Indoor Evaluation of a Temperature-Controlled Gel Intelligent Diversion System
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Technical Mechanism
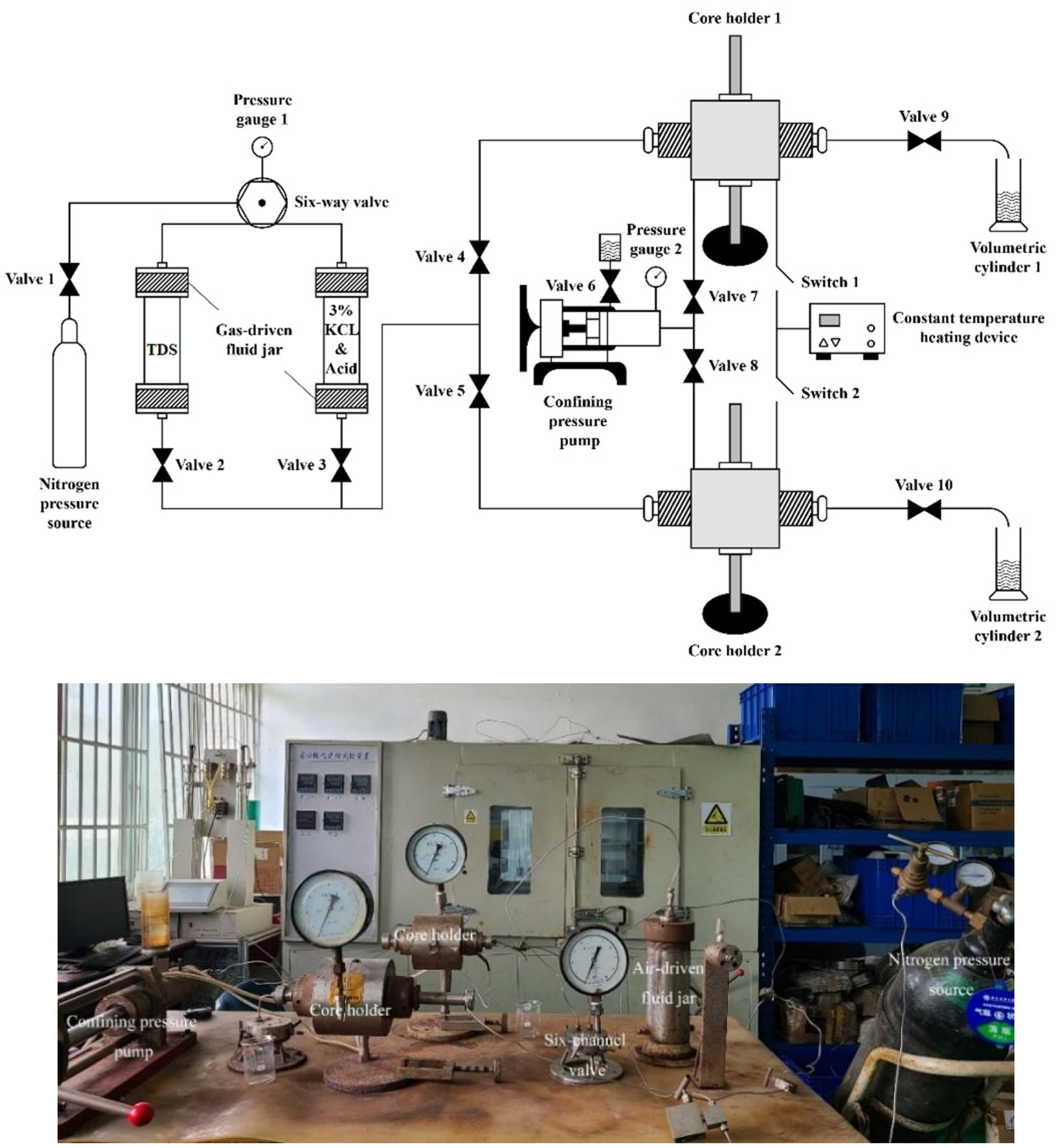
2.3. Experimental Equipment
2.4. Experimental Methods
- Preparation: Prepare 50 mL of TDS and 1000 mL of a 3% KCl solution (with dye, hereinafter referred to as the base solution).
- Core Setup: Place a group of cores in the core holder and apply a confining pressure slightly higher than the displacement pressure to prevent the cores from slipping during the displacement process.
- Pre-washing: Inject the base solution to pre-wash the cores and record the flow rates of the two cores after the pressure stabilizes.
- Injection of TDS: Inject TDS into the system and record the pressure and flow. When colorless droplets appear at the outlet, close the nitrogen bottle, open the vent valve, and release the pressure.
- Core Heating: Heat the cores to a constant temperature of 70 °C for 45 min to allow the TDS to fully solidify.
- Injection of Acid: Inject acid into the core and record the pressure and flow rates.
- Degradation of TDS: Set the temperature to 85 °C and heat the system for another 40 min to degrade the TDS.
- Final Injection: Inject the base fluid again and record the pressure and flow rates.
- The core was placed in the holder and secured with a confining pressure (as in the core parallel flow experiment).
- The core was pre-flushed with the base fluid.
- TDS was injected into the cores and heated to allow curing.
- The base fluid was injected using an advection pump to gradually increase the pressure.
- The pressure and flow rate were monitored. When the flow rate suddenly increased, the corresponding pressure value at that moment was recorded as the static breakthrough pressure.
