Tribological Behavior and Mechanism of Silane-Bridged h-BN/MoS2 Hybrid Filling Epoxy Solid Lubricant Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
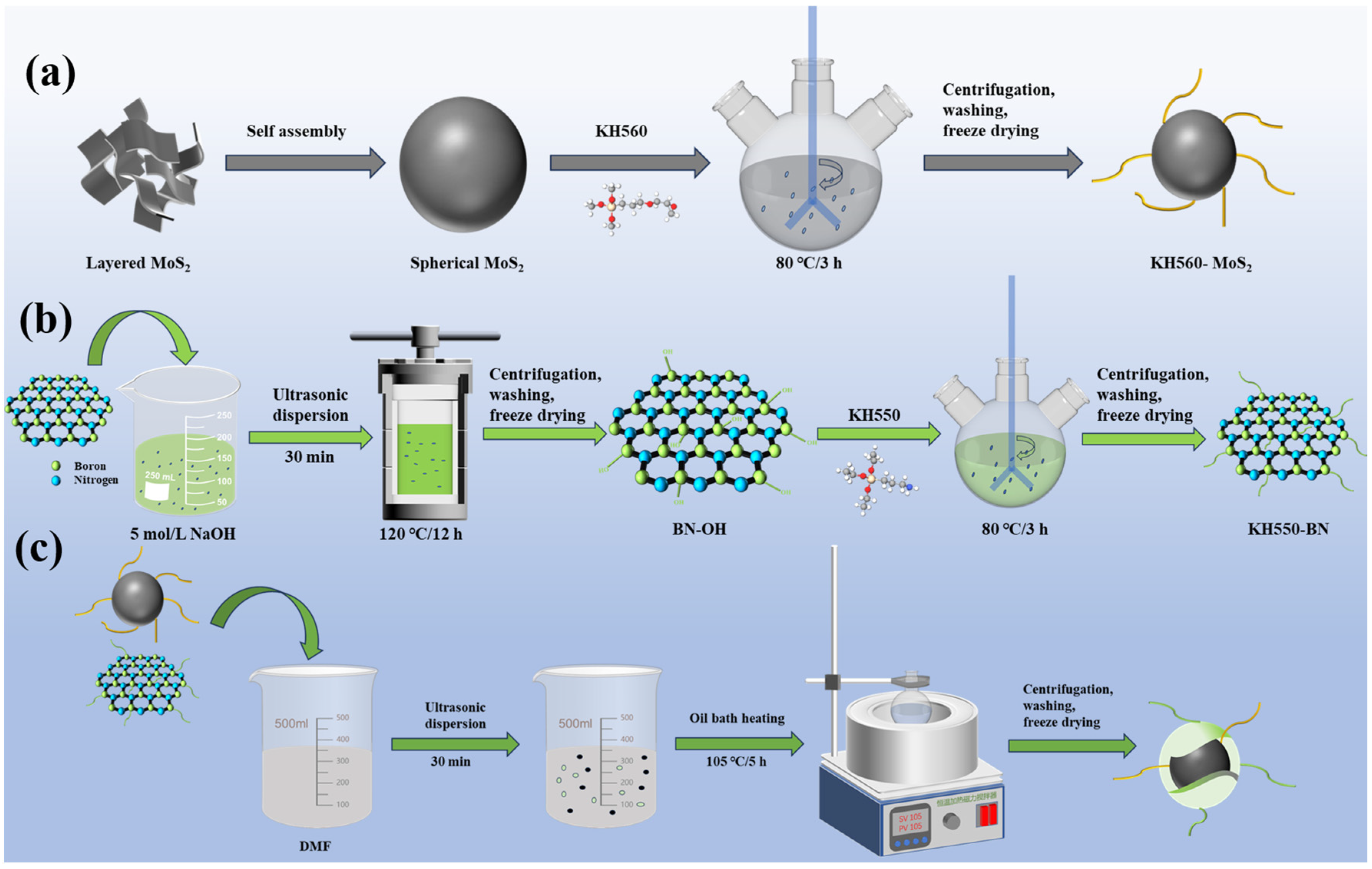
2.1.1. Preparation of Spherical MoS2
2.1.2. Preparation of KH560-MoS2
2.1.3. Preparation of KH550-BN
2.1.4. Preparation of h-BN/MoS2 Hybrid
2.2. Preparation of h-BN/MoS2 Epoxy Resin Composite Coating
2.3. Characterization
2.4. Tribological Properties Tests
3. Results and Discussion
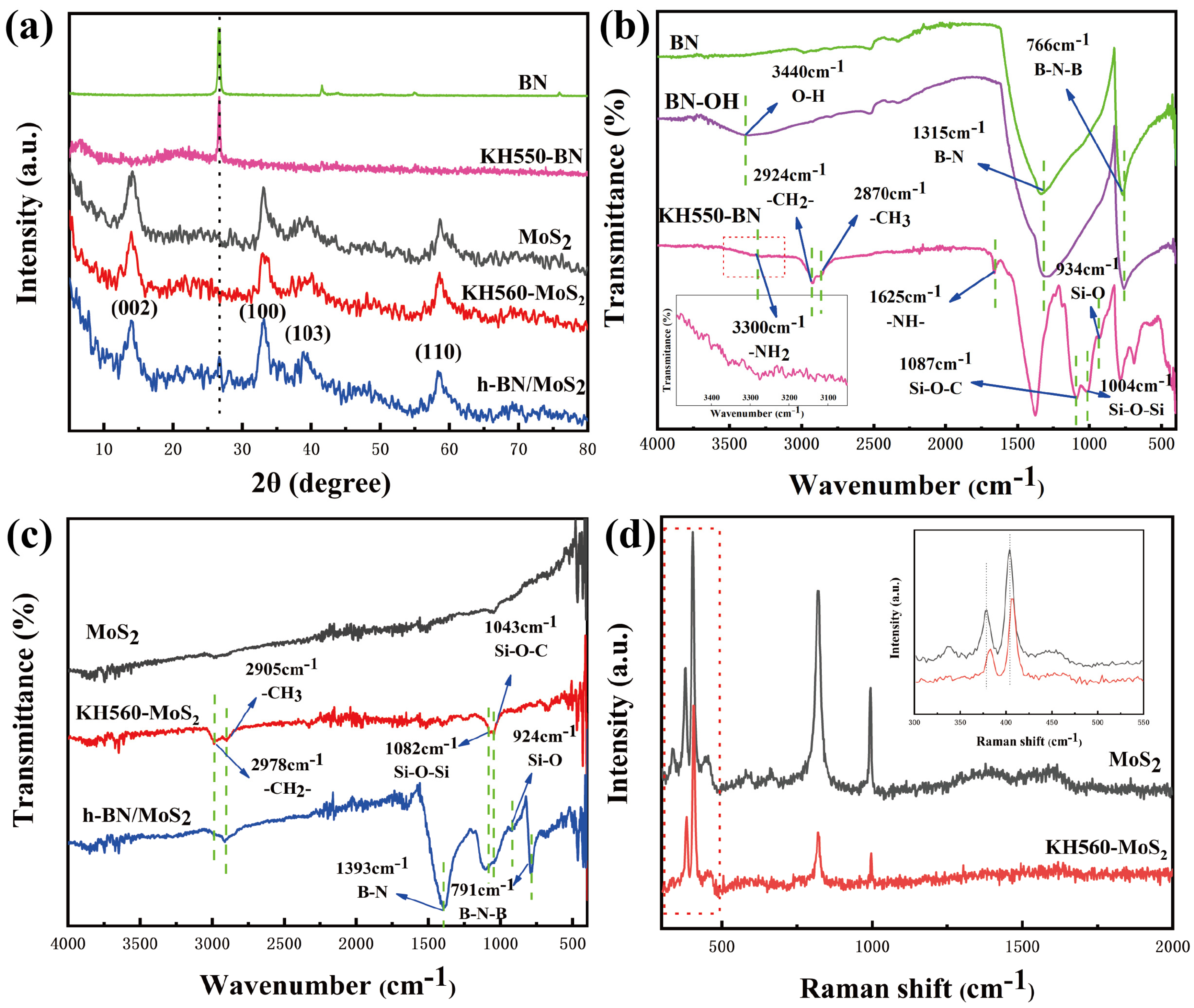
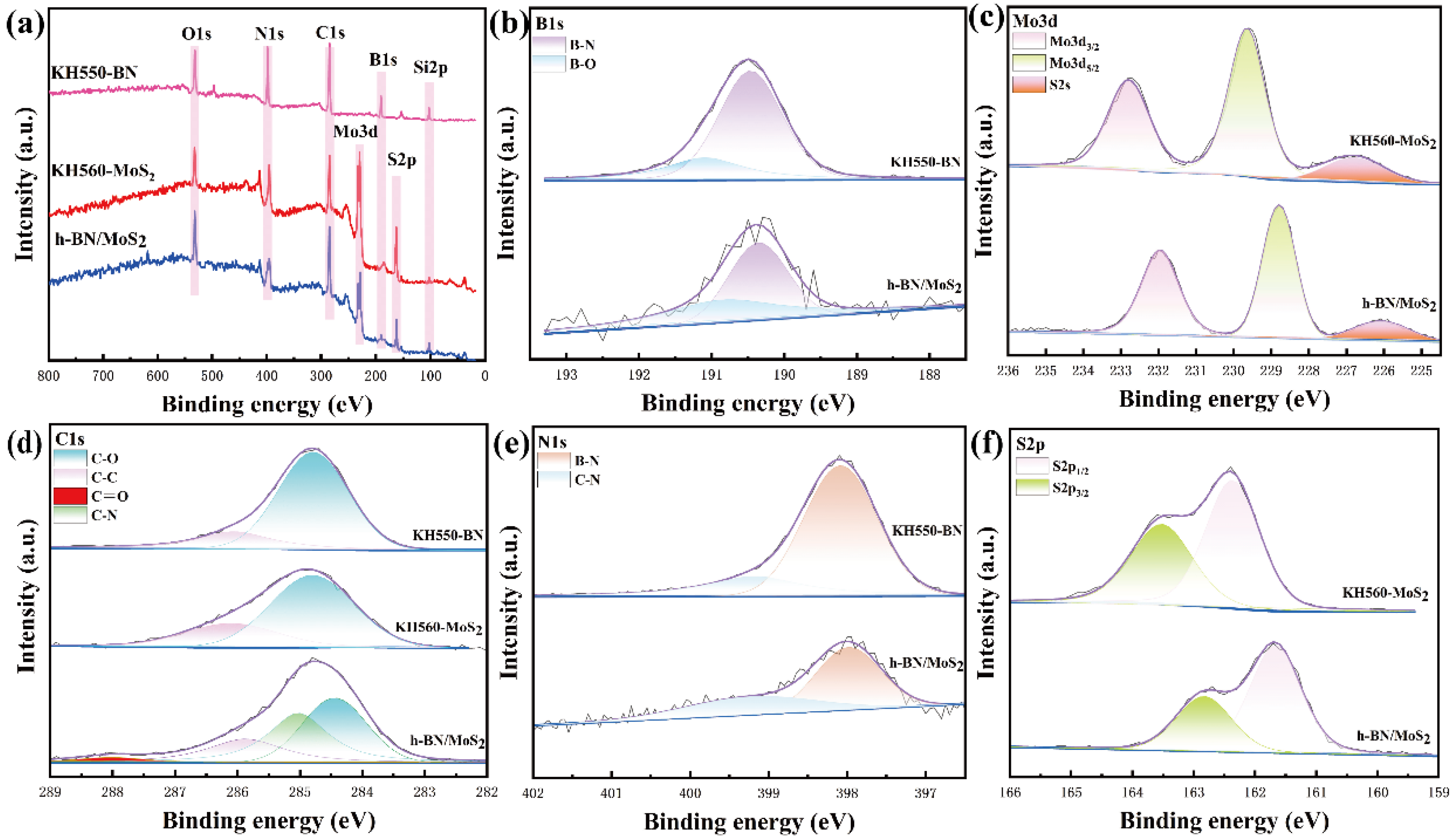
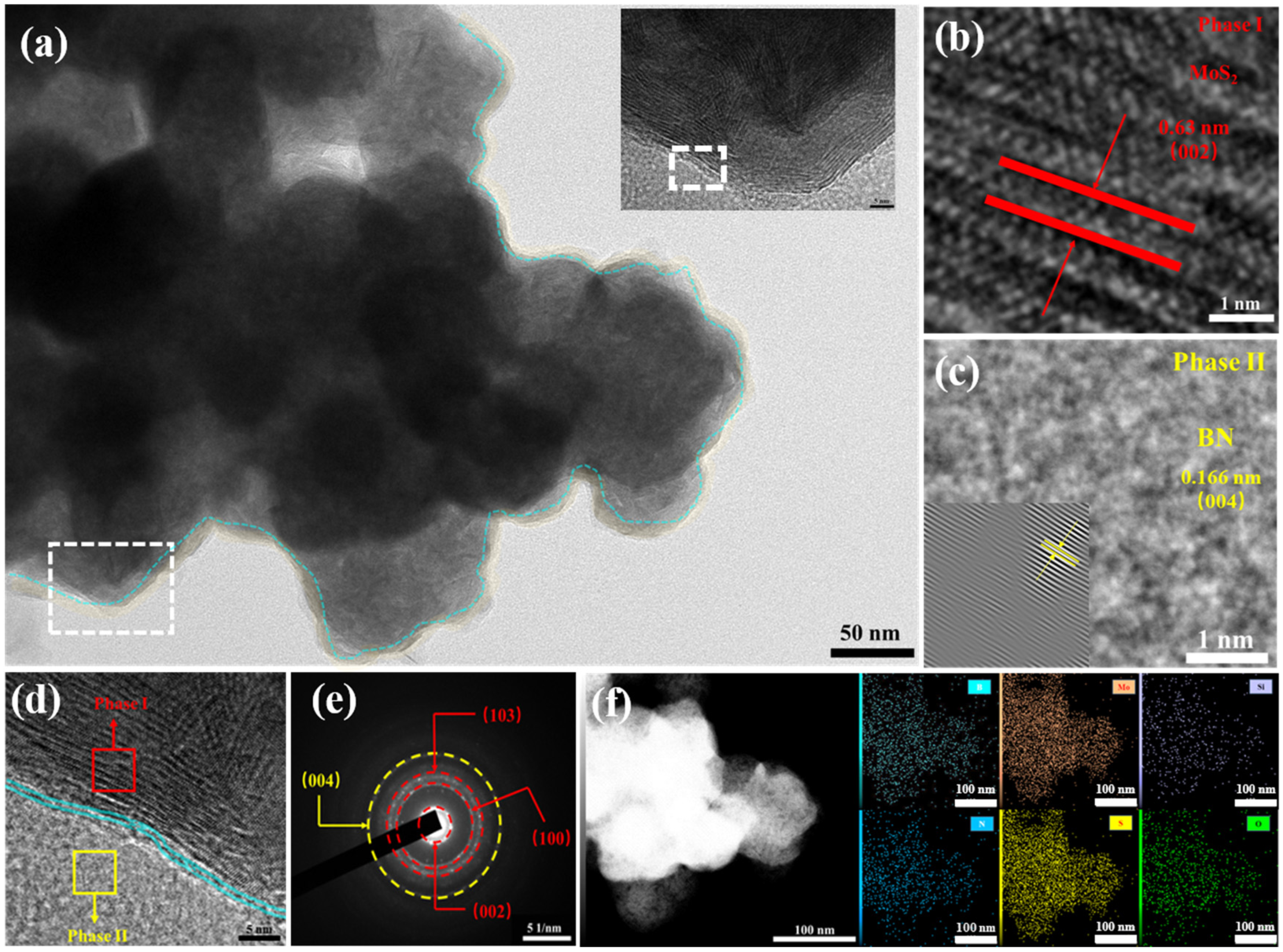
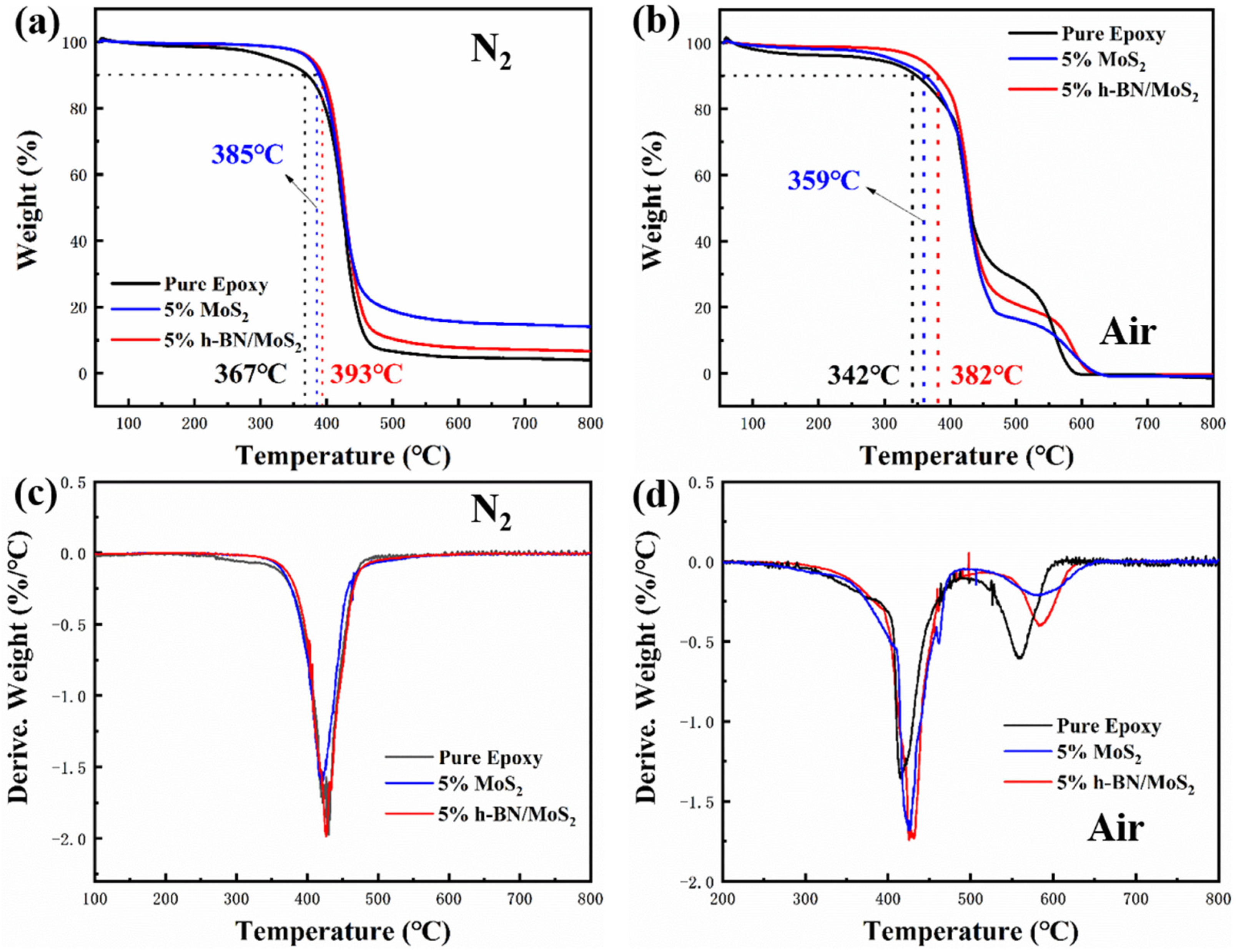
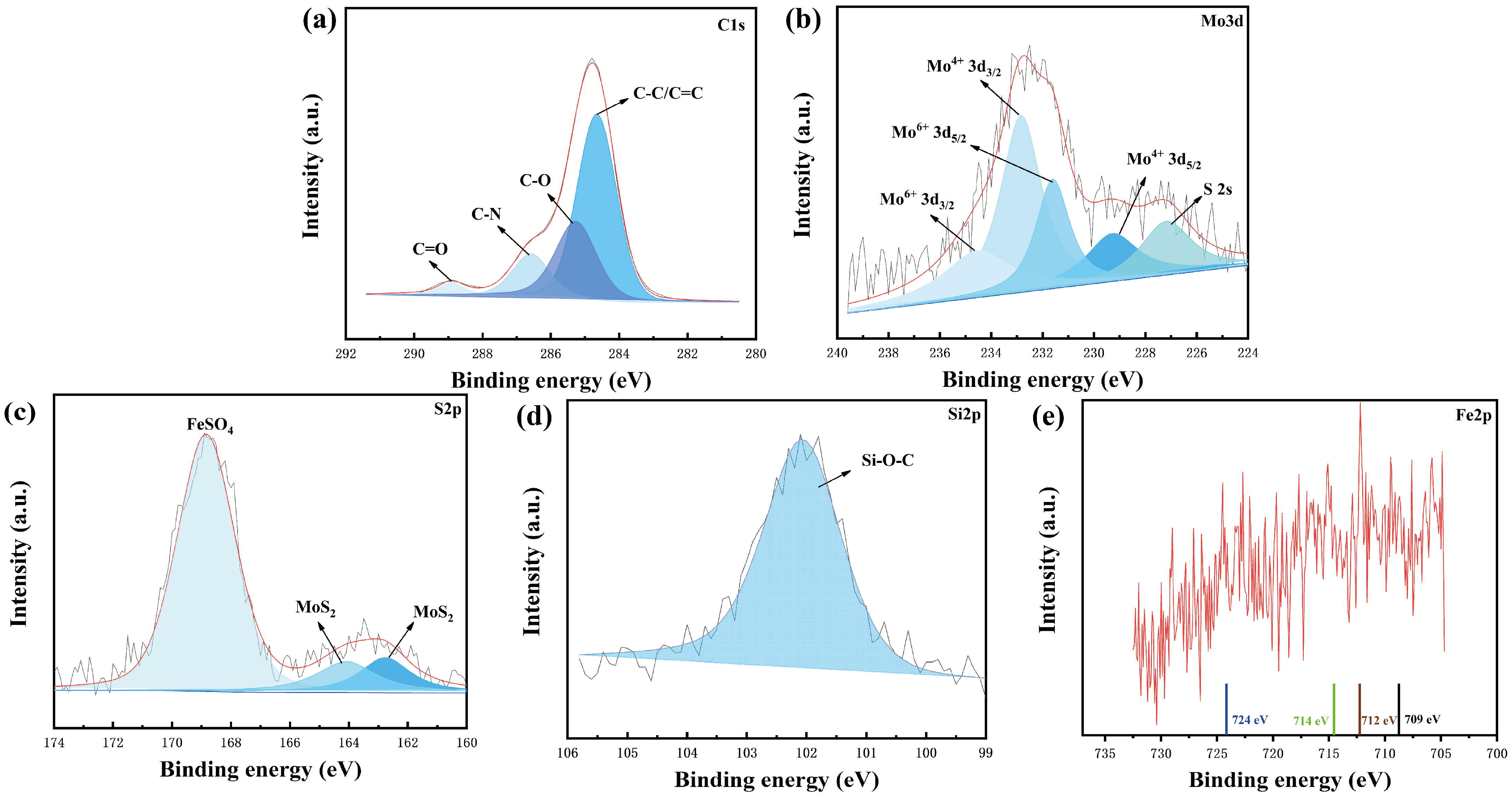
3.1. Characterization of Materials
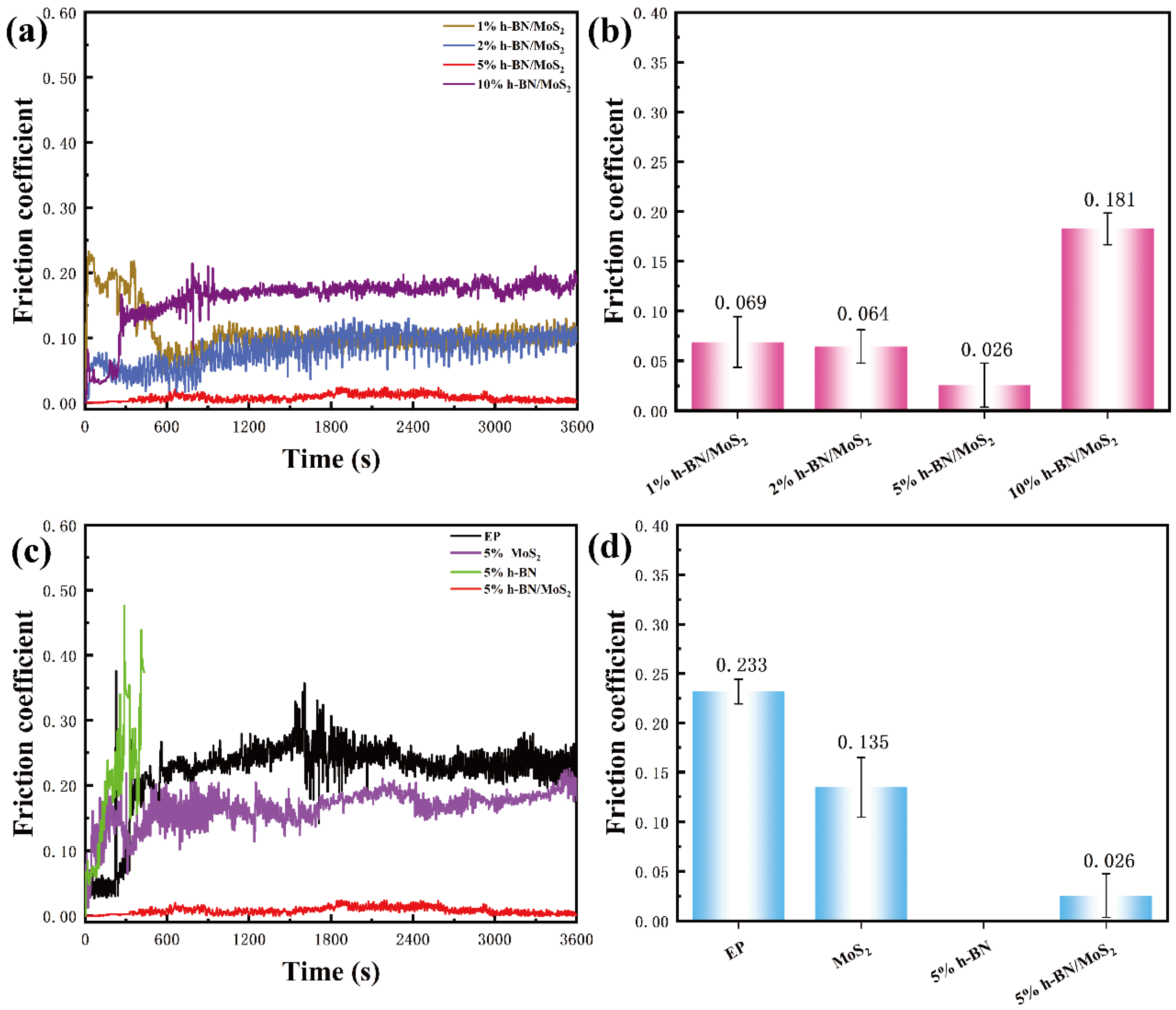
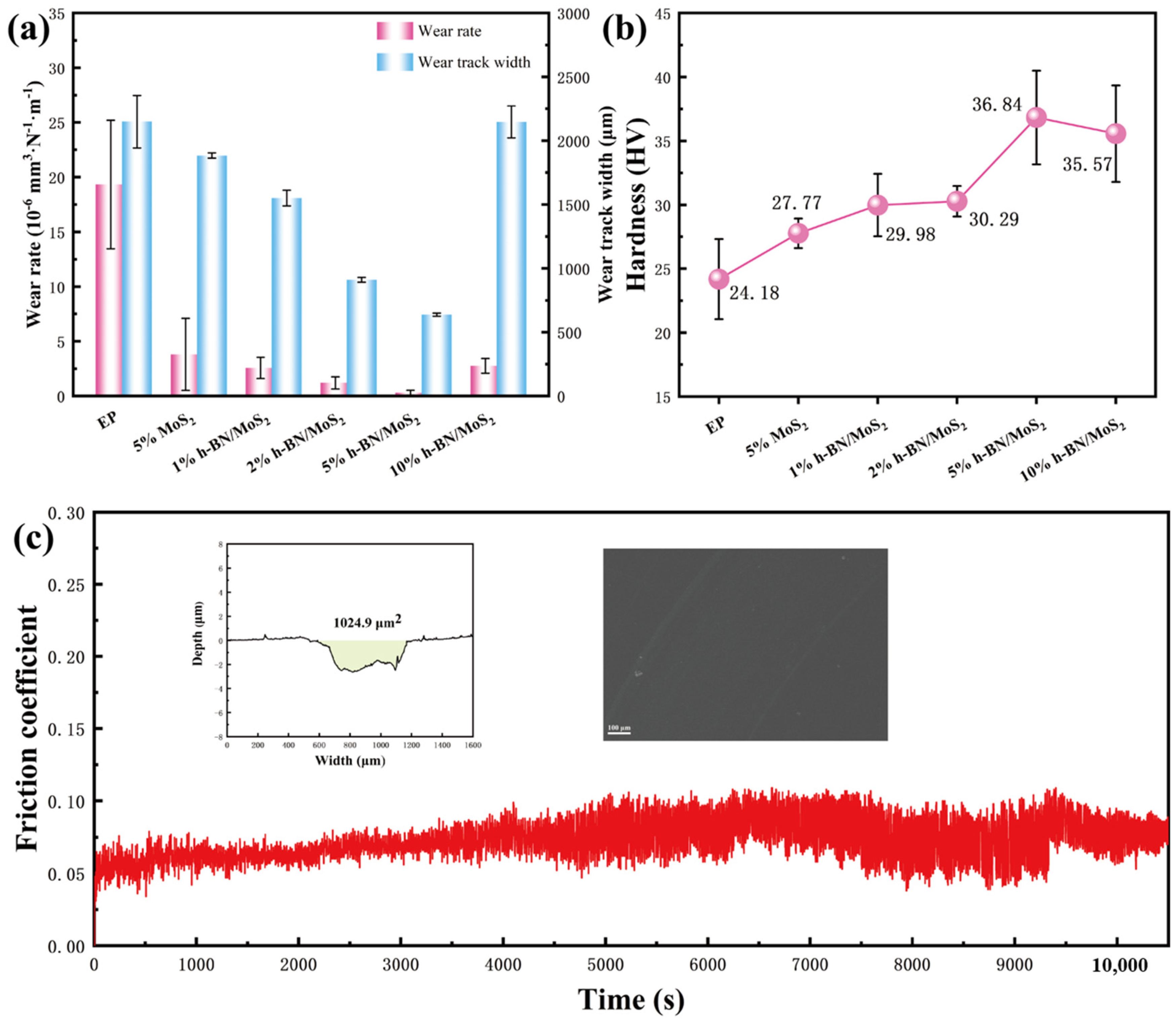
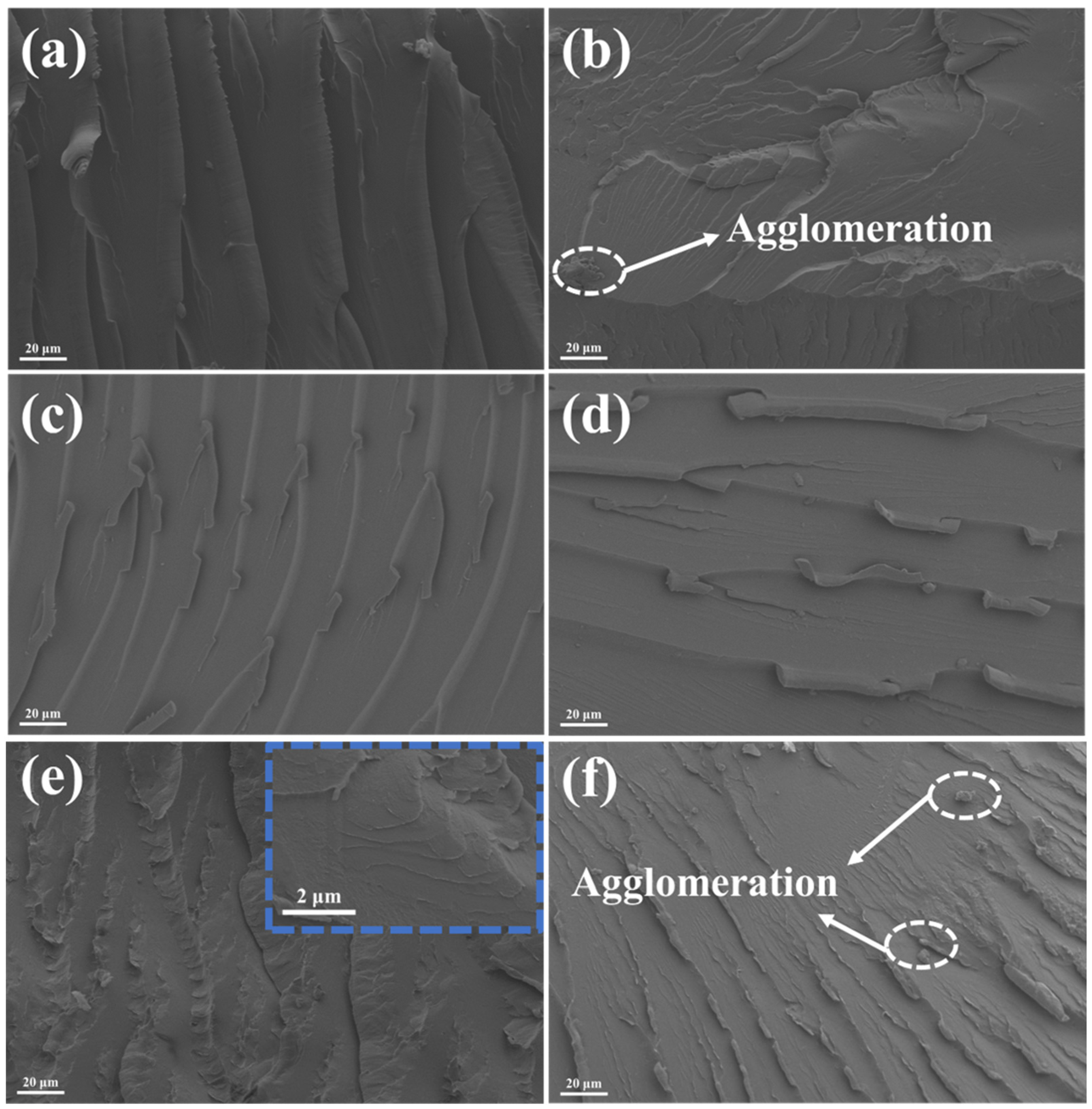
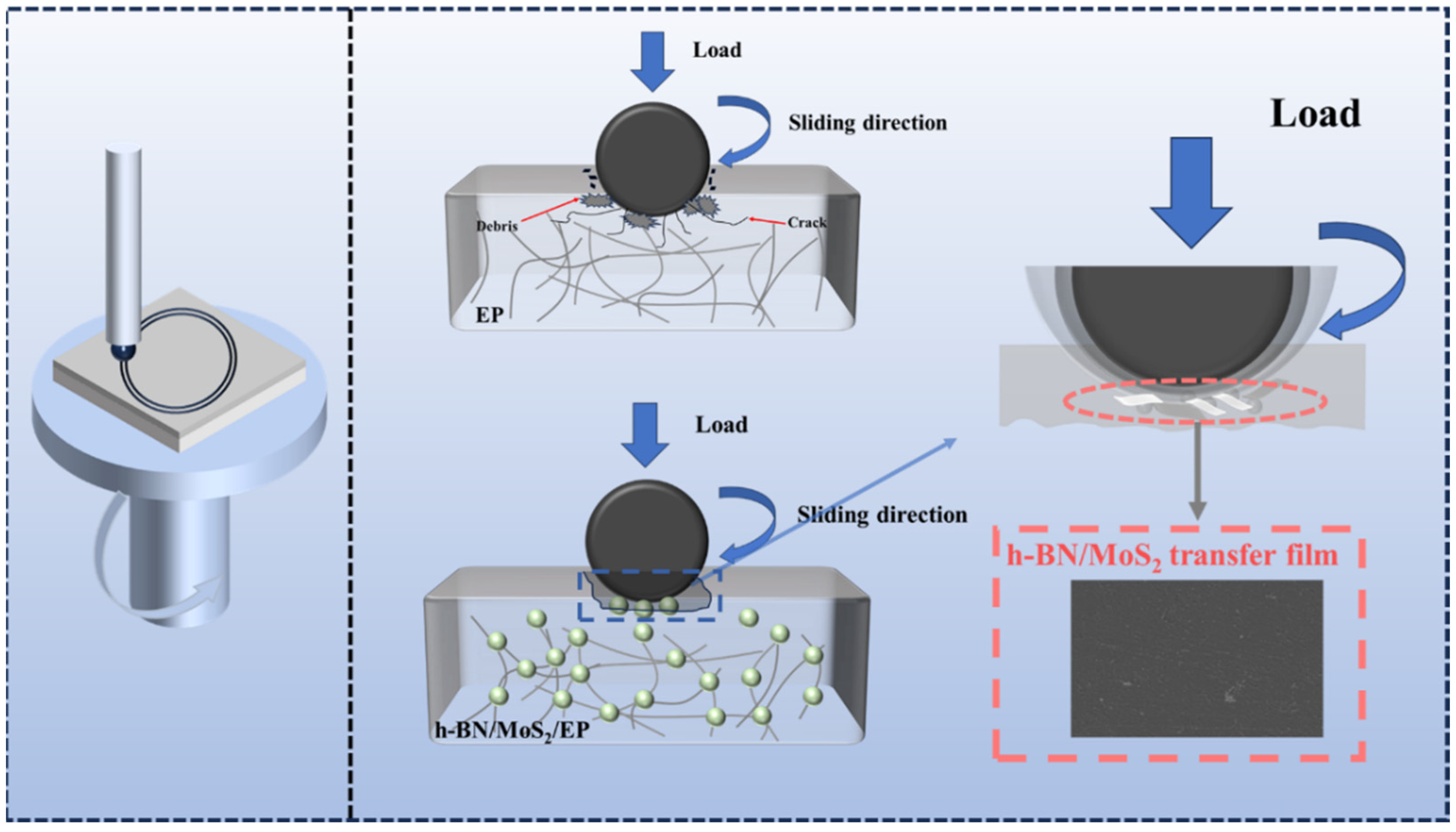
3.2. Mechanical and Tribological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Kurdi, A.; Kan, W.H.; Chang, L. Tribological behaviour of high performance polymers and polymer composites at elevated temperature. Tribol. Int. 2019, 130, 94–105. [Google Scholar] [CrossRef]
- Wan, H.; Jia, Y.; Ye, Y.; Xu, H.; Cui, H.; Chen, L.; Zhou, H.; Chen, J. Tribological behavior of polyimide/epoxy resin-polytetrafluoroethylene bonded solid lubricant coatings filled with in situ-synthesized silver nanoparticles. Prog. Org. Coat. 2017, 106, 111–118. [Google Scholar] [CrossRef]
