Controllable Hydrothermal Synthesis of 1D β-Ga2O3 for Solar-Blind Ultraviolet Photodetection
Abstract
:1. Introduction
2. Experiment
2.1. Materials Growth and Device Construction
2.2. Characterization Methods
3. Results and Discussion
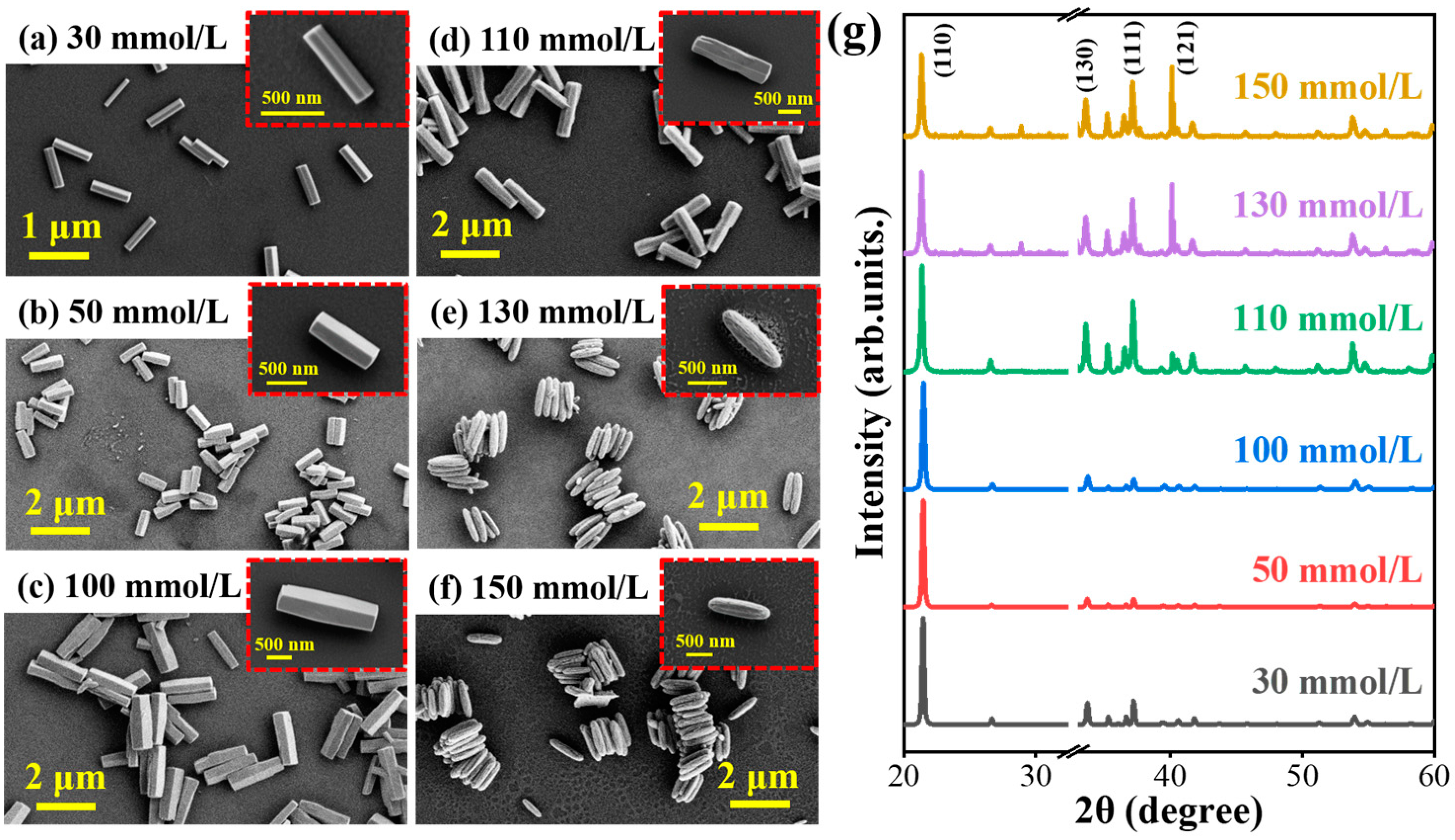
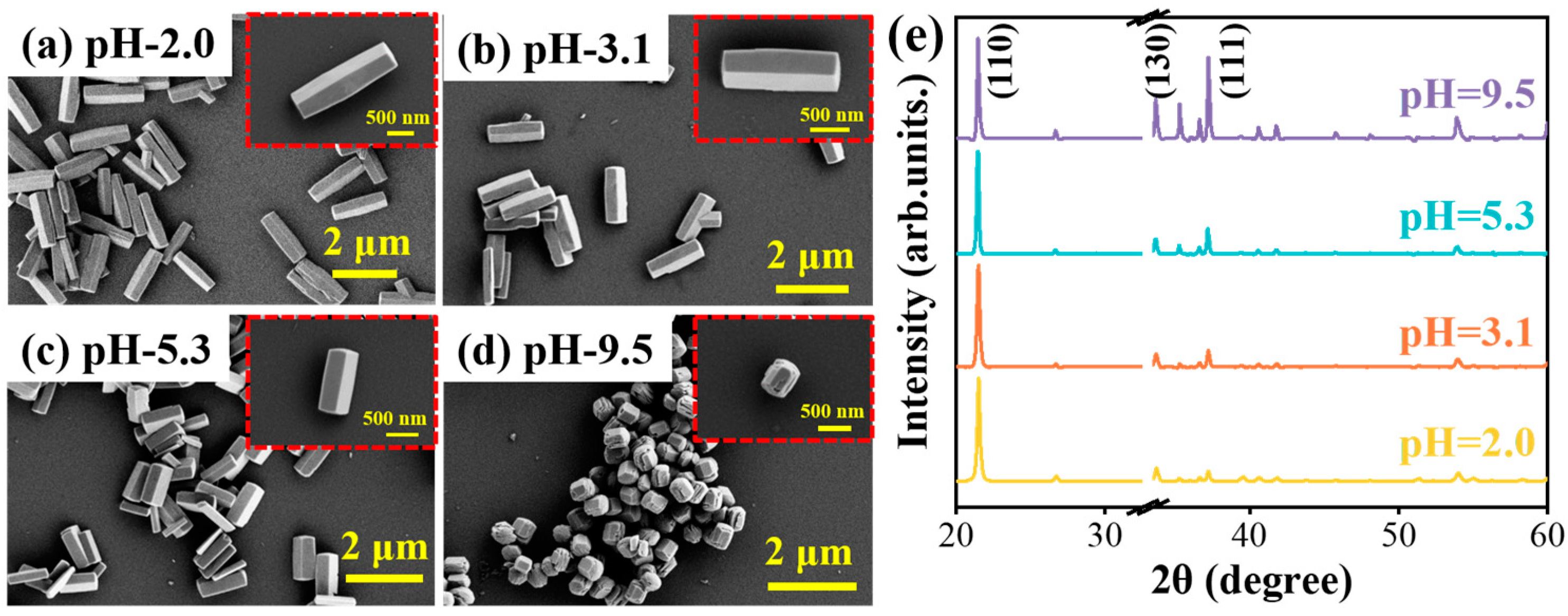
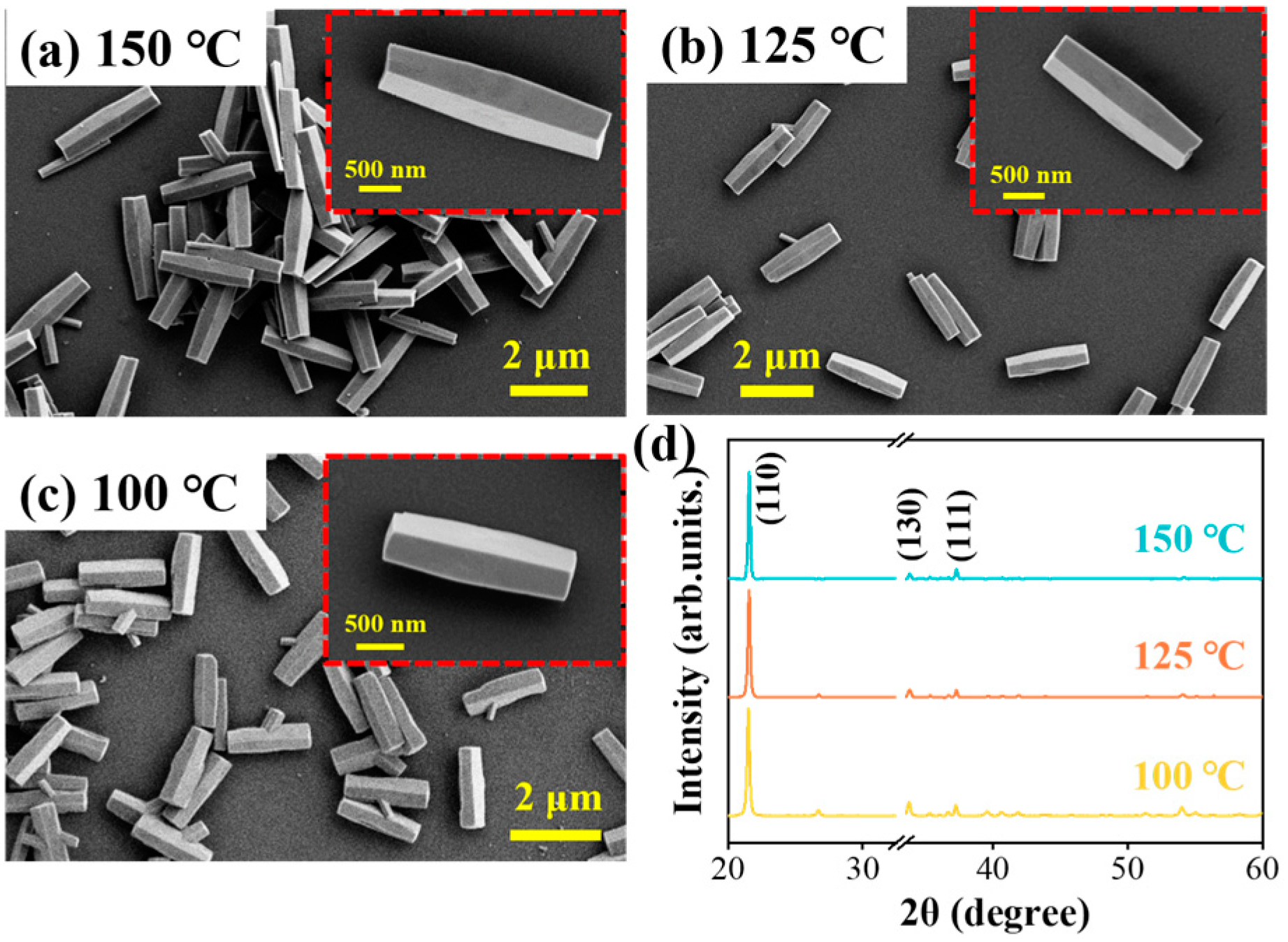
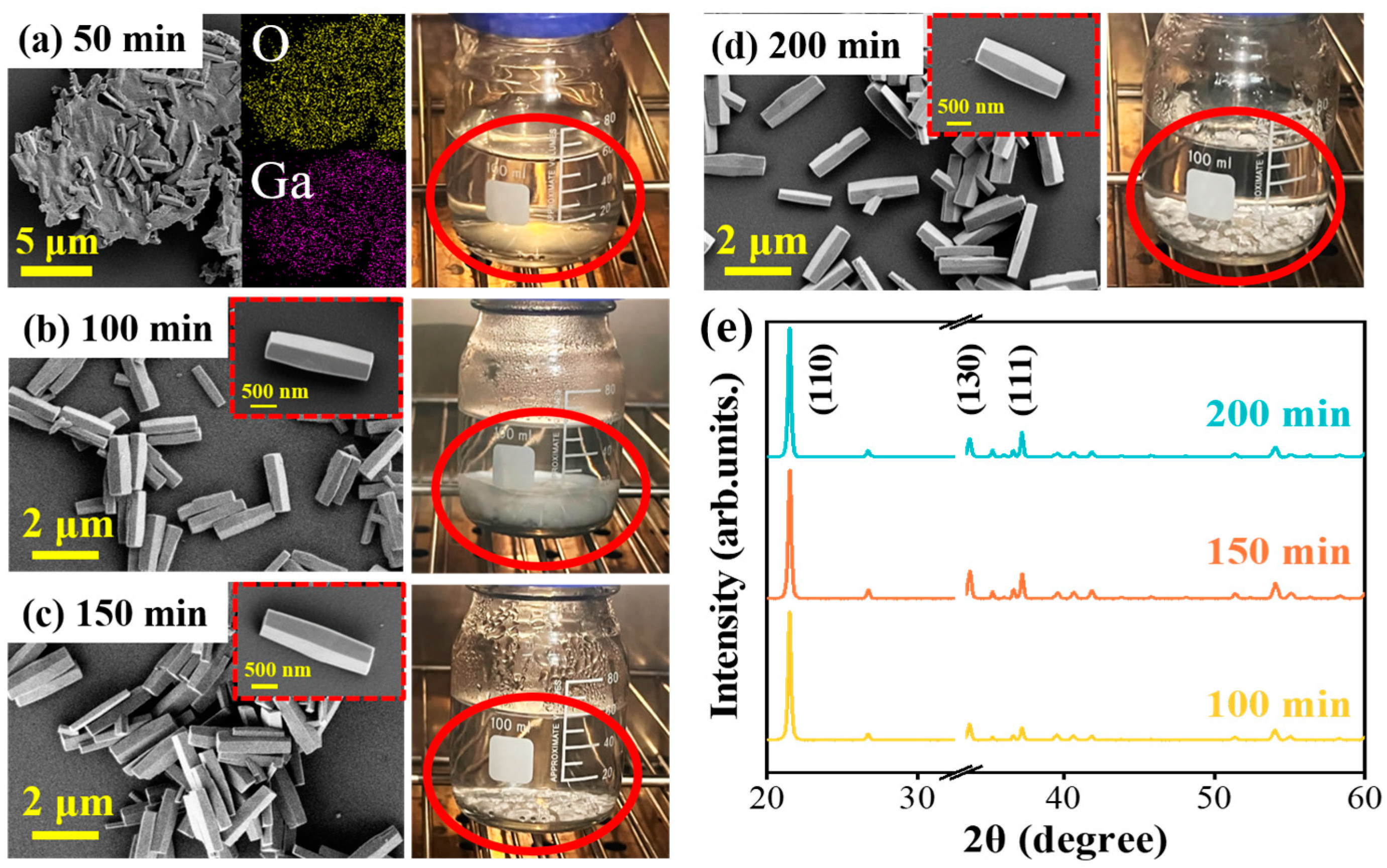
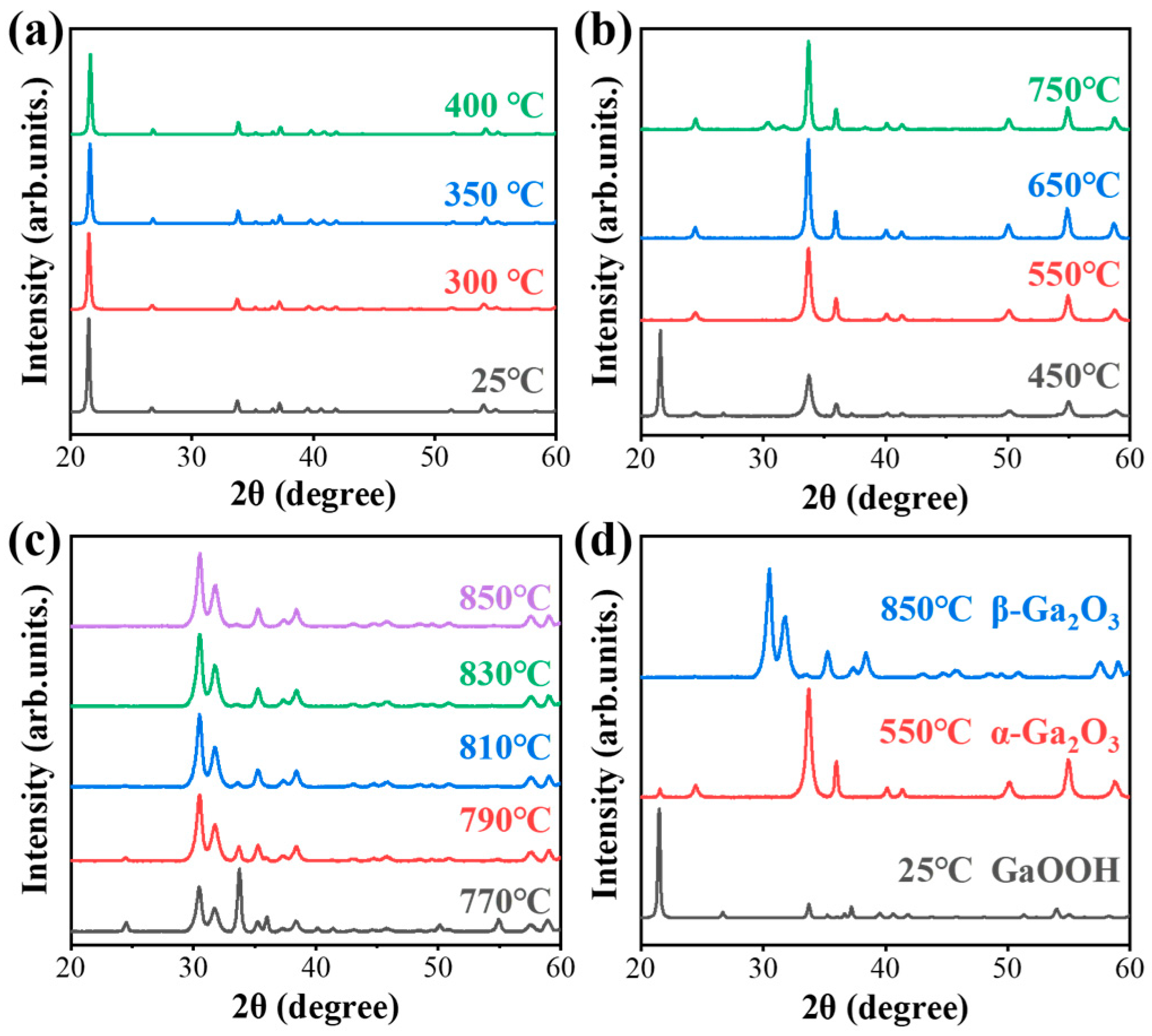
3.1. Controllable Growth Process of GaOOH
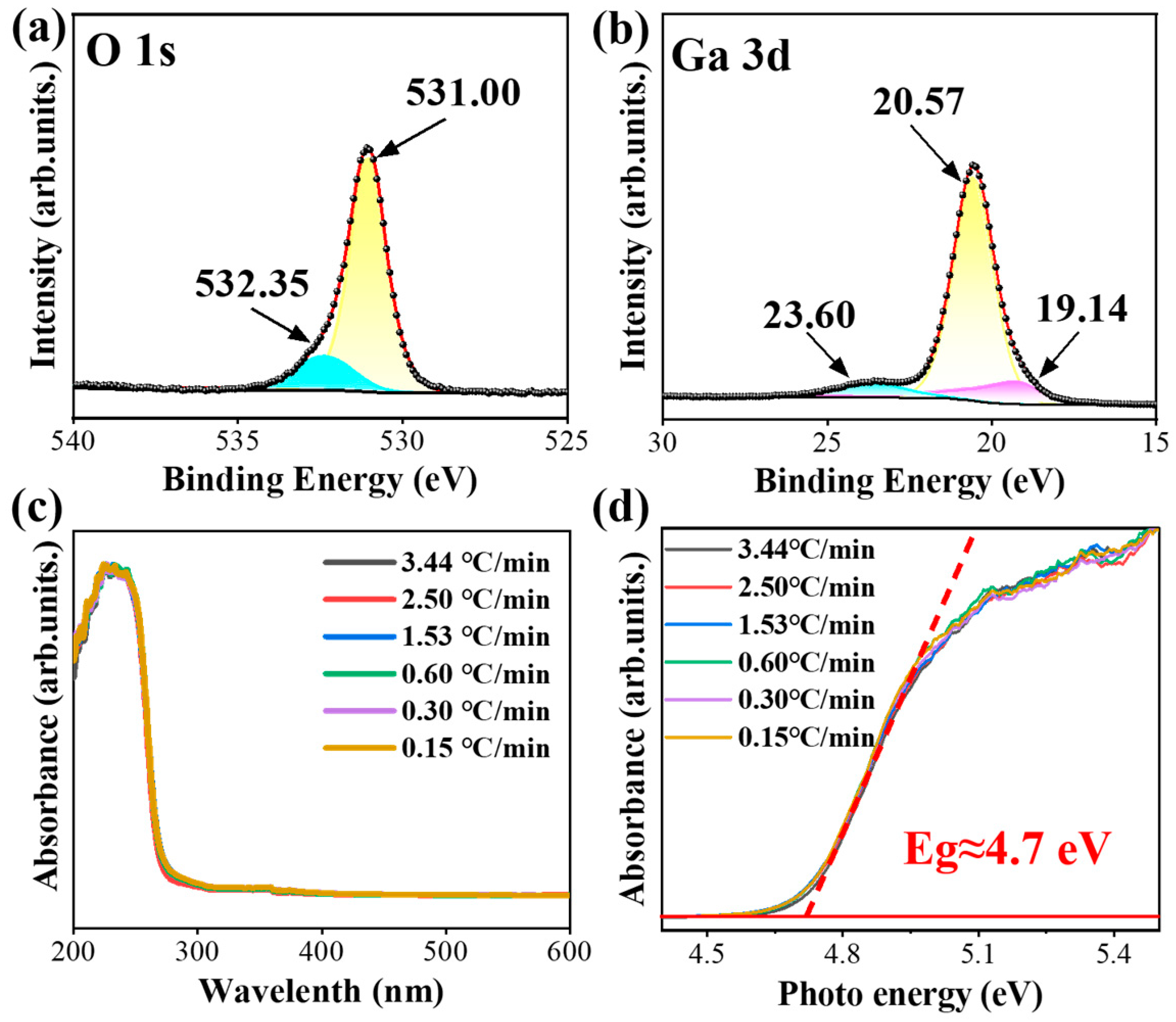
3.2. Thermal Annealing GaOOH for β-Ga2O3
3.3. Solar-Blind UV Photodetection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Zhou, S.; Xiong, Y.; Tang, J.; Wang, X.; Li, X.; Pang, D.; Li, H.; Zhang, H.; Ye, L.; et al. Self-powered Pt/a-Ga2O3/ITO vertical Schottky junction solar-blind photodetector with excellent detection performance. Opt. Express 2023, 31, 28200–28211. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.; Polyakov, A.; Krymov, V.; Saranin, D.; Chernykh, A.; Vasilev, A.; Schemerov, I.; Romanov, A.; Matros, N.; Kochkova, A.; et al. Trap States in p-NiO/n-Ga2O3 Heterojunctions on Czochralski β-Ga2O3 Crystals. ECS J. Solid State Sci. Technol. 2024, 13, 123004. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, J.; Ding, K.; Tang, Y.; Zhang, H.; Ye, L.; Pang, D.; Li, H.; Li, W. High-Performance Broad-Spectrum UV Photodetectors with Uniform Response: Engineering β-Ga2O3:Si/GaN:Si Heterojunctions via Thermal Oxidation for Optoelectronic Logic Gate and Multispectral Imaging. Small 2024, 2406447. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, Y.; Liu, L.; Berbille, A.; Wang, K.; Han, G.; Zhu, L.; Wang, Z. A self-powered Ag/β-Ga2O3 photodetector with broadband response from 200 to 980 nm based on the photovoltaic and pyro-phototronic effects. J. Mater. Sci. Technol. 2024, 206, 125–134. [Google Scholar] [CrossRef]
- Yu, C.; Li, H.; Ding, K.; Huang, L.; Zhang, H.; Pang, D.; Xiong, Y.; Yang, P.-A.; Fang, L.; Li, W.; et al. Flexible and Self-Powered Photoelectrochemical-Type Solar-Blind Photodetectors Based on Ag Nanowires-Embedded Amorphous Ga2O3 Films. Adv. Opt. Mater. 2024, 12, 2400116. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Zhou, H.; Xing, H.; Wang, L.; Zhang, W.; Tang, K.; Huang, J.; Wang, L. High-Performance Ga2O3 Solar-Blind Photodetector Based on Thermal Oxidized Ga Buffer-Layer. ACS Appl. Mater. Interfaces 2024, 16, 63808–63817. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Z.; Zhao, E.; Wei, S.; Jiao, C.; Xin, M.; Wang, K.; Zhai, R.; Ye, W.; Wang, H.; et al. Improved photoresponse performance of self-powered solar-blind UV photodetectors based on n-Si/n-Ga2O3/p-Li:NiO dual-junction. Appl. Phys. Lett. 2024, 125, 233501. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, B.; Hu, G.; Fang, X.; Wang, H.; Wang, L.; Luo, J.; Han, X.; Wang, X.; Pan, C.; et al. Piezo-Phototronic Effect Modulated Deep UV Photodetector Based on ZnO-Ga2O3 Heterojuction Microwire. Adv. Funct. Mater. 2018, 28, 1706379. [Google Scholar] [CrossRef]