3. Results
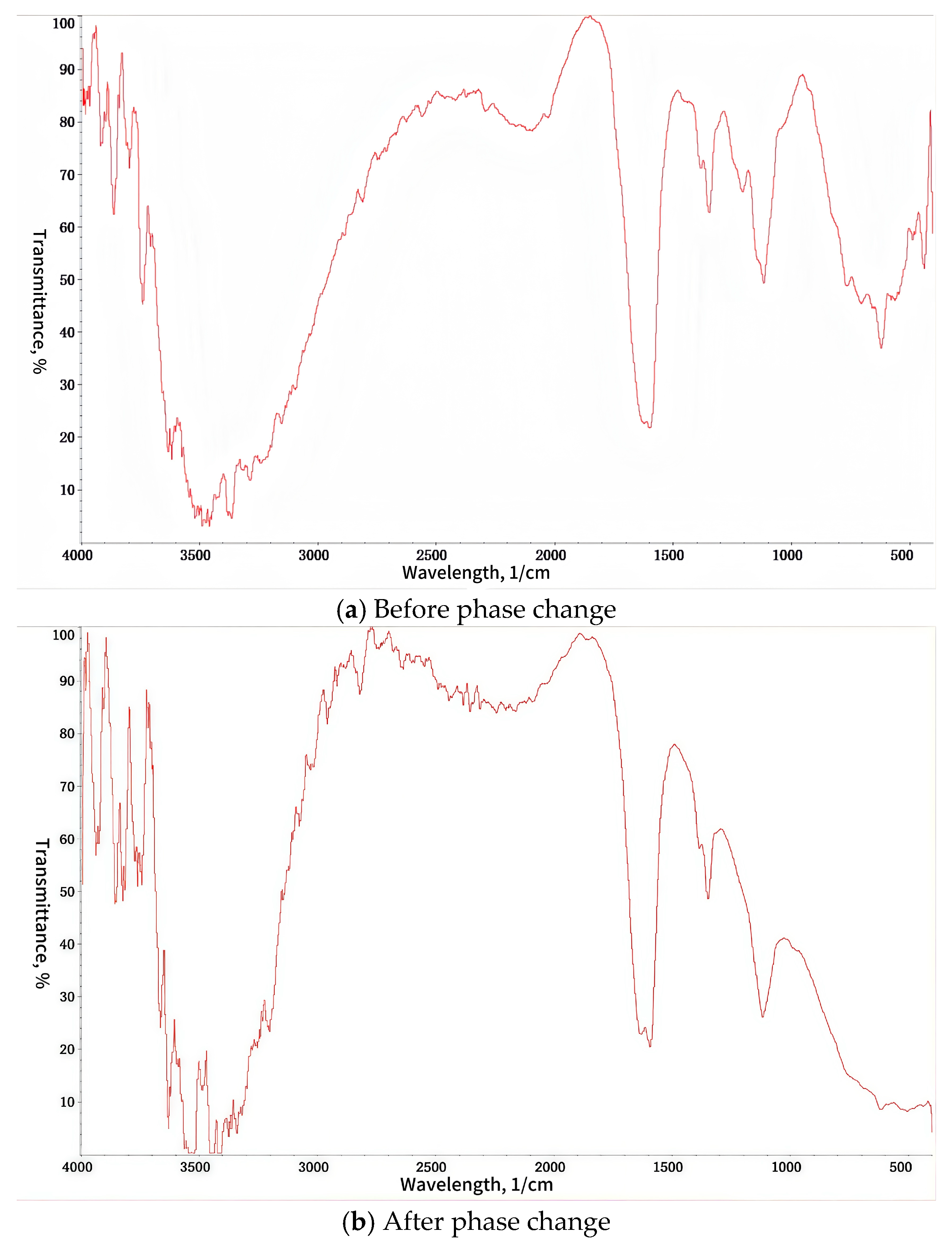
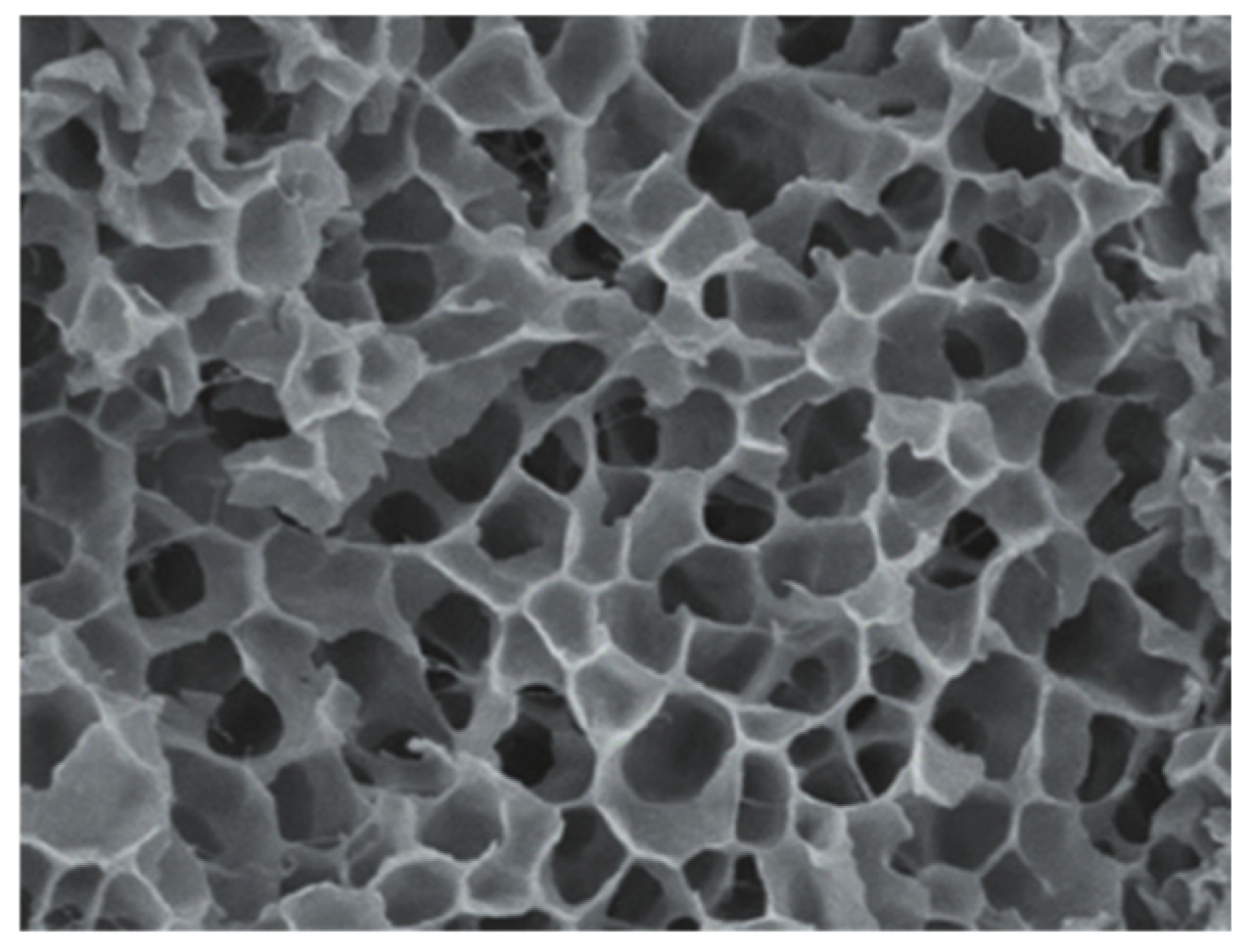
3.1. Characterization Analysis
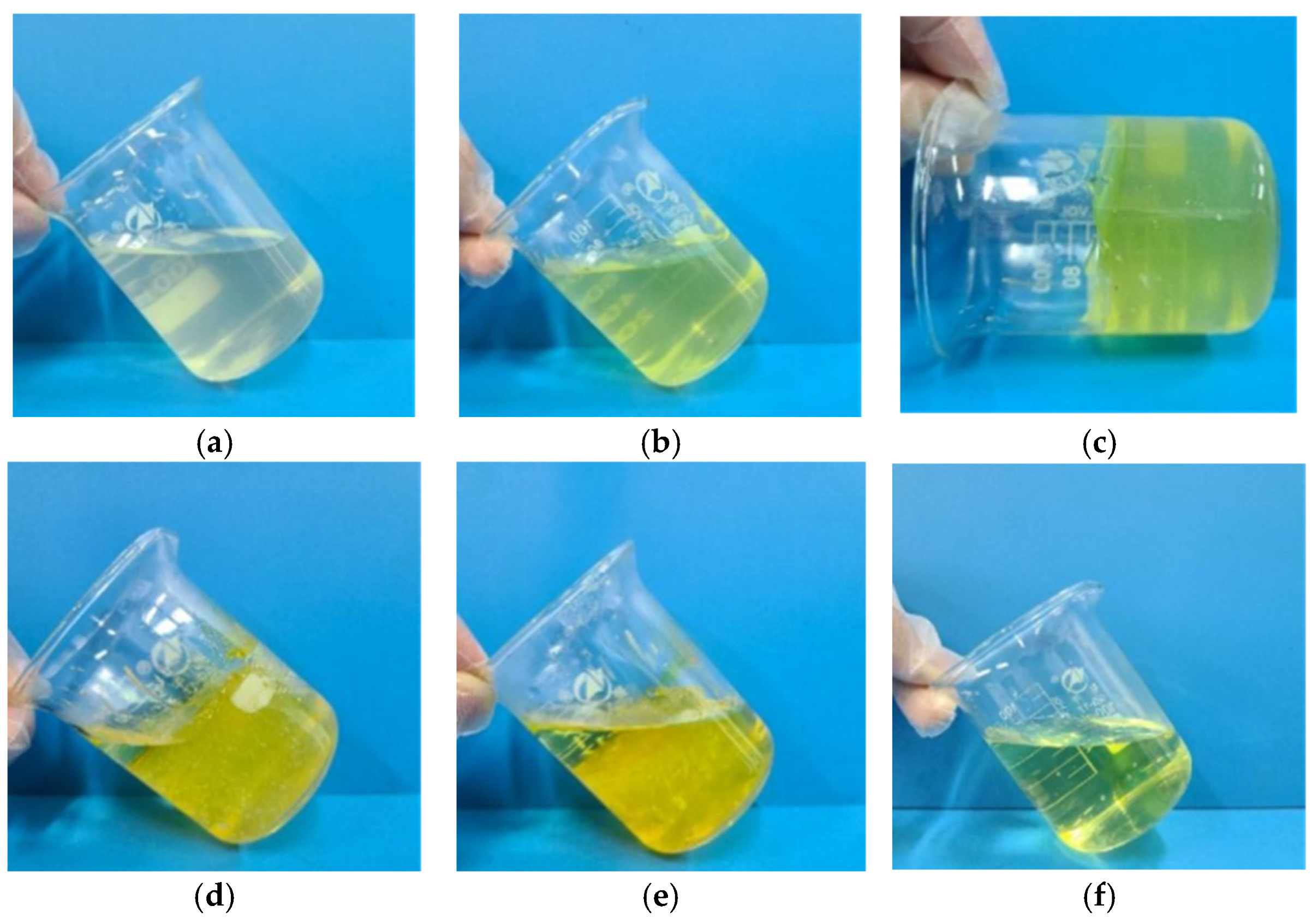
3.2. Compatibility
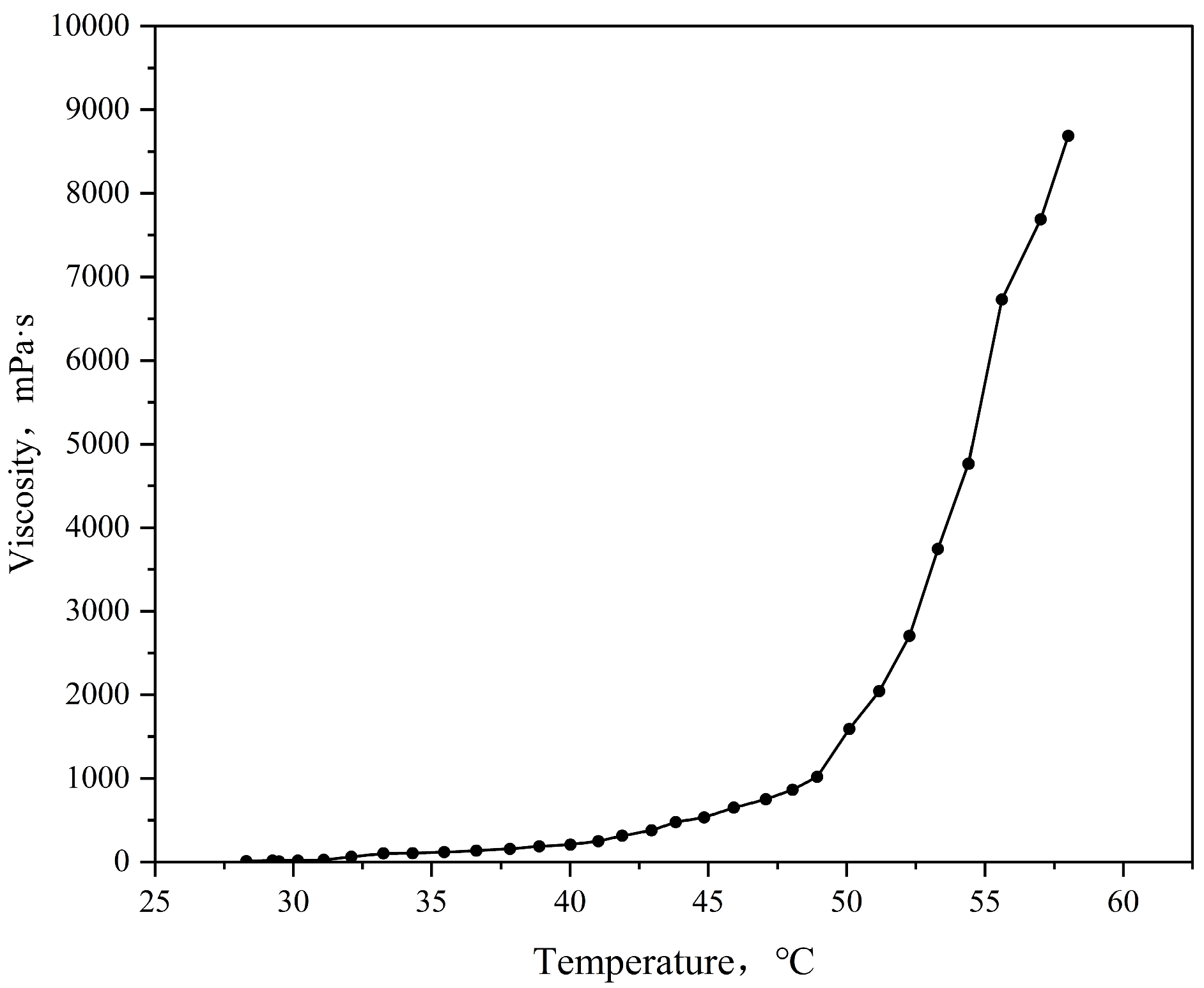
3.3. Viscosity–Temperature Characteristics of TDS
3.4. Phase Change Performance of TDS
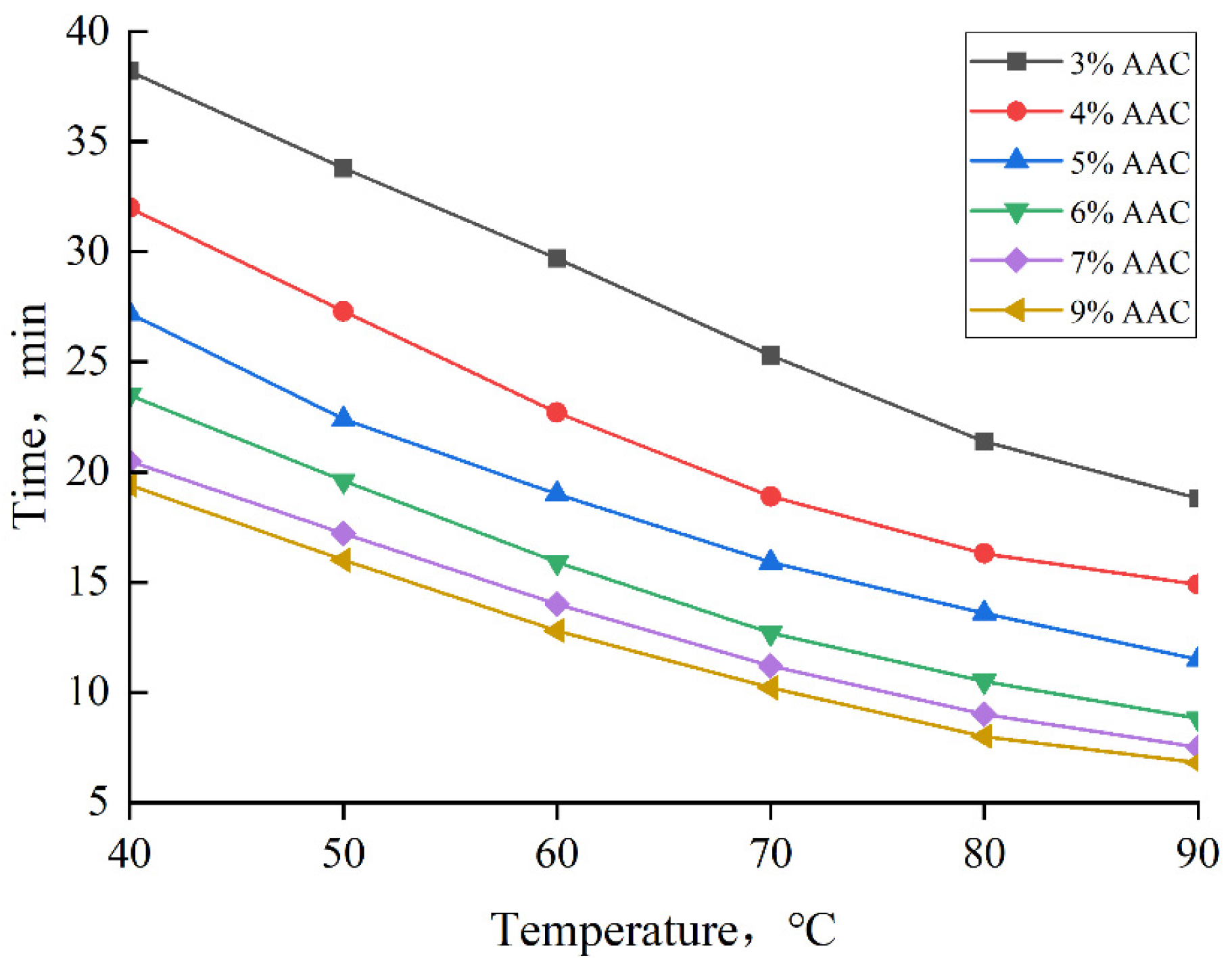
3.5. Curing Temperature and Curing Time
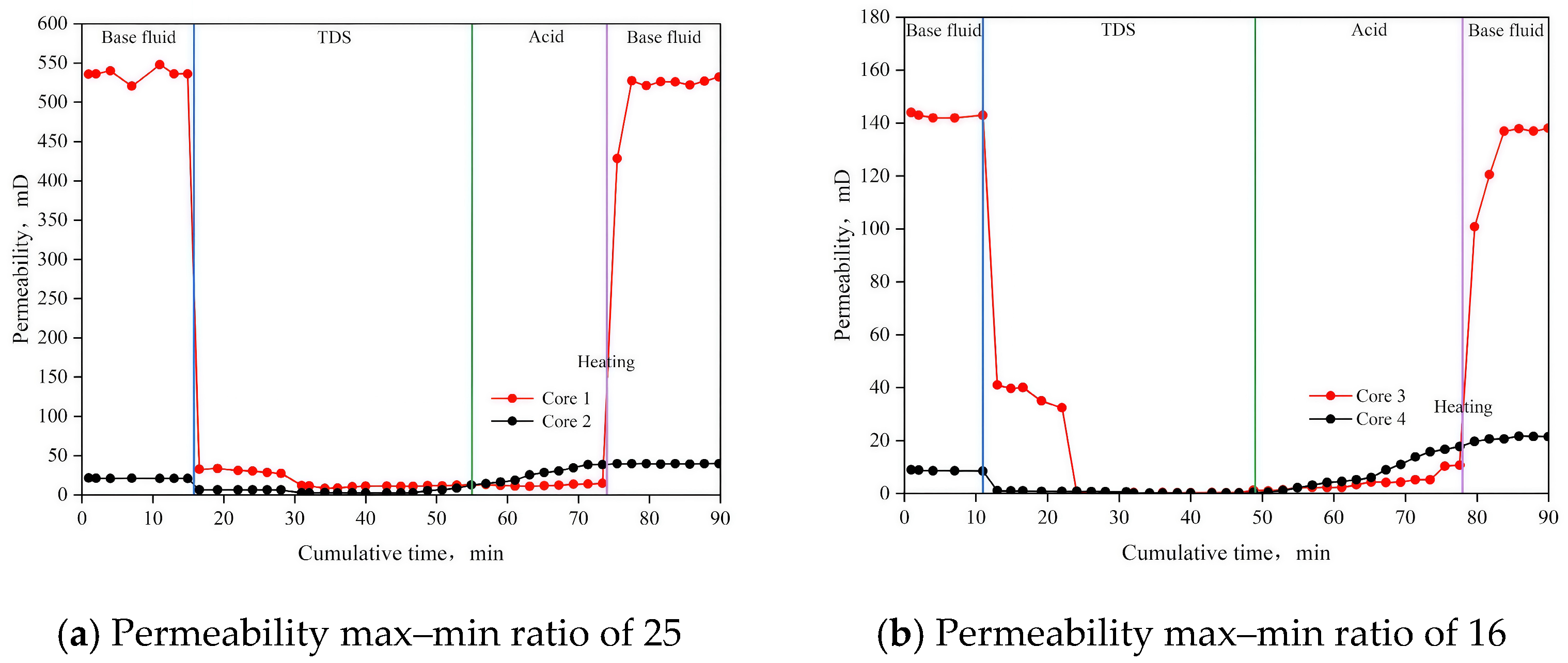
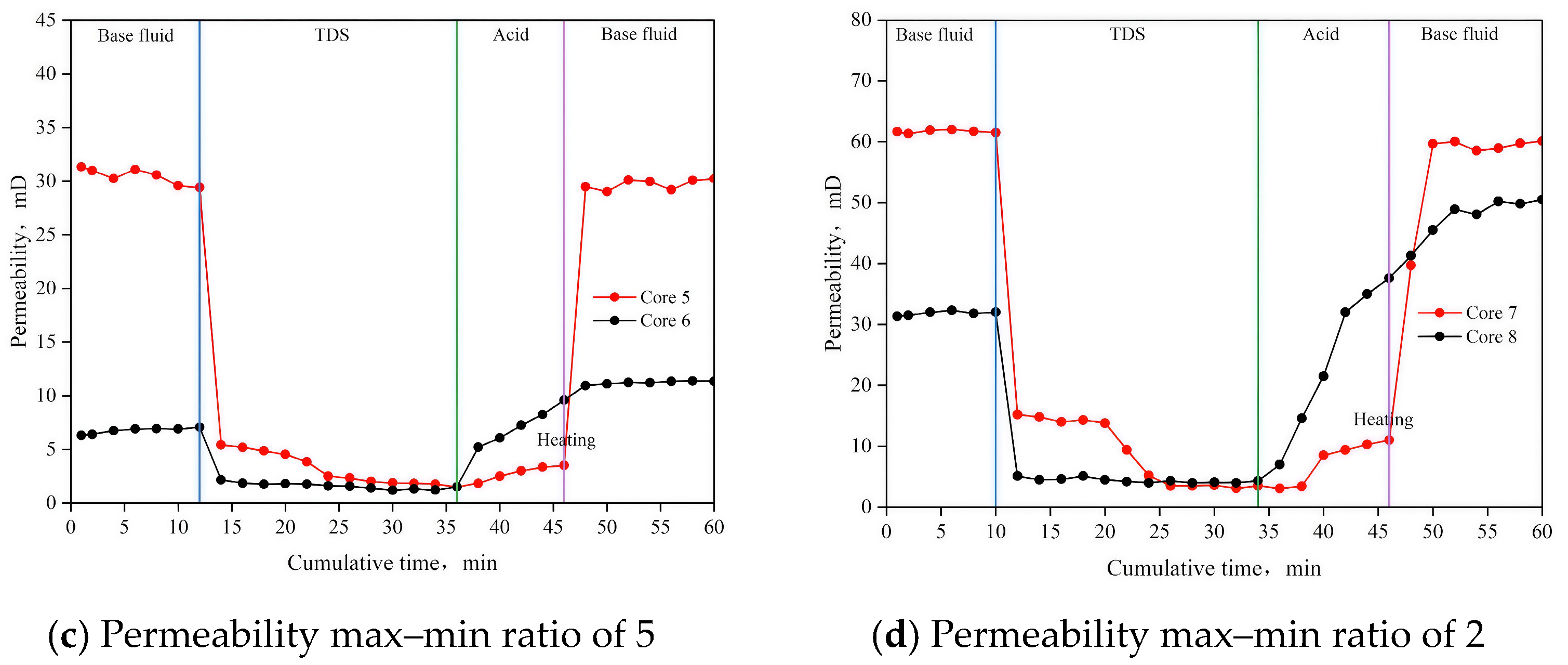
3.6. Parallel Flow Displacement Experiment
3.7. Plugging Rate and Plugging Strength
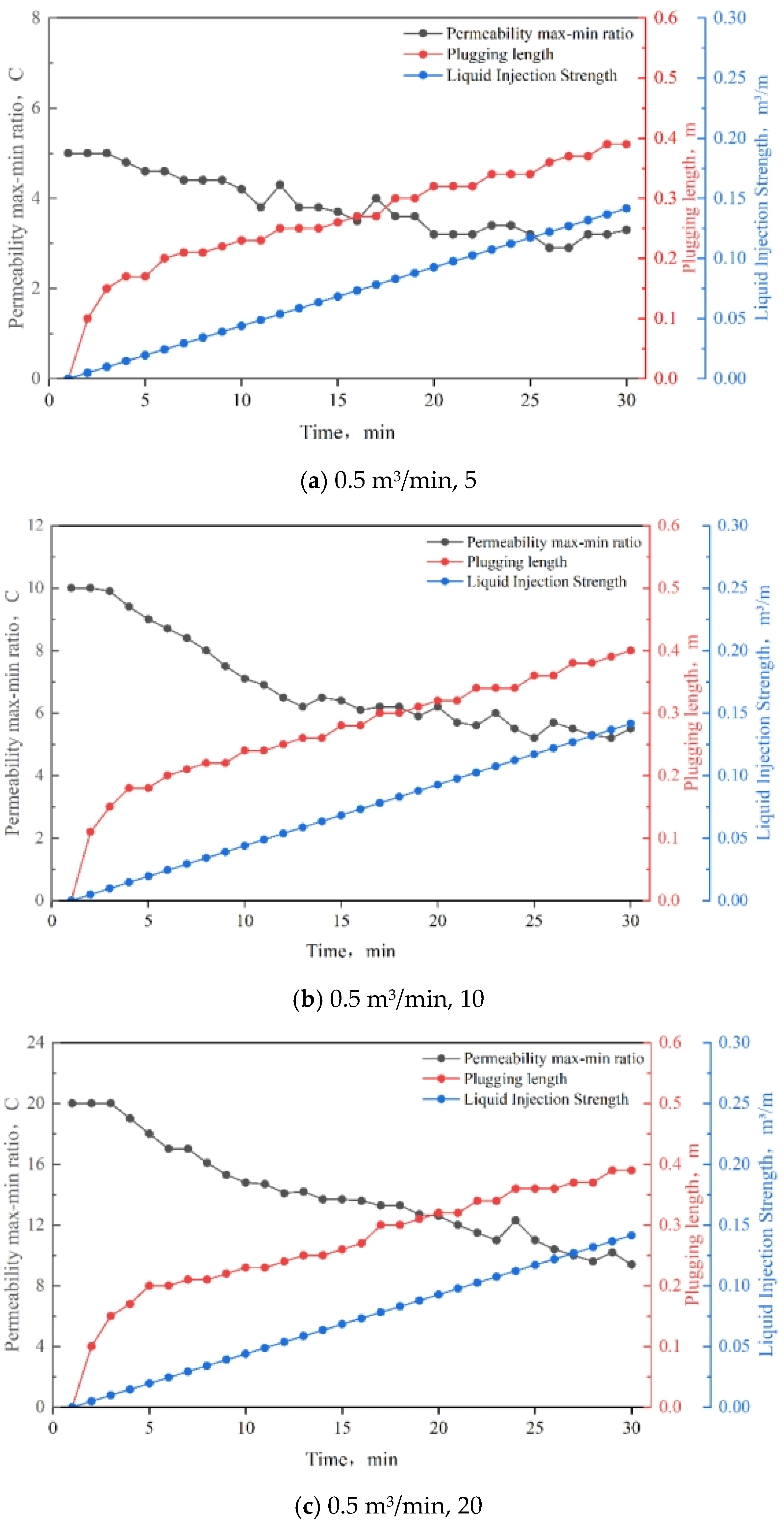
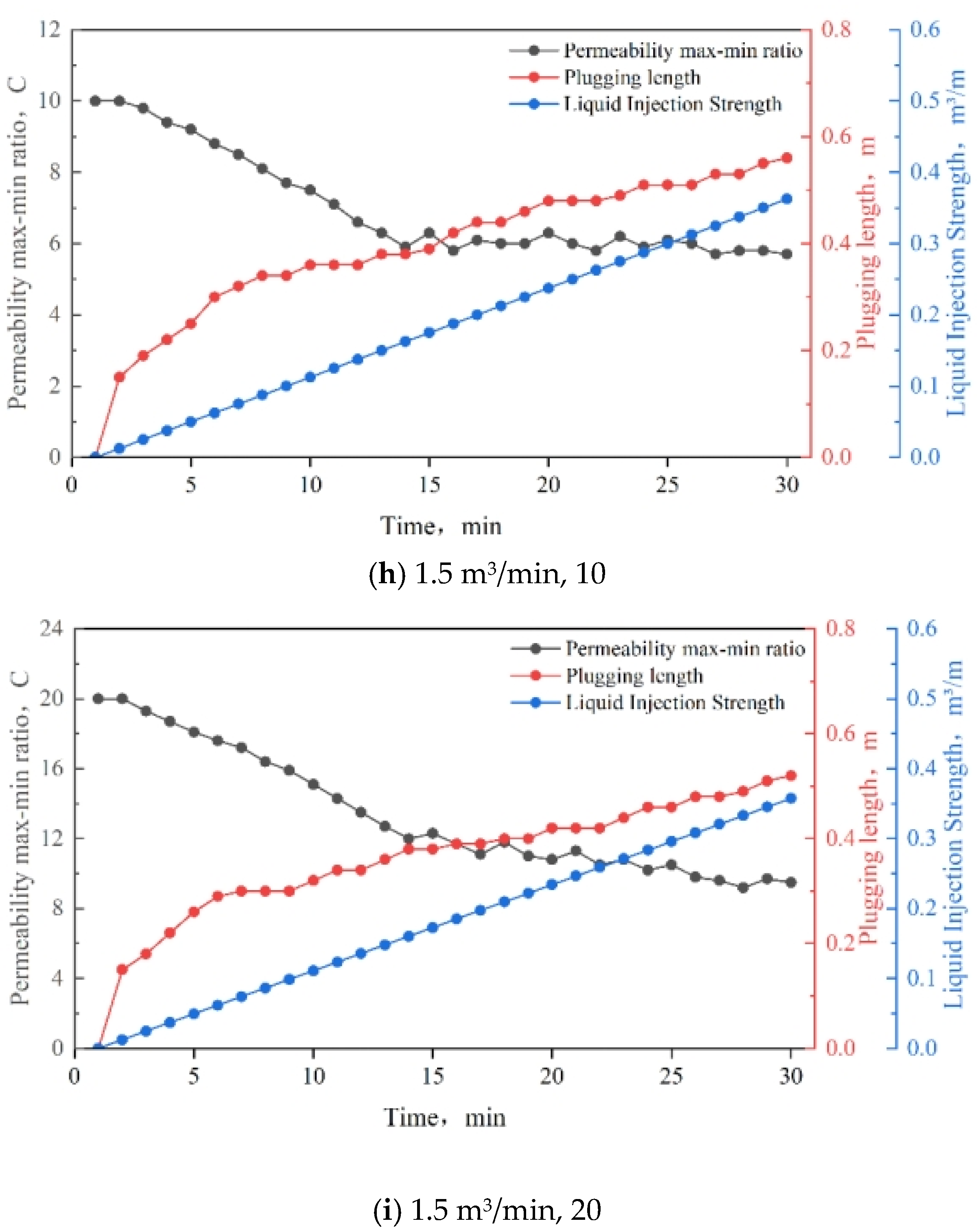
3.8. Injection Strength
3.9. Slug Design and Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDS | temperature-controlled diversion system |
| IDBPRT | Intelligent Diversion and Balanced Plugging Removal Technology |
| ACC | auxiliary agent concentration |
| gel-forming temperature | |
| COFs | covalent organic frameworks |
| permeability ratio after and before treatment | |
| the permeability after treatment | |
| the permeability before treatment | |
| NMR | nuclear magnetic resonance |
| SEM | scanning electron microscope |
| injection strength | |
| V | displacement |
| he | effective absorption thickness |
| L | plugging length |
| L′ | mixed-phase band length |
| porosity | |
| α | safety factor |
| n | number of temporary plugging |
