Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets
Abstract
:1. Introduction
2. Results and Discussion
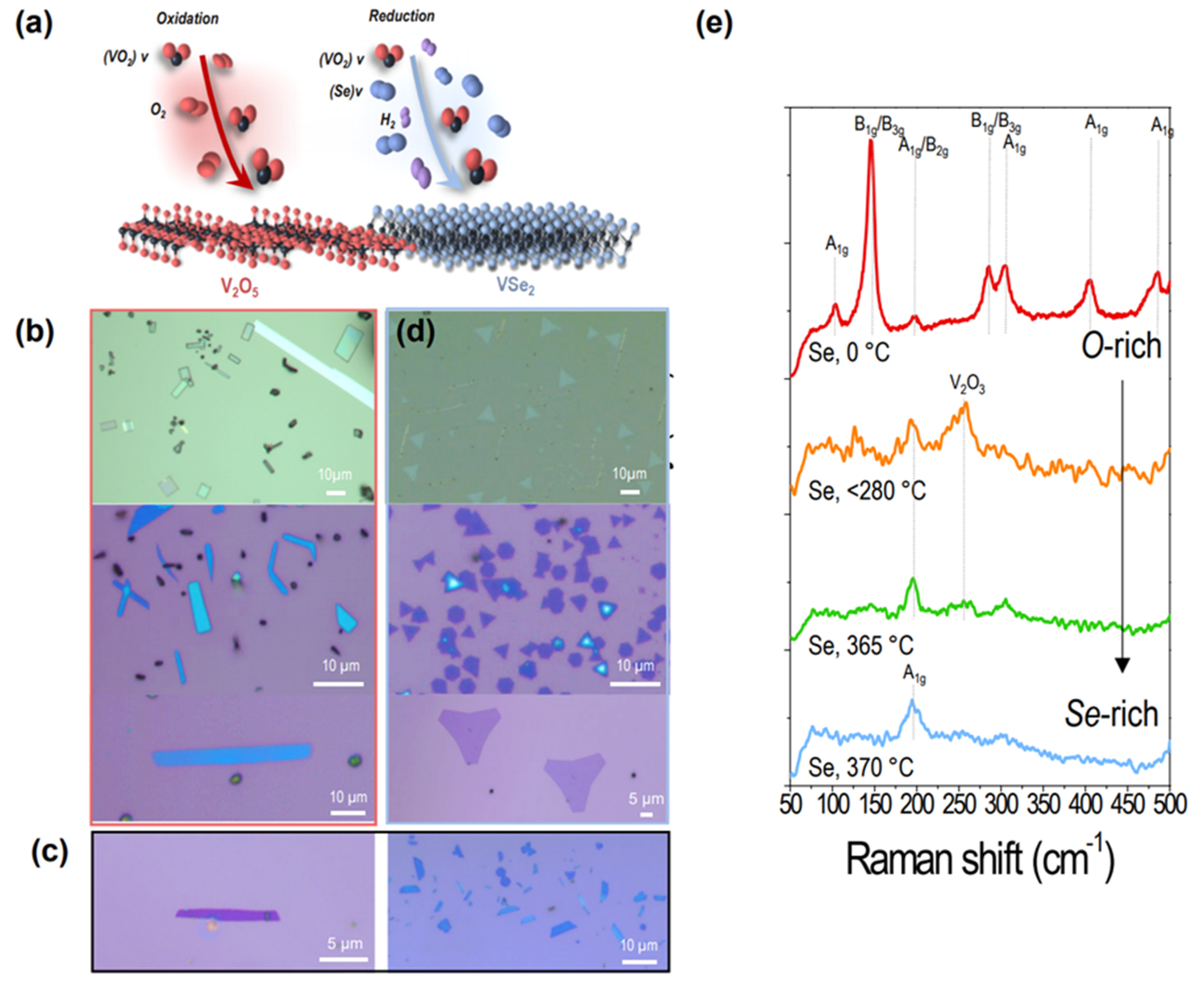
2.1. Vapor-Phase Control of Selenium for Synthesis of O-Rich and Se-Rich Crystals
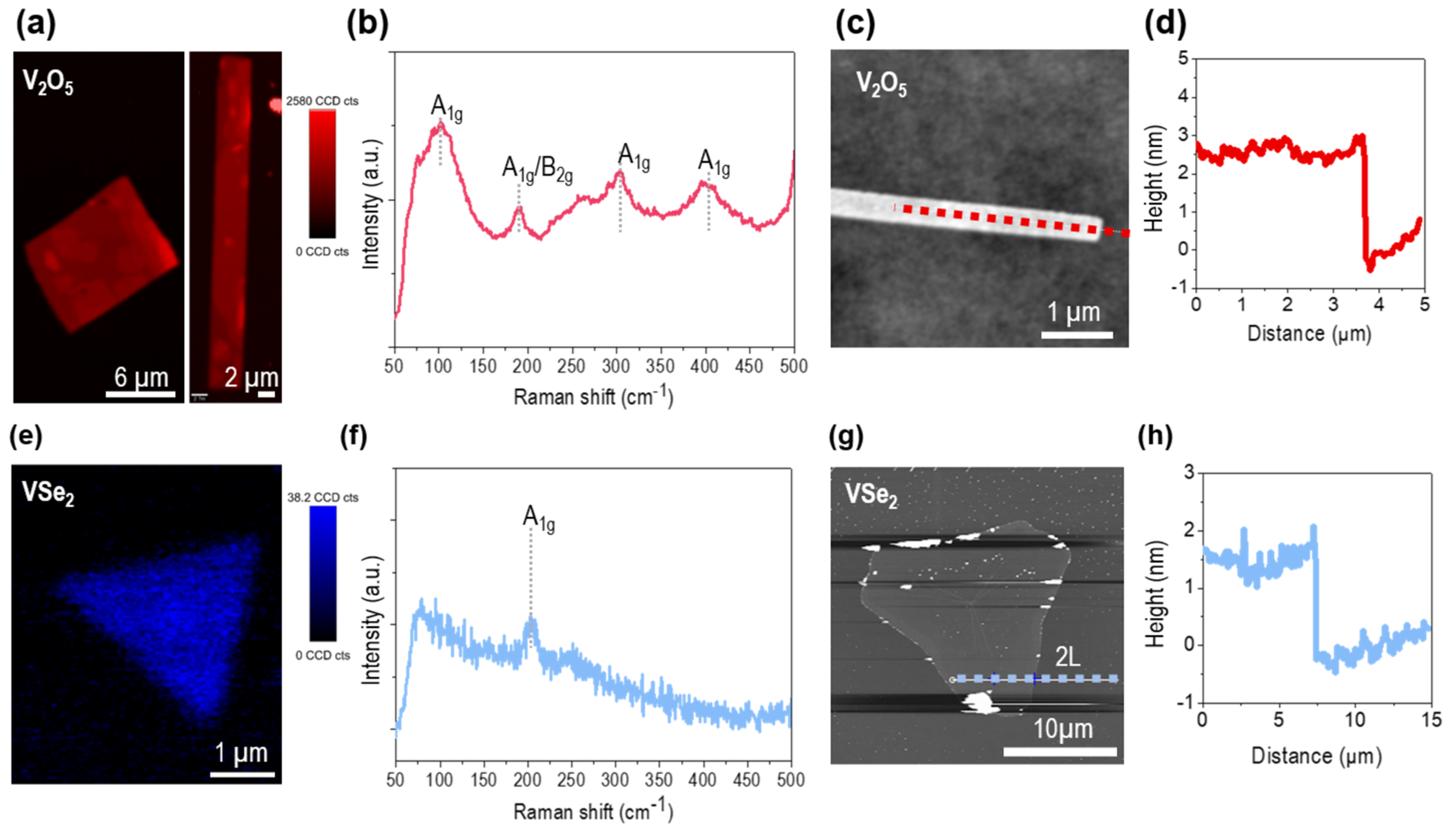
2.2. Material Characterization of Atomically Thin VSe2 and V2O5 Nanosheets
2.3. Crystal Structure of Orthorhombic V2O5 and Trigonal VSe2
3. Materials and Methods
3.1. Chemical Vapor Deposition (CVD) of Atomically Thin Vanadium Compounds
3.2. Gradual Selenium Control Mechanism
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Xian, C.; Wang, J.; Liu, B.; Ling, L.; Zhang, L.; Cao, L.; Qu, Z.; Xiong, Y. Anisotropic anomalous Hall effect in triangular itinerant ferromagnet Fe3GeTe2. Phys. Rev. B 2017, 96, 134428. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Smirnov, M. Raman Microspectrometry Study of Electrochemical Lithium Intercalation into Sputtered Crystalline V2O5 Thin Films. J. Phys. Chem. C 2012, 116, 7181–7187. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High Electron Mobility and Quantum Oscillations in Non-Encapsulated Ultrathin Semiconducting Bi2O2Se. Nat. Nanotechnol. 2017, 12, 530–534. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, B.; Guo, Z.; Su, G.; Gao, R.; Wang, W.; Cao, L. Colloidal synthesis of VSe2single-layer nanosheets as novel electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2016, 52, 9228–9231. [Google Scholar] [CrossRef]
- Bharathi, G.; Hong, S. Prospects of Band Structure Engineering in MXenes for Active Switching MXetronics: Computational Insights and Experimental Approaches. Materials 2024, 18, 104. [Google Scholar] [CrossRef]
- Truong Hoang, Q.; Huynh, K.A.; Nguyen Cao, T.G.; Kang, J.H.; Dang, X.N.; Ravichandran, V.; Kang, H.C.; Lee, M.; Kim, J.E.; Ko, Y.T. Piezocatalytic 2D WS2 Nanosheets for Ultrasound-Triggered and Mitochondria-Targeted Piezodynamic Cancer Therapy Synergized with Energy Metabolism-Targeted Chemotherapy. Adv. Mater. 2023, 35, 18–2300437. [Google Scholar]
