Tattoo Ink Metal Nanoparticles: Assessment of Toxicity In Vitro and with a Novel Human Ex Vivo Model
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Measurements of Metal NP Certified Reference Standards
3.2. Presence of Metal NPs in Tattoo Inks
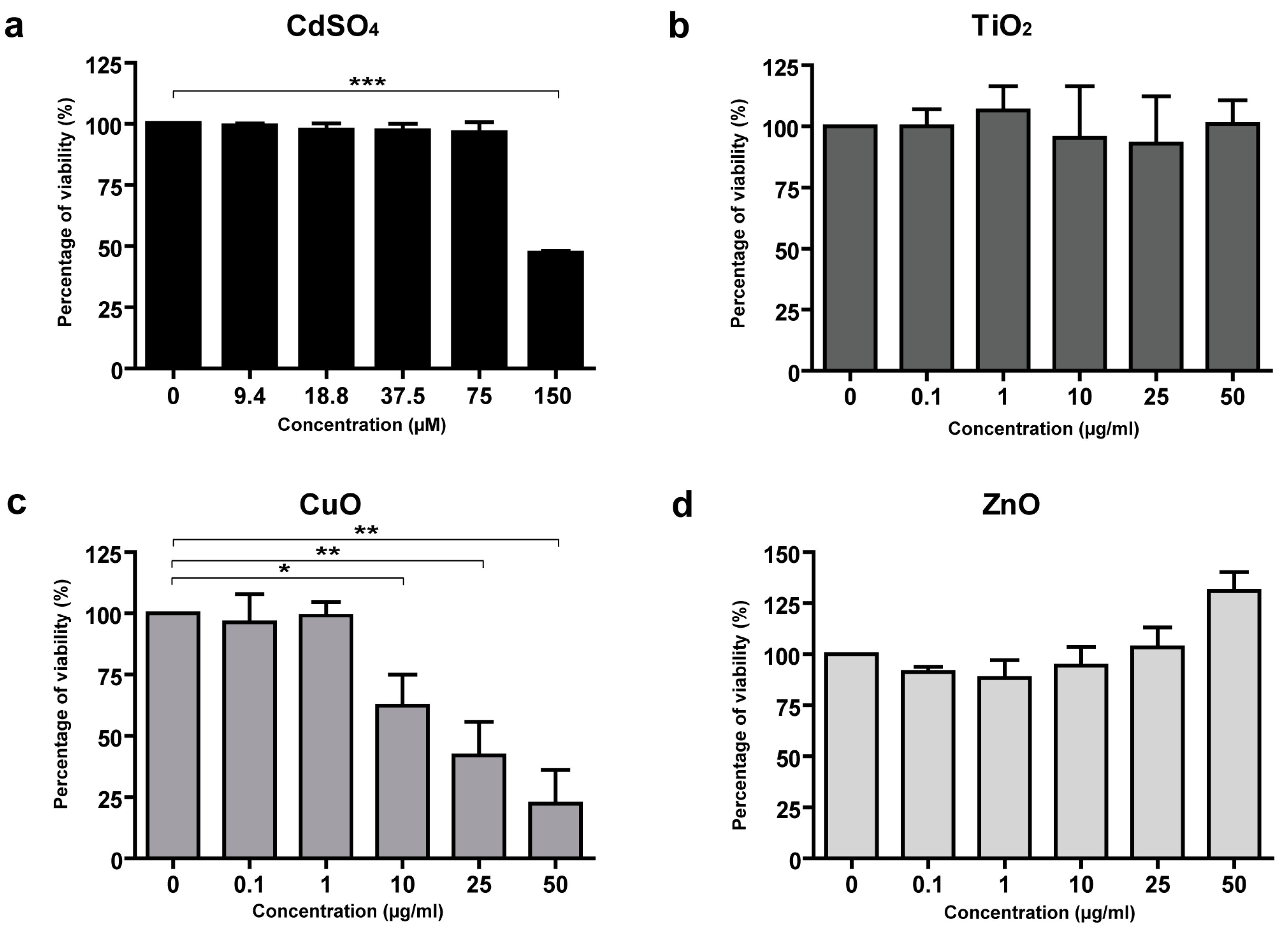
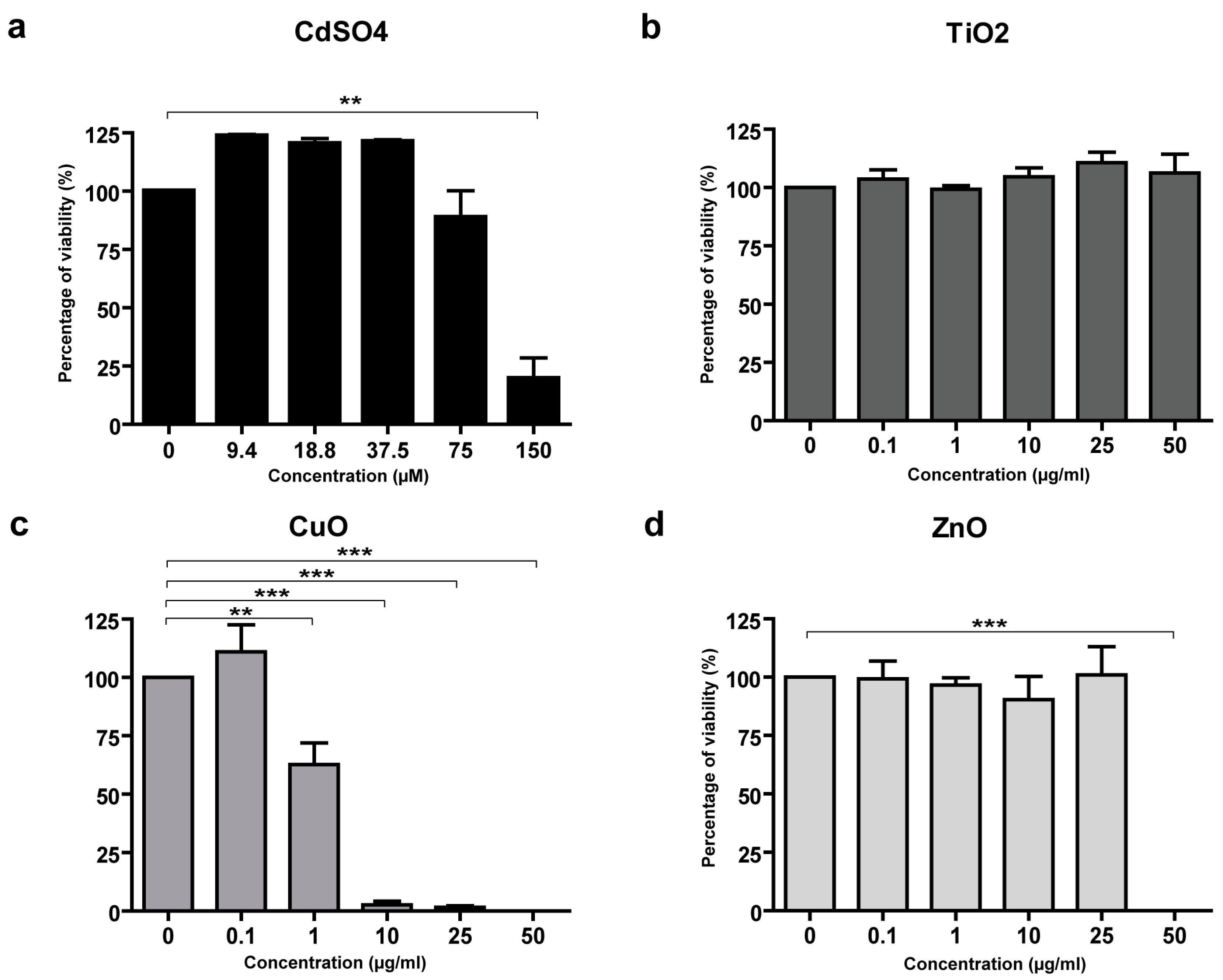
3.3. Toxicity of CuO and ZnO NPs in Skin Cells
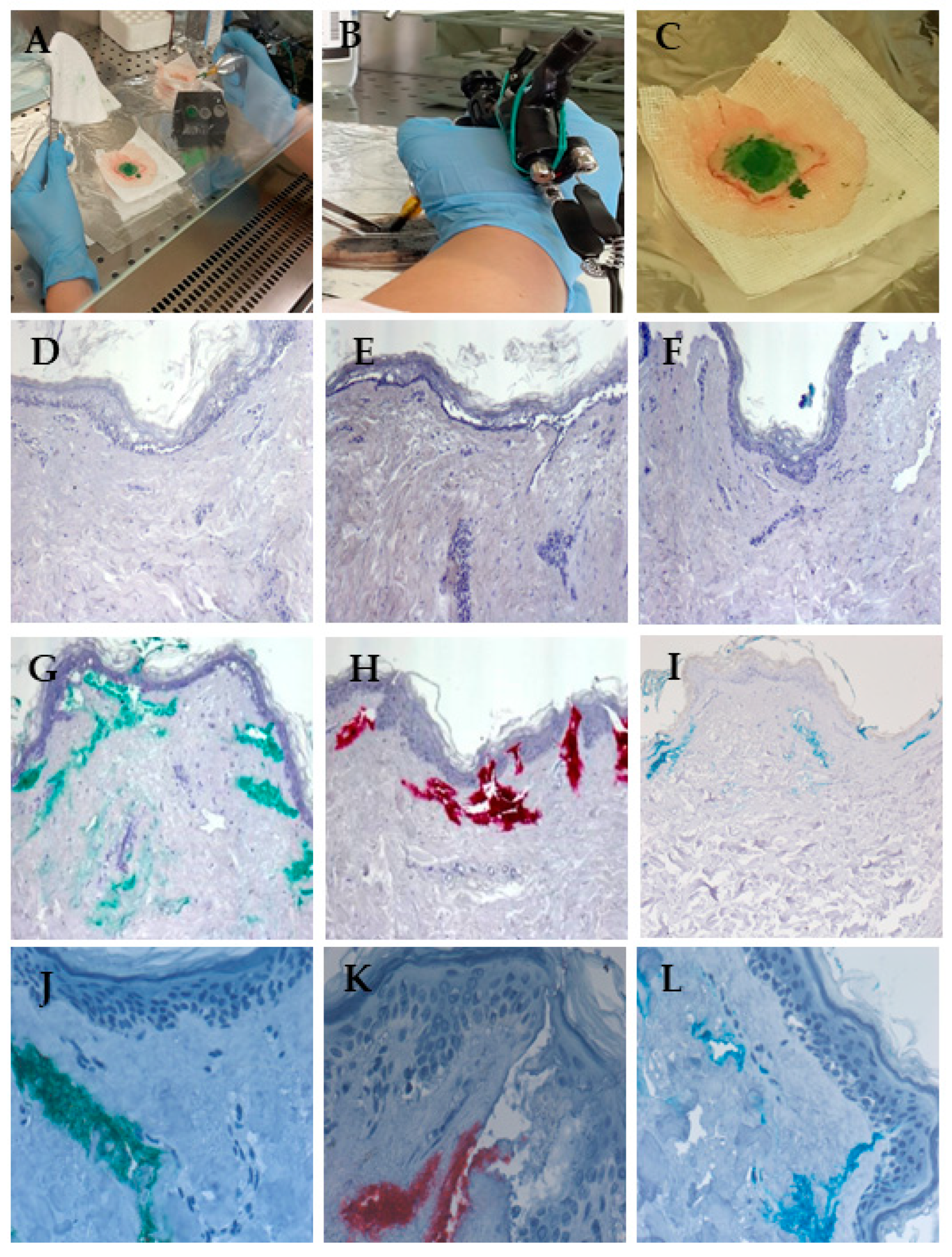
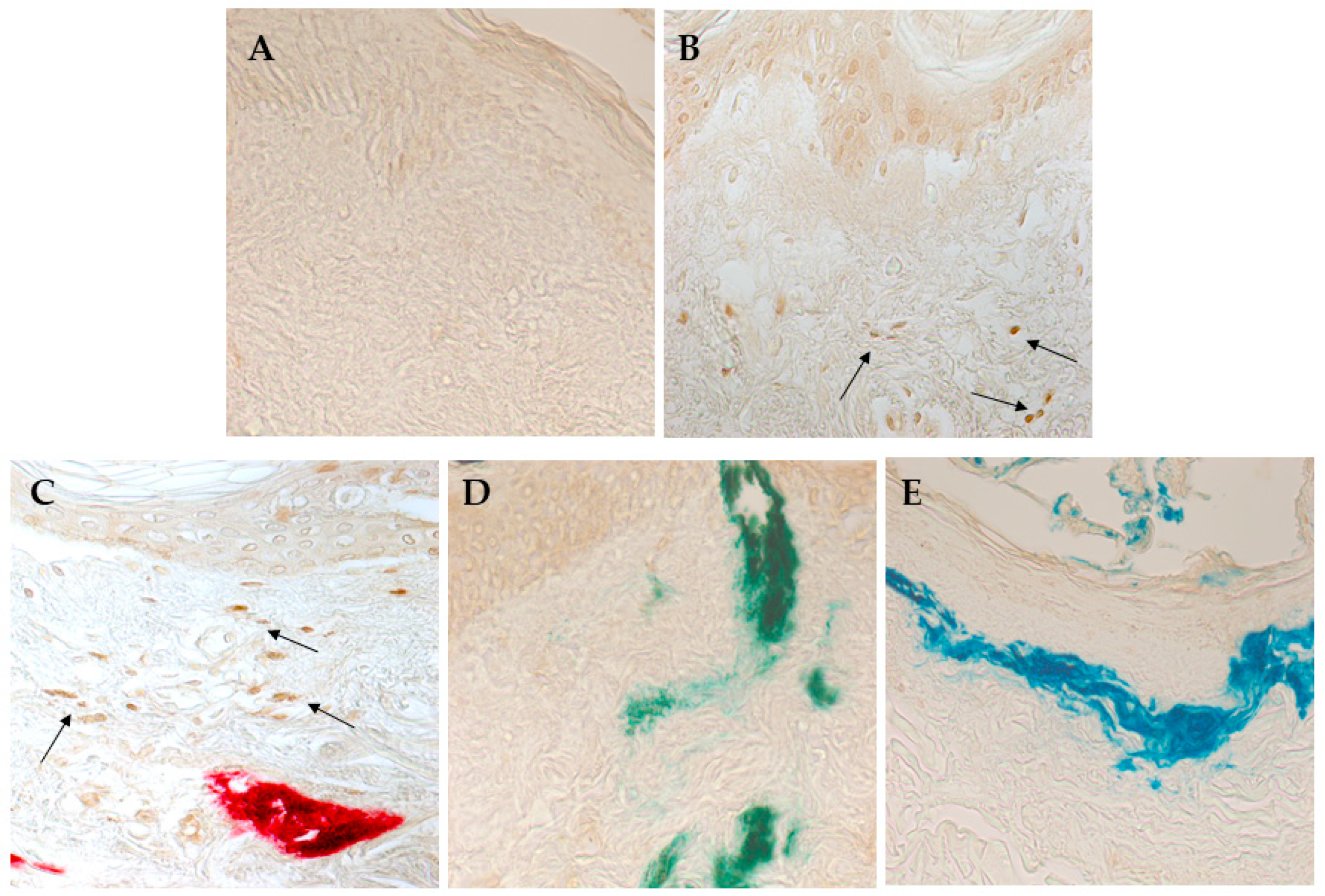
3.4. Red Ink Tattooing Caused Damages to the Skin
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giulbudagian, M.; Schreiver, I.; Singh, A.V.; Laux, P.; Luch, A. Safety of tattoos and permanent make-up: A regulatory view. Arch. Toxicol. 2020, 94, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Battisini, B.; Bäumler, W.; Blass Rico, A.M.; Bocca, B.; Brungs, C.; Famele, M.; Foerster, M.; Gutsche, B.; Houben, V.; et al. Lessons learned in a decade: Medical-toxicological view of tattooing. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Sordi, D.; Pagnanelli, G.; Cavan, I.A.; Mazzanti, C. Tattoo and warts: Efficacy of topical immunotherapy. Eur. J. Dermatol. 2017, 27, 322–323. [Google Scholar] [CrossRef]
- Veasey, J.V.; Erthal, A.L.N.; Lellis, R.F. In vivo and ex vivo dermoscopy of lesions from implantation of human papillomavirus in tattoos: Report of two cases. An. Bras. Dermatol. 2020, 95, 78–81. [Google Scholar] [CrossRef] [PubMed]
- de Cuyper, C.; Lodewick, E.; Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Laux, P.; Luch, A. Are metals involved in tattoo-related hypersensitivity reactions? A case report. Contact Dermat. 2017, 77, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kluger, N. An update on cutaneous complications of permanent tattooing. Expert. Rev. Clin. Immunol. 2019, 15, 1135–1143. [Google Scholar] [CrossRef]
