Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of WO3-x/TiO2
2.2. SERS Performance
2.3. Photocatalytic Performance
3. Results and Discussion
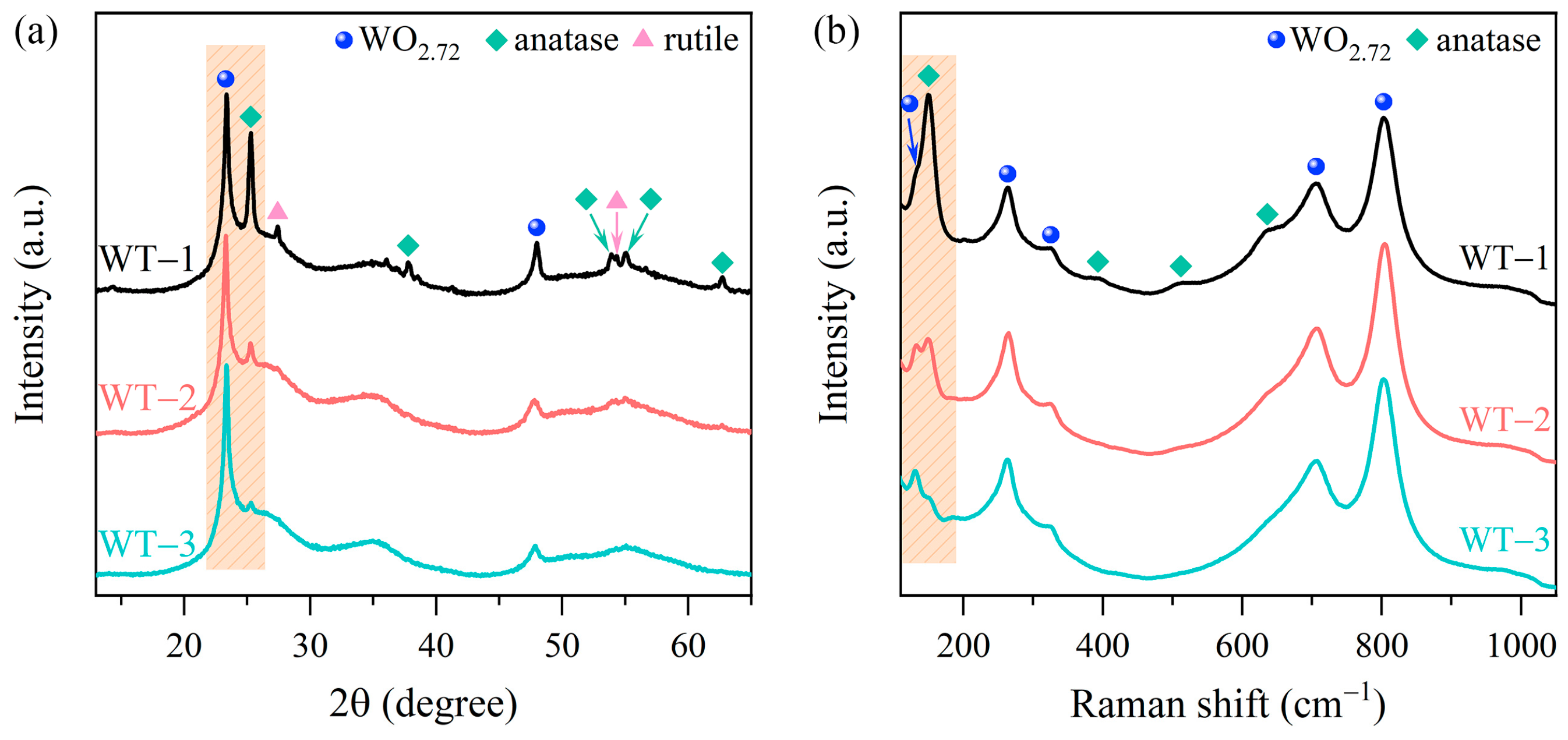
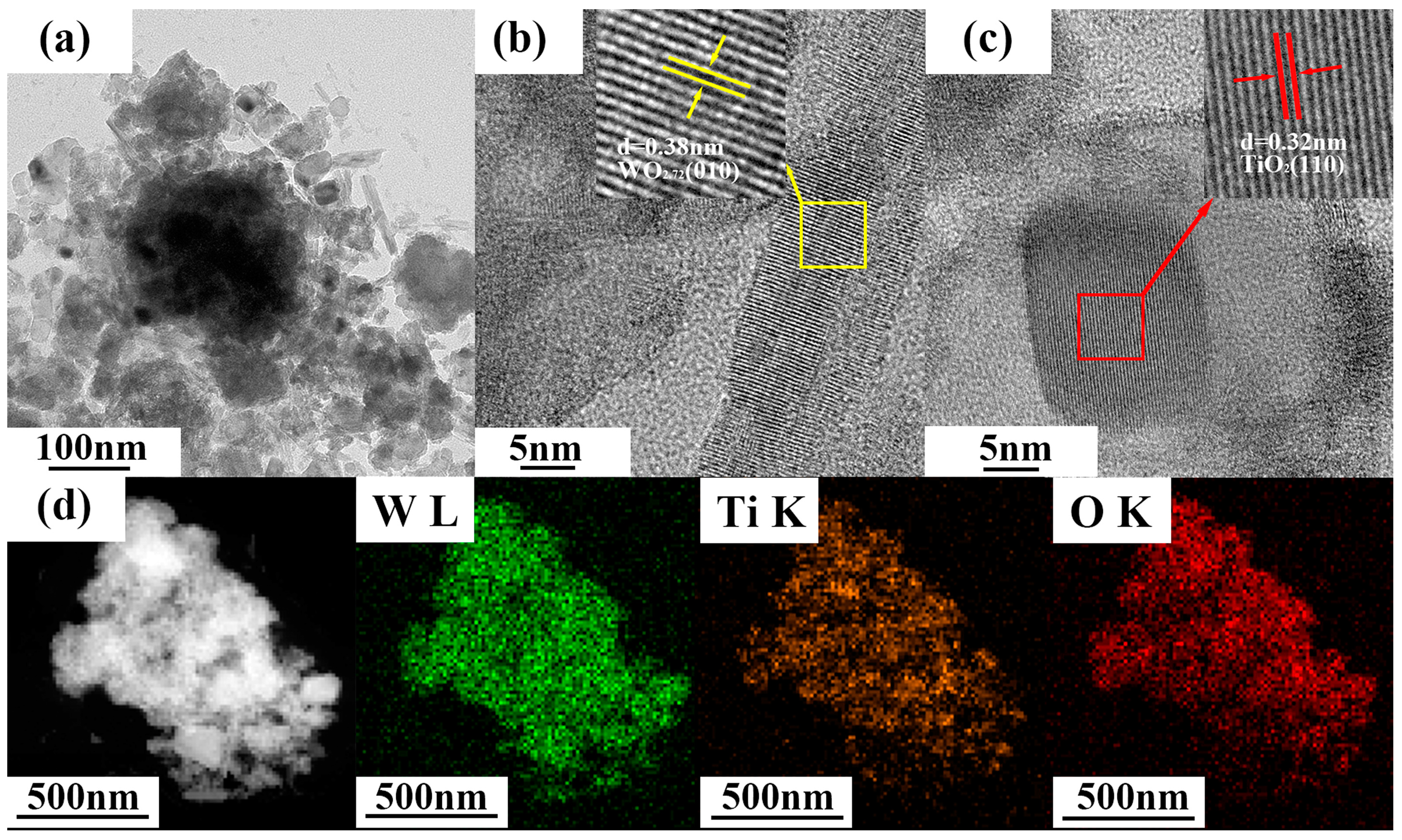
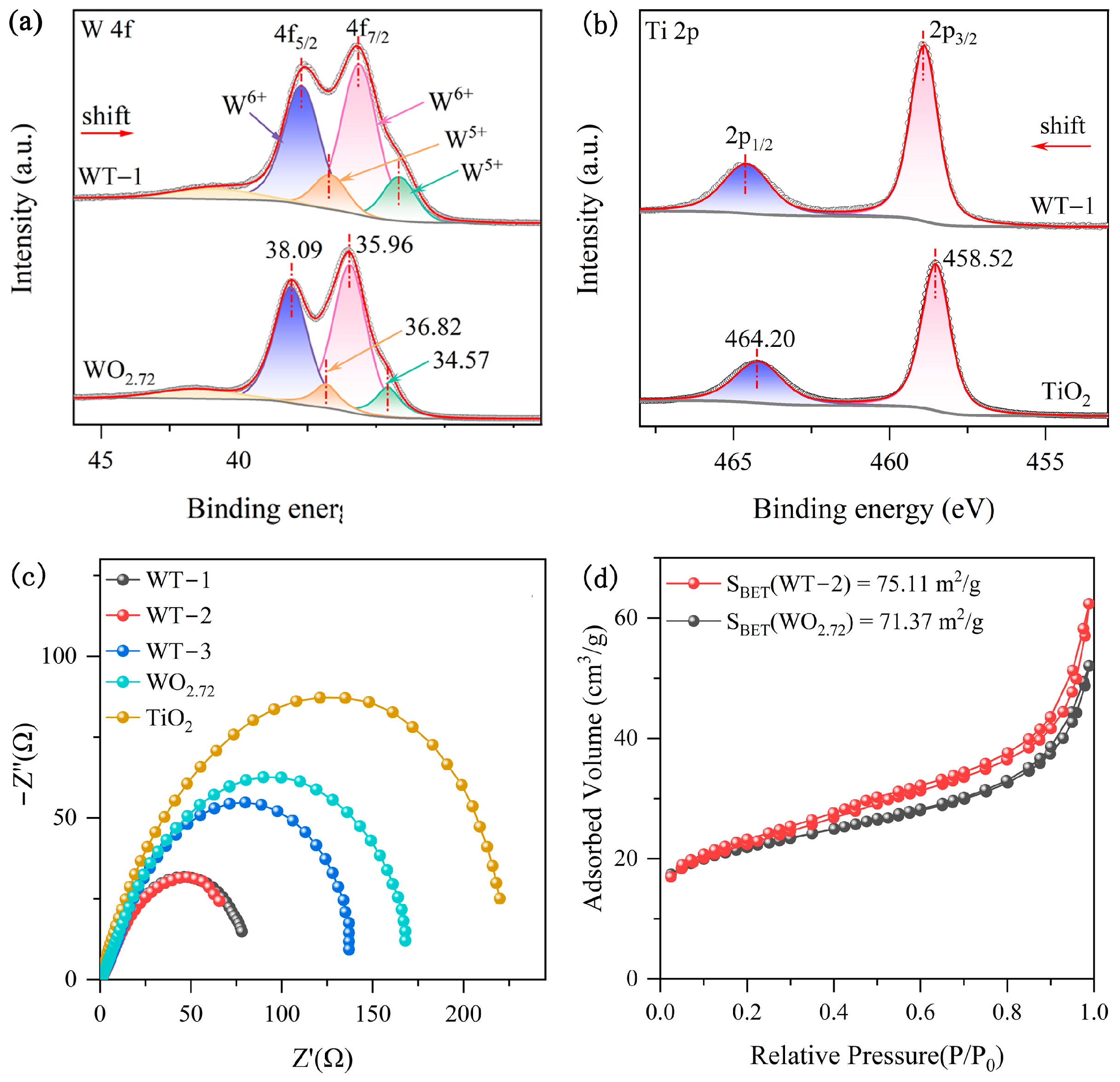
3.1. Characterization of WO3-x/TiO2
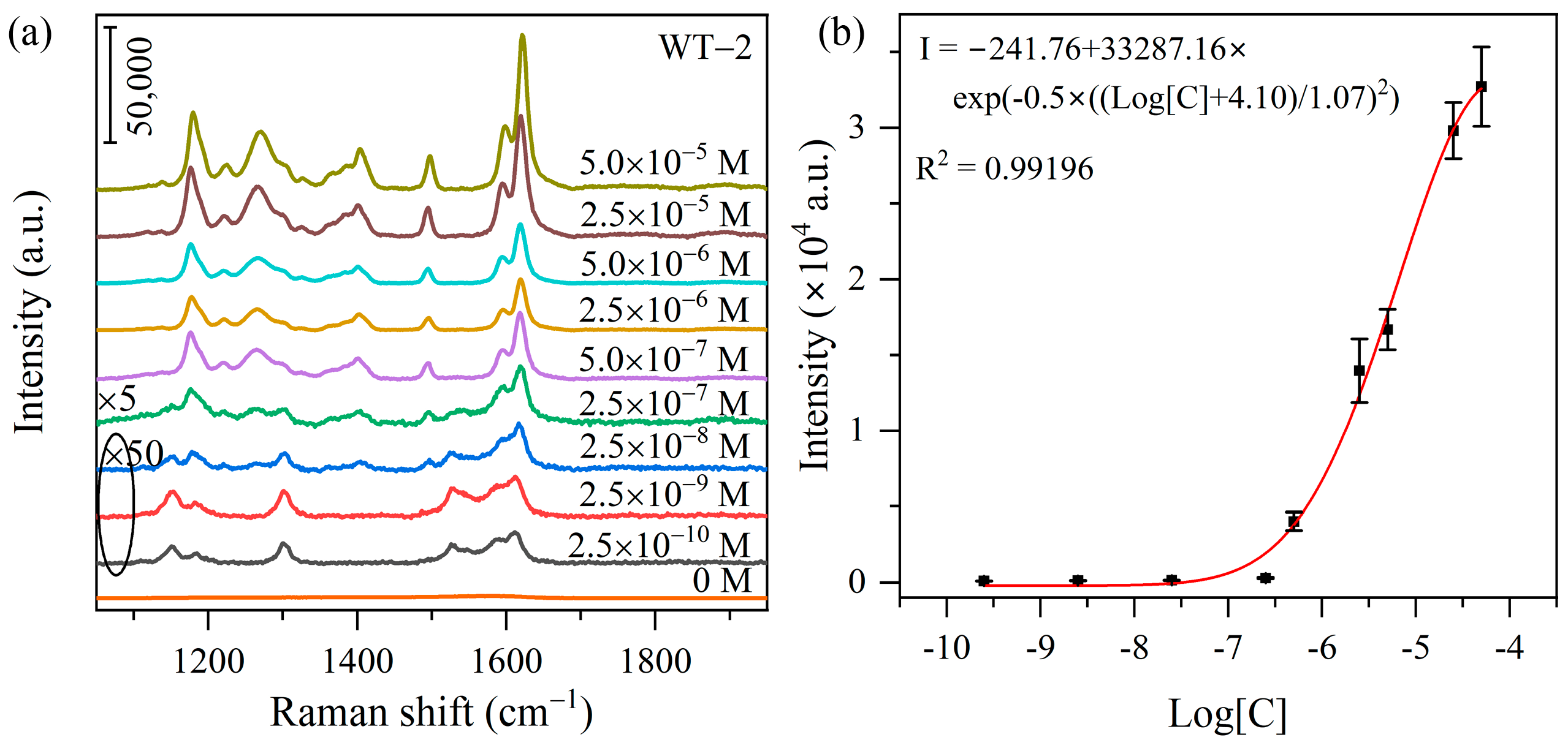
3.2. SERS Activity
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, J.; You, E.-M.; Hu, R.; Wu, D.-Y.; Liu, G.-K.; Yang, Z.-L.; Zhang, H.; Gu, Y.; Wang, Y.-H.; Wang, X.; et al. Surface-enhanced Raman spectroscopy: A half-century historical perspective. Chem. Soc. Rev. 2025, 54, 1453–1551. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Cui, Q.; Li, S.; Li, H.; Xu, L.; Tian, Y.; Ma, H.; Zhang, M.; Liu, X.; Chen, M. Aligned TiO2 nanorod arrays decorated with closely interconnected Au/Ag nanoparticles: Near-infrared SERS active sensor for monitoring of antibiotic molecules in water. Sens. Actuators B Chem. 2022, 350, 130848. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mahdi Mousavi, S.; Amiri, A.; Tian, J.; Cao, R.; Wang, X. Surface-Enhanced Raman Spectroscopy Substrates for Food Safety and Quality Analysis. J. Agric. Food Chem. 2022, 70, 5463–5476. [Google Scholar] [CrossRef]
- Zappalà, G.; Soufi, G.; Dumont, E.; Molander, N.; Slipets, R.; Thamdrup, L.H.E.; Andersson, P.O.; Rindzevicius, T.; Boisen, A. SERS-integrated centrifugal microfluidic platform for the detection and quantification of Chemical Warfare Agents in single-component solution and mixtures. Sens. Actuators B Chem. 2025, 422, 136698. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kim, J.; Choi, K.; Nam, J.-M. Plasmonic Dual-Gap Nanodumbbells for Label-Free On-Particle Raman DNA Assays. Adv. Mater. 2023, 35, 2208250. [Google Scholar] [CrossRef]
- Sun, L.; Cao, C.; Zhi, Y.; Shan, Y.; Zhang, H.; Dou, B.; Zhang, L.; Huang, W. Au–Ag Nanoparticles with Controllable Morphologies for the Surface-Enhanced Raman Scattering Detection of Trace Thiram. ACS Appl. Nano Mater. 2023, 6, 4253–4261. [Google Scholar] [CrossRef]
