Adsorption of Macrolide Antibiotics by Aged Microplastics of Different Sizes: Mechanisms and Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. MPs Aging Experiment
2.3. MPs Characterization Method
2.4. Antibiotic Adsorption Experiment
2.4.1. Adsorption Kinetics Experiment
2.4.2. Adsorption Isothermal Experiment
2.5. Adsorption Model
3. Results and Discussion
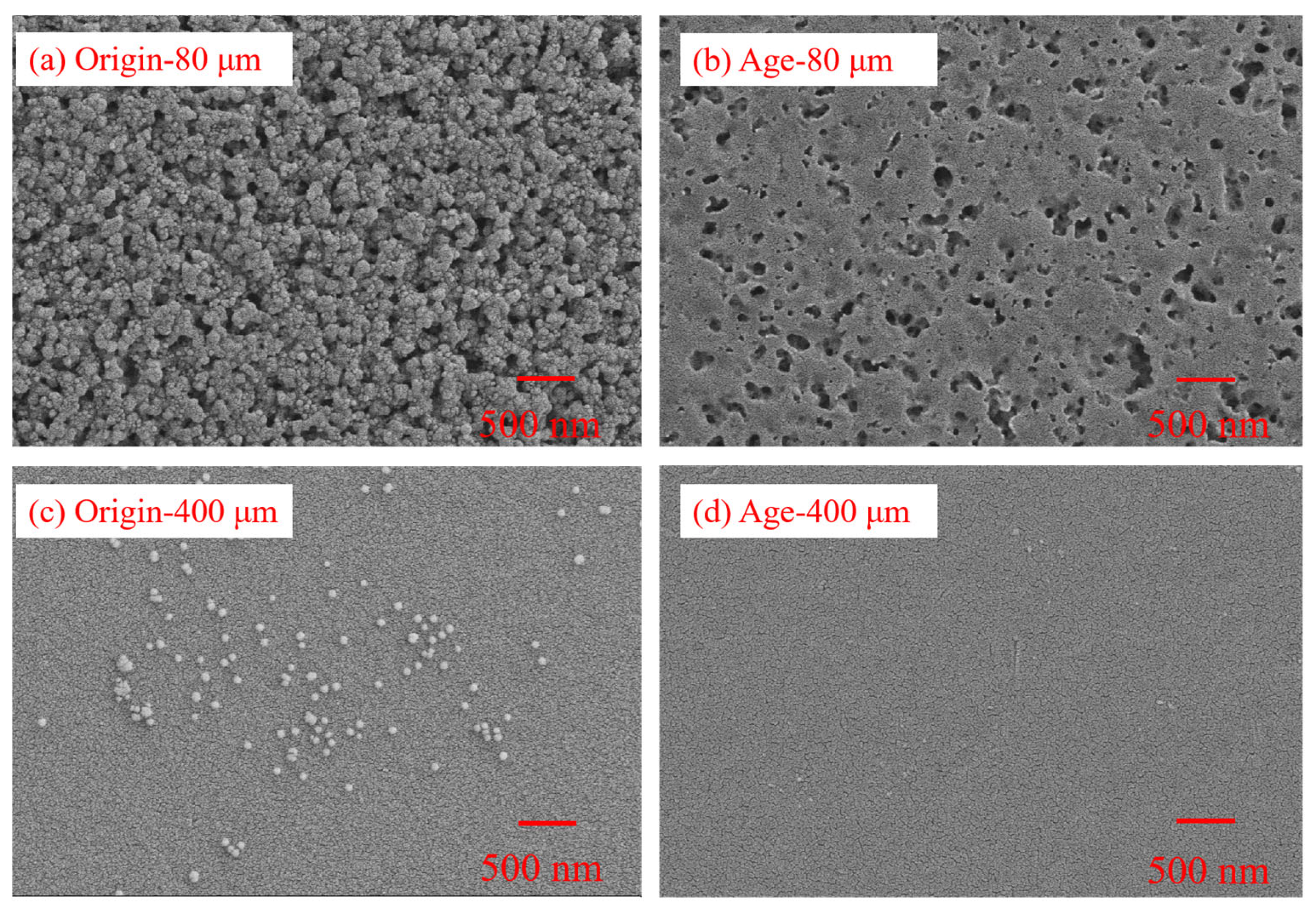
3.1. Changes in Physical Properties of Pristine and Aged PS MPs
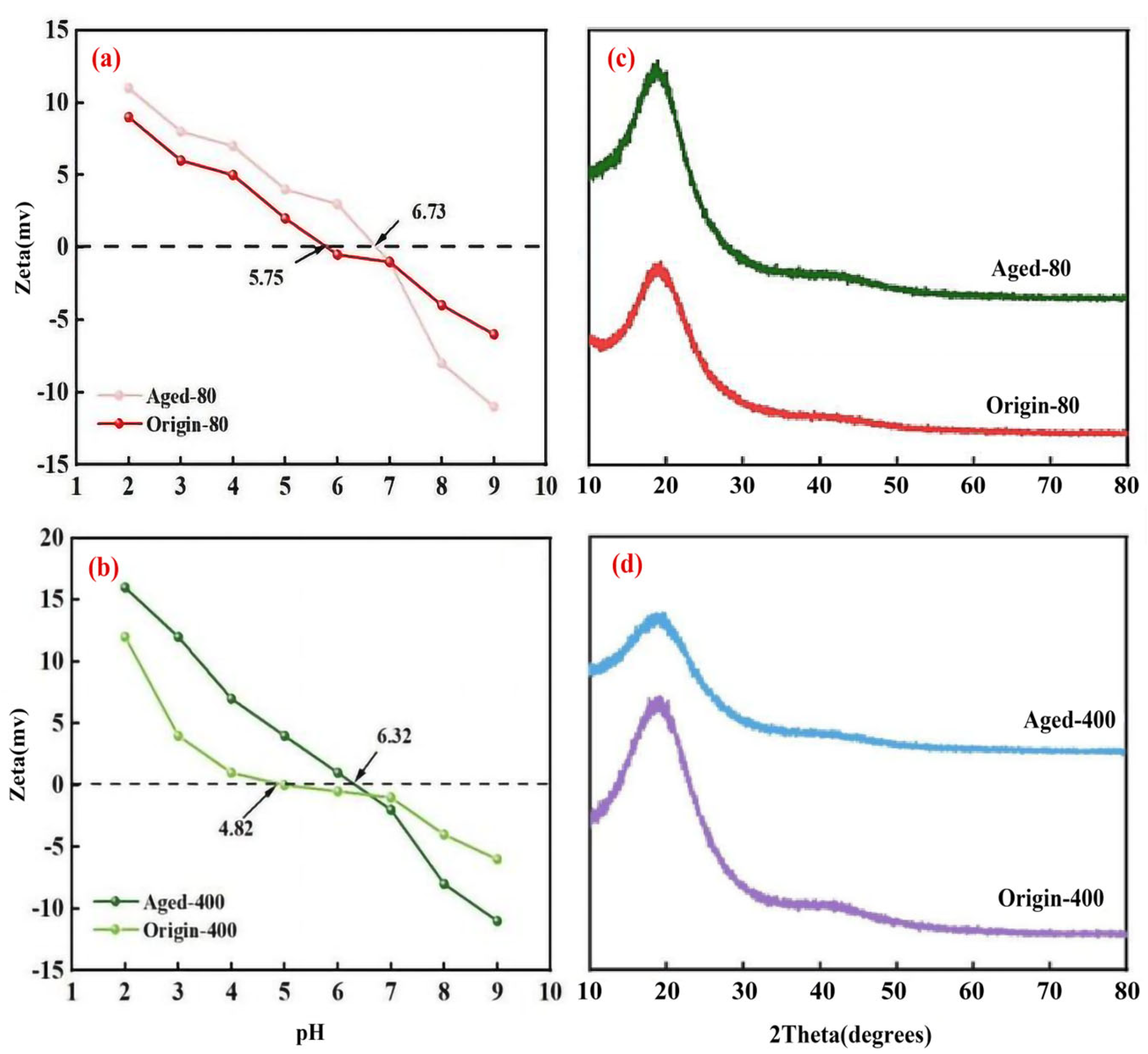
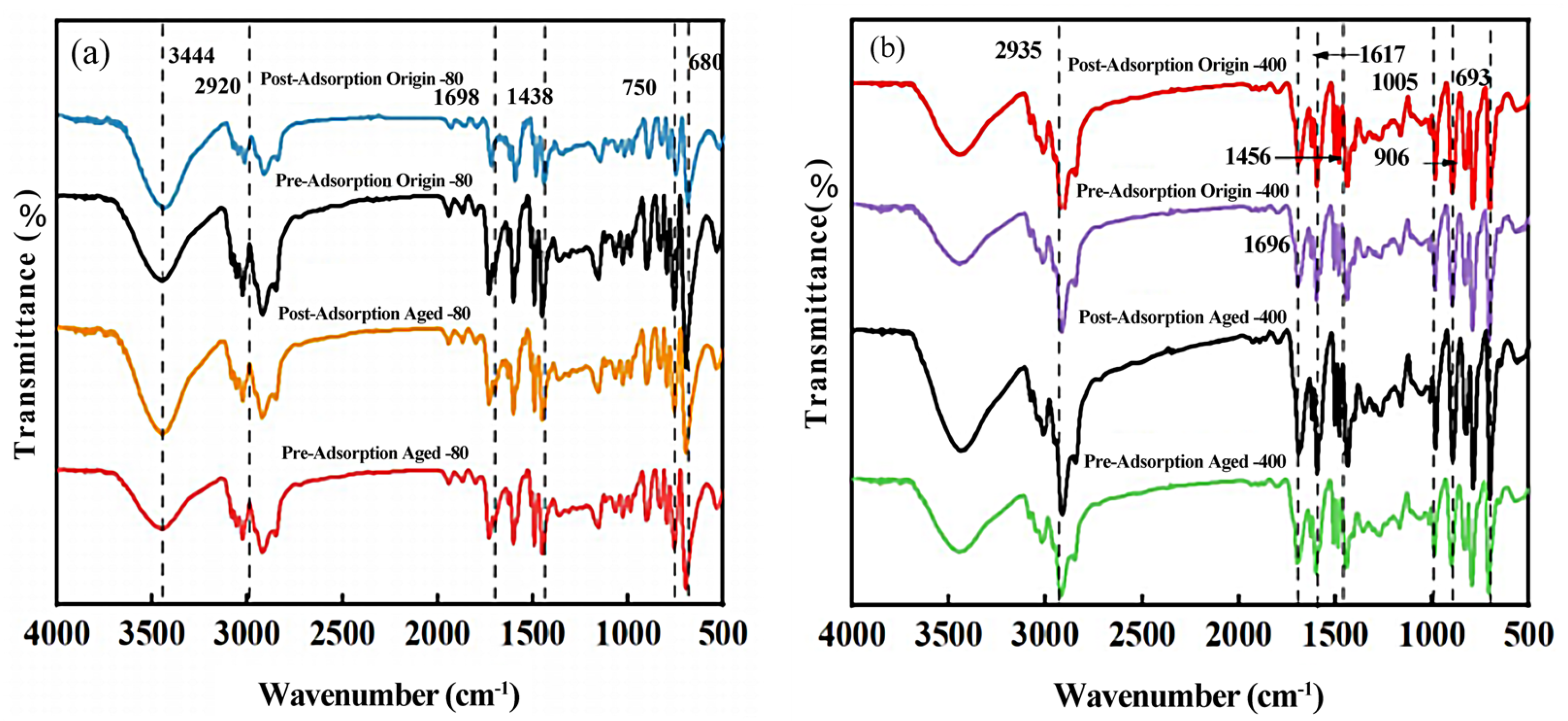
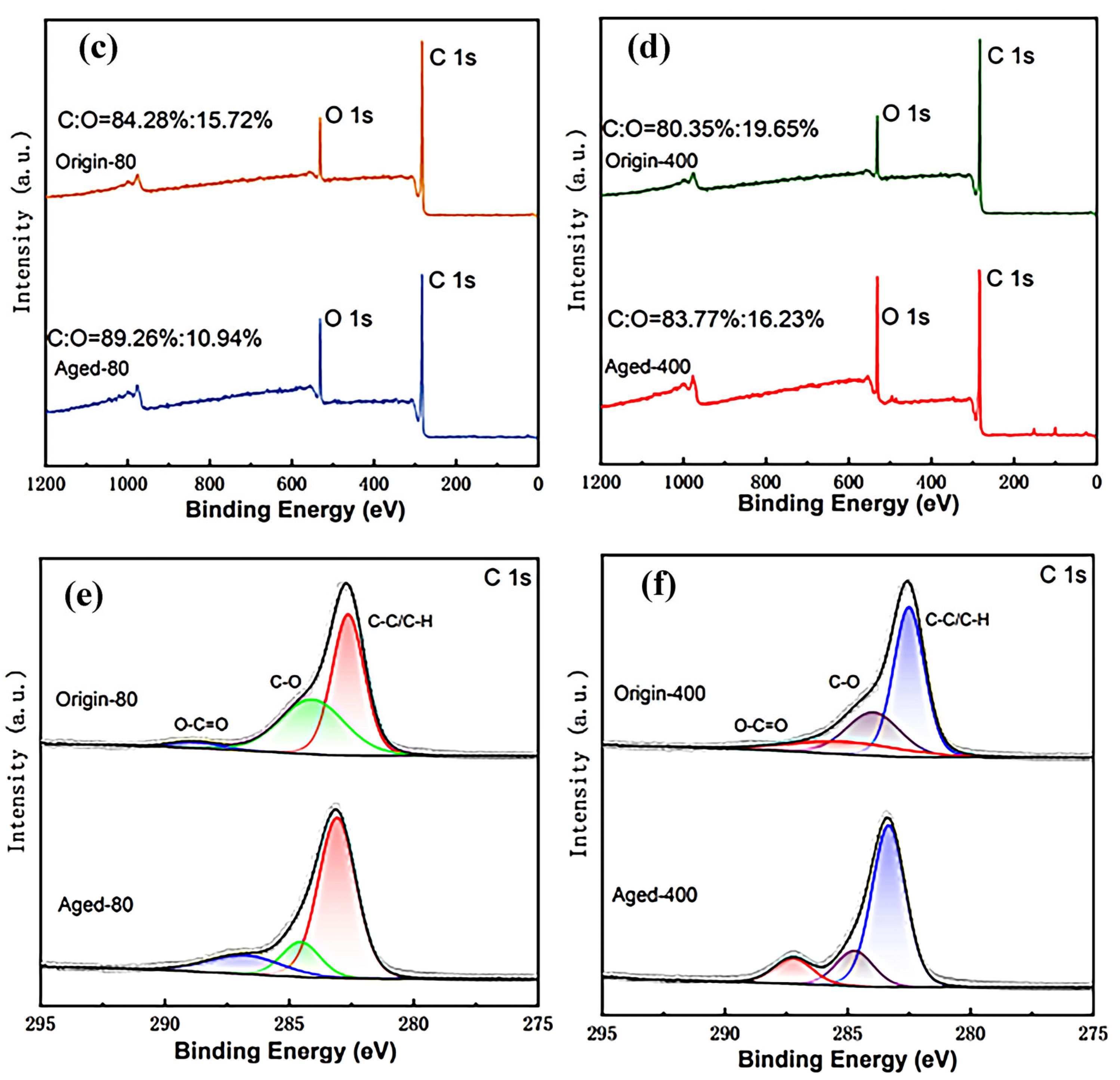
3.2. Changes in Chemical Properties of Pristine and Aged PS MPs
3.3. Adsorption Behavior
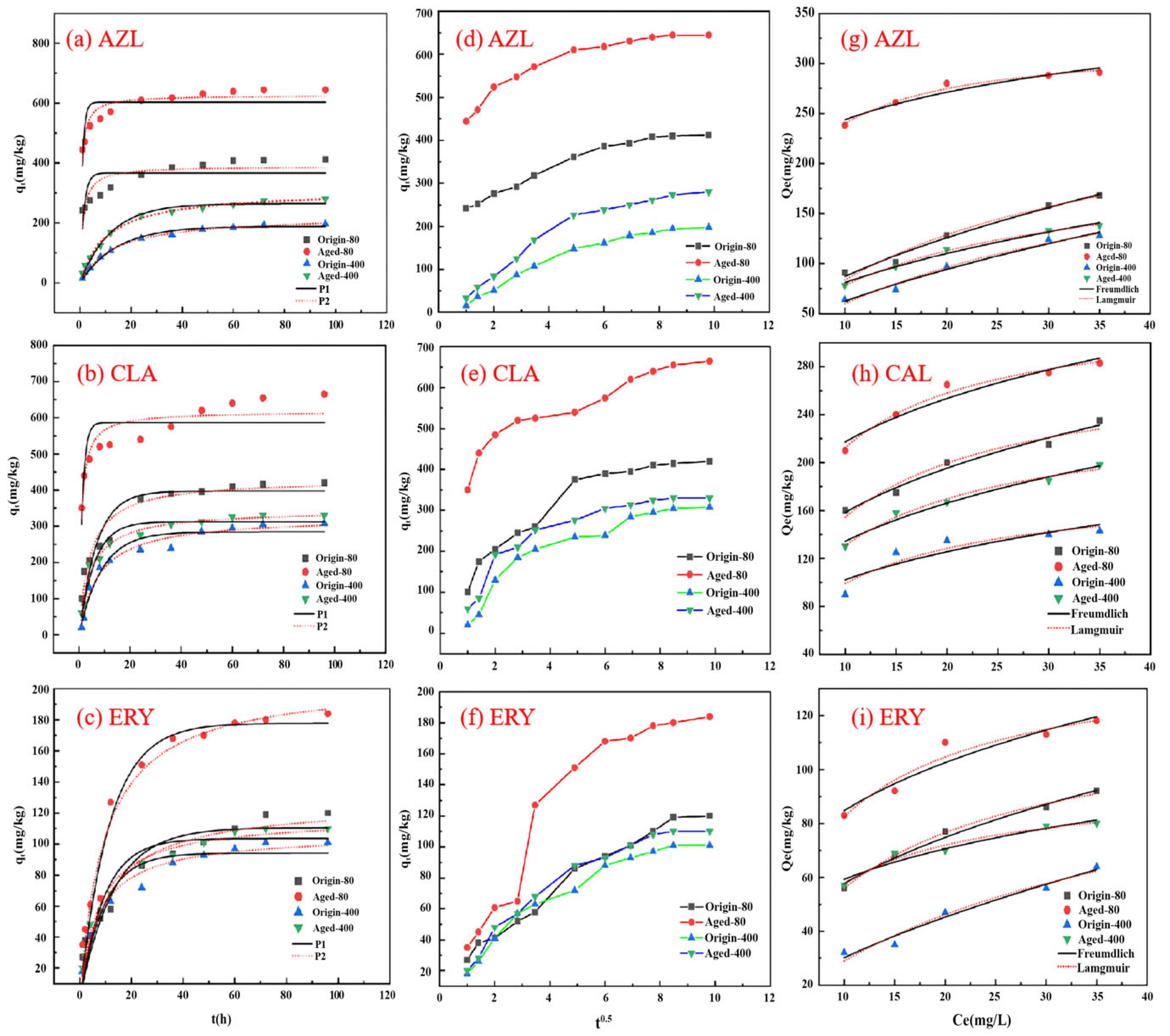
3.3.1. Adsorption Kinetics
3.3.2. Adsorption Isotherm Behavior
3.4. The Effects of Environmental Factors
3.4.1. Ionic Strength
3.4.2. pH
3.4.3. HA
3.5. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A.S. Metals and Marine Microplastics: Adsorption from the Environment versus Addition during Manufacture, Exemplified with Lead. Water Res. 2020, 173, 115577. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced Desorption of Persistent Organic Pollutants from Microplastics under Simulated Physiological Conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Liang, Y.; Kim, M.; Byun, J.; Choi, H. Microplastics with Adsorbed Contaminants: Mechanisms and Treatment. Environ. Chall. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, L.; Fan, W.; Yang, C.; Li, E.; Du, Y.; Wang, X. Inconsistent Seasonal Variation of Antibiotics between Surface Water and Groundwater in the Jianghan Plain: Risks and Linkage to Land Uses. J. Environ. Sci. 2021, 109, 102–113. [Google Scholar] [CrossRef]