- Tang, R.; Li, G.; Hu, X.; Gao, N.; Li, J.; Huang, K.; Kang, J.; Zhang, R. Micro-Nanoarchitectonics of Ga2O3/GaN Core-Shell Rod Arrays for High-Performance Broadband Ultraviolet Photodetection. Crystals 2023, 13, 366. [Google Scholar] [CrossRef]
- Zeng, C.; Ma, Y.; Kong, M.; Zhang, X.; Lin, W.; Cui, Q.; Sun, Y.; Zhang, X.; Chen, T.; Zhang, X.; et al. Ultraviolet photodetector based on vertical β-Ga2O3 nanowire array on GaN substrate. Mater. Res. Express 2021, 8, 055903. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, F.; Chen, H.; Zheng, L.; Su, L.; Zhao, D.; Fang, X. An Ultrahigh Responsivity (9.7 mA W−1) Self-Powered Solar-Blind Photodetector Based on Individual ZnO–Ga2O3 Heterostructures. Adv. Funct. Mater. 2017, 27, 1700264. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Zhang, Z.-F.; Yang, X.; He, G.-H.; Lin, C.-N.; Chen, X.-X.; Zang, J.-H.; Zhao, W.-B.; Chen, Y.-C.; Zhang, L.-L.; et al. High-performance solar-blind photodetector arrays constructed from Sn-doped Ga2O3 microwires via patterned electrodes. Nano Res. 2022, 15, 7631–7638. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, J.; Ding, K.; Ye, L.; Pang, D.; Li, H.; Zhang, H.; Tang, Y.; Li, W. Catalyst-Free Polymorphic β-Ga2O3 Nanomaterials for Solar-Blind Optoelectronic Devices: Applications in Imaging and Neural Communication. Small Methods 2024, 2401473. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.; Xu, S. Synthesis and mechanism of a new environment-friendly flame retardant (anhydrous magnesium carbonate) by hydrothermal method. Adv. Powder Technol. 2022, 33, 103776. [Google Scholar] [CrossRef]
- Ren, M.; Shen, P.; Tao, Y.; Poon, C.-s. Development of highly carbonation-effective calcium silicates (β-C2S): Phase evolution, microstructure, and carbonation mechanisms. Cem. Concr. Res. 2024, 181, 107542. [Google Scholar] [CrossRef]
- Suvaci, E.; Özel, E. Hydrothermal Synthesis. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 59–68. [Google Scholar]
- Xu, C.; Kang, J.; Zhao, Y.; Zhu, L.; Zhang, J.; Wei, B.; Wang, H. Comparison of carbon dots prepared from collagen peptides using conventional hydrothermal and microwave methods. New J. Chem. 2023, 47, 3159–3166. [Google Scholar] [CrossRef]
- Shi, F.; Qiao, H. Preparations, properties and applications of gallium oxide nanomaterials—A review. Nano Sel. 2022, 3, 348–373. [Google Scholar] [CrossRef]
- Gong, G.; Gou, L. Microstructure and properties of Cu-doped β-Ga2O3 rod prepared with liquid metallic gallium. J. Eur. Ceram. Soc. 2025, 45, 116916. [Google Scholar] [CrossRef]
- Babu, A.; Rao, N.M. Structural, Optical, and Magnetic Studies of Nickel-Doped β-Ga2O3 Monoclinic and Spinel Polycrystalline Powders. J. Electron. Mater. 2024, 53, 7026–7034. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wang, D.; Ni, S.; Gao, S.; Wang, J. Solar-blind ultraviolet photodetection of an α-Ga2O3 nanorod array based on photoelectrochemical self-powered detectors with a simple, newly-designed structure. J. Mater. Chem. C 2019, 7, 6867–6871. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of β-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Pilliadugula, R.; Krishnan, N.G. Effect of pH dependent morphology on room temperature NH3 sensing performances of β-Ga2O3. Mater. Sci. Semicond. Process. 2020, 112, 105007. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Wahed, M.S.M.A.; Ahmmad, B.; Kuroda, Y.; Suri, R.P.S.; Wu, J.J.; Sillanpää, M.E.T. Controlled Fabrication of α-GaOOH and α-Ga2O3 Self-Assembly and Its Superior Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 44–53. [Google Scholar] [CrossRef]
- Dulda, A. Morphology Controlled Synthesis of α-GaO(OH) Nanoparticles: Thermal Conversion to Ga2O3 and Photocatalytic Properties. Adv. Mater. Sci. Eng. 2016, 2016, 3905625. [Google Scholar] [CrossRef]
- Li, G.; Peng, C.; Li, C.; Yang, P.; Hou, Z.; Fan, Y.; Cheng, Z.; Lin, J. Shape-Controllable Synthesis and Morphology-Dependent Luminescence Properties of GaOOH:Dy3+ and β-Ga2O3:Dy3+. Inorg. Chem. 2010, 49, 1449–1457. [Google Scholar] [CrossRef]
- Taş, A.C.; Majewski, P.J.; Aldinger, F. Synthesis of Gallium Oxide Hydroxide Crystals in Aqueous Solutions with or without Urea and Their Calcination Behavior. J. Am. Ceram. Soc. 2002, 85, 1421–1429. [Google Scholar] [CrossRef]