References
- Yang, L. Application of temporary plugging steering technology in fracturing seam network of tight oil straight wells. W. Prospect. Eng. 2023, 35, 64–66, 71. [Google Scholar] [CrossRef]
- Xiong, C.; Shi, Y.; Zhou, F.; Liu, X.; Yang, X.-F.; Yang, X.-T. High efficiency reservoir stimulation based on temporary plugging and diverting for deep reservoirs. Pet. Explor. Dev. 2018, 45, 948–954. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Y.; Liu, P.; Zhao, L.; Ding, D.; Feng, Y. Research and application of composite acid fracturing technology with fiber diversion temporary plugging in ultra-deep carbonate horizontal wells. Pet. Geol. Recovery Effic. 2015, 22, 117–121. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Wang, M.; Zhang, X.; Zhou, C.; Hu, Q. Experimental evaluation and process optimization of temporary plugging to acid-frac in fractured carbonate reservoirs. Chem. Eng. Oil Gas 2021, 50, 90–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, B.; He, C.; Zhang, R.; Li, Y.; Sun, H.; Kang, R.; Cheng, X.; Gao, Y. Volume fracturing technology based on geo-engineering integration for ultra-high temperature and ultra-deep heterogeneous carbonate reservoir. Acta Pet. Sin. 2018, 39, 92–100. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Yang, Z.; Zhu, J.; Xie, S.; Liu, Z.; Gao, C. Progress and prospect of sandstone diversion acidification technology. In Proceedings of the The 32nd National Annual Conference on Natural Gas, Chongqing, China, 12 November 2020; Committee of China’s Petroleum Institute: Chengdu, China, 2020. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, W.; Zou, J.; Huang, T.; Wang, X. Application of Temporary Plugging Steering Acid Fracturing in Tahe Oilfield. Spec. Oil Gas Reserv. 2019, 26, 146–150. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, X.; Ci, J.; Huang, Y.; Ding, D. The Multistage Temporary Plugging Acid-fracturing Technology for the Open-hole Horizontal Wells in the Marine Reservoir. J. Chongqing Univ. Sci. Technol. Nat. Sci. Ed. 2015, 17, 69–72. [Google Scholar] [CrossRef]
- Da, Y.; Li, J.; Wang, F.; Huang, T.; Xue, X.; Yu, J. Fracturing Technologies with Profile Control and Water Shutoff for Medium and High Water-Cut Wells in Ultra-Low Permeability Reservoirs of Changqing Oilfield. Pet. Drill. Tech. 2022, 50, 74–79. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, S.; Song, Z.; Wang, E.; Yang, Q.; Wu, W. Study and application of online diversion acidizing technology in injection wells in ultra-low permeability reservoirs. Oil Gas Geol. 2019, 40, 430–435. [Google Scholar] [CrossRef]
- Li, Z. Research on the Mechanism of Water-Sensitive Self-Adhesive Emulsion Temporary Plugging Steering Acidification. Master’s Thesis, China University of Petroleum (East China), Dongying, China, 2020. [Google Scholar]