- Jing, C.; Yunhua, L.; Feng, Y.; Linfang, Y.; Lixin, C.; Bao, H.; Wang, Z.; Yu, Z.; Li, J. Potential Risk of Antibiotic Contamination to Soil and Crops from Livestock and Poultry Manure and Its Countermeasures. Anim. Husb. Feed Sci. 2020, 41, 50. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Feng, L.; Zhang, J.; Li, Y.; Lu, T.; Qian, H. Adverse Effects of Levofloxacin and Oxytetracycline on Aquatic Microbial Communities. Sci. Total Environ. 2020, 734, 139499. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, L.; Qin, S.; Yang, B.; Chen, J.; Jiang, Z.; Feng, Y. The Correlation between Quinolone Antibiotics and Microbial Community Structure and Diversity in Baiyangdian Lake. Acta Sci. Circumstantiae 2020, 40, 574–584. [Google Scholar]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Driscoll, S.K. Microplastics as Vectors for Bioaccumulation of Hydrophobic Organic Chemicals in the Marine Environment: A State-of-the-Science Review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wang, S.; Huang, T.; Li, M.; Zhu, Z.; Liu, G. Sorption of Fluoroquinolones to Nanoplastics as Affected by Surface Functionalization and Solution Chemistry. Environ. Pollut. 2020, 262, 114347. [Google Scholar] [CrossRef]
- Jie, Y.; Long, C.; Jiangli, Y.; Dongmei, Z. Effects of Different Soil Environmental Factors on Tetracycline Adsorption of Microplastics. J. Agro-Environ. Sci. 2019, 38, 2503–2510. [Google Scholar] [CrossRef]