- Kluger, N.; Koljonen, V. Tattoos, inks, and cancer. Lancet Oncol. 2012, 13, e161–e168. [Google Scholar] [CrossRef]
- Ricci, F.; Paradisi, A.; Maier, S.A.; Kovacs, M.; Podda, M.; Peris, K.; Abeni, D. Melanoma and tattoos: A case report and review of the literature. Eur. J. Dermatol. 2018, 28, 50–55. [Google Scholar] [CrossRef]
- Forte, G.; Petrucci, F.; Cristaudo, A.; Bocca, B. Market survey on toxic metals contained in tattoo inks. Sci. Total Environ. 2009, 407, 5997–6002. [Google Scholar] [CrossRef] [PubMed]
- Battistini, B.; Petrucci, F.; De Angelis, I.; Failla, C.M.; Bocca, B. Quantitative analysis of metals and metal-based nano- and submicron-particles in tattoo inks. Chemosphere 2020, 245, 125667. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Josefsson, L.; Meschnark, S.; Lind, M.L.; Emmer, Å.; Goessler, W.; Hedberg, Y.S. Analytical survey of tattoo inks-A chemical and legal perspective with focus on sensitizing substances. Contact Dermat. 2021, 85, 340–353. [Google Scholar] [CrossRef]
- Bocca, B.; Senofonte, O.; Petrucci, F. Hexavalent chromium in tattoo inks: Dermal exposure and systemic risk. Contact Dermat. 2018, 79, 218–225. [Google Scholar] [CrossRef]
- Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials 2019, 9, 888. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Villanova, J.; Laux, P.; Dreiack, N.; Penning, R.; Tucoulou, R.; Cotte, M.; et al. Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci. Rep. 2017, 7, 11395. [Google Scholar] [CrossRef]
- Bäumler, W. Absorption, distribution, metabolism and excretion of tattoo colorants and ingredients in mouse and man: The known and the unknown. Curr. Probl. Dermatol. 2015, 48, 176–184. [Google Scholar] [CrossRef]