- Dong, H.; Qiao, Y.; Peng, S.; Li, Y.; Zhen, Y.; Tan, W.; Cheng, Q.; Wang, Y. 2D material/epoxy composite coatings, a perspective from the regulation of 2D materials. Prog. Org. Coat. 2023, 183, 107817. [Google Scholar] [CrossRef]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Chen, X.; Zhang, C.; Luo, J. Preparation and Tribological Properties of Self-Lubricating Epoxy Resins with Oil-Containing Nanocapsules. ACS Appl. Mater. Interfaces 2022, 14, 18954–18964. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Gao, S.; Chen, Q.; Peng, L.; Liu, K.; Wei, X. Superlubricity between MoS2 Monolayers. Adv. Mater. 2017, 29, 1701474. [Google Scholar] [CrossRef]
- Cai, M.; Fan, X.; Yan, H.; Li, Y.; Song, S.; Li, W.; Li, H.; Lu, Z.; Zhu, M. In situ assemble Ti3C2Tx MXene@MgAl-LDH heterostructure towards anticorrosion and antiwear application. Chem. Eng. J. 2021, 419, 130050. [Google Scholar] [CrossRef]
- An, D.; Wang, Z.; Qin, L.; Wu, Y.; Lu, S.; Yang, H.; Ma, Z.; Mawignon, F.J.; Liu, J.; Hao, L.; et al. Preparation of MXene/EP coating for promising anticorrosion and superlow friction properties. Prog. Org. Coat. 2023, 183, 107779. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Chen, J.; Alhashmialameer, D.; Wang, S.; Mahmoud, M.H.H.; Mersal, G.A.M.; Huang, J.; Zhang, Q.; Zhao, G.; et al. Enhanced mechanical, thermal, and tribological performance of 2D-laminated molybdenum disulfide/RGO nanohybrid filling phenolic resin composites. Adv. Compos. Hybrid Mater. 2022, 5, 1206–1220. [Google Scholar] [CrossRef]