- Shen, X.-C.; Li, D.-C.; Sima, X.-F.; Cheng, H.-Y.; Jiang, H. The Effects of Environmental Conditions on the Enrichment of Antibiotics on Microplastics in Simulated Natural Water Column. Environ. Res. 2018, 166, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Zhao, X.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Duan, M.; Wu, F. Polystyrene Nanoplastics Toxicity to Zebrafish: Dysregulation of the Brain–Intestine–Microbiota Axis. ACS Nano 2022, 16, 8190–8204. [Google Scholar] [CrossRef]
- Wu, P.; Kang, G.; Li, X.; Lu, L.; Zhou, H.; Zhang, S.; Bu, Y.; Zhang, H. Revealing the Adsorption Kinetics of Microplastics towards Hydrophobic Antibiotic: New Insights into the Microplastics Aging Behavior and Aquatic Environmental Factors. J. Environ. Chem. Eng. 2025, 13, 115444. [Google Scholar] [CrossRef]
- Gao, L.; Fu, D.; Zhao, J.; Wu, W.; Wang, Z.; Su, Y.; Peng, L. Microplastics Aged in Various Environmental Media Exhibited Strong Sorption to Heavy Metals in Seawater. Mar. Pollut. Bull. 2021, 169, 112480. [Google Scholar] [CrossRef]
- De Castro, A.A.; Assis, L.C.; Da Cunha, E.F.F.; Ramalho, T.C.; La Porta, F.A. New in Silico Insights into the Application of (Hydroxy)Chloroquine with Macrolide Antibiotic Co-Crystals against the SARS-CoV-2 Virus. COVID 2022, 2, 230–243. [Google Scholar] [CrossRef]
- Xu, P.; Guo, J.; Ma, D.; Guo, W.; Zhang, Z.; Chen, C. Sorption of Polybrominated Diphenyl Ethers by Virgin and Aged Microplastics. Environ. Sci. 2020, 41, 1329–1337. [Google Scholar]
