The Composition of the Dispersion Medium Determines the Antibacterial Properties of Copper (II) Oxide Nanoparticles Against Escherichia coli Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Dispersed Systems of Nanoparticles
- (1)
- CuO-CD in water, in the physiological saline solution, and in LB broth;
- (2)
- CuO-CD in water, in the physiological saline solution, and in LB broth + Triton X-100;
- (3)
- CuO-CD in water, in the physiological saline solution, and in LB broth + SDS;
- (4)
- CuO-EE in water, in the physiological saline solution, and in LB broth;
- (5)
- CuO-EE in water, in the physiological saline solution, and in LB broth + Triton X-100;
- (6)
- CuO-EE in water, in the physiological saline solution, and in LB broth + SDS;
- (7)
- CuO-CS in water, in the physiological saline solution, and in LB broth;
- (8)
- CuO-CS in water, in the physiological saline solution, and in LB broth + Triton X-100;
- (9)
- CuO-CS in water, in the physiological saline solution, and in LB broth + SDS.
2.3. Microbiological Analysis
2.4. Bactericidal Test
2.5. Statistical Analysis
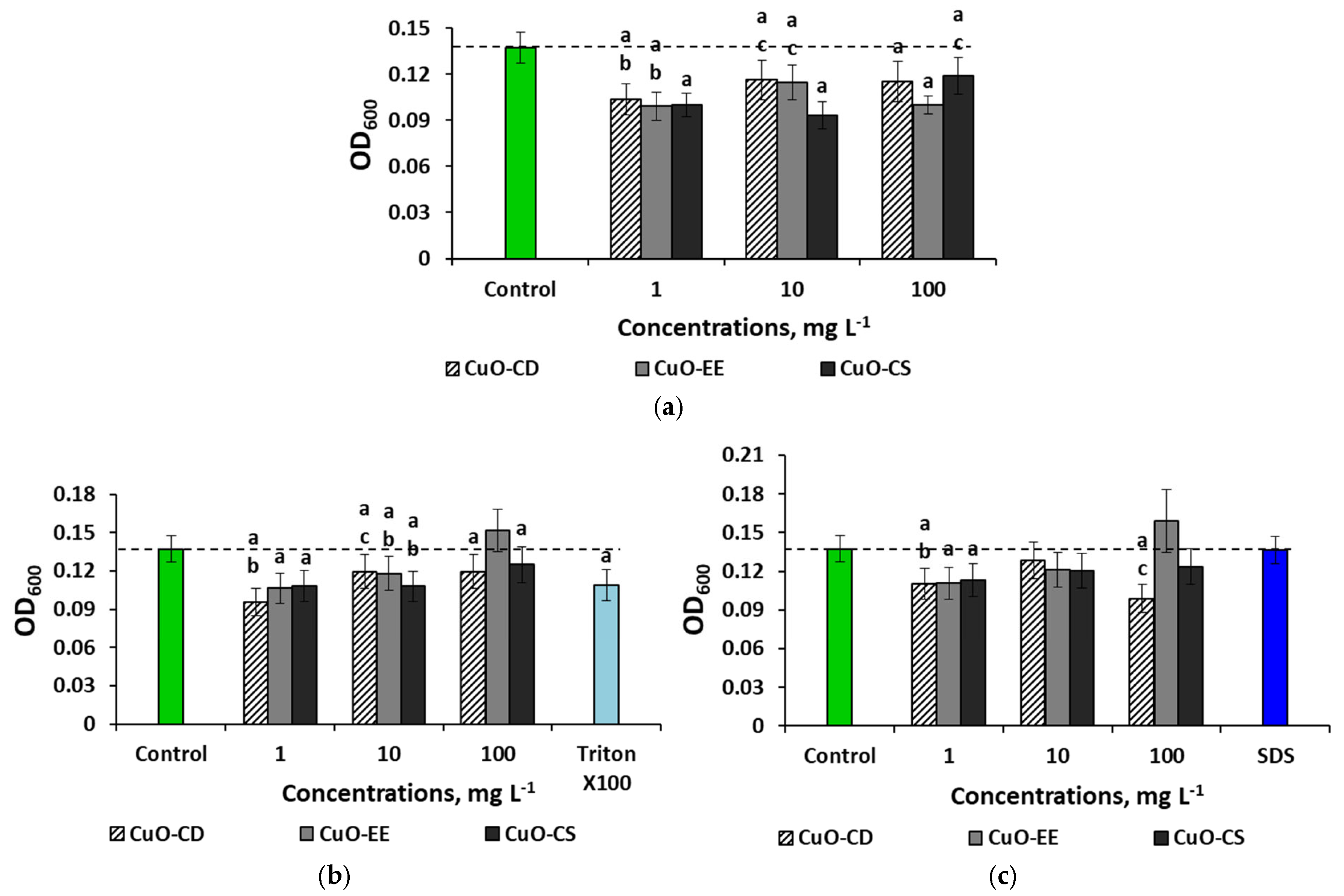
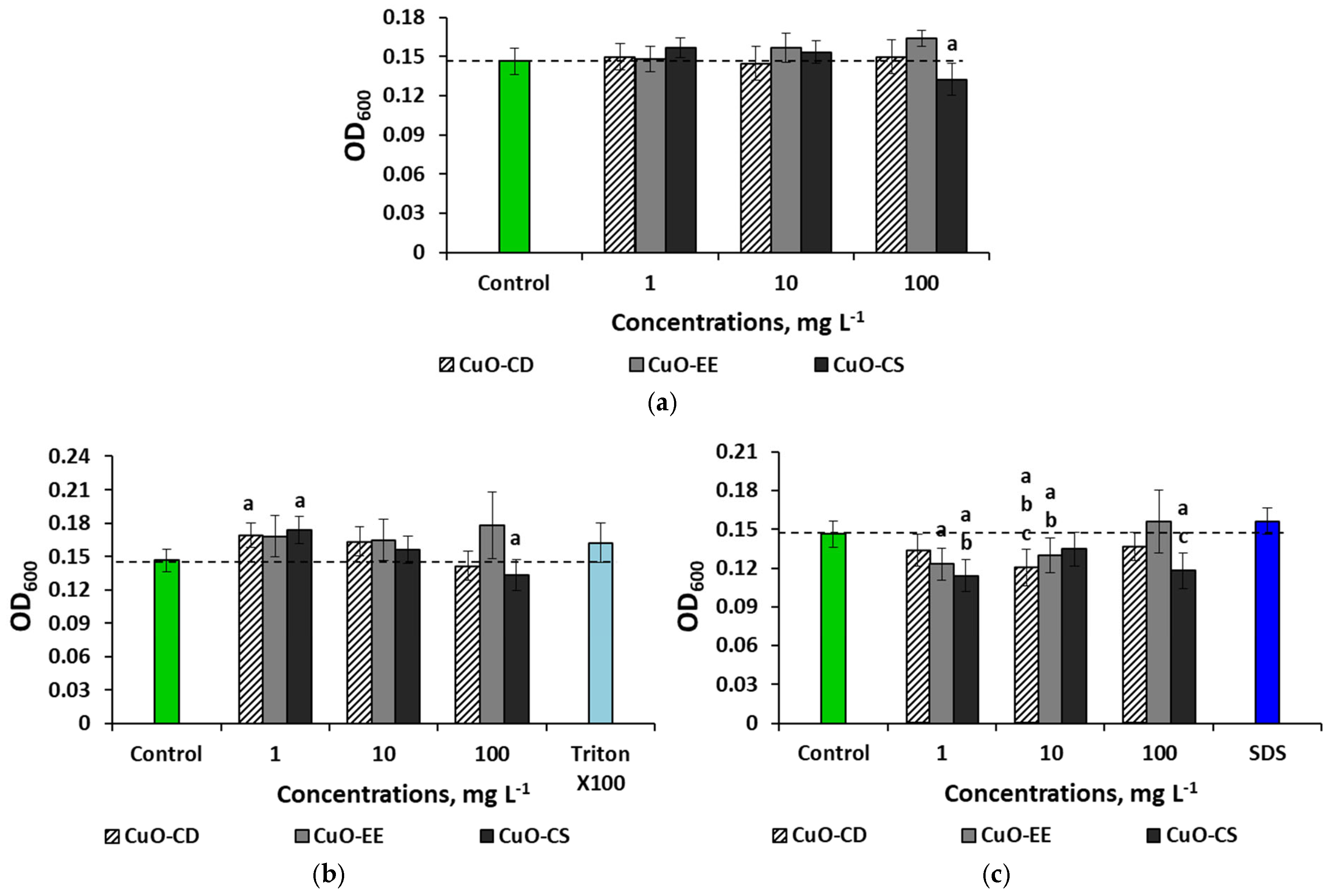
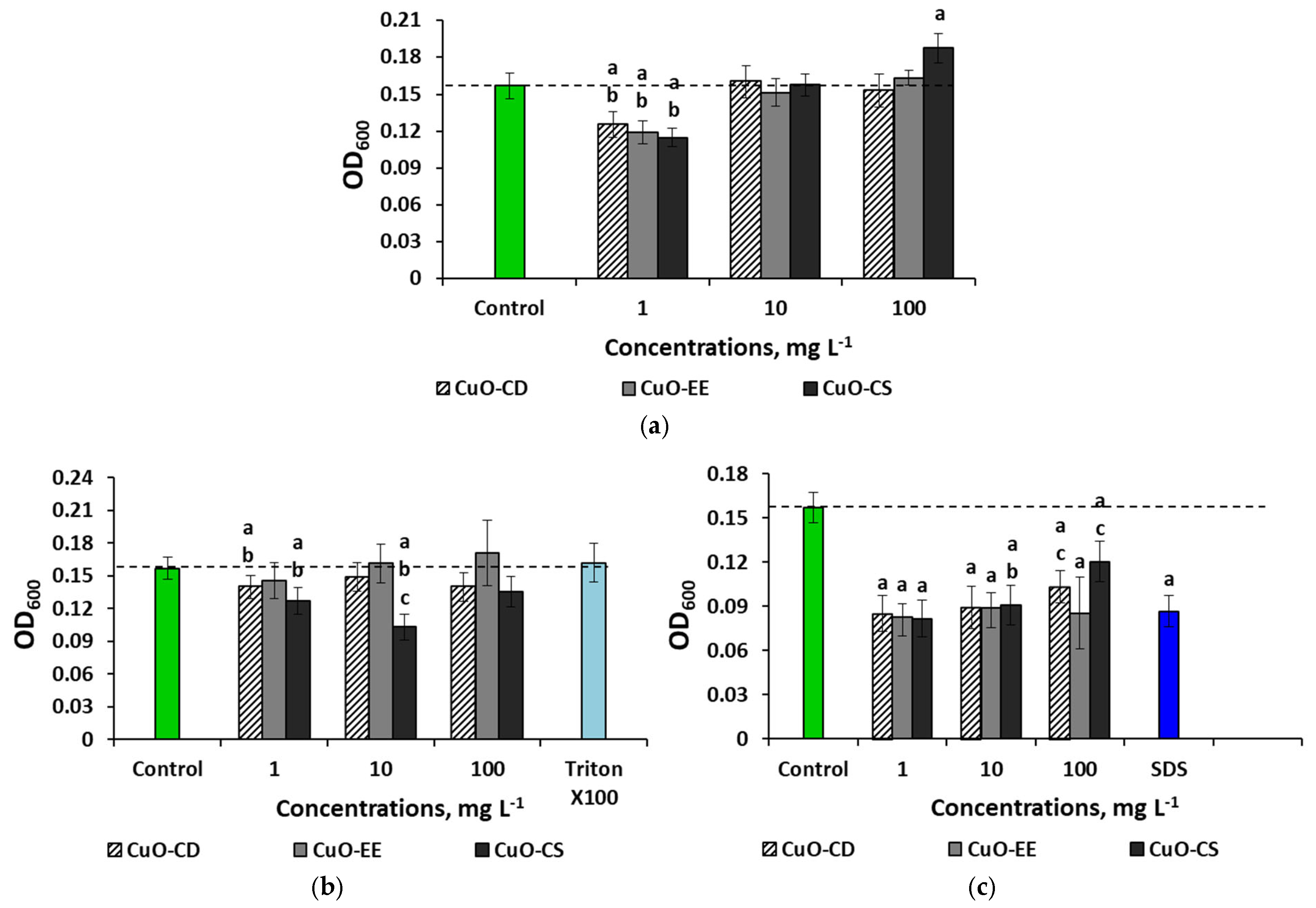
3. Results
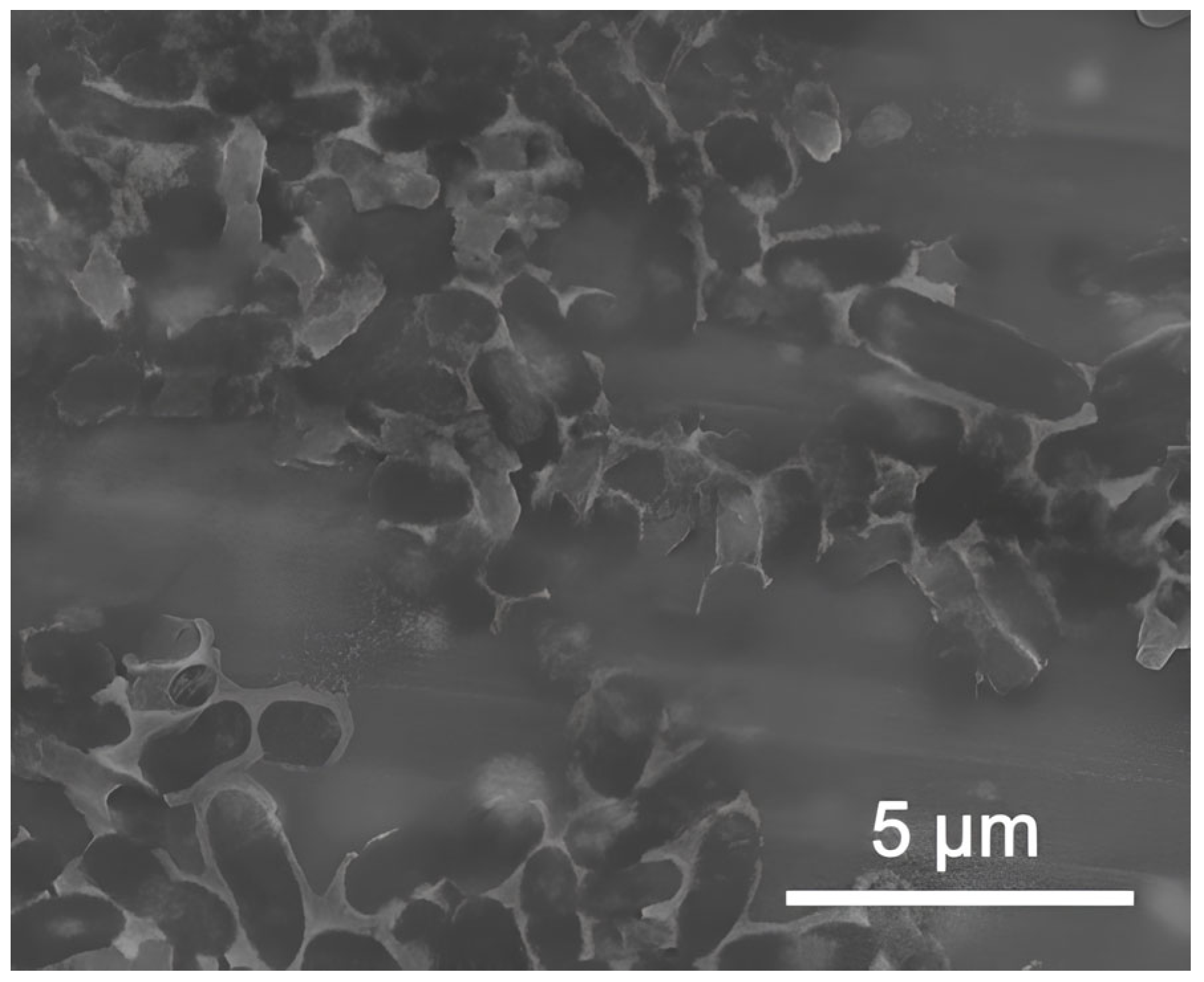
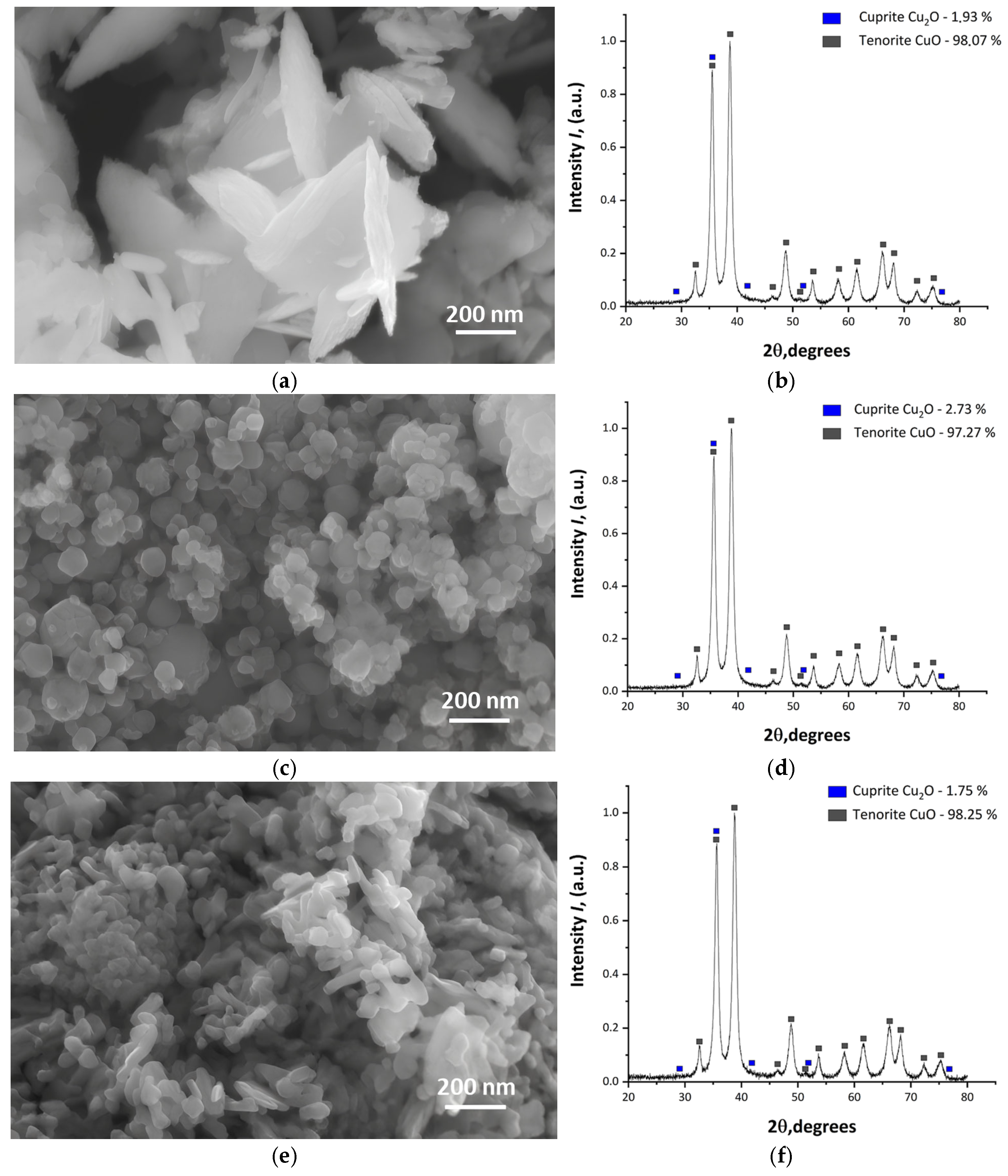
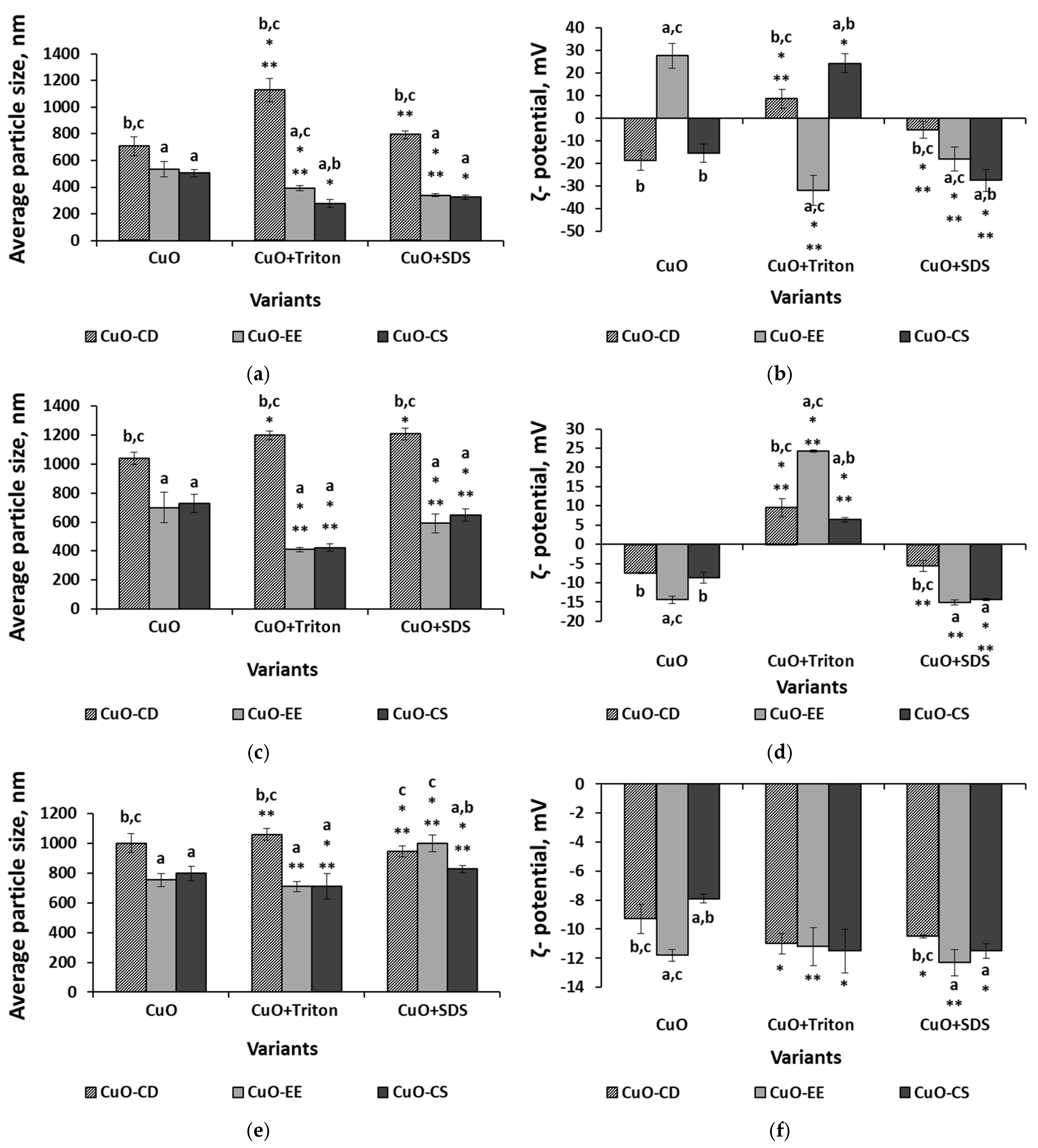
3.1. Analysis of Nanoparticles and Their Dispersions
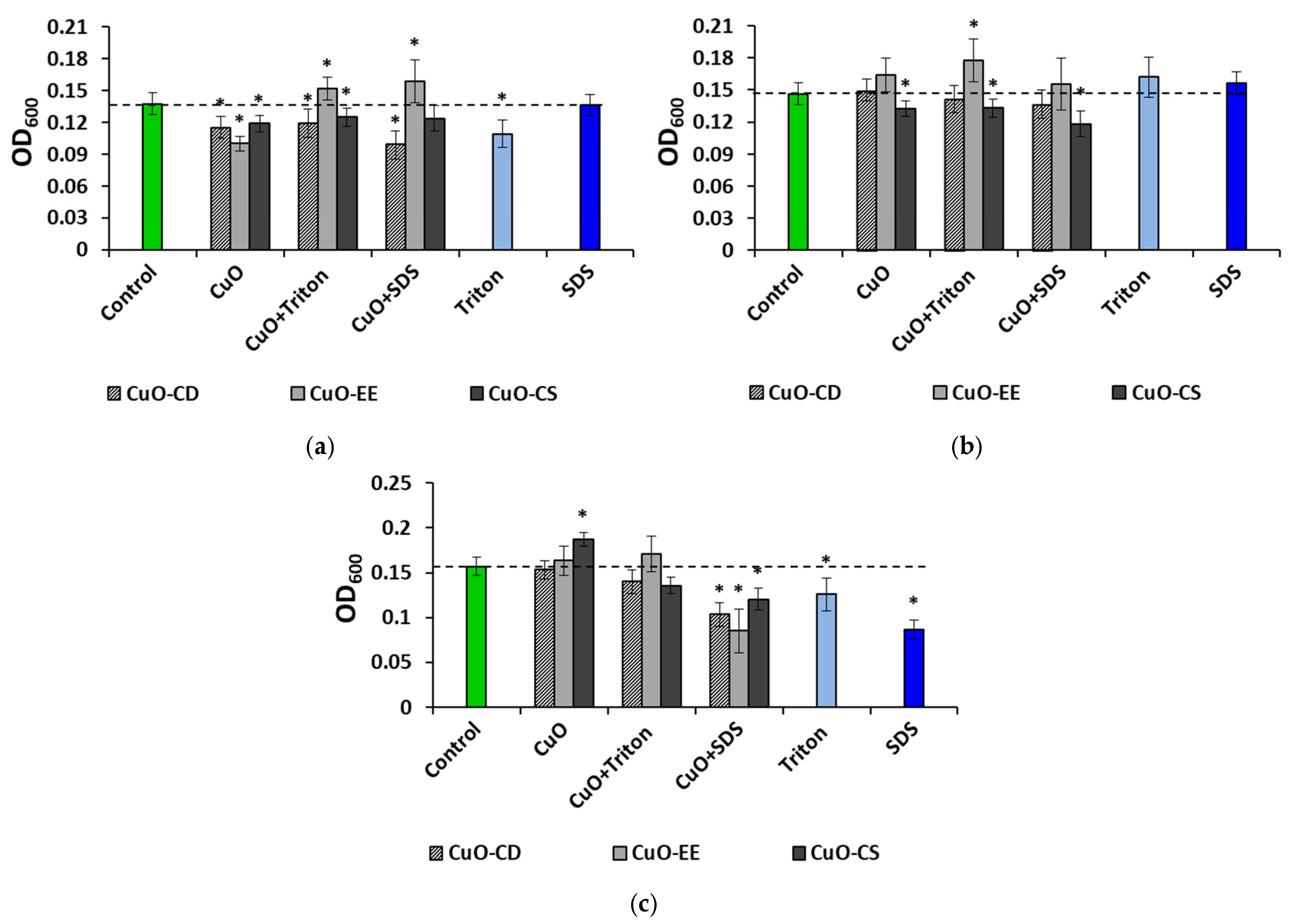
3.2. Effect of Dispersed Systems of CuO NPs at a Concentration of 100 mg L−1 on Bacteria
3.3. Concentration Effects of CuO NPs
4. Discussion
4.1. Dispersion Medium Effects
4.2. Stabilizer Effects
4.3. Sise and Shape Effects of CuO NPs
4.4. Concentration Effects of CuO NPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajulo, S.; Awosile, B. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 January 2025).
- McBryde, E.S.; Bradley, L.C.; Whitby, M.; McElwain, D.L. An investigation of contact transmission of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2004, 58, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Hancharova, M.; Halicka-Stępień, K.; Dupla, A.; Lesiak, A.; Sołoducho, J.; Cabaj, J. Antimicrobial activity of metal-based nanoparticles: A mini-review. BioMetals 2024, 37, 773–801. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Rezić, I.; Meštrović, E. Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review. Coatings 2023, 13, 1830. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Klapiszewska, I.; Parus, A.; Balicki, S.; Kornaus, K.; Gapiński, B.; Wieczorowski, M.; Wilk, K.A.; Jesionowski, T.; Klapiszewski, Ł. Antimicrobial action and chemical and physical properties of CuO-doped engineered cementitious composites. Sci. Rep. 2023, 13, 10404. [Google Scholar] [CrossRef]
