Fluorescence Quenching of Graphene Quantum Dots from Orange Peel for Methyl Orange Detection
Abstract
1. Introduction
2. Experiment and Facilities
2.1. Experimental Facilities
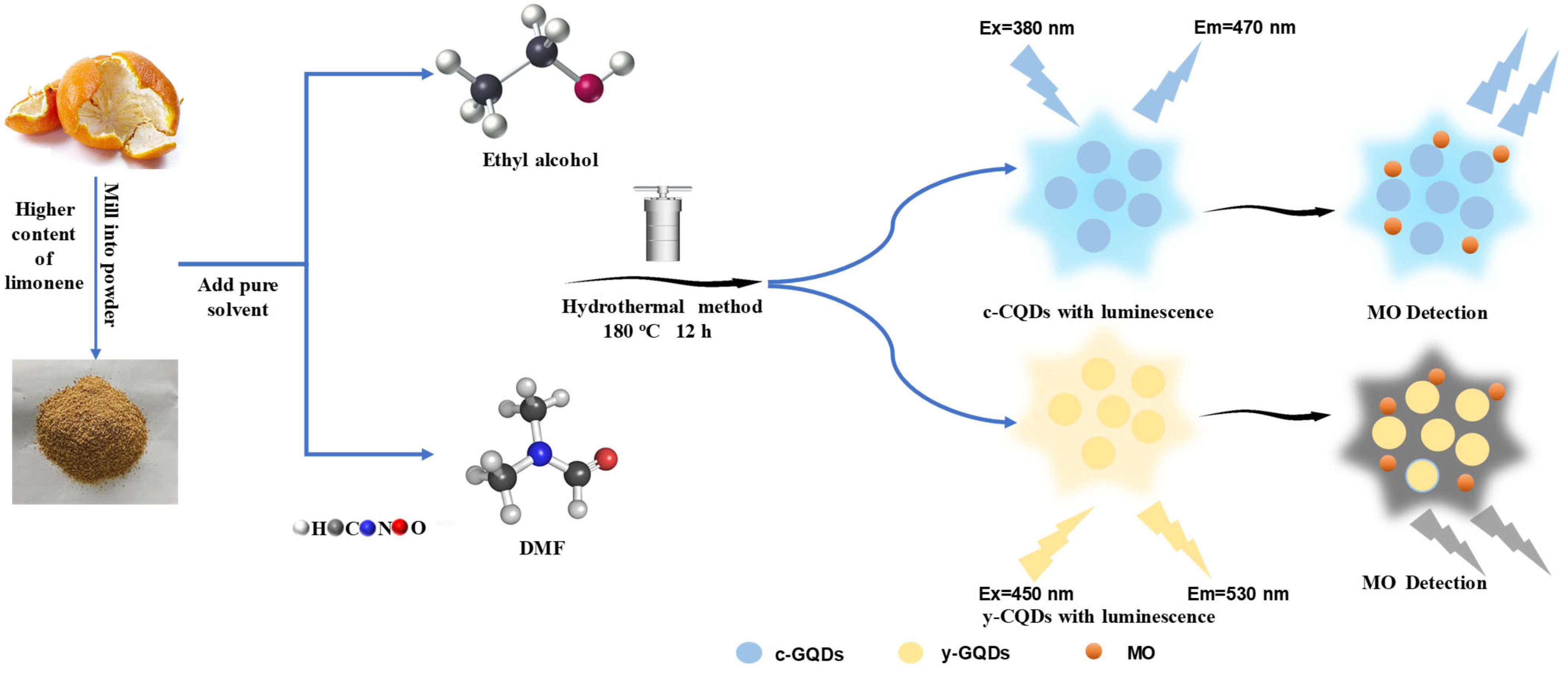
2.2. Preparation of GQDs
2.3. Dye Detection
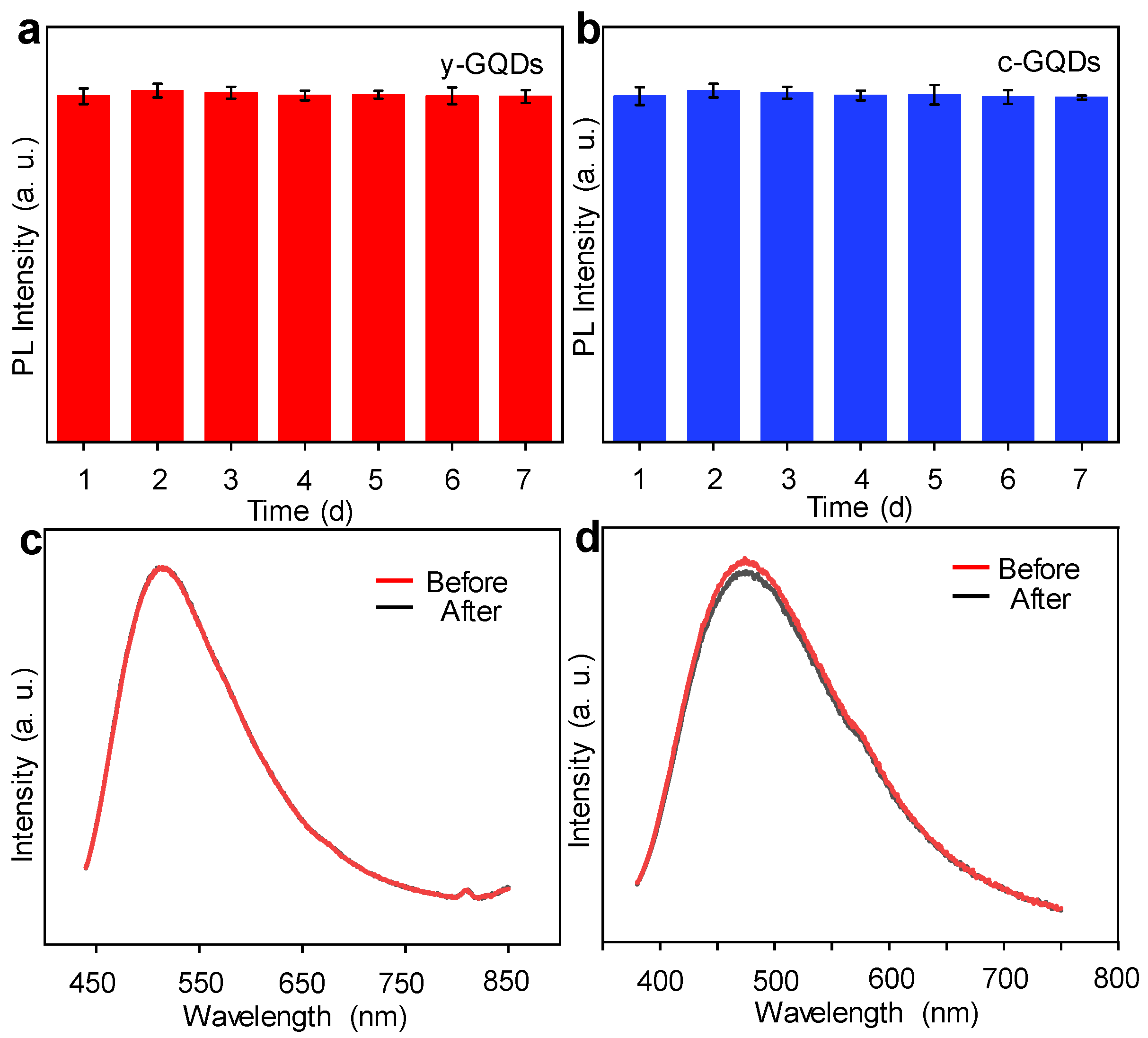
3. Results and Discussion
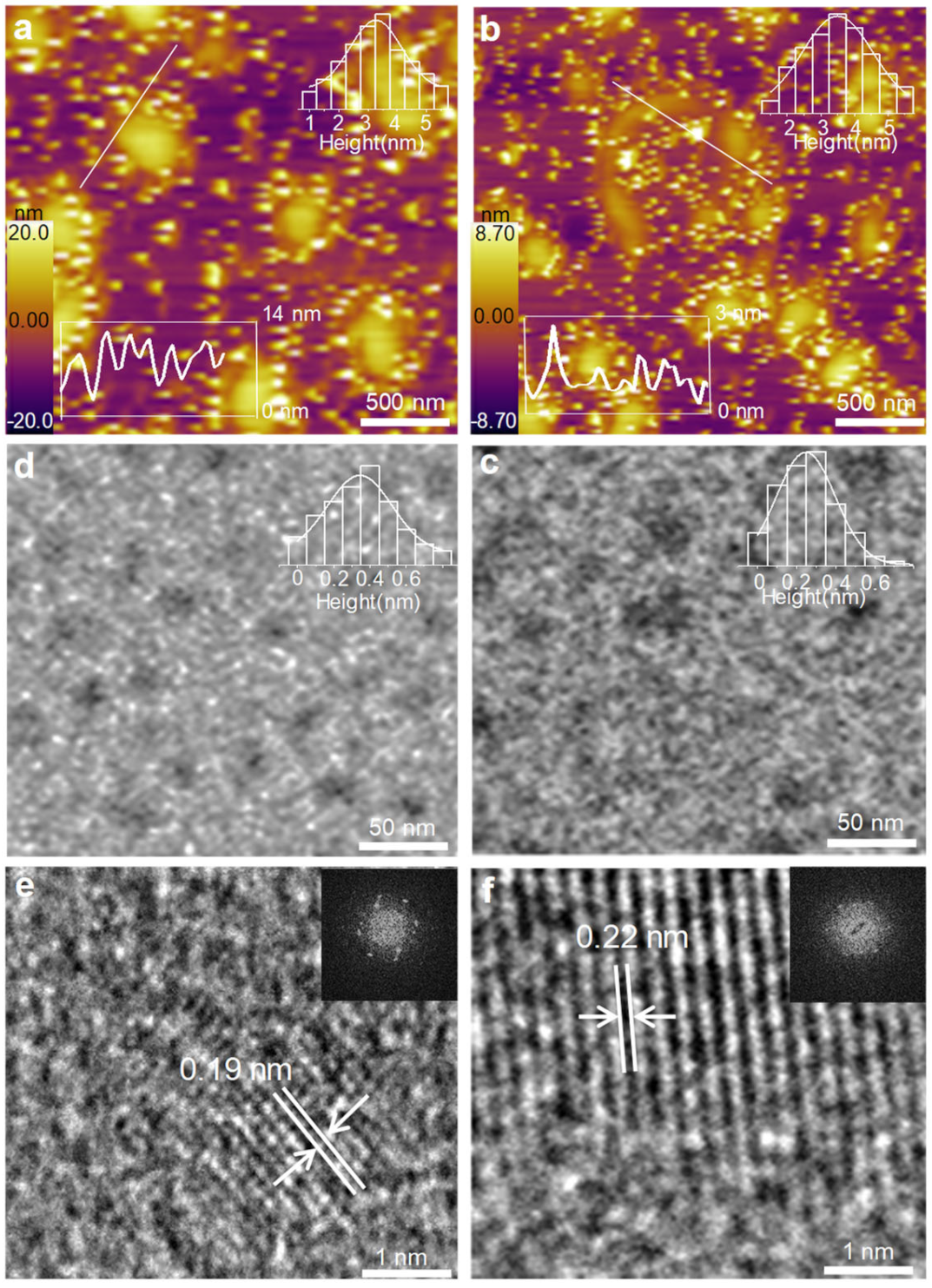
3.1. Topography Characterization of GQDs
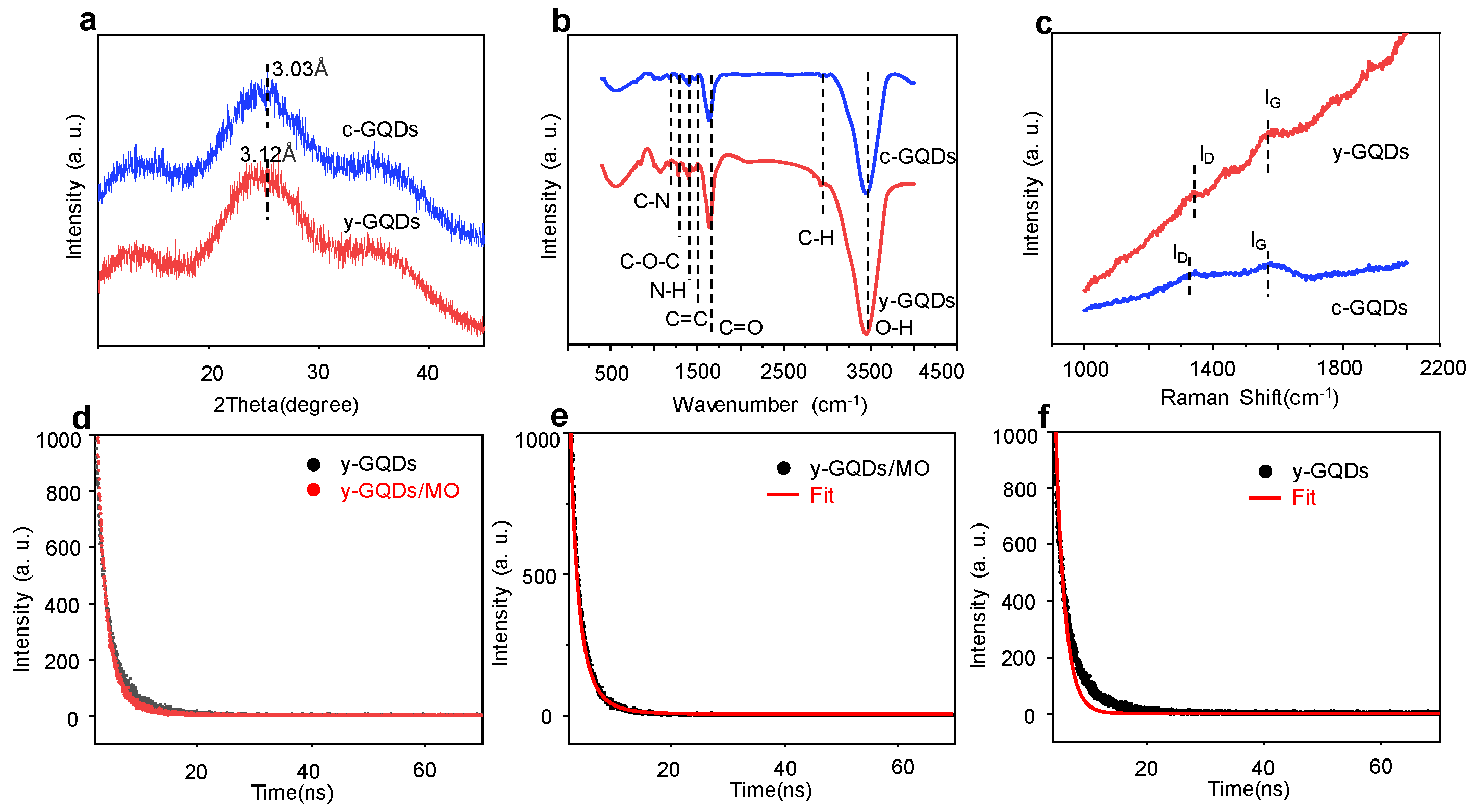
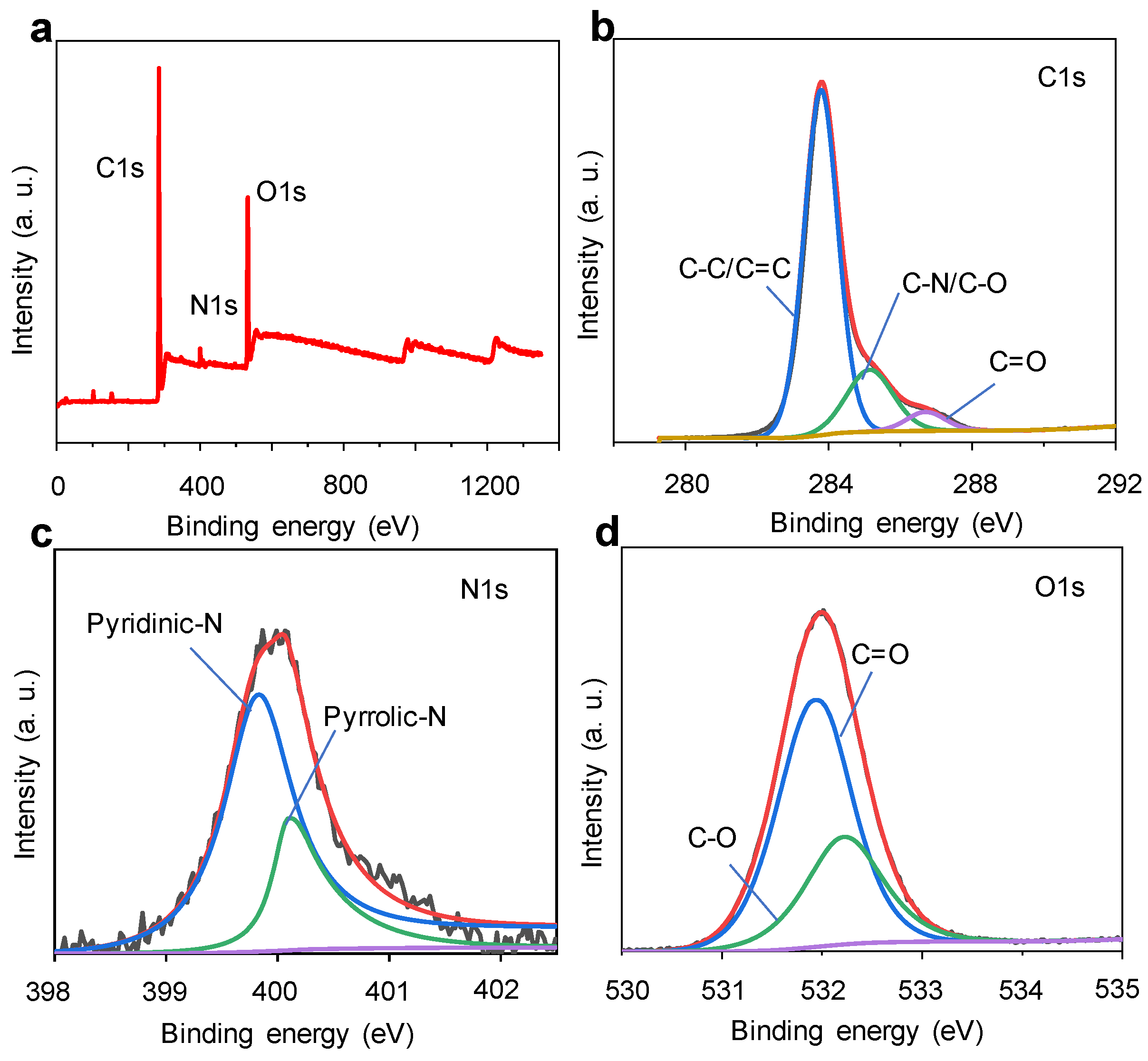
3.2. Structural Characterization of GQDs
3.3. The Optical Properties Characterization of GQDs
3.4. The Application of GQDs in Dye Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, J.; Han, G.; Ren, J.; Liu, C.; Wang, J.; Wang, X. Single-layered graphene quantum dots with self-passivated layer from xylan for visual detection of trace chromium(Vl). Chem. Eng. J. 2022, 435, 131833. [Google Scholar] [CrossRef]
- Hu, X.-H.; Zhang, R.; Wu, Z.; Xiong, S. Concentrated Solar Induced Graphene. ACS Omega 2022, 7, 27263–27271. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.M.; Hasanin, M.S.; Suleiman, W.B.; Helal, E.E.-H.; Hashem, A.H. Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: Antimicrobial, antioxidant, and anticancer activities. Biomass Convers. Biorefinery 2022, 14, 6987–6998. [Google Scholar] [CrossRef]
- Gong, D.; Guo, J.; Wang, F.; Zhang, J.; Song, S.; Feng, B.; Zhang, X.; Zhang, W. Green construction of metal- and additive-free citrus peel-derived carbon dot/g-C3N4 photocatalysts for the high-performance photocatalytic decomposition of sunset yellow. Food Chem. 2023, 425, 136470. [Google Scholar] [CrossRef]