- Mu, M.; Tang, X.; Wang, L.; Zhang, X.; Liu, H.; Jiang, M.; Jiang, D.; Zhang, Y. Progress of Fabrications, Properties and Applications of Chemical Temporary Plugging Systems. Oilfield Chem. 2022, 39, 735–744, 760. [Google Scholar] [CrossRef]
- Zhou, F.; Yuan, L.; Liu, X.; Wang, B.; Li, M.; Li, B. Advances and key techniques of temporary plugging and diverting fracturing. Pet. Sci. Bull. 2022, 7, 365–381. [Google Scholar] [CrossRef]
- Mao, J.; Lu, W.; Zhang, Z.; Song, Z.; Zhao, J.; Zhang, J.; Wang, L. Research and development of temporary plugging diverting technology for reservoir Re-stimulation. Appl. Chem. Ind. 2018, 47, 2202–2206, 2211. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, D.; Feng, P.; Yang, G.; Zhou, F.; Shao, S. Latest research progress of carbonate formation mechanical diversion stimulation technology. Unconv. Oil Gas 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Zou, H.; Zhu, H.; Tang, X. Application of coiled tubing withdrawing acidizing with diverting acid system in Ahdeb oil field, Iraq. Oil Drill. Prod. Technol. 2014, 36, 88–91. [Google Scholar] [CrossRef]