- Takele Assefa, E.; Shumi, G.; Mohammed Gendo, K.; Kenasa, G.; Roba, N. Review on green synthesis, characterization, and antibacterial activity of CuO nanoparticles using biomolecules of plant extract. Results Chem. 2024, 8, 101606. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.; Dwivedi, N.; Kumar, P.; Venkatesh, M.; Yadav, A.K.; Mishra, D.; Solanki, P.; Verma, N.K.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Recent advances in copper and copper-derived materials for antimicrobial resistance and infection control. Curr. Opin. Biomed. Eng. 2022, 24, 100408. [Google Scholar] [CrossRef]
- Karampoor, M.R.; Atapour, M.; Bahrami, A. Preparation of an anti-bacterial CuO-containing polyurea-formaldehyde/linseed oil self-healing coating. Prog. Org. Coat. 2023, 184, 107879. [Google Scholar] [CrossRef]
- Culpepper, J.D.; Frutos, A.G.; Yehl, J.B.; Chang, T.; Lahiri, J. Colorless copper-containing coatings with high antimicrobial efficacy and formulation versatility. RSC Appl. Interfaces 2025, 2, 484–495. [Google Scholar] [CrossRef]
- Graham, C.; Mezzadrelli, A.; Senaratne, W.; Pal, S.; Thelen, D.; Hepburn, L.; Mazumder, P.; Pruneri, V. Towards transparent and durable copper-containing antimicrobial surfaces. Commun. Mater. 2024, 5, 39. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Bhat, K.U.; Bhat Panemangalore, D. Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Ali, A.Y.; Alani, A.-A.K.; Ahmed, B.O.; Hamid, L.L. Effect of biosynthesized silver nanoparticle size on antibacterial and anti-biofilm activity against pathogenic multi-drug resistant bacteria. OpenNano 2024, 20, 100213. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Reznickova, A.; Nguyenova, H.Y.; Zaruba, K.; Strasakova, J.; Kolska, Z.; Michalcova, A.; Prusa, F.; Kvitek, O.; Slepicka, P.; Sajdl, P.; et al. Grafting of silver nanospheres and nanoplates onto plasma activated PET: Effect of nanoparticle shape on antibacterial activity. Vacuum 2022, 203, 111268. [Google Scholar] [CrossRef]
- Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Pereira, E.; Teixeira, P.; Poças, F. Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials 2024, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.A.-Z.; Eissa, N.G.; Shen, Y.; Hunstad, D.A.; Wooley, K.L.; Elsabahy, M. Morphologic design of nanostructures for enhanced antimicrobial activity. J. Nanobiotechnol. 2022, 20, 536. [Google Scholar] [CrossRef]
- Su, C.; Huang, K.; Li, H.-H.; Lu, Y.-G.; Zheng, D.-L. Antibacterial Properties of Functionalized Gold Nanoparticles and Their Application in Oral Biology. J. Nanomater. 2020, 2020, 5616379. [Google Scholar] [CrossRef]
- Alameen, A.S.; Undre, S.B.; Undre, P.B. Functionalization of ZnO nanoparticles and their antimicrobial activity: In vitro. Nano-Struct. Nano-Objects 2024, 39, 101314. [Google Scholar] [CrossRef]