- Zhang, H.; Xu, X.; Tang, X.; Kong, F. Investigation of the Adsorption of Norfloxacin by Biodegradable and Non-Biodegradable Microplastics Aged by Chemical Oxidation. J. Polym. Environ. 2023, 31, 3894–3906. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; Su, F.; He, X.; Elumalai, V. Adsorption Behavior of Aged Polybutylene Terephthalate Microplastics Coexisting with Cd(II)-Tetracycline. Chemosphere 2022, 301, 134789. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, P.; Qu, G.; Jing, J.; Zhang, T.; Shi, H.; Zhao, Y. Insight into the Characteristics and Sorption Behaviors of Aged Polystyrene Microplastics through Three Type of Accelerated Oxidation Processes. J. Hazard. Mater. 2021, 407, 124836. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Li, H.; Chen, Z.; Liu, X. Preparation and Characterization of Stearic Acid/Expanded Graphite Composites as Thermal Energy Storage Materials. Energy 2010, 35, 4622–4626. [Google Scholar] [CrossRef]
- Marchessault, R.H. Application of Infra-Red Spectroscopy to Cellulose and Wood Polysaccharides. Pure Appl. Chem. 1962, 5, 107–130. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, S.; Zhang, X.; Iqbal, M.S.; Yang, Z.; Xi, Y.; Xiang, X. Accelerated Aging Behavior of Degradable and Non-Degradable Microplastics via Advanced Oxidation and Their Adsorption Characteristics towards Tetracycline. Ecotoxicol. Environ. Saf. 2024, 284, 116864. [Google Scholar] [CrossRef]
- Lin, W.; Wang, S.; Zhang, X.; Hou, Y.; He, W.; Zhang, S.; Zhang, J. Effect of Aging on Adsorption of Tetracycline by Microplastics and the Mechanisms. Environ. Sci. 2022, 43, 4511–4521. [Google Scholar]