- Fan, X.; Li, X.; Zhao, Z.; Yue, Z.; Feng, P.; Ma, X.; Li, H.; Ye, X.; Zhu, M. Heterostructured rGO/MoS2 nanocomposites toward enhancing lubrication function of industrial gear oils. Carbon 2022, 191, 84–97. [Google Scholar] [CrossRef]
- Ju, H.; Wang, R.; Ding, N.; Yu, L.; Xu, J.; Ahmed, F.; Zuo, B.; Geng, Y. Improvement on the oxidation resistance and tribological properties of molybdenum disulfide film by doping nitrogen. Mater. Des. 2020, 186, 108300. [Google Scholar] [CrossRef]
- Song, Y.; Rong, C.; Feng, X.; Liu, Y.; Zhang, Y. Heterostructured rGO/MoS2 to improve friction and wear performance of epoxy resins. Polym. Compos. 2023, 44, 8010–8020. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Li, X.; Dong, Z.; Jia, Y.; Li, C. Tribological properties of epoxy-based self-lubricating composite coating enhanced by 2D/2D h-BN/MoS2 hybrid. Prog. Org. Coat. 2020, 147, 105767. [Google Scholar] [CrossRef]
- Zhao, B.; Ding, W.; Kuang, W.; Fu, Y. Microstructure and tribological property of self-lubrication CBN abrasive composites containing molybdenum disulfide. Ind. Lubr. Tribol. 2019, 71, 712–717. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Xu, H.; Chen, L.; An, Y.; Gao, M.; Zhou, H.; Chen, J. Hydroxylated hexagonal boron nitride nanoplatelets enhance the mechanical and tribological properties of epoxy-based composite coatings. Prog. Org. Coat. 2022, 165, 106731. [Google Scholar] [CrossRef]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong Oxidation Resistance of Atomically Thin Boron Nitride Nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Xu, B.; Sun, Q.; Zhang, W.; Zhang, Y.; Meng, F. Synthesis, structure and antioxidant performance of boron nitride (hexagonal) layers coating on carbon nanotubes (multi-walled). Appl. Surf. Sci. 2018, 450, 284–291. [Google Scholar] [CrossRef]