- Singh, A.K.; Sri, S.; Garimella, L.B.V.S.; Dhiman, T.K.; Sen, S.; Solanki, P.R. Graphene Quantum Dot-Based Optical Sensing Platform for Aflatoxin B1 Detection via the Resonance Energy Transfer Phenomenon. ACS Appl. Bio Mater. 2022, 5, 1179–1186. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Kumar, J.V.; Rhim, J.-W. Fluorescent carbon quantum dots for food contaminants detection applications. J. Environ. Chem. Eng. 2024, 12, 111999. [Google Scholar] [CrossRef]
- Kolhe, P.; Roberts, A.; Gandhi, S. Fabrication of an ultrasensitive electrochemical immunosensor coupled with biofunctionalized zero-dimensional graphene quantum dots for rapid detection of cephalexin. Food Chem. 2023, 398, 133846. [Google Scholar] [CrossRef]
- Gozali Balkanloo, P.; Mohammad Sharifi, K.; Poursattar Marjani, A. Graphene quantum dots: Synthesis, characterization, and application in wastewater treatment: A review. Mater. Adv. 2023, 4, 4272–4293. [Google Scholar] [CrossRef]
- Kadyan, P.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Mohan, S.; Yadav, M.; Dalal, S.; Ramniwas, S.; Kumar Kataria, S.; Arasu, T.; et al. Comprehensive Review on Synthesis, Applications, and Challenges of Graphene Quantum Dots (GQDs). J. Nanomater. 2023, 2023, 1–26. [Google Scholar] [CrossRef]