- Alsing, K.K.; Johannesen, H.H.; Hansen, R.H.; Mårtensson, N.L.; Persson, D.P.; Qvortrup, K.; Wulf, H.C.; Lerche, C.M. Biodistribution of iron oxide tattoo pigment: An experimental murine study. Exp. Dermatol. 2024, 33, e15183. [Google Scholar] [CrossRef]
- Minghetti, P.; Musazzi, U.M.; Dorati, R.; Rocco, P. The safety of tattoo inks: Possible options for a common regulatory framework. Sci. Total Environ. 2019, 651, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Karadagli, S.S.; Cansever, I.; Armagan, G.; Sogut, O. Are Some Metals in Tattoo Inks Harmful to Health? An Analytical Approach. Chem. Res. Toxicol. 2023, 36, 104–111. [Google Scholar] [CrossRef]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control Release 2019, 299, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Caimi, S.; Failla, C.M.; Dellambra, E.; Lulli, D.; Carbone, M.L.; Scatozza, F.; De Angelis, I.; Battistini, B. A SP-ICP-MS protocol for the detection of metal nanoparticle composition and size in tattooed ex vivo human skin explants. J. Phys. Conf. Ser. 2023, 2579, 012008. [Google Scholar] [CrossRef]
- Nelissen, I.; Haase, A.; Anguissola, S.; Rocks, L.; Jacobs, A.; Willems, H.; Riebeling, C.; Luch, A.; Piret, J.P.; Toussaint, O.; et al. Improving Quality in Nanoparticle-Induced Cytotoxicity Testing by a Tiered Inter-Laboratory Comparison Study. Nanomaterials 2020, 10, 1430. [Google Scholar] [CrossRef]
- Panacchia, L.; Dellambra, E.; Bondanza, S.; Paterna, P.; Maurelli, R.; Paionni, E.; Guerra, L. Nonirradiated human fibroblasts and irradiated 3T3-J2 murine fibroblasts as a feeder layer for keratinocyte growth and differentiation in vitro on a fibrin substrate. Cells Tissues Organs 2010, 191, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Battistini, B.; Vetrani, I.; Reppuccia, L.; Fedele, M.; Ruggieri, F.; Bocca, B.; Iavicoli, I. The