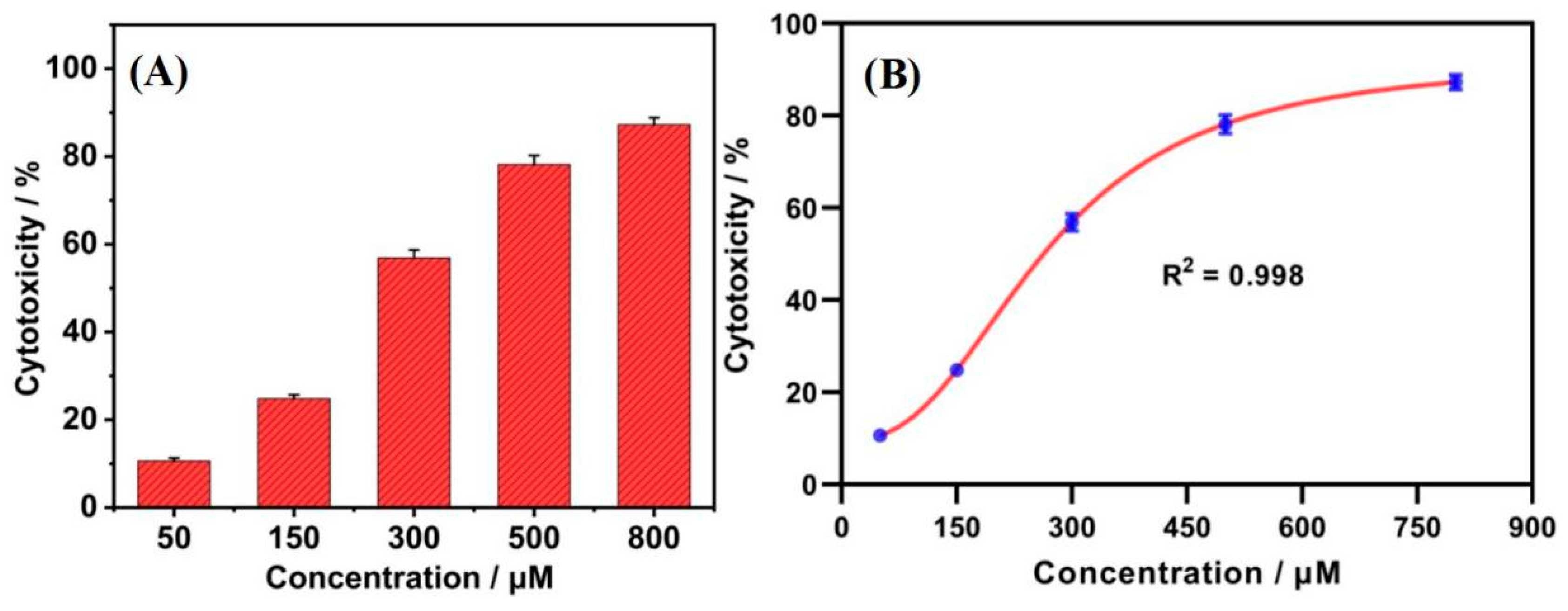
3.3. Toxicity of Tetracycline to HepG2 Cells
3.4. Repeatability of the Gr/IL/GCE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mengting, Z.; Kurniawan, T.A.; Yanping, Y.; Dzarfan Othman, M.H.; Avtar, R.; Fu, D.; Hwang, G.H. Fabrication, characterization, and application of ternary magnetic recyclable Bi2WO6/BiOI@Fe3O4 composite for photodegradation of tetracycline in aqueous solutions. J. Environ. Manag. 2020, 270, 110839. [Google Scholar] [CrossRef] [PubMed]
- Odetokun, I.A.; Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [Google Scholar]
- Li, S.; He, Y.; Kong, F.; Sun, W.; Hu, J. Photolytic Degradation of Tetracycline in the Presence of Ca(II) and/or Humic Acid. Water 2020, 12, 2078. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 2190–4707. [Google Scholar]
- Wang, Q.; Zhao, W.-M. Optical methods of antibiotic residues detections: A comprehensive review. Sens. Actuators B Chem. 2018, 269, 238–256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Cui, Z.; Liu, S.; Xu, J.; Jia, C.; Chen, Y.; Wang, L.; Sun, J.; Zhang, D.; et al. Chemical staining enhanced Enzyme-linked immunosorbent assay for sensitive determination of Clenbuterol in food. Food Chem. 2023, 400, 134012. [Google Scholar] [CrossRef]
- Che, H.; Nie, Y.; Tian, X.; Li, Y. New method for morphological identification and simultaneous quantification of multiple tetracyclines by a white fluorescent probe. J. Hazard. Mater. 2023, 441, 129956. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nur Azizah, P.; Nur Hasanah, A.; Binti Asman, S. Analytical method for monitoring tetracycline residues in various samples: A focus on environmental and health implications. Microchem. J. 2024, 206, 111408. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cai, Z.; Chen, M.; Cheng, F. A novel electrochemical sensor for formaldehyde based on palladium nanowire arrays electrode in alkaline media. Electrochim. Acta 2012, 68, 172–177. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Szymanski, C.; Wang, Z.; Zhou, Y.; Ma, X.; Yu, J.; Evans, J.; Orr, G.; Liu, S.; Zhu, Z.; et al. Chemical imaging of molecular changes in a hydrated single cell by dynamic secondary ion mass spectrometry and super-resolution microscopy. Integr. Biol. 2016, 8, 635–644. [Google Scholar] [CrossRef]
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xing, Y.; Yuan, X.; Wu, G.; Zhu, X.; Wu, D. Cytotoxicity and action mechanisms of polycyclic aromatic hydrocarbons by a miniature electrochemical detection system. Biomed. Microdevices 2021, 23, 1572–8781. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Zhao, Y.; Ye, C.; Zhou, S.; Guo, X.; Zhang, C.; Li, J.; Wu, D. A label-free approach to detect cell viability/cytotoxicity based on intracellular xanthine/guanine by electrochemical method. J. Pharmacol. Toxicol. Methods 2019, 100, 106625. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Xu, J.; Wang, C.; Wang, Y.; Yuan, D.; Chew, J.W. Electrosorption performance on graphene-based materials: A review. RSC Adv. 2023, 13, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Ajayan, J.; Radhika, J.M.; Sivasankari, B.; Tayal, S.; Saravanan, M. A comprehensive review on graphene FET bio-sensors and their emerging application in DNA/RNA sensing & rapid COVID-19 detection. Measurement 2023, 206, 112202. [Google Scholar]