- Chen, B.-R.; Sun, W.; Kitchaev, D.A.; Mangum, J.S.; Thampy, V.; Garten, L.M.; Ginley, D.S.; Gorman, B.P.; Stone, K.H.; Ceder, G.; et al. Understanding crystallization pathways leading to manganese oxide polymorph formation. Nat. Commun. 2018, 9, 2553. [Google Scholar] [CrossRef]
- Balitsky, D.V.; Philippot, E.; Papet, P.; Balitsky, V.S.; Pey, F. Comparative crystal growth of GaPO4 crystals in the retrograde and direct solubility range by hydrothermal methods of temperature gradient. J. Cryst. Growth 2005, 275, e887–e894. [Google Scholar] [CrossRef]
- Yadav, H.; Sinha, N.; Goel, S.; Kumar, B. Eu-doped ZnO nanoparticles for dielectric, ferroelectric and piezoelectric applications. J. Alloys Compd. 2016, 689, 333–341. [Google Scholar] [CrossRef]
- Moharram, A.H.; Mansour, S.A.; Hussein, M.A.; Rashad, M. Direct Precipitation and Characterization of ZnO Nanoparticles. J. Nanomater. 2014, 2014, 716210. [Google Scholar] [CrossRef]
- Chen, C.; Yu, B.; Liu, J.; Dai, Q.; Zhu, Y. Investigation of ZnO films on Si<111> substrate grown by low energy O+ assisted pulse laser deposited technology. Mater. Lett. 2007, 61, 2961–2964. [Google Scholar] [CrossRef]
- Fiedler, A.; Ramsteiner, M.; Galazka, Z.; Irmscher, K. Raman scattering in heavily donor doped β-Ga2O3. Appl. Phys. Lett. 2020, 117, 152107. [Google Scholar] [CrossRef]
- Gao, Y.H.; Bando, Y.; Sato, T.; Zhang, Y.F.; Gao, X.Q. Synthesis, Raman scattering and defects of β-Ga2O3 nanorods. Appl. Phys. Lett. 2002, 81, 2267–2269. [Google Scholar] [CrossRef]