endocrine disrupting effects of nanoplastic exposure: A systematic review. Toxicol. Ind. Health 2023, 39, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Zijno, A.; Cavallo, D.; Di Felice, G.; Ponti, J.; Barletta, B.; Butteroni, C.; Corinti, S.; De Berardis, B.; Palamides, J.; Ursini, C.L.; et al. Use of a common European approach for nanomaterials’ testing to support regulation: A case study on titanium and silicon dioxide representative nanomaterials. J. Appl. Toxicol. 2020, 40, 1511–1525. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Rossner, P., Jr.; Cervena, T.; Echalar, B.; Palacka, K.; Milcova, A.; Novakova, Z.; Sima, M.; Simova, Z.; Vankova, J.; Holan, V. Metal Nanoparticles with Antimicrobial Properties: The Toxicity Response in Mouse Mesenchymal Stem Cells. Toxics 2023, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Mahmoud, N.N.; Hashemi, F.; Hajipour, M.J.; Farvadi, F.; Mahmoudi, M. Misinterpretation in Nanotoxicology: A Personal Perspective. Chem. Res. Toxicol. 2016, 29, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Ong, K.J.; MacCormack, T.J.; Clark, R.J.; Ede, J.D.; Ortega, V.A.; Felix, L.C.; Dang, M.K.; Ma, G.; Fenniri, H.; Veinot, J.G.; et al. Widespread nanoparticle-assay interference: Implications for nanotoxicity testing. PLoS ONE 2014, 9, e90650. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef]
- Lee, P.L.; Chen, B.C.; Gollavelli, G.; Shen, S.Y.; Yin, Y.S.; Lei, S.L.; Jhang, C.L.; Lee, W.R.; Ling, Y.C. Development and validation of TOF-SIMS and CLSM imaging method for cytotoxicity study of ZnO nanoparticles in HaCaT cells. J. Hazard. Mater. 2014, 277, 3–12. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Sengupta, S.; Shukla, R.K.; Kumar, A. ZnO nanoparticles-associated mitochondrial stress-induced apoptosis and G2/M arrest in HaCaT cells: A mechanistic approach. Mutagenesis 2019, 34, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Sozer Karadagli, S.; Kaftan, G.; Cansever, I.; Armagan, G.; Sogut, O. Tattoo inks: Evaluation of cellular responses and analysis of some trace metals. Biometals 2024, 37, 495–505. [Google Scholar] [CrossRef] [PubMed]
- van der Bent, S.A.S.; de Winter, R.W.; Wolkerstorfer, A.; Rustemeyer, T. Red tattoo reactions, a prospective cohort on clinical aspects. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e384–e386. [Google Scholar] [CrossRef]
- van der Bent, S.; Oyen, E.; Rustemeyer, T.; Jaspars, L.; Hoekzema, R. Histopathology of Red Tattoo Reactions. Am. J. Dermatopathol. 2021, 43, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Maxim, E.; Higgins, H.; D’Souza, L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int. J. Womens Dermatol. 2017, 3, 228–230. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef]
- Arl, M.; Nogueira, D.J.; Schveitzer Köerich, J.; Mottim Justino, N.; Schulz Vicentini, D.; Gerson Matias, W. Tattoo inks: Characterization and in vivo and in vitro toxicological evaluation. J. Hazard. Mater. 2019, 364, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, R.; Fogliano, C.; Rienzi, M.; Siciliano, A.; Salvatore, M.M.; De Tommaso, G.; Benvenuto, G.; Galdiero, E.; Guida, M. Comparative Toxicological Evaluation of Tattoo Inks on Two Model Organisms. Biology 2021, 10, 1308. [Google Scholar] [CrossRef]
- Hering, H.; Zoschke, C.; Kühn, M.; Gadicherla, A.K.; Weindl, G.; Luch, A.; Schreiver, I. TatS: A novel in vitro tattooed human skin model for improved pigment toxicology research. Arch. Toxicol. 2020, 94, 2423–2434. [Google Scholar] [CrossRef]