- Kundu, A.; Maity, B.; Basu, S. Orange Pomace-Derived Fluorescent Carbon Quantum Dots: Detection of Dual Analytes in the Nanomolar Range. ACS Omega 2023, 8, 22178–22189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, T.; Ge, S.; Fan, W.; Wang, H.; Zhang, Z.; Lichtfouse, E.; Van Tran, T.; Liew, R.K.; Rezakazemi, M.; et al. Synthesis and applications of carbon quantum dots derived from biomass waste: A review. Environ. Chem. Lett. 2023, 21, 3393–3424. [Google Scholar] [CrossRef]
- Fu, S.; Tang, B.; Wang, Z.; An, G.; Zhang, M.; Wang, K.; Liu, W.; Guo, H.; Zhang, B.; Wang, L. Hydrophobic carbon quantum dots with Lewis-Basic nitrogen sites for electrocatalyst CO2 reduction to CH4. Chem. Eng. J. 2024, 500, 157207. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/graphene quantum dots/graphene oxide film. Anal. 2021, 146, 4000–4009. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Li, J.; Dong, J.; Liu, H.; Zhao, J.; Luo, X.; Huo, D.; Hou, C. B-doped graphene quantum dots array as fluorescent sensor platforms for plasticizers detection. Sens. Actuators B Chem. 2023, 376, 132989. [Google Scholar] [CrossRef]
- Tang, S.; Chen, D.; Yang, Y.; Wang, C.; Li, X.; Wang, Y.; Gu, C.; Cao, Z. Mechanisms behind multicolor tunable Near-Infrared triple emission in graphene quantum dots and ratio fluorescent probe for water detection. J. Colloid Interface Sci. 2022, 617, 182–192. [Google Scholar] [CrossRef]