- Norouzi, N.; Ong, Y.; Damle, V.G.; Habibi Najafi, M.B.; Schirhagl, R. Effect of medium and aggregation on antibacterial activity of nanodiamonds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110930. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Vasyukova, I.; Muratov, D.S.; Rybkin, I.; Bratashov, D.; Lapanje, A.; Il’inikh, I.; Kolesnikov, E.; Kuznetsov, D. Effect of GO on bacterial cells: Role of the medium type and electrostatic interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 275–281. [Google Scholar] [CrossRef]
- Zakharova, O.; Kolesnikov, E.; Vishnyakova, E.; Strekalova, N.; Gusev, A. Antibacterial activity of ZnO nanoparticles: Dependence on particle size, dispersion media and storage time. IOP Conf. Ser. Earth. Environ. Sci. 2019, 226, 012062. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Muratov, D.S.; Vorobeva, N.S.; Sarker, M.; Rybkin, I.; Bratashov, D.; Kolesnikov, E.; Lapanje, A.; Kuznetsov, D.V.; et al. Medium-Dependent Antibacterial Properties and Bacterial Filtration Ability of Reduced Graphene Oxide. Nanomaterials 2019, 9, 1454. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawi, D.M.; Selim, M.M.; Hamdi, N.; Hassan, S.A.; Ezzat, A. Studies on the influence of the physicochemical characteristics of nanostructured copper, zinc and magnesium oxides on their antibacterial activities. J. Environ. Chem. Eng. 2018, 6, 5608–5615. [Google Scholar] [CrossRef]
- Motshekga, S.C. Structural and antibacterial properties of copper oxide nanoparticles: A study on the effect of calcination temperature. Nano Express 2024, 5, 015011. [Google Scholar] [CrossRef]
- Moniri Javadhesari, S.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110011. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, L.; Zhu, X.; Huang, W.; Wang, J. Size-dependent oxidative stress effect of nano/micro-scaled polystyrene on Karenia mikimotoi. Mar. Pollut. Bull. 2020, 154, 111074. [Google Scholar] [CrossRef]
- Laha, D.; Pramanik, A.; Laskar, A.; Jana, M.; Pramanik, P.; Karmakar, P. Shape-dependent bactericidal activity of copper oxide nanoparticle mediated by DNA and membrane damage. Mater. Res. Bull. 2014, 59, 185–191. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Ahmadi, S. Green synthesis and morphology dependent antibacterial activity of copper oxide nanoparticles. J. Nanostruct. 2019, 9, 163–171. [Google Scholar] [CrossRef]
- Zhu, Q.; Fan, Z.; Zeng, L.; Li, C.; Ye, C.; Zheng, R. Effect of sodium dodecyl sulfate (SDS) on microbial community structure and function in lake–terrestrial ecotones: A simulation experiment. Environ. Technol. Innov. 2024, 34, 103594. [Google Scholar] [CrossRef]
- Sharma, P.; Vaiwala, R.; Parthasarathi, S.; Patil, N.; Verma, A.; Waskar, M.; Raut, J.S.; Basu, J.K.; Ayappa, K.G. Interactions of Surfactants with the Bacterial Cell Wall and Inner Membrane: Revealing the Link between Aggregation and Antimicrobial Activity. Langmuir 2022, 38, 15714–15728. [Google Scholar] [CrossRef]