- Shahid, Z.; Fu, B.; Rehman, S.; Ahmad, Z.; Chen, J.; Lu, X. Potential-resolved real-time ultrasensitive sensing of bisphenol compounds based on ionic liquid coupled Zr-MOF@GO nanosensor. Microchem. J. 2024, 207, 111888. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; He, R.; Feng, L.; Lv, C.; Zhang, S.; Zhao, X.; Zhao, G. Sensitive electrochemical detection of As(III) in soil based on CoFe2O4/AuNPs/IL nanocomposite modified electrode: Insight into the sensing mechanism of a dual electrocatalysis system. Microchem. J. 2024, 203, 110849. [Google Scholar] [CrossRef]
- Asha, S.; Vijayalakshmi, K.P.; George, B.K. Electronic structural studies of pyrrolidinium-based ionic liquids for electrochemical application. Int. J. Quantum Chem. 2019, 119, 0020–7608. [Google Scholar] [CrossRef]
- Al-Salman, R.; Sommer, H.; Brezesinski, T.; Janek, J. Template-Free Electrochemical Synthesis of High Aspect Ratio Sn Nanowires in Ionic Liquids: A General Route to Large-Area Metal and Semimetal Nanowire Arrays? Chem. Mater. 2015, 27, 3830–3837. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, S.A.; Golding, J.; Deacon, G.B. Ionic liquids based on imidazolium, ammonium and pyrrolidinium salts of the dicyanamide anion. Green Chem. 2002, 4, 444–448. [Google Scholar] [CrossRef]
- Gusain, R.; Mungse, H.P.; Kumar, N.; Ravindran, T.R.; Pandian, R.; Sugimura, H.; Khatri, O.P. Covalently attached graphene–ionic liquid hybrid nanomaterials: Synthesis, characterization and tribological application. J. Mater. Chem. A 2016, 4, 926–937. [Google Scholar] [CrossRef]
- Jalili, F.; Jalalvand, A.R. A novel and intelligent molecularly imprinted enzymatic biosensor for biosensing of human serum albumin in the presence of gamma-globulin, and glucose as uncalibrated interference. Sens. Bio-Sens. Res. 2023, 42, 22141804. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Ma, Q.; Liu, R.; Liu, X. Layer-by-layer assembled ionic-liquid functionalized graphene–polyaniline nanocomposite with enhanced electrochemical sensing properties. J. Mater. Chem. C 2014, 2, 4818. [Google Scholar] [CrossRef]
- Berciaud, S.; Ryu, S.; Brus, L.E.; Heinz, T.F. Probing the Intrinsic Properties of Exfoliated Graphene: Raman Spectroscopy of Free-Standing Monolayers. Nano Lett. 2009, 9, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Aranburu, N.; Otaegi, I.; Guerrica-Echevarria, G. Using an Ionic Liquid to Reduce the Electrical Percolation Threshold in Biobased Thermoplastic Polyurethane/Graphene Nanocomposites. Polymers 2019, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Hauk, V.M.; Macherauch, E. A Useful Guide For X-Ray Stress Evaluation (XSE). Adv. X-Ray Anal. 1984, 27, 81–99. [Google Scholar]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef]
- Pantokratoras, A. Comment on the papers: Journal of Molecular Liquids 224 (2016) 1341–1347, Journal of Molecular Liquids 222 (2016) 854–862 and Journal of Molecular Liquids 225 (2017) 569–576. J. Mol. Liq. 2017, 238, 1–2. [Google Scholar] [CrossRef]
- Guo, C.X.; Lei, Y.; Li, C.M. Porphyrin Functionalized Graphene for Sensitive Electrochemical Detection of Ultratrace Explosives. Electroanalysis 2010, 23, 885–893. [Google Scholar] [CrossRef]