- Lin, C.; Marquardt, Y.; Rütten, S.; Liao, L.; Rahimi, K.; Haraszti, T.; Baron, J.M.; Bartneck, M. Macrophage-like rapid uptake and toxicity of tattoo ink in human monocytes. Immunology 2024, 171, 388–401. [Google Scholar] [CrossRef]


| DLS | |
|---|---|
| Instrument | Zetasizer (Malvern Panalytical, Malvern, UK) |
| Record number | 10 |
| Temperature | 25 °C |
| Dispersant refractive index/viscosity (cP) | Water, 1.330/0.8872 cP; DMEM, 1.330/0.9400 cP |
| Material refractive index/absorption | Ag, 0.18/0.010; Al2O3, 1.75/0.430; Au, 0.20/0.500; Cr2O3, 2.50/0.800; CuO, 0.86/0.737; Fe2O3, 2.94/5.200; TiO2, 2.49/0.100; ZnO, 2.00/0.002; polystyrene, 1.59/0.01 |
| SP ICP-MS | |
| Instrument | iCAP-Q (Thermo Fisher, Waltham, MA USA) |
| Nebulizer | Quartz concentric |
| Spray chamber | Quartz cyclonic |
| Sample uptake rate | 0.33 mL/min |
| RF power | 1450 W |
| Masses | 107Ag, 197Au, 27Al, 52Cr, 63Cu, 56Fe, 48Ti, 66Zn |
| Acquisition mode and time | Q-Cell in KED (4.8 mL/min He), 60 s |
| Nebulization efficiency | 4% |
| Dwell time | 5 ms |
| NPs | Certified Diameter | Dispersant | Time | DLS | SP ICP-MS | |
|---|---|---|---|---|---|---|
| (nm) | (h) | Z-Average (nm) | PDI | Diameter (nm) | ||
| Ag | 20 nm | H2O | 0 | 24.9 | 0.104 | 27.3 ± 1.1 |
| DMEM | 0 | 20.2 | 0.377 | 23.7 ± 2.0 | ||
| DMEM | 24 | 15.7 | 0.238 | 20.3 ± 1.3 | ||
| Al2O3 | 30 nm | H2O | 0 | 166.1 | 0.140 | 38.4 ± 8.2 |
| DMEM | 0 | 196.3 | 0.600 | 45.4 ± 9.6 | ||
| DMEM | 24 | 185.1 | 0.726 | 34.3 ± 9.7 | ||
| Au | 5 nm | H2O | 0 | 27.3 | 0.375 | 14.4 ± 2.3 |
| DMEM | 0 | 23.2 | 0.526 | 14.8 ± 2.1 | ||
| DMEM | 24 | 17.2 | 0.450 | 13.9 ± 2.3 | ||
| Cr2O3 | 60 nm | H2O | 0 | 181.1 | 0.466 | 60.1 ± 35.0 |
| DMEM | 0 | 134.5 | 0.717 | 61.1 ± 37.5 | ||