- He, X.; Jin, S.; Fan, W. Synergistic In Vitro Antimicrobial Activity of Triton X-100 and Metformin against Enterococcus faecalis in Normal and High-Glucose Conditions. Microorganisms 2022, 10, 124. [Google Scholar] [CrossRef]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Luo, Y.; Liu, R.; Fan, W.; Fan, B. Triton X-100 enhanced antibacterial effect of photodynamic therapy against Enterococcus faecalis infection: An in vitro study. Colloids Surf. B Biointerfaces 2024, 240, 113978. [Google Scholar] [CrossRef]
- He, X.; Xv, S.; Liu, R.; Duan, M.; Fan, W.; Fan, B. Triton X-100 counteracts antibiotic resistance of Enterococcus faecalis: An in vitro study. J. Dent. 2024, 146, 105046. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Peng, N.; Cui, A.; Liu, Y.; Peng, X.; Huang, L.; Ed-Dra, A.; He, F.; Li, Y.; Yang, S.; et al. Sodium dodecyl sulfate-coated silver nanoparticles accelerate antimicrobial potentials by targeting amphiphilic membranes. mLife 2024, 3, 551–564. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO Nanoparticle Interaction with Human Epithelial Cells: Cellular Uptake, Location, Export, and Genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface Characteristics, Copper Release, and Toxicity of Nano- and Micrometer-Sized Copper and Copper(II) Oxide Particles: A Cross-Disciplinary Study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Godymchuk, A.Y.; Gusev, A.A.; Gulchenko, S.I.; Vasyukova, I.A.; Kuznetsov, D.V. Considerable Variation of Antibacterial Activity of Cu Nanoparticles Suspensions Depending on the Storage Time, Dispersive Medium, and Particle Sizes. BioMed Res. Int. 2015, 2015, 412530. [Google Scholar] [CrossRef]
- Godymchuk, A.; Frolov, G.; Gusev, A.; Zakharova, O.; Yunda, E.; Kuznetsov, D.; Kolesnikov, E. Antibacterial Properties of Copper Nanoparticle Dispersions: Influence of Synthesis Conditions and Physicochemical Characteristics. IOP Conf. Ser. Earth Environ. Sci. 2015, 98, 012033. [Google Scholar] [CrossRef]
- Tuttle, A.R.; Trahan, N.D.; Son, M.S. Growth and Maintenance of Escherichia coli Laboratory Strains. Curr. Protoc. 2021, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Modanlou, N.; Hosseini, S.; Abbasi, M. Synthesis and Characterization of CuO Nanoparticles by the Chemical Liquid Deposition Method and Investigation of its Catalytic Effect on the Thermal Decomposition of Ammonium Perchlorate. Cent. Eur. J. Energ. Mater. 2017, 14, 152–168. [Google Scholar] [CrossRef]
- Kayser, A.; Weber, J.; Hecht, V.; Rinas, U. Metabolic Flux Analysis of Escherichia coli in Glucose-Limited Continuous Culture. I. Growth-Rate-Dependent Metabolic Efficiency at Steady State. Microbiology 2005, 151, 693–706. [Google Scholar] [CrossRef]