- Belmonte, G.K.; Charles, G.; Strumia, M.C.; Weibel, D.E. Permanent Hydrophilic Modification of Polypropylene and Poly(Vinyl Alcohol) Films by Vacuum Ultraviolet Radiation. Appl. Surf. Sci. 2016, 382, 93–100. [Google Scholar] [CrossRef]
- Huang, W.; Deng, J.; Liang, J.; Xia, X. Comparison of Lead Adsorption on the Aged Conventional Microplastics, Biodegradable Microplastics and Environmentally-Relevant Tire Wear Particles. Chem. Eng. J. 2023, 460, 141838. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption Properties of Tylosin on Four Different Microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef]
- Kaina, Z.; Li, J.; Li, X.; Zhang, H. Mechanisms and Kinetics of Oxytetracycline Adsorption-Desorption onto Microplastics. Environ. Chem. 2017, 36, 2531–2540. [Google Scholar]
- Xiong, Y.; Zhao, J.; Li, L.; Wang, Y.; Dai, X.; Yu, F.; Ma, J. Interfacial Interaction between Micro/Nanoplastics and Typical PPCPs and Nanoplastics Removal via Electrosorption from an Aqueous Solution. Water Res. 2020, 184, 116100. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, Y.; Liu, J.; Li, Y.; Li, Q.; He, J. Adsorption and Desorption Behaviors of Antibiotics on TWP and PVC Particles Before and After Aging. Environ. Sci. 2021, 42, 1901–1912. [Google Scholar]
- Costigan, E.; Collins, A.; Hatinoglu, M.D.; Bhagat, K.; MacRae, J.; Perreault, F.; Apul, O. Adsorption of Organic Pollutants by Microplastics: Overview of a Dissonant Literature. J. Hazard. Mater. Adv. 2022, 6, 100091. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics Play a Minor Role in Tetracycline Sorption in the Presence of Dissolved Organic Matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Mejías, C.; Martín, J.; Martín-Pozo, L.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of Macrolide Antibiotics and a Metabolite onto Polyethylene Terephthalate and Polyethylene Microplastics in Aquatic Environments. Antibiotics 2024, 13, 408. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Kothawala, D.N.; Dittmar, T.; Tranvik, L.J. Persistence of Dissolved Organic Matter in Lakes Related to Its Molecular Characteristics. Nat. Geosci. 2015, 8, 454–457. [Google Scholar] [CrossRef]
- Guo, C.; Wang, L.; Lang, D.; Qian, Q.; Wang, W.; Wu, R.; Wang, J. UV and Chemical Aging Alter the Adsorption Behavior of Microplastics for Tetracycline. Environ. Pollut. 2023, 318, 120859. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Li, J.; Hao, M.; Fan, Z.; Sun, L.; Jiang, L.; Lu, C. Adsorption Behavior and Mechanism of Tetracycline on Microplastics Subjected to Peroxymonosulfate and Alkaline Aging. Sep. Purif. Technol. 2025, 354, 128956. [Google Scholar] [CrossRef]

| Models | Antibiotic | AZI | CLA | ERY | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS-MPs | Original-80 | Aged-80 | Original-400 | Aged-400 | Original-80 | Aged-80 | Original-400 | Aged-400 | Original-80 | Aged-80 | Original-400 | Aged-400 | |
| Pseudo-first-order | K1 (min−1) | 0.675 | 1.046 | 0.069 | 0.083 | 0.144 | 0.734 | 0.112 | 0.166 | 0.076 | 0.087 | 0.108 | 0.107 |