- Anantha, P.; Raj, P.; Zheng, P.; Tanwar, S.; Barman, I. Gold nanoprism enhanced SERS aptasensor for simultaneous detection of thrombin and VEGF. Sens. Actuators B Chem. 2025, 423, 136811. [Google Scholar] [CrossRef]
- Husain, S.; Mutalik, C.; Yougbaré, S.; Chen, C.-Y.; Kuo, T.-R. Plasmonic Au@Ag Core–Shell nanoisland Film for Photothermal Inactivation and Surface-Enhanced Raman Scattering Detection of Bacteria. Nanomaterials 2024, 14, 695. [Google Scholar] [CrossRef] [PubMed]
- Kaja, S.; Nag, A. Bimetallic Ag–Cu Alloy Microflowers as SERS Substrates with Single-Molecule Detection Limit. Langmuir 2021, 37, 13027–13037. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hao, W.; Shang, Y.; Wang, X.; Qiu, D.; Ma, G.; Chen, C.; Li, S.; Guo, L. Direct Experimental Observation of Facet-Dependent SERS of Cu2O Polyhedra. Small 2018, 14, 1703274. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef]
- Singh, N.S.; Mayanglambam, F.; Nemade, H.B.; Giri, P.K. Plasma-Treated Graphene Surfaces for Trace Dye Detection Using Surface-Enhanced Raman Spectroscopy. ACS Appl. Nano Mater. 2022, 5, 6352–6364. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, T.; Shao, M.; Ji, C.; Li, C.; Zhang, C.; Li, Z. Pyroelectrically Driven Charge Transfer and its Advantages on SERS and Self-Cleaning Property. Laser Photonics Rev. 2024, 19, 2401152. [Google Scholar] [CrossRef]
- Jiang, Y.; Cong, S.; Song, G.; Sun, H.; Zhang, W.; Yao, W.; Zhao, Z. Defective cuprous oxide as a selective surface-enhanced Raman scattering sensor of dye adulteration in Chinese herbal medicines. J. Raman Spectrosc. 2021, 52, 1265–1274. [Google Scholar] [CrossRef]
