Progress in Modeling and Applications of Solid Electrolyte Interphase Layers for Lithium Metal Anodes
Abstract
1. Introduction
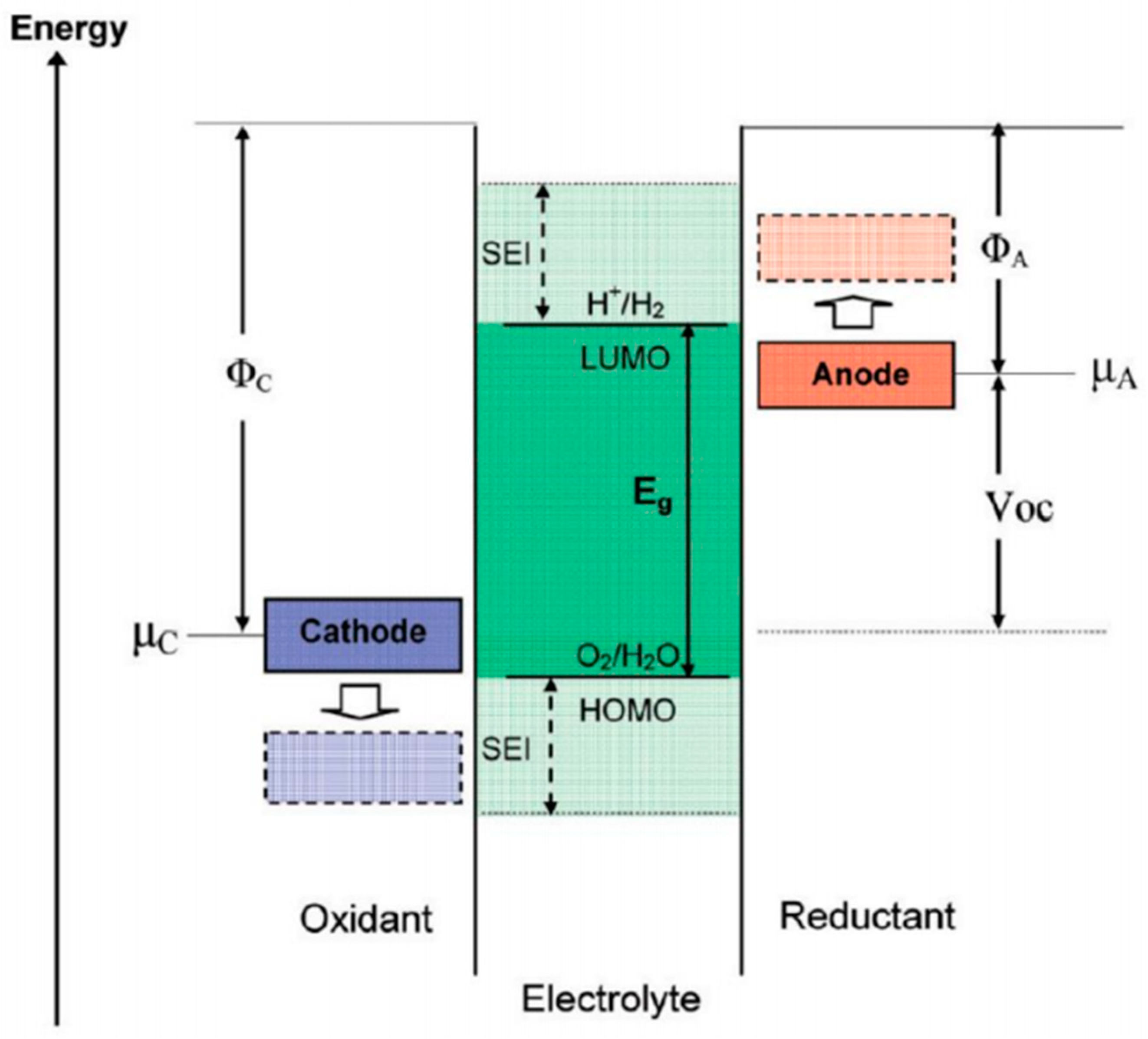
2. SEI Formation Mechanism
3. Overview of SEI Modeling Development
3.1. Development of Composition