- Sun, G.; Bi, J.; Wang, W.; Zhang, J. One-pot synthesis of reduced graphene oxide@boron nitride nanosheet hybrids with enhanced oxidation-resistant properties. Appl. Surf. Sci. 2017, 426, 1249–1255. [Google Scholar] [CrossRef]
- Hu, K.H.; Wang, Y.R.; Hu, X.G.; Wo, H.Z. Preparation and characterisation of ball-like MoS2 nanoparticles. Mater. Sci. Technol. 2007, 23, 242–246. [Google Scholar] [CrossRef]
- Wan, P.; Zhao, N.; Qi, F.; Zhang, B.; Xiong, H.; Yuan, H.; Liao, B.; Ouyang, X. Synthesis of PDA-BN@f-Al2O3 hybrid for nanocomposite epoxy coating with superior corrosion protective properties. Prog. Org. Coat. 2020, 146, 105713. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Q.; Xie, Z.; Tan, C.; Chen, P.; Zeng, Y.; Wang, F.; Liu, H.; Liu, Y.; Liu, G.; et al. Carbon nitride modified hexagonal boron nitride interface as highly efficient blue LED light-driven photocatalyst. Appl. Catal. B Environ. 2018, 238, 410–421. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J.; Luo, J. Enhancement of friction performance enabled by a synergetic effect between graphene oxide and molybdenum disulfide. Carbon 2019, 154, 266–276. [Google Scholar] [CrossRef]