- Kang, H.E.; Ko, J.; Song, S.G.; Yoon, Y.S. Recent progress in utilizing carbon nanotubes and graphene to relieve volume expansion and increase electrical conductivity of Si-based composite anodes for lithium-ion batteries. Carbon 2024, 219, 118800. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.-G.; Kim, N.D.; Kim, M.J. Arc discharge synthesis of graphene with enhanced boron doping concentration for electrochemical applications. Appl. Surf. Sci. 2023, 637, 157825. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Seo, T.H.; Kim, Y.H.; Suh, E.-K.; Bae, S.; Hwang, J.Y.; Kim, J.; Kang, Y.; Kim, M.J.; Ahn, S. Two-Dimensional Stacked Composites of Self-Assembled Alkane Layers and Graphene for Transparent Gas Barrier Films with Low Permeability. Nano Lett. 2022, 22, 286–293. [Google Scholar]
- Yoon, H.; Hong, S. Highly improved photocurrent of a flexible MoS2 photodetector via a backside Al metal mirror and its in- and outward folding states. RSC Adv. 2024, 14, 34979–34984. [Google Scholar] [CrossRef]
- Selvam, S.P.; Phan, L.M.; Cho, S. SARS-CoV-2 N Gene-Targeted Anodic Stripping Voltammetry Sensor Using a Novel CoS-NGQD/Pt@Pd Platform and Au-DNA-CdTe QD Probe. Adv. Mater. Technol. 2023, 8, 2201344. [Google Scholar]
- Jung, S.-C.; Jang, W.; Beom, B.; Won, J.-K.; Jeong, J.; Choi, Y.-J.; Moon, M.-K.; Cho, E.-S.; Chang, K.-A.; Han, J.-H. Synthesis of Highly Porous Graphene Oxide–PEI Foams for Enhanced Sound Absorption in High-Frequency Regime. Polymers 2024, 16, 2983. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.H.; Kwon, S.J.; Jeon, Y.; Cho, E. Selective Surface Treatment Using Atmospheric Ar Plasma Jet for Aluminum-doped Zinc Oxide Based Transparent and Flexible Electronics. Adv. Mater. Interfaces 2023, 10, 2300592. [Google Scholar] [CrossRef]
- Bae, D.; Jung, U.; Lee, H.; Yoo, H.; Moon, S.Y.; Lee, K.-H.; Kim, M.J. Synthesis of Double-Walled Boron Nitride Nanotubes from Ammonia Borane by Thermal Plasma Methods. ACS Omega 2023, 8, 21514–21521. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef]
- Lee, C.S.; Han, H.J.; Ahn, J.H.; Jin, G. Area-selective deposition of lateral van der Waals semiconductor heterostructures. Cell Rep. Phys. Sci. 2024, 5, 102254. [Google Scholar]
- Kezilebieke, S.; Huda, N.; Dreher, P.; Manninen, I.; Zhou, Y.; Sainio, J.; Mansell, R.; Ugeda, M.M.; van Dijken, S.; Komsa, H.-P.; et al. Electronic and magnetic characterization of epitaxial VSe2 monolayers on superconducting NbSe2. Commun. Phys. 2020, 3, 116. [Google Scholar] [CrossRef]