- He, R.; Lai, H.; Wang, S.; Chen, T.; Xie, F.; Chen, Q.; Liu, P.; Chen, J.; Xie, W. Few-layered vdW MoO3 for sensitive, uniform and stable SERS applications. Appl. Surf. Sci. 2020, 507, 145116. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, M.; Chen, G.; Fan, C.; Li, M.; Gu, X.; Cong, S.; Zhao, Z.; Fu, L.; Fang, X.; et al. Photodegradable CuS SERS Probes for Intraoperative Residual Tumor Detection, Ablation, and Self-Clearance. ACS Appl. Mater. Interfaces 2019, 11, 23436–23444. [Google Scholar] [CrossRef]
- Pan, T.; Song, G.; Cong, S.; Chen, Z.; Chen, J.; Zhao, Z. Hydroxyl Group-Abundant TiO2 Semiconductor SERS Sensor toward Polymerization Inhibitor Sensing. J. Phys. Chem. C 2020, 124, 20530–20537. [Google Scholar] [CrossRef]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef] [PubMed]
- Ushkov, A.; Dyubo, D.; Belozerova, N.; Kazantsev, I.; Yakubovsky, D.; Syuy, A.; Tikhonowski, G.V.; Tselikov, D.; Martynov, I.; Ermolaev, G.; et al. Tungsten Diselenide Nanoparticles Produced via Femtosecond Ablation for SERS and Theranostics Applications. Nanomaterials 2025, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, X.; Wang, Z.; Yuan, M.; Zhao, Z.; Ye, X.; Shang, S.; Song, Z.; Huang, L.; Liu, Y.; et al. Geometric and Electronic Structure Modulation to Optimize the Charge Transfer of TiO2 for Ultrasensitive and Stable SERS Sensing. Inorg. Chem. 2024, 63, 17608–17616. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, X.; Yi, M.; Chi, Y.; Mao, Z.; Yang, B.; Jung, Y.M. Lithium-doped ZrO2 nanoparticles for SERS-based norfloxacin drug detection. Spectrochim. Acta Part A 2025, 326, 125239. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, P.; Dong, Q.; Wang, D.; Zhang, D.; Tang, L.; Wang, L.; Jin, S.; Ni, D.; Yu, Z. Facile Reduction Method Synthesis of Defective MoO2–x Nanospheres Used for SERS Detection with High Chemical Enhancement. Anal. Chem. 2019, 91, 8683–8690. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Wu, P.; Jiang, H.; Tang, J. Nonstoichiometric tungsten oxide nanosheets with abundant oxygen vacancies for defects-driven SERS sensing. J. Raman Spectrosc. 2022, 53, 1880–1889. [Google Scholar] [CrossRef]