- Rong, X.; Cui, B.; Feng, P.; Zhang, Q.; Chen, J. Intelligent Diversion, Water Cut Control and Plugging Removal Technology for Carbonate Reservoir—Case Study of S Oilfield in Iraq. Ind. Technol. Innov. 2023, 10, 85–95. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, D. Experimental study and effect evaluation of the migration of temporary plugging ball in horizontal wells. J. Northeast. Pet. Univ. 2023, 47, 55–66. [Google Scholar] [CrossRef]
- Zeng, F.; Hu, D.; Zhang, Y.; Liu, X.; Guo, J.; Qu, Y.; Chen, A. Optimization and application of particle parameters of multi-scale temporary plugging agent. Pet. Geol. Oilfield Dev. Daqing 2023, 42, 57–66. [Google Scholar] [CrossRef]
- Wu, W. Synthesis and Properties of Oleic Acid Amide Polyoxyethylene Ether Carboxylate. Adv. Fine Petrochem. 2019, 20, 18–20, 33. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Wang, C.; Xu, W.; Liu, C. Research of Blocking Removal Technology of Shunting Multi-hydrogen acid with Intralayer CO2 Generation in Offshore Oil Fields. Offshore Oil 2024, 44, 82–87. [Google Scholar] [CrossRef]
- Xie, K.; Li, Q.; Yuan, S.; Lu, X. Adaptation between the Hydrophobically Associating Polymer and Bohai Reservoir Heterogeneity. Oilfield Chem. 2015, 32, 102–107. [Google Scholar] [CrossRef]
- He, C.; Chen, H.; Zhao, H.; Liu, H. Study on rheological of VES self-diversion acid system. Oil Gas Geol. Recovery 2010, 17, 104–107, 118. [Google Scholar] [CrossRef]