- Alonso-Orts, M.; Sánchez, A.M.; Nogales, E.; Méndez, B. Ge incorporation in gallium oxide nanostructures grown by thermal treatment. J. Mater. Sci. 2020, 55, 11431–11438. [Google Scholar] [CrossRef]
- Song, P.; Wu, Z.; Shen, X.; Kang, J.; Fang, Z.; Zhang, T.-Y. Self-consistent growth of single-crystalline (01)β-Ga2O3 nanowires using a flexible GaN seed nanocrystal. CrystEngComm 2017, 19, 625–631. [Google Scholar] [CrossRef]
- Das, B.; Das, B.; Sankar Das, N.; Pal, S.; Kumar Das, B.; Sarkar, S.; Kumar Chattopadhyay, K. Novel Ag2O-Ga2O3 type II p-n heterojunction as an efficient water cleanser for green cleaning technology. Appl. Surf. Sci. 2020, 515, 145958. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Galazka, Z.; Alamé, S.; Skuridina, D.; Vogt, P.; Esser, N. Surface properties of annealed semiconducting β-Ga2O3 (100) single crystals for epitaxy. Appl. Surf. Sci. 2015, 349, 368–373. [Google Scholar] [CrossRef]
- Guo, T.; Ling, C.; Zhang, T.; Li, H.; Li, X.; Chang, X.; Zhu, L.; Zhao, L.; Xue, Q. High-performance WO3−x-WSe2/SiO2/n-Si heterojunction near-infrared photodetector via a homo-doping strategy. J. Mater. Chem. C 2018, 6, 5821–5829. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Pan, Z.-Q.; Wang, Y.-Y.; Ge, C.-W.; Yu, Y.-Q.; Xu, J.-Y.; Wang, L.; Luo, L.-B. Core–shell silicon nanowire array–Cu nanofilm Schottky junction for a sensitive self-powered near-infrared photodetector. J. Mater. Chem. C 2016, 4, 10804–10811. [Google Scholar] [CrossRef]