| DMEM | 24 | 86.4 | 0.727 | 59.3 ± 17.9 | ||
| CuO | 25–55 nm | H2O | 0 | 227.1 | 0.203 | 43.3 ± 11.7 |
| DMEM | 0 | 243.0 | 0.338 | 41.3 ± 7.9 | ||
| DMEM | 24 | 192.4 | 0.467 | 40.3 ± 5.5 | ||
| Fe2O3 | 30 nm | H2O | 0 | 165.9 | 0.142 | 43.6 ± 12.5 |
| DMEM | 0 | 300.5 | 0.358 | 49.4 ± 10.2 | ||
| DMEM | 24 | 287.5 | 0.314 | 47.7 ± 11.3 | ||
| TiO2 | <100 nm | H2O | 0 | 310.3 | 0.489 | 89.6 ± 12.2 |
| DMEM | 0 | 100.7 | 0.743 | 103.9 ± 20.2 | ||
| DMEM | 24 | 80.6 | 0.975 | 99.9 ± 19.1 | ||
| ZnO | 30–40 nm | H2O | 0 | 432.1 | 1.000 | 31.6 ± 4.5 |
| DMEM | 0 | 43.7 | 0.740 | 37.1 ± 3.8 | ||
| DMEM | 24 | 31.9 | 0.461 | 35.6 ± 4.6 | ||
| Bright Red | Light Green | Mario’s Blue | ||||
|---|---|---|---|---|---|---|
| DLS | Z-Average (nm) | PDI | Z-Average (nm) | PDI | Z-Average (nm) | PDI |
| 206.7 | 0.347 | 193.4 | 0.176 | 192.8 | 0.238 | |
| SP ICP-MS | Diameter (nm) | Diameter (nm) | Diameter (nm) | |||
| Ag | nd | nd | nd | |||
| Al2O3 | 137.8 ± 20.1 | 115.2 ± 13.8 | 115.7 ± 13.1 | |||
| Au | nd | nd | nd | |||
| Cr2O3 | 55.0 ± 4.5 | 62.3 ± 4.0 | 45.2 ± 7.3 | |||
| CuO | nd | 45.8 ± 13.4 | 57.5 ± 9.2 | |||
| Fe2O3 | 75.5 ± 8.5 | 77.2 ± 11.0 | 77.5 ± 11.6 | |||
| TiO2 | 166.1 ± 34.2 | 260.2 ± 30.5 | 228.4 ± 53.6 | |||
| ZnO | 26.2 ± 5.9 | nd | nd | |||
| Bright Red | Light Green | Mario’s Blue | ||
|---|---|---|---|---|
| Nanoparticles | Dispersant | Measured Diameter | Measured Diameter | Measured Diameter |
| (nm) | (nm) | (nm) | ||
| Ag | Skin | nd | nd | nd |
| KBM | nd | nd | nd | |
| Al2O3 | Skin | 50.2 ± 8.9 | 49.8 ± 5.0 | 46.1 ± 4.1 |
| KBM | 175.7 ± 18.3 | 190.9 ± 12.8 | 136.0 ± 13.4 | |
| Au | Skin | nd | nd | nd |
| KBM | nd | nd | nd | |
| Cr2O3 | Skin | 50.2 ± 7.4 | 52.2 ± 6.5 | 41.1 ± 7.0 |
| KBM | 52.1 ± 8.0 | 50.0 ± 6.7 | 40.2 ± 7.2 | |