- Efremova, L.V.; Vasilchenko, A.S.; Rakov, E.G.; Deryabin, D.G. Toxicity of Graphene Shells, Graphene Oxide, and Graphene Oxide Paper Evaluated with Escherichia coli Biotests. BioMed Res. Int. 2015, 2015, 869361. [Google Scholar] [CrossRef]
- Puente, J.L.; Finlay, B.B. Chapter 9-Pathogenic Escherichia coli. In Principles of Bacterial Pathogenesis; Groisman, E.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 387–456. [Google Scholar]
- Zakharova, O.V.; Gusev, A.A.; Abourahma, J.; Vorobeva, N.S.; Sokolov, D.V.; Muratov, D.S.; Kuznetsov, D.V.; Sinitskii, A. Nanotoxicity of ZrS3 Probed in a Bioluminescence Test on E. coli Bacteria: The Effect of Evolving H2S. Nanomaterials 2020, 10, 1401. [Google Scholar] [CrossRef]
- Lebedev, S.V.; Gavrish, I.A.; Galaktionova, L.V.; Korotkova, A.M.; Sizova, E.A. Assessment of the toxicity of silicon nanooxide in relation to various components of the agroecosystem under the conditions of the model experiment. Environ. Geochem. Health 2019, 41, 769–782. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Belova, V.V.; Baranchikov, P.A.; Kostyakova, A.A.; Muratov, D.S.; Grigoriev, G.V.; Chebotaryova, S.P.; Kuznetsov, D.V.; Gusev, A.A. The Conditions Matter: The Toxicity of Titanium Trisulfide Nanoribbons to Bacteria E. coli Changes Dramatically Depending on the Chemical Environment and the Storage Time. Int. J. Mol. Sci. 2023, 24, 8299. [Google Scholar] [CrossRef]
- Yudina, N.Y.; Kozlova, T.N.; Bogachikhin, D.A.; Kosarenina, M.M.; Arlyapov, V.A.; Alferov, S.V. Electrochemical Biosensors for Express Analysis of the Integral Toxicity of Polymer Materials. Biosensors 2023, 13, 1011. [Google Scholar] [CrossRef]
- Shoshin, D.E.; Sizova, E.A.; Kamirova, A.M. Morphological changes and luminescence of Escherichia coli in contact with Mn2O3 and Co3O4 ultrafine particles as components of a mineral feed additive. Vet. World 2024, 17, 1880–1888. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Aleshina, E.S.; Efremova, L.V. Application of the Inhibition of Bacterial Bioluminescence Test for Assessment of Toxicity of Carbon-Based Nanomaterials. Microbiology 2012, 81, 492–497. [Google Scholar] [CrossRef]
- Sirichai, S.; Gorret, N.; Delvigne, F.; Uribelarrea, J.-L.; Molina-Jouve, C. Real-time monitoring of metabolic shift and transcriptional induction of YciG::LuxCDABE E. coli reporter strain to a glucose pulse of different concentrations. J. Biotechnol. 2012, 157, 379–390. [Google Scholar] [CrossRef]
- Bazhenov, S.V.; Novoyatlova, U.S.; Scheglova, E.S.; Prazdnova, E.V.; Mazanko, M.S.; Kessenikh, A.G.; Kononchuk, O.V.; Gnuchikh, E.Y.; Liu, Y.; Al Ebrahim, R.; et al. Bacterial Lux-Biosensors: Constructing, Applications, and Prospects. Biosens. Bioelectron. 2023, 13, 100323. [Google Scholar] [CrossRef]