- Das, S.; Mohapatra, N. Tunable Physical Properties of VSe2 hexagonal disks. J. Phys. Conf. Ser. 2023, 2518, 012010. [Google Scholar] [CrossRef]
- Song, D.; Zhou, Y.; Zhang, M.; He, X.; Li, X. Structural and Transport Properties of 1T-VSe2 Single Crystal Under High Pressures. Front. Mater. 2021, 8, 710849. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Kan, C.-M.; He, D.; Li, Z.; Hao, Q.; Zhao, H.; Wu, C.; Jin, C.; Cui, X. Structural Phase Transition of Multilayer VSe2. ACS Appl. Mater. Interfaces 2020, 12, 25143–25149. [Google Scholar] [CrossRef]
- Yao, J.; Li, Y.; Massé, R.C.; Uchaker, E.; Cao, G. Revitalized interest in vanadium pentoxide as cathode material for lithium-ion batteries and beyond. Energy Storage Mater. 2018, 11, 205–259. [Google Scholar]
- Natarajan, S.; Kim, S.J.; Aravindan, V. Restricted lithiation into a layered V2O5 cathode towards building “rocking-chair” type Li-ion batteries and beyond. J. Mater. Chem. A 2020, 8, 9483–9495. [Google Scholar]
- De Jesus, L.R.; Andrews, J.L.; Parija, A.; Banerjee, S. Defining Diffusion Pathways in Intercalation Cathode Materials: Some Lessons from V2O5 on Directing Cation Traffic. ACS Energy Lett. 2018, 3, 915–931. [Google Scholar]
- Yue, Y.; Liang, H. Micro- and Nano-Structured Vanadium Pentoxide (V2O5) for Electrodes of Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602545. [Google Scholar]
- Liu, M.; Su, B.; Tang, Y.; Jiang, X.; Yu, A. Recent Advances in Nanostructured Vanadium Oxides and Composites for Energy Conversion. Adv. Energy Mater. 2017, 7, 1700885. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Antonatos, N.; Luxa, J.; Děkanovský, L.; Ashtiani, S.; Fomekong, R.L.; Sofer, Z. Two-dimensional VSe2 nanoflakes as a promising sensing electrocatalyst for nitrobenzene determination in water samples. Electrochim. Acta 2024, 475, 143653. [Google Scholar] [CrossRef]
- Najafi, L.; Oropesa-Nuñez, R.; Bellani, S.; Martín-García, B.; Pasquale, L.; Serri, M.; Drago, F.; Luxa, J.; Sofer, Z.; Sedmidubský, D.; et al. Topochemical Transformation of Two-Dimensional VSe2 into Metallic Nonlayered VO2 for Water Splitting Reactions in Acidic and Alkaline Media. ACS Nano 2022, 16, 351–367. [Google Scholar]