- Nair, R.V.; Chandran, P.R.; Mohamed, A.P.; Pillai, S. Sulphur-doped graphene quantum dot based fluorescent turn-on aptasensor for selective and ultrasensitive detection of omethoate. Anal. Chim. Acta 2021, 1181, 338893. [Google Scholar] [CrossRef]
- Xie, L.; Liang, C.; Wu, Y.; Wang, K.; Hou, W.; Guo, H.; Wang, Z.; Lam, Y.M.; Liu, Z.; Wang, L. Isomerization Engineering of Oxygen-Enriched Carbon Quantum Dots for Efficient Electrochemical Hydrogen Peroxide Production. Small 2024, 20, e2401253. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Yang, Y.; Tang, S.; Li, X.; Xie, F.; Wang, G.; Guo, Q. Synthesis of multi-color fluorine and nitrogen co-doped graphene quantum dots for use in tetracycline detection, colorful solid fluorescent ink, and film. J. Colloid Interface Sci. 2021, 602, 689–698. [Google Scholar] [CrossRef]
- Hu, B.; Huang, K.; Tang, B.; Lei, Z.; Wang, Z.; Guo, H.; Lian, C.; Liu, Z.; Wang, L. Graphene Quantum Dot-Mediated Atom-Layer Semiconductor Electrocatalyst for Hydrogen Evolution. Nano-Micro Lett. 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Liu, H.; Deng, Z.; Zhang, Z.; Lin, W.; Zhang, M.; Wang, H. Graphene quantum dots as metal-free nanozymes for chemodynamic therapy of cancer. Matter 2024, 7, 977–990. [Google Scholar] [CrossRef]
- Anusuya, T.; Kumar, V.; Kumar, V. Hydrophilic graphene quantum dots as turn-off fluorescent nanoprobes for toxic heavy metal ions detection in aqueous media. Chemosphere 2021, 282, 131019. [Google Scholar] [CrossRef] [PubMed]