- Fan, X.; Gan, C.; Feng, P.; Ma, X.; Yue, Z.; Li, H.; Li, W.; Zhu, M. Controllable preparation of fluorinated boron nitride nanosheets for excellent tribological behaviors. Chem. Eng. J. 2022, 431, 133482. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Wu, Y.; Wang, D.; Pan, L. Effect of functionalization on thermal conductivity of hexagonal boron nitride/epoxy composites. Int. J. Heat Mass Transf. 2024, 219, 124844. [Google Scholar] [CrossRef]
- Shen, H.; Wu, Z.; Dou, R.; Jiao, L.; Chen, G.; Lin, M.; Huang, W.; Liu, Q.; Chen, H. The effect of modified carbon-doped boron nitride on the mechanical, thermal and γ-radiation stability of silicone rubber composites. Polym. Degrad. Stab. 2023, 218, 110542. [Google Scholar] [CrossRef]
- Songfeng, E.; Ye, X.; Wang, M.; Huang, J.; Ma, Q.; Jin, Z.; Ning, D.; Lu, Z. Enhancing the tribological properties of boron nitride by bioinspired polydopamine modification. Appl. Surf. Sci. 2020, 529, 147054. [Google Scholar]
- Jing, Y.; Wang, P.; Yang, Q.; He, Y.; Bai, Y. Molybdenum disulfide with poly(dopamine) and epoxy groups as an efficiently anticorrosive reinforcers in epoxy coating. Synth. Met. 2020, 259, 116249. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Zhong, F.; Chen, J. Co-modification of polydopamine and KH560 on g-C3N4 nanosheets for enhancing the corrosion protection property of waterborne epoxy coating. React. Funct. Polym. 2020, 146, 104405. [Google Scholar] [CrossRef]
- Yu, Z.; Ye, J.; Chen, W.; Xu, S.; Huang, F. Fabrication of few-layer molybdenum disulfide/reduced graphene oxide hybrids with enhanced lithium storage performance through a supramolecule-mediated hydrothermal route. Carbon 2017, 114, 125–133. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, P.; Yang, Q.; He, Y.; Bai, Y. The effect of a functionalized defect-rich molybdenum disulfide nanosheets on anticorrosion performance of epoxy coating. Mater. Res. Express 2019, 6, 086473. [Google Scholar] [CrossRef]
- Hou, K.; Wang, J.; Yang, Z.; Ma, L.; Wang, Z.; Yang, S. One-pot synthesis of reduced graphene oxide/molybdenum disulfide heterostructures with intrinsic incommensurateness for enhanced lubricating properties. Carbon 2017, 115, 83–94. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Xia, Y.; Li, H.; He, Z. Two-dimensional hybrid materials: MoS2-RGO nanocomposites enhanced the barrier properties of epoxy coating. Appl. Surf. Sci. 2018, 444, 511–521. [Google Scholar] [CrossRef]
- Song, H.; Wang, B.; Zhou, Q.; Xiao, J.; Jia, X. Preparation and tribological properties of MoS2/graphene oxide composites. Appl. Surf. Sci. 2017, 419, 24–34. [Google Scholar] [CrossRef]
- Furlan, K.P.; de Mello, J.D.B.; Klein, A.N. Self-lubricating composites containing MoS2: A review. Tribol. Int. 2018, 120, 280–298. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, H.; Qi, F.; Zhao, N.; Zhang, B.; Ouyang, X. Functionalized Modified BN@F-SiC Particle-Incorporating Epoxy: An Effective Hydrophobic Antiwear and Anticorrosion Coating Material. Ind. Eng. Chem. Res. 2021, 60, 8430–8441. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, G.; Li, W.; Zhao, H.; Wang, L. Noncovalent exfoliation of graphene and its multifunctional composite coating with enhanced anticorrosion and tribological performance. J. Alloys Compd. 2018, 747, 60–70. [Google Scholar] [CrossRef]