- Cao, L.; Zhu, J.; Li, Y.; Xiao, P.; Zhang, Y.; Zhang, S.; Yang, S. Ultrathin single-crystalline vanadium pentoxide nanoribbon constructed 3D networks for superior energy storage. J. Mater. Chem. A 2014, 2, 13136–13142. [Google Scholar] [CrossRef]
- Zhai, T.; Liu, H.; Li, H.; Fang, X.; Liao, M.; Li, L.; Zhou, H.; Koide, Y.; Bando, Y.; Golberg, D. Centimeter-Long V2O5 Nanowires: From Synthesis to Field-Emission, Electrochemical, Electrical Transport, and Photoconductive Properties. Adv. Mater. 2010, 22, 2547. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Liu, Y.; Huang, B.; Wu, R.; Zhang, Z.; Zhao, B.; Ma, H.; Dang, W.; Wei, Z.; et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nature 2020, 579, 368–374. [Google Scholar]
- Xu, K.; Chen, P.; Li, X.; Wu, C.; Guo, Y.; Zhao, J.; Wu, X.; Xie, Y. Ultrathin Nanosheets of Vanadium Diselenide: A Metallic Two-Dimensional Material with Ferromagnetic Charge-Density-Wave Behavior. Angew. Chem. Int. Ed. 2013, 52, 10477–10481. [Google Scholar] [CrossRef] [PubMed]
- Sugai, S.; Murase, K.; Uchida, S.; Tanaka, S. Investigation of the charge density waves in IT-VSe2 by raman scattering. J. De Phys. Colloq. 1981, 42, 740–742. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Lucazeau, G. Infrared and Raman study of amorphous V2O5. J. Raman Spectrosc. 1982, 12, 68. [Google Scholar] [CrossRef]

| Sample | Raman Shift (cm−1) | Vibration Mode |
|---|---|---|
| VSe2 | 200.3 | A1g |
| 2L-VSe2 | 206.1 | A1g |
| V2O5 | 103.7 | A1g |
| V2O5 | 145.6 | B1g/B3g |
| V2O5 | 196.8 | A1g/B2g |
| V2O5 | 284.7 | B1g/B3g |
| V2O5 | 304.4 | A1g |
| V2O5 | 405.4 | A1g |
| V2O5 | 484.1 | A1g |
| 3 nm thick V2O5 | 102.3 | A1g |
| 3 nm thick V2O5 | 190.2 | A1g/B2g |
| 3 nm thick V2O5 | 303.2 | A1g |
| 3 nm thick V2O5 | 401.5 | A1g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G. Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. Nanomaterials 2025, 15, 548. https://doi.org/10.3390/nano15070548
Jin G. Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. Nanomaterials. 2025; 15(7):548. https://doi.org/10.3390/nano15070548
Chicago/Turabian StyleJin, Gangtae. 2025. "Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets" Nanomaterials 15, no. 7: 548. https://doi.org/10.3390/nano15070548
APA StyleJin, G. (2025). Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. Nanomaterials, 15(7), 548. https://doi.org/10.3390/nano15070548