- Vlasenko, L.V.; Nechitailo, K.S. Bacterial luminescent biosensors in the system for assessing the mechanisms of antibacterial activity of carbon-based nanomaterials. IOP Conf. Ser. Earth Environ. Sci. 2022, 979, 012056. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Galhano, J.; Duarte, M.P.; Capelo-Martínez, J.L.; Lodeiro, C.; Oliveira, E. Validation of a Standard Luminescence Method for the Fast Determination of the Antimicrobial Activity of Nanoparticles in Escherichia coli. Nanomaterials 2022, 12, 2164. [Google Scholar] [CrossRef]
- Naqvi, Q.U.; Kanwal, A.; Qaseem, S.; Naeem, M.; Ali, S.R.; Shaffique, M.; Maqbool, M. Size-dependent inhibition of bacterial growth by chemically engineered spherical ZnO nanoparticles. J. Biol. Phys. 2019, 45, 147–159. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Wang, Z.; Huang, X.; Li, J.; Yao, C.; Zhou, N.; Shen, J. Insight into the effect of particle size distribution differences on the antibacterial activity of carbon dots. J. Colloid Interface Sci. 2021, 584, 505–519. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Pamies, R.; Cifre, J.G.H.; Espín, V.F.; Collado-González, M.; Baños, F.G.D.; de la Torre, J.G. Aggregation behaviour of gold nanoparticles in saline aqueous media. J. Nanopart. Res. 2014, 16, 2376. [Google Scholar] [CrossRef]
- Liu, J.; Legros, S.; Ma, G.; Veinot, J.G.C.; von der Kammer, F.; Hofmann, T. Influence of surface functionalization and particle size on the aggregation kinetics of engineered nanoparticles. Chemosphere 2012, 87, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.; Coglitore, D.; Curran, J.M.; Gilliland, D.; Macko, P.; Whelan, M.; Worth, A.; Patterson, E.A. The influence of inter-particle forces on diffusion at the nanoscale. Sci. Rep. 2019, 9, 12689. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Prodan, G.; Matei, C.; Moscalu, F.; Diacon, A. The influence of Triton X-100 surfactant on the morphology and properties of zinc sulfide nanoparticles for applications in azo dyes degradation. Mater. Chem. Phys. 2017, 193, 316–328. [Google Scholar] [CrossRef]
- Chadha, R.; Sharma, R.; Maiti, N.; Ballal, A.; Kapoor, S. Effect of SDS concentration on colloidal suspensions of Ag and Au nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 664–670. [Google Scholar] [CrossRef]
- Li, X.; Yoneda, M.; Shimada, Y.; Matsui, Y. Effect of surfactants on the aggregation and stability of TiO2 nanomaterial in environmental aqueous matrices. Sci. Total Environ. 2017, 574, 176–182. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef]
- He, Q. Investigation of Stabilization Mechanisms for Colloidal Suspension Using Nanoparticles. Ph.D. Dissertation, University of Louisville, Louisville, KY, USA, 2014. [Google Scholar]