- Alyamni, N.; Abot, J.L.; Zestos, A.G. Perspective—Advances in Voltammetric Methods for the Measurement of Biomolecules. ECS Sens. Plus 2024, 3, 027001. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, M.; Gao, P.; Zhang, G.; Shi, L.; Yuan, M.; Shuang, S. The synthesis of high bright silver nanoclusters with aggregation-induced emission for detection of tetracycline. Sens. Actuators B Chem. 2021, 326, 129009. [Google Scholar] [CrossRef]
- Kushikawa, R.T.; Silva, M.R. Angelo ACD, Teixeira MFS: Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens. Actuators B Chem. 2016, 228, 207–213. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Qi, J.; Miao, Q.; Deng, D.; He, H.; Yan, X.; Luo, L. A paper-based ratiometric fluorescence sensor based on carbon dots modified with Eu3+ for the selective detection of tetracycline in seafood aquaculture water. Analyst 2024, 149, 1571–1578. [Google Scholar] [CrossRef]
- Al Nimer, A.; Kawde, A.-N.; Elgamouz, A.; Shehadi, I.; AbdelHamid, A. Copper enhanced guanine electrochemical signal for nucleic acids detection in municipal tertiary wastewater. Arab. J. Chem. 2023, 16, 105091. [Google Scholar] [CrossRef]
- Guo, J.; Xin, J.; Wang, J.; Li, Z.; Yang, J.; Yu, X.; Yan, M.; Mo, J. A high-efficiency and selective fluorescent assay for the detection of tetracyclines. Sci. Rep. 2024, 14, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, H.; Zhang, Z.; Ma, S.; Feng, Q.; Wang, J.; Wu, G.; Ng, H.Y. Cytotoxicity evaluation of organophosphorus flame retardants using electrochemical biosensors and elucidation of associated toxic mechanisms. Water Res. 2024, 265, 122262. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhou, S.; Wu, G.; Wang, C.; Yuan, X.; Feng, Q.; Zhu, X.; Qu, J. A sensitive electrochemical sensor for bisphenol F detection and its application in evaluating cytotoxicity. Microchem. J. 2021, 168, 106414. [Google Scholar] [CrossRef]


| Modified Electrode | Linear Detection Range (μM) | Detection Limit (μM) | Sensitivity (mA.L/mol) |
|---|---|---|---|
| MIOPPy-AuNP/SPCE [33] | 1~20 | 0.65 | - |
| Ag NCs [34] | 1.12~230 | 0.47 | - |
| PtNPs/C/GCE [35] | 9.99~44.01 | 4.28 | 45.4 |
| CDs-Eu@paper [36] | 0.1~100 | 0.03 | - |
| clay-CPE [37] | 0.1~45 | 0.16 | 1100 |
| Fluorescent assay [38] | 10~350 | 1.70 | - |
| Gr/IL/GCE | 10~500 | 2.06 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.; Huang, L.; Wang, C.; Zuo, R.; Liu, J. Preparation of Electrochemical Sensors Based on Graphene/Ionic Liquids and the Quantitative Detection and Toxicity Evaluation of Tetracycline. Nanomaterials 2025, 15, 263. https://doi.org/10.3390/nano15040263
Lai M, Huang L, Wang C, Zuo R, Liu J. Preparation of Electrochemical Sensors Based on Graphene/Ionic Liquids and the Quantitative Detection and Toxicity Evaluation of Tetracycline. Nanomaterials. 2025; 15(4):263. https://doi.org/10.3390/nano15040263
Chicago/Turabian StyleLai, Meidan, Linzhe Huang, Chengzhi Wang, Rui Zuo, and Jun Liu. 2025. "Preparation of Electrochemical Sensors Based on Graphene/Ionic Liquids and the Quantitative Detection and Toxicity Evaluation of Tetracycline" Nanomaterials 15, no. 4: 263. https://doi.org/10.3390/nano15040263
APA StyleLai, M., Huang, L., Wang, C., Zuo, R., & Liu, J. (2025). Preparation of Electrochemical Sensors Based on Graphene/Ionic Liquids and the Quantitative Detection and Toxicity Evaluation of Tetracycline. Nanomaterials, 15(4), 263. https://doi.org/10.3390/nano15040263