and Structure Model for SEI
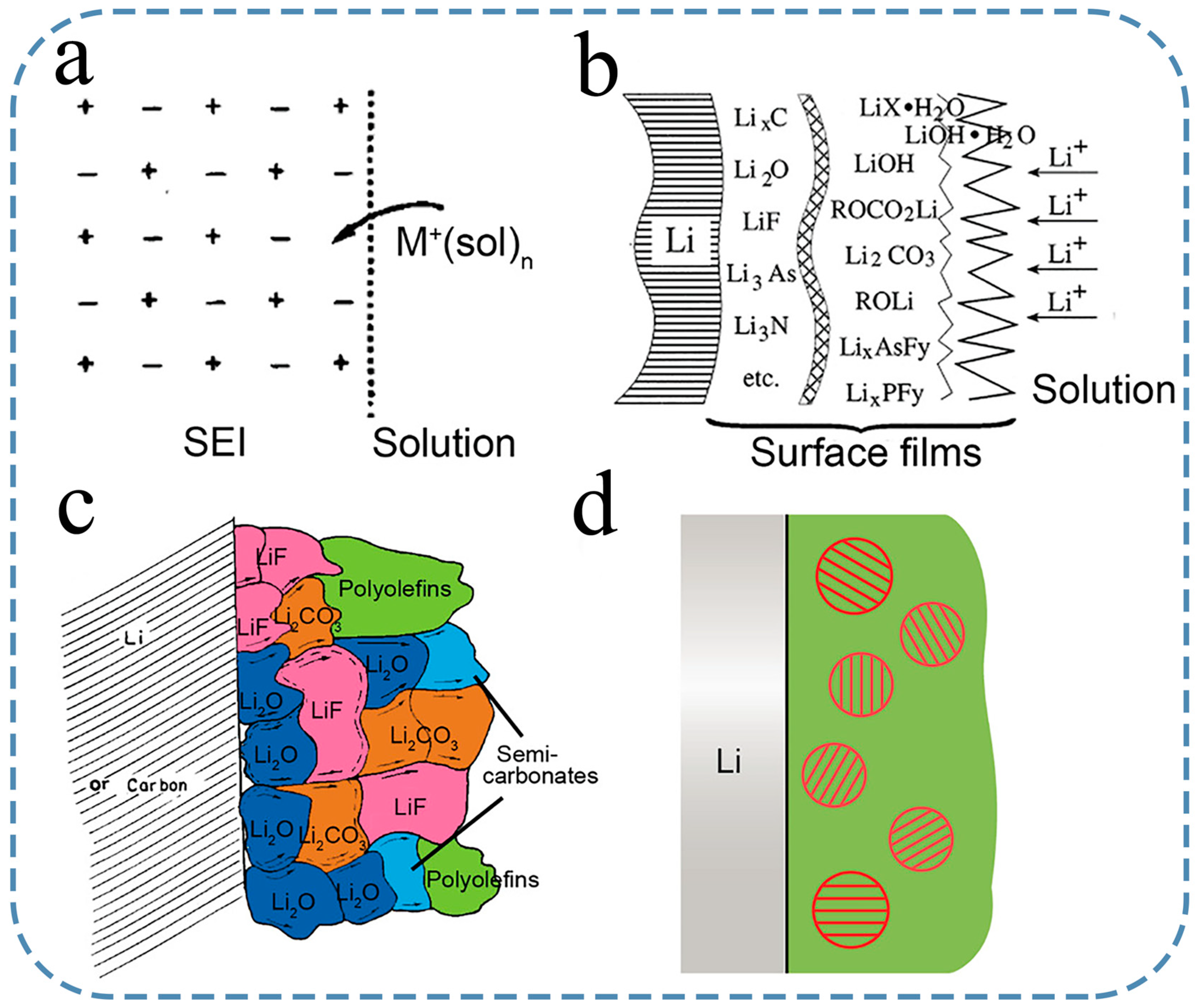
3.2. Development of SEI Growth Prediction Model