| CuO | Skin | nd | 43.7 ± 8.3 | 53.2 ± 9.9 |
| KBM | nd | 46.6 ± 6.1 | 44.3 ± 7.2 | |
| Fe2O3 | Skin | 39.4 ± 5.2 | 33.6 ± 3.9 | 36.9 ± 6.0 |
| KBM | 74.9 ± 8.9 | 79.0 ± 9.5 | 84.9 ± 11.6 | |
| TiO2 | Skin | 118.4 ± 23.7 | 162.8 ± 35.4 | 177.2 ± 17.6 |
| KBM | 165.7 ± 44.7 | 233.9 ± 37.7 | 210.4 ± 34.3 | |
| ZnO | Skin | 22.7 ± 5.9 | nd | nd |
| KBM | 26.2 ± 6.2 | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistini, B.; Lulli, D.; Bocca, B.; Carbone, M.L.; Ramondino, C.; Caimi, S.; Capone, A.; Nicodemi, E.M.; Dellambra, E.; De Angelis, I.; et al. Tattoo Ink Metal Nanoparticles: Assessment of Toxicity In Vitro and with a Novel Human Ex Vivo Model. Nanomaterials 2025, 15, 270. https://doi.org/10.3390/nano15040270
Battistini B, Lulli D, Bocca B, Carbone ML, Ramondino C, Caimi S, Capone A, Nicodemi EM, Dellambra E, De Angelis I, et al. Tattoo Ink Metal Nanoparticles: Assessment of Toxicity In Vitro and with a Novel Human Ex Vivo Model. Nanomaterials. 2025; 15(4):270. https://doi.org/10.3390/nano15040270
Chicago/Turabian StyleBattistini, Beatrice, Daniela Lulli, Beatrice Bocca, Maria Luigia Carbone, Carmela Ramondino, Stefano Caimi, Alessio Capone, Ezio Maria Nicodemi, Elena Dellambra, Isabella De Angelis, and et al. 2025. "Tattoo Ink Metal Nanoparticles: Assessment of Toxicity In Vitro and with a Novel Human Ex Vivo Model" Nanomaterials 15, no. 4: 270. https://doi.org/10.3390/nano15040270
APA StyleBattistini, B., Lulli, D., Bocca, B., Carbone, M. L., Ramondino, C., Caimi, S., Capone, A., Nicodemi, E. M., Dellambra, E., De Angelis, I., & Failla, C. M. (2025). Tattoo Ink Metal Nanoparticles: Assessment of Toxicity In Vitro and with a Novel Human Ex Vivo Model. Nanomaterials, 15(4), 270. https://doi.org/10.3390/nano15040270