| qe1 (mg/kg) | 366.87 | 602.9 | 188.81 | 264.78 | 397.34 | 586.28 | 284.39 | 312.58 | 110.59 | 177.88 | 94.204 | 103.643 | |
| R2 | 0.481 | 0.592 | 0.988 | 0.986 | 0.884 | 0.663 | 0.952 | 0.954 | 0.881 | 0.956 | 0.937 | 0.946 | |
| Pseudo-second-order | K2 × 10−4 | 0.0024 | 0.0029 | 3.444 | 3.105 | 5.488 | 0.0018 | 4.001 | 6.352 | 7.82 | 5.09 | 0.001 | 0.001 |
| qe2 (mg/kg) | 389.66 | 626.19 | 225.03 | 309.48 | 429.57 | 616.78 | 326.43 | 344.79 | 127.01 | 205.49 | 106.58 | 117.11 | |
| R2 | 0.93 | 0.973 | 0.998 | 0.995 | 0.971 | 0.966 | 0.993 | 0.992 | 0.97 | 0.972 | 0.989 | 0.983 | |
| Weber–Morris | K1 (min−1) | 28.5 | 58.47 | 38.05 | 49.3 | 73.02 | 86.37 | 11.4 | 88.94 | 12.584 | 16.69 | 21.09 | 21.67 |
| C1 | 213.64 | 390.86 | / | / | 49.03 | 292.35 | / | / | 16.71 | 21.26 | / | 0.052 | |
| R12 | 0.973 | 0.946 | 0.989 | 0.996 | 0.892 | 0.867 | 0.971 | 0.878 | 0.942 | 0.895 | 0.996 | 0.948 | |
| K2 (min−1) | 22.22 | 23.73 | 20.2 | 23.156 | 26.98 | 28.98 | 18.53 | 20.79 | 19.68 | 84.87 | 30.1 | 38.47 | |
| C2 | 246.21 | 518.42 | 41.199 | 97.24 | 150.7 | 421.35 | 130.28 | 187.83 | 12.22 | 12.98 | 9.18 | 9.2 | |
| R22 | 0.977 | 0.966 | 0.984 | 0.95 | 0.802 | 0.908 | 0.881 | 0.977 | 0.934 | 0.945 | 0.967 | 0.954 | |
| K3 (min−1) | 1.89 | 2.15 | 5.43 | 8.8 | 4.74 | 11.65 | 2.15 | 5.86 | 2.93 | 4.39 | 0.86 | 1.72 | |
| C3 | 393.51 | 624.64 | 144.85 | 194.95 | 373.78 | 552.22 | 251.78 | 309.64 | 78.19 | 155.17 | 84.717 | 101.85 | |
| R32 | 0.974 | 0.601 | 0.817 | 0.906 | 0.974 | 0.926 | 0.801 | 0.601 | 0.688 | 0.999 | 0.601 | 0.601 | |