- Duprez, H.; Cances, S.; Omahen, A.; Masseroni, M.; Ruckriegel, M.J.; Adam, C.; Tong, C.; Garreis, R.; Gerber, J.D.; Huang, W.; et al. Spin-valley locked excited states spectroscopy in a one-particle bilayer graphene quantum dot. Nat. Commun. 2024, 15, 9717. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, S.; Wang, K.; Guo, H.; Zhang, J.; Wu, M.; Wang, L. Carbon quantum dot-mediated binary metal–organic framework nanosheets for efficient oxygen evolution at ampere-level current densities in proton exchange membrane electrolyzers. J. Mater. Chem. A 2024, 12, 31253–31261. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Gao, W.; Tang, B. Recent advancement in graphene quantum dots based fluorescent sensor: Design, construction and bio-medical applications. Coord. Chem. Rev. 2023, 478, 214966. [Google Scholar] [CrossRef]
- Zhu, L.; Li, D.; Lu, H.; Zhang, S.; Gao, H. Lignin-based fluorescence-switchable graphene quantum dots for Fe3+ and ascorbic acid detection. Int. J. Biol. Macromol. 2022, 194, 254–263. [Google Scholar] [CrossRef]
- Tam, T.V.; Hur, S.H.; Chung, J.S.; Choi, W.M. Novel paper- and fiber optic-based fluorescent sensor for glucose detection using aniline-functionalized graphene quantum dots. Sens. Actuators B Chem. 2021, 329, 129250. [Google Scholar] [CrossRef]
- Kang, S.; Han, H.; Lee, K.; Kim, K.M. Ultrasensitive Detection of Fe3+ Ions Using Functionalized Graphene Quantum Dots Fabricated by a One-Step Pulsed Laser Ablation Process. ACS Omega 2022, 7, 2074–2081. [Google Scholar] [CrossRef]