- Quan, Y.; Tang, X.-H.; Lu, W.; Huo, W.; Song, Z.; Shen, W.; Yang, M.; Huang, X.-J.; Liu, W.-Q. Amorphous/Crystal Heterostructure Coupled Oxygen Vacancies-Sensitized TiO2 with Conspicuous Charge-Transfer Resonance for Efficient SERS Detection of Chloramphenicol. Adv. Opt. Mater. 2023, 11, 2301609. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, L.; Ji, W.; Wang, W.; Du, J.; Yang, L.; Song, W.; Han, X.; Zhao, B. One plus one greater than Two: Ultrasensitive Surface-Enhanced Raman scattering by TiO2/ZnO heterojunctions based on Electron-Hole separation. Appl. Surf. Sci. 2022, 584, 152609. [Google Scholar] [CrossRef]
- He, C.; Jiang, L.; Yuan, R.; Yang, X. Facet junction of CeO2 with high SERS activity for sensitive detection of ATP. Sens. Actuators B Chem. 2023, 374, 132777. [Google Scholar] [CrossRef]
- Meng, W.; Kragt, A.J.J.; Gao, Y.; Brembilla, E.; Hu, X.; van der Burgt, J.S.; Schenning, A.P.H.J.; Klein, T.; Zhou, G.; van den Ham, E.R.; et al. Scalable Photochromic Film for Solar Heat and Daylight Management. Adv. Mater. 2024, 36, 2304910. [Google Scholar] [CrossRef]
- Wang, P.; Guo, S.; Hu, Z.; Li, T.; Pu, S.; Mao, H.; Cai, H.; Zhu, Z.; Li, H.-Y.; Liu, H. W18O49 sensitized with Pd nanoparticles for ultrasensitive ppb-level formaldehyde detection. Chem. Eng. J. 2023, 456, 140988. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Liu, B.; Guo, L.; Lu, N.; Wang, L.; Huang, J.; Liu, K.; Dong, B. IR-Driven Ultrafast Transfer of Plasmonic Hot Electrons in Nonmetallic Branched Heterostructures for Enhanced H2 Generation. Adv. Mater. 2018, 30, 1705221. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhang, C.; Ye, J.-J.; Zou, M.-Z.; Liu, C.-J.; Zhang, X.-Z. Near-Infrared Light Responsive Nanoreactor for Simultaneous Tumor Photothermal Therapy and Carbon Monoxide-Mediated Anti-Inflammation. ACS Cent. Sci. 2020, 6, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ismail, P.M.; Khan, M.; Dang, A.; Ali, S.; Zada, A.; Raziq, F.; Khan, I.; Khan, M.S.; Ateeq, M.; et al. Charge transfer in TiO2-based photocatalysis: Fundamental mechanisms to material strategies. Nanoscale 2024, 16, 4352–4377. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, Q.; Zhu, H.; Zhao, R.; Feng, L.; He, S.; Wang, W.; He, G.; Liu, B.; Yang, P. Thermoelectric Nanoheterojunction-Mediated Multiple Energy Conversion for Enhanced Cancer Therapy. ACS Nano 2024, 18, 34257–34271. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, Z.; Lu, T.; Liu, X.; Li, J.; Wang, C.; Yang, G.; Pan, L. Machine learning accelerated study for predicting the lattice constant and substitution energy of metal doped titanium dioxide. Ceram. Int. 2024, 50, 1079–1086. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, B.; Gao, R.; Zhang, X.; Cheng, X.; Huo, L.; Major, Z.; Xu, Y. Crosslinked WO3 nanonet for rapid detection of sulfur mustard gas simulant: Mechanism insights and sensing application. Sens. Actuators B Chem. 2023, 385, 133704. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hirakawa, H.; Togawa, Y.; Sugano, Y.; Ichikawa, S.; Hirai, T. Rutile Crystallites Isolated from Degussa (Evonik) P25 TiO2: Highly Efficient Photocatalyst for Chemoselective Hydrogenation of Nitroaromatics. ACS Catalysis 2013, 3, 2318–2326. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3 xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, T.; Wang, X.; Cheng, Z.; Xu, J. Coal mine gases sensors with dual selectivity at variable temperatures based on a W18O49 ultra-fine nanowires/Pd@Au bimetallic nanoparticles composite. Sens. Actuators B Chem. 2022, 354, 131004. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.; Guan, N.; Li, L.; Gong, J. Tungsten Oxide Single Crystal Nanosheets for Enhanced Multichannel Solar Light Harvesting. Adv. Mater. 2015, 27, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Mikami, M.; Nakamura, S.; Kitao, O.; Arakawa, H. Lattice dynamics and dielectric properties of TiO2 anatase: A first-principles study. Phys. Rev. B 2002, 66, 155213. [Google Scholar] [CrossRef]