| Langmuir | Qm (mg/kg) | 278.7 | 322.06 | 244.75 | 202.32 | 281.71 | 328.74 | 182.08 | 241.71 | 121.56 | 142.82 | 116.01 | 95.027 |
| Kl (L/mg) | 0.042 | 0.289 | 0.032 | 0.062 | 0.121 | 0.182 | 0.119 | 0.117 | 0.081 | 0.136 | 0.033 | 0.155 | |
| R2 | 0.973 | 0.981 | 0.978 | 0.998 | 0.955 | 0.981 | 0.9884 | 0.983 | 0.996 | 0.945 | 0.96 | 0.967 | |
| Freundlich | KF (g/(mg·min)) | 25.68 | 170.73 | 15.81 | 29.15 | 79.049 | 130.11 | 51.541 | 66.402 | 24.35 | 45.01 | 7.756 | 33.23 |
| n | 0.532 | 0.154 | 0.598 | 0.444 | 0.302 | 0.223 | 0.298 | 0.307 | 0.376 | 0.275 | 0.592 | 0.252 | |
| R2 | 0.983 | 0.931 | 0.979 | 0.985 | 0.975 | 0.939 | 0.805 | 0.976 | 0.988 | 0.92 | 0.972 | 0.944 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Tan, J.; Sha, H.; Li, K.; Li, X. Adsorption of Macrolide Antibiotics by Aged Microplastics of Different Sizes: Mechanisms and Effects. Nanomaterials 2025, 15, 467. https://doi.org/10.3390/nano15060467
Li Q, Tan J, Sha H, Li K, Li X. Adsorption of Macrolide Antibiotics by Aged Microplastics of Different Sizes: Mechanisms and Effects. Nanomaterials. 2025; 15(6):467. https://doi.org/10.3390/nano15060467
Chicago/Turabian StyleLi, Qi, Jingnan Tan, Haichao Sha, Ke Li, and Xi Li. 2025. "Adsorption of Macrolide Antibiotics by Aged Microplastics of Different Sizes: Mechanisms and Effects" Nanomaterials 15, no. 6: 467. https://doi.org/10.3390/nano15060467
APA StyleLi, Q., Tan, J., Sha, H., Li, K., & Li, X. (2025). Adsorption of Macrolide Antibiotics by Aged Microplastics of Different Sizes: Mechanisms and Effects. Nanomaterials, 15(6), 467. https://doi.org/10.3390/nano15060467