- Revesz, I.A.; Hickey, S.M.; Sweetman, M.J. Metal ion sensing with graphene quantum dots: Detection of harmful contaminants and biorelevant species. J. Mater. Chem. B 2022, 10, 4346–4362. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, L.; Zhou, X.; Guo, Z.; Bian, H.; Dai, H. Highly fluorescent graphene quantum dots from biorefinery waste for tri-channel sensitive detection of Fe3+ ions. J. Hazard. Mater. 2021, 412, 125096. [Google Scholar] [CrossRef]
- Huynh, T.V.; Anh, N.T.N.; Darmanto, W.; Doong, R.-A. Erbium-doped graphene quantum dots with up- and down-conversion luminescence for effective detection of ferric ions in water and human serum. Sens. Actuators B Chem. 2021, 328, 129056. [Google Scholar] [CrossRef]
- Reagen, S.; Wu, Y.; Liu, X.; Shahni, R.; Bogenschuetz, J.; Wu, X.; Chu, Q.R.; Oncel, N.; Zhang, J.; Hou, X.; et al. Synthesis of Highly Near-Infrared Fluorescent Graphene Quantum Dots Using Biomass-Derived Materials for In Vitro Cell Imaging and Metal Ion Detection. ACS Appl. Mater. Interfaces 2021, 13, 43952–43962. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lim, S.F.; Baini, R.; Qu, Y.; Li, X. Visual Determination of Copper (II) Using a Biomass Sourced Carbon Quantum Dot (CQD) Ratiometric Fluorescent Probe. Anal. Lett. 2024, 58, 228–241. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Kurniawan, D.; Gholami, A.; Rahmanian, V.; Omidifar, N.; Chiang, W.-H. Recent Advances in Inflammatory Diagnosis with Graphene Quantum Dots Enhanced SERS Detection. Biosensors 2022, 12, 461. [Google Scholar] [CrossRef]