- Baek, W.; Chang, H.; Bootharaju, M.S.; Kim, J.H.; Park, S.; Hyeon, T. Recent Advances and Prospects in Colloidal Nanomaterials. JACS Au 2021, 1, 1849–1859. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Corti, D.S.; Franses, E.I. Effect of Triton X-100 on the stability of titania nanoparticles against agglomeration and sedimentation: A masked depletion interaction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 296–304. [Google Scholar] [CrossRef]
- Mao, Y.; Cates, M.E.; Lekkerkerker, H.N.W. Depletion force in colloidal systems. Phys. A Stat. Mech. Appl. 1995, 222, 10–24. [Google Scholar] [CrossRef]
- Lekkerkerker, H.; Tuinier, R.; Vis, M. Colloids and the Depletion Interaction, 2nd ed.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Vihodceva, S.; Šutka, A.; Sihtmäe, M.; Rosenberg, M.; Otsus, M.; Kurvet, I.; Smits, K.; Bikse, L.; Kahru, A.; Kasemets, K. Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri. Nanomaterials 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Saed, M.; Ayivi, R.D.; Wei, J.; Obare, S.O. Gold nanoparticles antibacterial activity: Does the surface matter? Colloid Interface Sci. Commun. 2024, 62, 100804. [Google Scholar] [CrossRef]
- Kundu, S. Gold Nanoparticles: Their Application as Antimicrobial Agents and Vehicles of Gene Delivery. Adv. Biotechnol. Microbiol. 2017, 4, 555658. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Ruan, J.; Zhuang, X. Using Positively Charged Magnetic Nanoparticles to Capture Bacteria at Ultralow Concentration. Nanoscale Res. Lett. 2019, 14, 195. [Google Scholar] [CrossRef]
- Garay-Jimenez, J.C.; Young, A.; Gergeres, D.; Greenhalgh, K.; Turos, E. Methods for purifying and detoxifying sodium dodecyl sulfate–stabilized polyacrylate nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 98–105. [Google Scholar] [CrossRef]
- Bae, E.; Park, H.-J.; Park, J.; Yoon, J.; Kim, Y.; Choi, K.; Yi, J. Effect of Chemical Stabilizers in Silver Nanoparticle Suspensions on Nanotoxicity. Bull. Korean Chem. Soc. 2011, 32, 613–619. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. The devil is in the details (or the surface): Impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J. Environ. Monit. 2011, 13, 1135–1144. [Google Scholar] [CrossRef]
- Bell, I.R.; Ives, J.A.; Jonas, W.B. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose-Response 2014, 12, 202–232. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 715, 136994. [Google Scholar] [CrossRef] [PubMed]
- Ketebo, A.A.; Din, S.U.; Lee, G.; Park, S. Mechanobiological Analysis of Nanoparticle Toxicity. Nanomaterials 2023, 13, 1682. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, O.V.; Gusev, A.A.; Altabaeva, Y.V.; Perova, S.Y. Biological Effects of Freshly Prepared and 24-h Aqueous Dispersions of Copper and Copper Oxide Nanoparticles on E. coli Bacteria. Nanotechnol. Russ. 2018, 13, 173–181. [Google Scholar] [CrossRef]