- García-Contreras, L.A.; Flores-Flores, J.O.; Arenas-Alatorre, J.Á.; Chávez-Carvayar, J.Á. Synthesis, characterization and study of the structural change of nanobelts of TiO2 (H2Ti3O7) to nanobelts with anatase, brookite and rutile phases. J. Alloy. Compd. 2022, 923, 166236. [Google Scholar] [CrossRef]
- Ma, T.; Li, B.; Tian, S.; Qian, J.; Zhou, L.; Liu, Q.; Liu, B.; Zhao, X.; Sankar, G. Reversible photochromic W18O49: Mechanism revealing and performance improvement for smart windows. Chem. Eng. J. 2023, 468, 143587. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Zhang, Y.; Feng, Z.; Zhao, F.; Wang, W. Light-responsive color switching of self-doped TiO2−x/WO3·0.33H2O hetero-nanoparticles for highly efficient rewritable paper. Nano Res. 2021, 14, 165–171. [Google Scholar] [CrossRef]
- Prakash, O.; Kumar, S.; Singh, P.; Deckert, V.; Chatterjee, S.; Ghosh, A.K.; Singh, R.K. Surface-enhanced Raman scattering characteristics of CuO: Mn/Ag heterojunction probed by methyl orange: Effect of Mn2+ doping. J. Raman Spectrosc. 2016, 47, 813–818. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y. Adsorption orientations and interactions of methyl orange on negatively and positively charged colloidal silver particles. J. Colloid Interface Sci. 2007, 305, 270–274. [Google Scholar] [CrossRef]
- Yu, T.-H.; Ho, C.-H.; Wu, C.-Y.; Chien, C.-H.; Lin, C.-H.; Lee, S. Metal–organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photodegradation of the aminoazobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2: Comparative mechanistic and kinetic investigations. J. Photochem. Photobiol. A 2000, 130, 35–47. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Chen, Z.; Wang, J.; Feng, H.; Peng, B.; Liu, Y.; Zhou, Y. Insight into the dual-channel charge-charrier transfer path for nonmetal plasmonic tungsten oxide based composites with boosted photocatalytic activity under full-spectrum light. Appl. Catal. B Environ. Energy 2018, 235, 225–237. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, Y.; Xu, Y.; Li, L.; Li, C.; Liu, X.; Niu, L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018, 350, 257–267. [Google Scholar] [CrossRef]
- Liu, C.-S.; Li, B.-H.; Chen, C.-H.; Peng, J.-W.; Lee, S. Enhancement in SERS intensities of azo dyes adsorbed on ZnO by plasma treatment. J. Raman Spectrosc. 2014, 45, 332–337. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Li, X.; Yu, J.; Wang, X.; Guo, L. Ultrasensitive SERS Detection by Defect Engineering on Single Cu2O Superstructure Particle. Adv. Mater. 2017, 29, 1604797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Chen, Z.-Y.; Wang, M.-Q.; Zhang, L.-Z.; Bao, S.-J. Interface engineered construction of porous g-C3N4/TiO2 heterostructure for enhanced photocatalysis of organic pollutants. Appl. Surf. Sci. 2018, 440, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Z.; Huang, W.; Lin, J.; Ma, G.; Li, S.; Guo, L. Remarkable SERS Activity Observed from Amorphous ZnO Nanocages. Angew. Chem. Int. Edit. 2017, 56, 9851–9855. [Google Scholar] [CrossRef]
- Zarei, A.; Shafiekhani, A. Surface-enhanced Raman scattering (SERS) of Methyl Orange on Ag-DLC nanoparticles. Mater. Chem. Phys. 2020, 242, 122559. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Akakuru, O.U.; Wang, X.; Yuan, B.; Chen, T.; Guo, L.; Wu, A. Low temperature-boosted high efficiency photo-induced charge transfer for remarkable SERS activity of ZnO nanosheets. Chem. Sci. 2020, 11, 9414–9420. [Google Scholar] [CrossRef]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, T.; Zhang, M.; Zhou, Y. Facile preparation of Cu2-xS supernanoparticles with an unambiguous SERS enhancement mechanism. Chem. Eng. J. 2022, 434, 134457. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Lai, W.; Yang, X.; Meng, J.; Su, L.; Gu, C.; Jiang, T.; Pun, E.Y.B.; Shao, L.; et al. Irreversible accumulated SERS behavior of the molecule-linked silver and silver-doped titanium dioxide hybrid system. Nat. Commun. 2020, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qayum, A.; Jiao, X.; Wang, T.; Chen, D. Photo-reduced WO3/PAN nanofiber membranes with deposited Ag nanoparticles as efficient SERS substrates. Appl. Surf. Sci. 2021, 568, 150936. [Google Scholar] [CrossRef]
- Yin, W.; An, S.; Cheng, T.; Jiang, L.; Cao, Y. Enhancing SERS sensitivity of semiconductors through constructing CuO@TiO2 heterojunctions via atomic layer deposition. Appl. Surf. Sci. 2024, 672, 160820. [Google Scholar] [CrossRef]
- Wei, J.; Yu, K.; Yu, Y.; Li, S.; Yu, H.; Li, B.; Cui, Y.; Abdul, Q.; Chen, Q.; Hao, Z.; et al. Photo-reduced TiO2@WO3 electrospun nanofibers for efficient SERS and photoelectrochemical performances. Compos. Commun. 2024, 46, 101847. [Google Scholar] [CrossRef]
- Korkmaz, I.; Sakir, M.; Sarp, G.; Salem, S.; Torun, I.; Volodkin, D.; Yavuz, E.; Onses, M.S.; Yilmaz, E. Fabrication of superhydrophobic Ag@ZnO@Bi2WO6 membrane disc as flexible and photocatalytic active reusable SERS substrate. J. Mol. Struct. 2021, 1223, 129258. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Gong, Y.; Niu, L.; Li, C. Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance. Nanomaterials 2025, 15, 521. https://doi.org/10.3390/nano15070521
He X, Gong Y, Niu L, Li C. Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance. Nanomaterials. 2025; 15(7):521. https://doi.org/10.3390/nano15070521
Chicago/Turabian StyleHe, Xunfei, Yinyan Gong, Lengyuan Niu, and Can Li. 2025. "Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance" Nanomaterials 15, no. 7: 521. https://doi.org/10.3390/nano15070521
APA StyleHe, X., Gong, Y., Niu, L., & Li, C. (2025). Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance. Nanomaterials, 15(7), 521. https://doi.org/10.3390/nano15070521