- He, X.; Li, S.; Wu, J.; Chen, Y.; Zhang, L.; Sheng, X. One-Pot Fabrication of an MXene-ZrP@PDA Heterojunction for Enhanced Corrosion/Wear Resistance of Waterborne Epoxy Coatings. Ind. Eng. Chem. Res. 2022, 61, 12576–12589. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, N.; Wang, C.; Wang, T.; Wang, Q.; Li, S.; Chen, S. MoS2/CF synergistic reinforcement on tribological properties of NBR/PU/EP interpenetrating polymer networks. Tribol. Int. 2022, 167, 107384. [Google Scholar] [CrossRef]
- Shao, X.; Wang, L.; Yang, Y.; Yang, T.; Deng, G.; He, Y.; Dong, L.; Wang, H.; Yang, J. Influence of preload on the tribological performance of MoS2/GO composite lubricating coating. Tribol. Int. 2023, 181, 108306. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, M.; Guo, Z.; Chen, H.; Yan, H.; Ren, F.; Jin, Y.; Sun, Z.; Ren, P. Synergistic effect of novel hyperbranched polysiloxane and Ti3C2Tx MXene/MoS2 hybrid filler towards desirable mechanical and tribological performance of bismaleimide composites. Compos. Part B Eng. 2023, 248, 110374. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Lin, Y.; Xue, X.; Yuan, Q.; Zhang, W.; Bao, Y.; Ma, J. Tribological properties of bismaleimide-based self-lubricating composite enhanced by MoS2 quantum dots/graphene hybrid. Compos. Commun. 2021, 28, 100922. [Google Scholar] [CrossRef]