- Khairy, T.; Amin, D.H.; Salama, H.M.; Elkholy, I.M.A.; Elnakib, M.; Gebreel, H.M.; Sayed, H.A.E. Antibacterial activity of green synthesized copper oxide nanoparticles against multidrug-resistant bacteria. Sci. Rep. 2024, 14, 25020. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Motlhalamme, T.; More, G.K.; Mohale, K.C.; Maaza, M. Antimicrobial, antioxidant, and cytotoxic properties of biosynthesized copper oxide nanoparticles (CuO-NPs) using Athrixia phylicoides DC. Heliyon 2023, 9, e15265. [Google Scholar] [CrossRef]
| CuO NPs, Concentrations, mg L−1 | Stabilizers and Types of Media | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No Stabilizer | Triton X-100 | SDS | |||||||
| Water | Physiological Saline Solution | LB Broth | Water | Physiological Saline Solution | LB Broth | Water | Physiological Saline Solution | LB Broth | |
| CuO-CD | |||||||||
| 1 | − | 0 | − | − | + | − | − | 0 | − |
| 10 | − | 0 | 0 | − | + | 0 | 0 | − | − |
| 100 | − | 0 | 0 | − | + | 0 | − | 0 | − |
| CuO-EE | |||||||||
| 1 | − | 0 | − | − | 0 | 0 | − | − | − |
| 10 | − | 0 | 0 | 0 | 0 | 0 | 0 | − | − |
| 100 | − | 0 | 0 | - | 0 | 0 | 0 | 0 | − |
| CuO-CS | |||||||||
| 1 | − | 0 | − | − | 0 | − | − | − | − |
| 10 | − | 0 | 0 | − | 0 | − | 0 | 0 | − |
| 100 | − | − | + | − | − | 0 | 0 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, O.V.; Gusev, A.A.; Baranchikov, P.A.; Chebotaryova, S.P.; Razlivalova, S.S.; Koiava, E.Y.; Kataranova, A.A.; Grigoriev, G.V.; Strekalova, N.S.; Krutovsky, K.V. The Composition of the Dispersion Medium Determines the Antibacterial Properties of Copper (II) Oxide Nanoparticles Against Escherichia coli Bacteria. Nanomaterials 2025, 15, 469. https://doi.org/10.3390/nano15060469
Zakharova OV, Gusev AA, Baranchikov PA, Chebotaryova SP, Razlivalova SS, Koiava EY, Kataranova AA, Grigoriev GV, Strekalova NS, Krutovsky KV. The Composition of the Dispersion Medium Determines the Antibacterial Properties of Copper (II) Oxide Nanoparticles Against Escherichia coli Bacteria. Nanomaterials. 2025; 15(6):469. https://doi.org/10.3390/nano15060469
Chicago/Turabian StyleZakharova, Olga V., Alexander A. Gusev, Peter A. Baranchikov, Svetlana P. Chebotaryova, Svetlana S. Razlivalova, Elina Y. Koiava, Anna A. Kataranova, Gregory V. Grigoriev, Nataliya S. Strekalova, and Konstantin V. Krutovsky. 2025. "The Composition of the Dispersion Medium Determines the Antibacterial Properties of Copper (II) Oxide Nanoparticles Against Escherichia coli Bacteria" Nanomaterials 15, no. 6: 469. https://doi.org/10.3390/nano15060469
APA StyleZakharova, O. V., Gusev, A. A., Baranchikov, P. A., Chebotaryova, S. P., Razlivalova, S. S., Koiava, E. Y., Kataranova, A. A., Grigoriev, G. V., Strekalova, N. S., & Krutovsky, K. V. (2025). The Composition of the Dispersion Medium Determines the Antibacterial Properties of Copper (II) Oxide Nanoparticles Against Escherichia coli Bacteria. Nanomaterials, 15(6), 469. https://doi.org/10.3390/nano15060469