- Michenzi, C.; Proietti, A.; Rossi, M.; Espro, C.; Bressi, V.; Vetica, F.; Simonis, B.; Chiarotto, I. Carbon nanodots from orange peel waste as fluorescent probes for detecting nitrobenzene. RSC Sustain. 2024, 2, 933–942. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Hemalatha, M.; Venkatasubbu, D.G.; Abimanyu, S.; Aravind, M.; Rathinsabapathi, P. Label-free detection of ampicillin in milk and water using fluorescence carbon dots synthesised from Citrus Limetta peel. Diam. Relat. Mater. 2025, 152, 111892. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, S.; Wang, H.; Wang, H.; Gao, M.; Wang, X. Construction of an “ON–OFF” fluoroprobe using ionic liquids-modified orange peel-based carbon quantum dots for selective/sensitive permanganate assay in waters and the underlying quenching mechanisms. Anal. Bioanal. Chem. 2023, 415, 4753–4766. [Google Scholar] [CrossRef]
- Gholipour, A.; Rahmani, S. The Green Synthesis of Carbon Quantum Dots through One-step Hydrothermal Approach by Orange Juice for Rapid, and Accurate Detection of Dopamine. J. Fluoresc. 2023, 34, 2665–2677. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Priya, S.S.; Balakrishnan, K.; Rameshkumar, P.; Kannan, K.; Mahalakshmi, K.; Gayathri, V.; Vinitha, G. Synthesis of fluorescent carbon quantum dots from Manihot esculenta waste peels for nonlinear optical and biological applications. Chem. Phys. Impact 2024, 8, 100515. [Google Scholar] [CrossRef]