- Beysengulov, N.R.; Rees, D.G.; Tayurskii, D.A.; Kono, K. Viscosifying steering determination criterion of viscoelastic surfactant self-steering acid. Spec. Oil Gas Reserv. 2018, 25, 150–154. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, J.; Li, M.; Zhang, G. Indoor Evaluation of Deep Plugging Removal of Steering Sour Oil Formation in Yanchang Oilfield. Contemp. Chem. Ind. 2024, 53, 2119–2123. [Google Scholar] [CrossRef]
- Mao, J.; Wang, C.; Zhang, H.; Zhang, W.; Yang, X.; Zhao, J.; Chen, S. Development and performance evaluation of cationic VES diverting acid system. Chem. Eng. Oil Gas 2019, 48, 65–69. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Wang, Q.; Zhao, P.; Wang, H. Viscoelastic oil displacement system with remarkable salt tolerance via mixed zwitterionic surfactants. J. China Univ. Pet. Ed. Nat. Sci. 2019, 43, 165–170. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y. Research on Comprehensive Management Technology of Oilfield Contenting High Water in Bohai Sea; Beijing Chemical Industry Press: Beijing, China, 2020; ISBN 978-7-122-37641-1. [Google Scholar]
- Zhang, J.; Wang, G.; Wang, L.; Deng, J.; Chen, C.; Zhang, G. Comprehensive treatment technologies for the stripped and deficient wells of Bohai heavy oil field in high water cut stage. Oil Drill. Prod. Technol. 2021, 43, 687–692. [Google Scholar] [CrossRef]
- SY/T 6385-2016; Porosity and Permeability Measurement Under Overburden Pressure. National Energy Administration: Beijing, China, 2016.