| Structure | a (Å) | b (Å) | c (Å) | β(°) | |
|---|---|---|---|---|---|
| GaOOH | orthorhombic | 4.58 | 9.75 | 2.98 | - |
| α-Ga2O3 | hexagonal | 4.99 | - | 13.49 | - |
| β-Ga2O3 | monoclinic | 12.23 | 3.04 | 5.80 | 103.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Wang, X.; Huang, C.; Ma, Y.; Qin, F.; Lu, W.; Zhu, G.; Shi, Z.; Cui, Q.; Xu, C. Controllable Hydrothermal Synthesis of 1D β-Ga2O3 for Solar-Blind Ultraviolet Photodetection. Nanomaterials 2025, 15, 402. https://doi.org/10.3390/nano15050402
Mao L, Wang X, Huang C, Ma Y, Qin F, Lu W, Zhu G, Shi Z, Cui Q, Xu C. Controllable Hydrothermal Synthesis of 1D β-Ga2O3 for Solar-Blind Ultraviolet Photodetection. Nanomaterials. 2025; 15(5):402. https://doi.org/10.3390/nano15050402
Chicago/Turabian StyleMao, Lingfeng, Xiaoxuan Wang, Chaoyang Huang, Yi Ma, Feifei Qin, Wendong Lu, Gangyi Zhu, Zengliang Shi, Qiannan Cui, and Chunxiang Xu. 2025. "Controllable Hydrothermal Synthesis of 1D β-Ga2O3 for Solar-Blind Ultraviolet Photodetection" Nanomaterials 15, no. 5: 402. https://doi.org/10.3390/nano15050402
APA StyleMao, L., Wang, X., Huang, C., Ma, Y., Qin, F., Lu, W., Zhu, G., Shi, Z., Cui, Q., & Xu, C. (2025). Controllable Hydrothermal Synthesis of 1D β-Ga2O3 for Solar-Blind Ultraviolet Photodetection. Nanomaterials, 15(5), 402. https://doi.org/10.3390/nano15050402