- Liu, C.; Yin, Q.; Zhang, W.; Bao, Y.; Li, P.; Hao, L.; Ma, J. Tribological properties of graphene-modified with ionic liquids and carbon quantum dots/ bismaleimide composites. Carbon 2021, 183, 504–514. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, Y.; Wang, W.; Yan, L.; Luo, J. An investigation on the tribological properties of multilayer graphene and MoS2 nanosheets as additives used in hydraulic applications. Tribol. Int. 2016, 97, 14–20. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Jing, H.; Yu, L.; Wu, Z.; Su, C.; Ji, Z.; Shu, J.; Tang, H.; Xia, M.; Xia, X.; et al. Tribological Behavior and Mechanism of Silane-Bridged h-BN/MoS2 Hybrid Filling Epoxy Solid Lubricant Coatings. Nanomaterials 2025, 15, 401. https://doi.org/10.3390/nano15050401
Peng X, Jing H, Yu L, Wu Z, Su C, Ji Z, Shu J, Tang H, Xia M, Xia X, et al. Tribological Behavior and Mechanism of Silane-Bridged h-BN/MoS2 Hybrid Filling Epoxy Solid Lubricant Coatings. Nanomaterials. 2025; 15(5):401. https://doi.org/10.3390/nano15050401
Chicago/Turabian StylePeng, Xiaoxiao, Haiyan Jing, Lan Yu, Zongdeng Wu, Can Su, Ziyu Ji, Junjie Shu, Hua Tang, Mingzhu Xia, Xifeng Xia, and et al. 2025. "Tribological Behavior and Mechanism of Silane-Bridged h-BN/MoS2 Hybrid Filling Epoxy Solid Lubricant Coatings" Nanomaterials 15, no. 5: 401. https://doi.org/10.3390/nano15050401
APA StylePeng, X., Jing, H., Yu, L., Wu, Z., Su, C., Ji, Z., Shu, J., Tang, H., Xia, M., Xia, X., Lei, W., & Hao, Q. (2025). Tribological Behavior and Mechanism of Silane-Bridged h-BN/MoS2 Hybrid Filling Epoxy Solid Lubricant Coatings. Nanomaterials, 15(5), 401. https://doi.org/10.3390/nano15050401