- Zhou, J.; Chen, X.; Zheng, Y. Heat-set gels and egg-like vesicles using two component gel system based on chiral calix[4]arenes. Chem. Commun. 2007, 2007, 5200–5202. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Lu, F.; Deng, Q. Tetraphenylethene-Based Covalent Organic Frameworks (COFs): Design, Synthesis and Applications. Prog. Chem. 2024, 36, 67–80. [Google Scholar] [CrossRef]
- Liu, P.; Ran, J.; Zhao, L.; Li, N.; Luo, Z.; Du, J.; Xu, K.; Chen, W. A New Type of Experimental Device for Acid-Liquid Partitioning in Dual Cores. CN Patent 206339468U, 18 July 2017. [Google Scholar]










| Core Samples | Core Number | Experiments | Core Length/cm | Core Diameter/cm | Permeability /m D |
|---|---|---|---|---|---|
| SZ36-1-J20S1 1-006A | 1 | Parallel Flow Displacement Experiments | 4.69 | 2.48 | 535.72 |
| SZ36-1-J20S1 1-006A | 2 | 4.99 | 2.51 | 21.36 | |
| SZ36-1-N12 3-027B | 3 | 2.69 | 2.52 | 143.90 | |
| SZ36-1-N12 3-031B | 4 | 2.72 | 2.51 | 8.89 | |
| SZ36-1-N12 3-029B | 5 | 4.98 | 2.53 | 31.34 | |
| SZ36-1-N12 3-032B | 6 | 5.04 | 2.52 | 6.31 | |
| SZ36-1-N12 3-028B | 7 | 2.60 | 2.52 | 61.65 | |
| SZ36-1-J20S1 1-017A | 8 | 2.53 | 2.50 | 31.33 | |
| Artificial Cores | 9 | Plugging Rate Tests | 4.99 | 2.50 | 533.57 |
| 10 | 5.03 | 2.53 | 142.16 | ||
| 11 | 5.01 | 2.49 | 40.79 | ||
| 12 | 4.87 | 2.50 | 139.70 | ||
| 13 | 4.95 | 2.51 | 252.68 | ||
| 14 | Plugging Strength Tests | 4.82 | 2.49 | 523.15 | |
| 15 | 2.69 | 2.51 | 141.37 | ||
| 16 | 5.02 | 2.52 | 246.71 |
| Name | Unit | Value |
|---|---|---|
| Geothermal Gradient | ℃/100 m | 2.8 |
| Formation Pressure Coefficient | MPa/100 m | 0.97 |
| Buried Depth | m | 1600 |
| Single Layer Thickness | m | 50 |
| Reservoir Porosity | % | 27 |
| Formation Crude Oil Viscosity | MPa·s | 15 |
| Organic Solvent Content (%) | PK01 Content (%) | PK02 Content (%) | Regulator Content (%) | P04 Content (%) | Temperature (°C) | Curing State |
|---|---|---|---|---|---|---|
| 96.7 | 3 | 0 | 0.3 | 2 | 90 | Partial Curing |
| 2.5 | 90 | Mostly Curing | ||||
| 3 | 90 | Mostly Curing | ||||
| 4 | 80 | Mostly Curing | ||||
| 5 | 60/70 | Partial Curing/Mostly Curing | ||||
| 6 | 50/60 | Partial Curing/Mostly Curing | ||||
| 7 | 50/60 | Mostly Curing | ||||
| 96.4 | 3 | 0.3 | 0.3 | 2 | 80/90 | Partial Curing/Mostly Curing (Able to sway) |
| 2.5 | 80/90 | Partial Curing/Mostly Curing | ||||
| 3 | 75/85 | Partial Curing/Mostly Curing | ||||
| 4 | 80 | Fully Curing | ||||
| 5 | 60 | Fully Curing | ||||
| 6 | 50 | Fully Curing | ||||
| 7 | 50 | Fully Curing |
| Experimental Group Number | Core Number | Initial Permeability, Pb/m D | Initial Permeability Max–Min Ratio/C | Permeability After Treatment, Pa/m D | Permeability Max–Min Ratio After Treatment/C | Permeability Ratio After and Before Treatment, Pab/% |
|---|---|---|---|---|---|---|
| 1 | Core1 | 535.72 | 25.08 | 525.89 | 13.26 | 98.17 |
| Core2 | 21.36 | 39.65 | 185.63 | |||
| 2 | Core3 | 143.90 | 16.19 | 137.39 | 6.41 | 95.48 |
| Core4 | 8.89 | 21.44 | 241.17 | |||
| 3 | Core5 | 31.34 | 4.97 | 29.83 | 2.65 | 95.18 |
| Core6 | 6.31 | 11.24 | 178.13 | |||
| 4 | Core7 | 61.65 | 1.97 | 59.50 | 1.2 | 96.51 |
| Core8 | 31.33 | 49.49 | 157.96 |
| Core Number | /m D | Average Permeability After Plugging, /m D | Plugging Rate, w/% |
|---|---|---|---|
| 1 | 533.72 | 10.47 | 98.04 |
| 2 | 142.16 | 3.39 | 97.62 |
| 3 | 40.79 | 1.74 | 95.73 |
| 4 | 139.70 | 6.98 | 95.00 |
| 5 | 252.68 | 12.18 | 95.18 |
| Slug Name | Function and Purpose |
|---|---|
| Pre-flushing fluid slug | Wellbore cleaning, increased permeability, pre-flush formations |
| Disposal fluid slug | Eliminate damage to high-permeability reservoirs |
| Displacement fluid slug | Separating the acid from the TDS |
| TDS fluid slug | Plugging high-permeability reservoirs |
| Displacement fluid slug | Separating the acid from the TDS |
| Disposal fluid slug | Eliminate damage to secondary permeability reservoirs |
| Displacement fluid slug | Separating the acid from the TDS |
| TDS fluid slug | Plugging secondary permeable reservoirs |
| Displacement fluid slug | Separating the acid from the TDS |
| Disposal fluid slug | Eliminate damage to low-permeability reservoirs |
| …… | …… |
| Displacement fluid slug | Protecting slug and preventing TDSfrom gumming up in the wellbore |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Wu, Q.; Chen, W.; Fu, H.; Xu, K.; Xi, H. Indoor Evaluation of a Temperature-Controlled Gel Intelligent Diversion System. Nanomaterials 2025, 15, 547. https://doi.org/10.3390/nano15070547
Luo Z, Wu Q, Chen W, Fu H, Xu K, Xi H. Indoor Evaluation of a Temperature-Controlled Gel Intelligent Diversion System. Nanomaterials. 2025; 15(7):547. https://doi.org/10.3390/nano15070547
Chicago/Turabian StyleLuo, Zhifeng, Qunlong Wu, Weiyu Chen, Haoran Fu, Kun Xu, and Haojiang Xi. 2025. "Indoor Evaluation of a Temperature-Controlled Gel Intelligent Diversion System" Nanomaterials 15, no. 7: 547. https://doi.org/10.3390/nano15070547
APA StyleLuo, Z., Wu, Q., Chen, W., Fu, H., Xu, K., & Xi, H. (2025). Indoor Evaluation of a Temperature-Controlled Gel Intelligent Diversion System. Nanomaterials, 15(7), 547. https://doi.org/10.3390/nano15070547