- Li, M.; Jiang, T.; Wang, X.; Chen, H.; Li, S.; Wei, F.; Ren, Z.; Yan, S.; Guo, X.; Tu, H. Preparation of highly oriented single crystal arrays of C8-BTBT by epitaxial growth on oriented isotactic polypropylene. J. Mater. Chem. C 2020, 8, 2155–2159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liu, Y.; Pang, X.; Huang, Y.; Dong, Z.; Niu, Q.; Xiong, Y.; Li, S.; Li, S.; Wang, L.; et al. Fluorescence Quenching of Graphene Quantum Dots from Orange Peel for Methyl Orange Detection. Nanomaterials 2025, 15, 376. https://doi.org/10.3390/nano15050376
Li W, Liu Y, Pang X, Huang Y, Dong Z, Niu Q, Xiong Y, Li S, Li S, Wang L, et al. Fluorescence Quenching of Graphene Quantum Dots from Orange Peel for Methyl Orange Detection. Nanomaterials. 2025; 15(5):376. https://doi.org/10.3390/nano15050376
Chicago/Turabian StyleLi, Weitao, Yang Liu, Xinglong Pang, Yuanhao Huang, Zeyun Dong, Qian Niu, Yuping Xiong, Shang Li, Shuai Li, Lei Wang, and et al. 2025. "Fluorescence Quenching of Graphene Quantum Dots from Orange Peel for Methyl Orange Detection" Nanomaterials 15, no. 5: 376. https://doi.org/10.3390/nano15050376
APA StyleLi, W., Liu, Y., Pang, X., Huang, Y., Dong, Z., Niu, Q., Xiong, Y., Li, S., Li, S., Wang, L., Guo, H., Cui, S., Hu, S., Li, Y., Cha, T., & Wang, L. (2025). Fluorescence Quenching of Graphene Quantum Dots from Orange Peel for Methyl Orange Detection. Nanomaterials, 15(5), 376. https://doi.org/10.3390/nano15050376