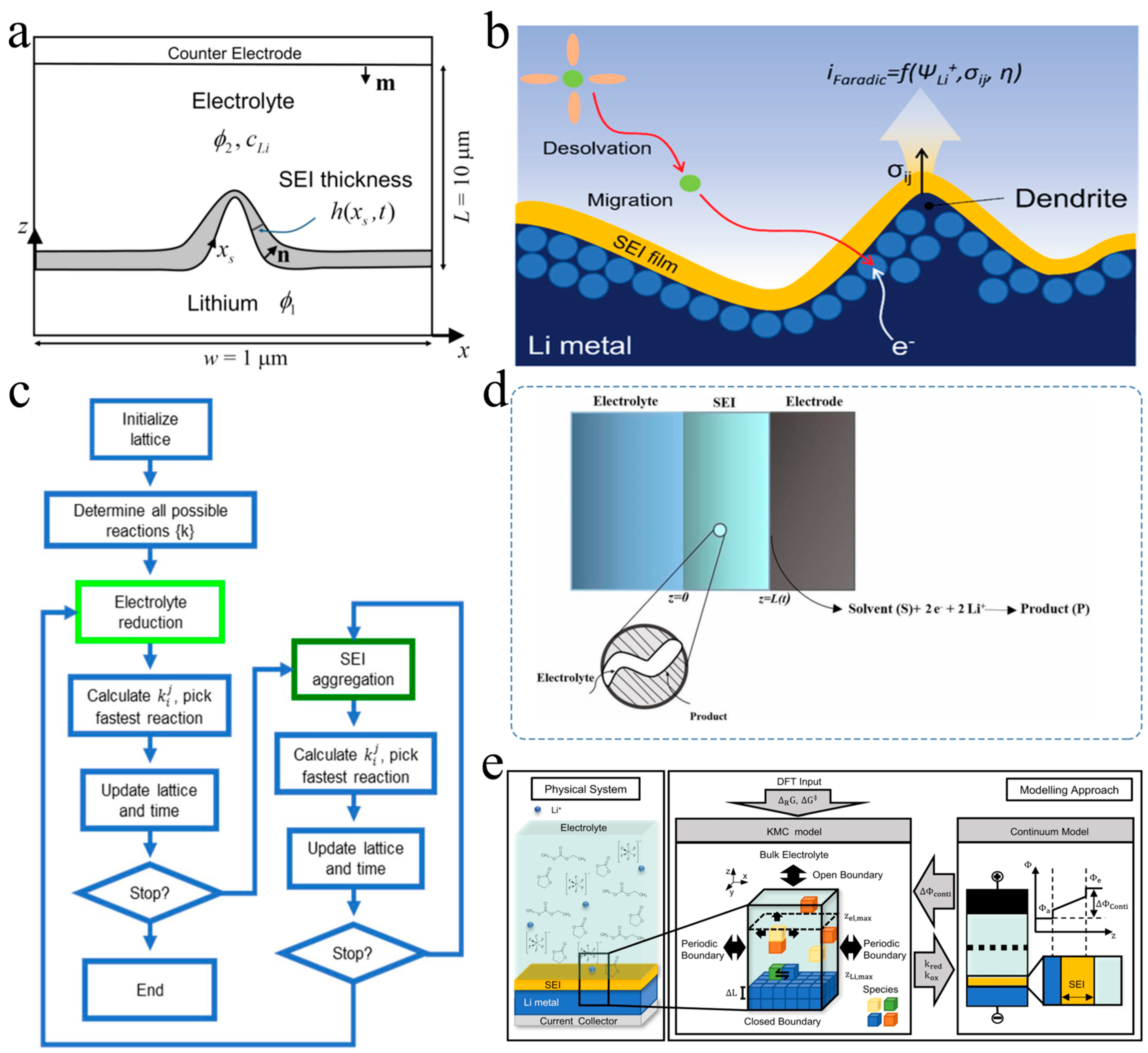
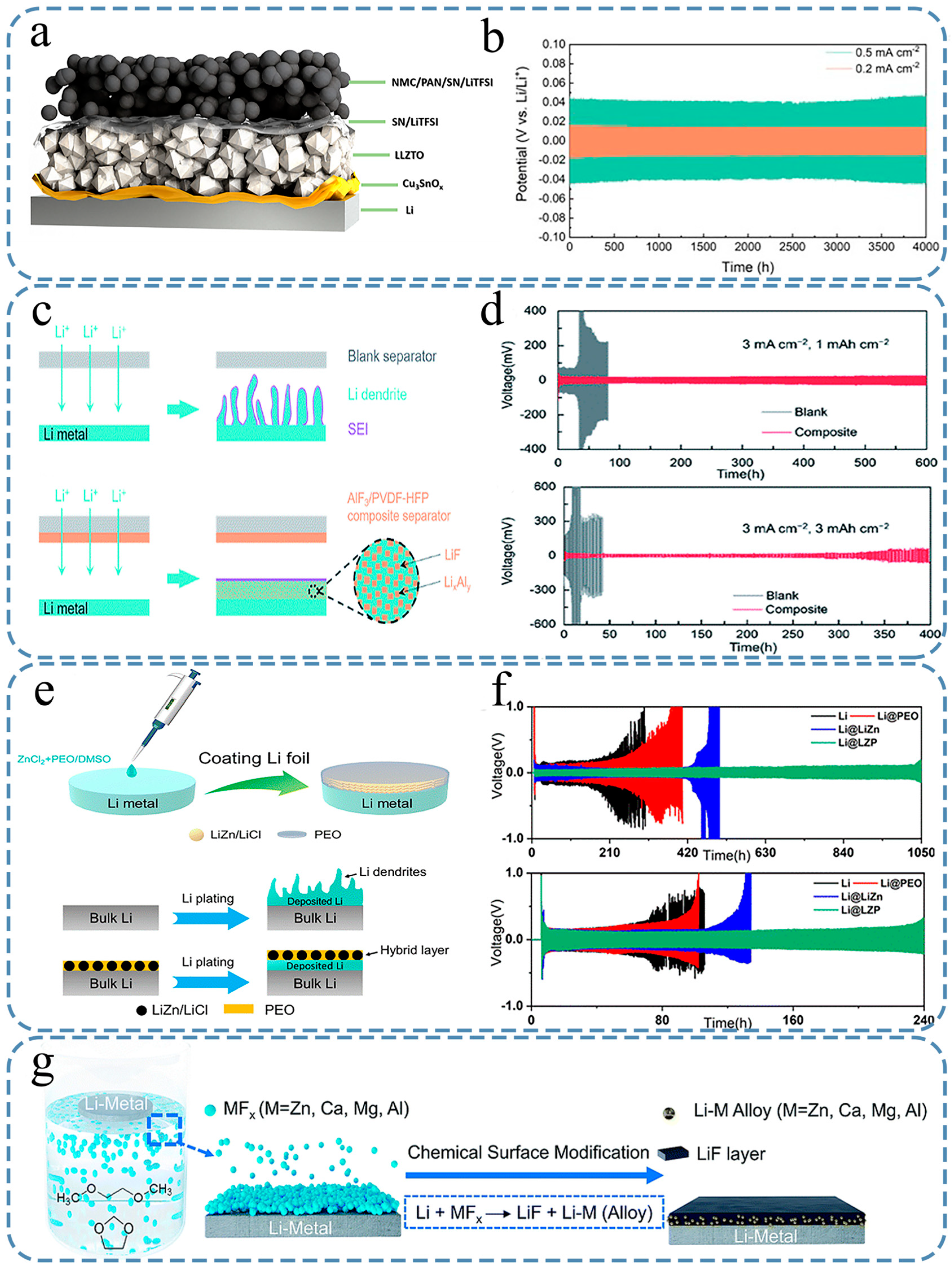
4. Construction of Functional SEIs
4.1. Artificial Inorganic SEI
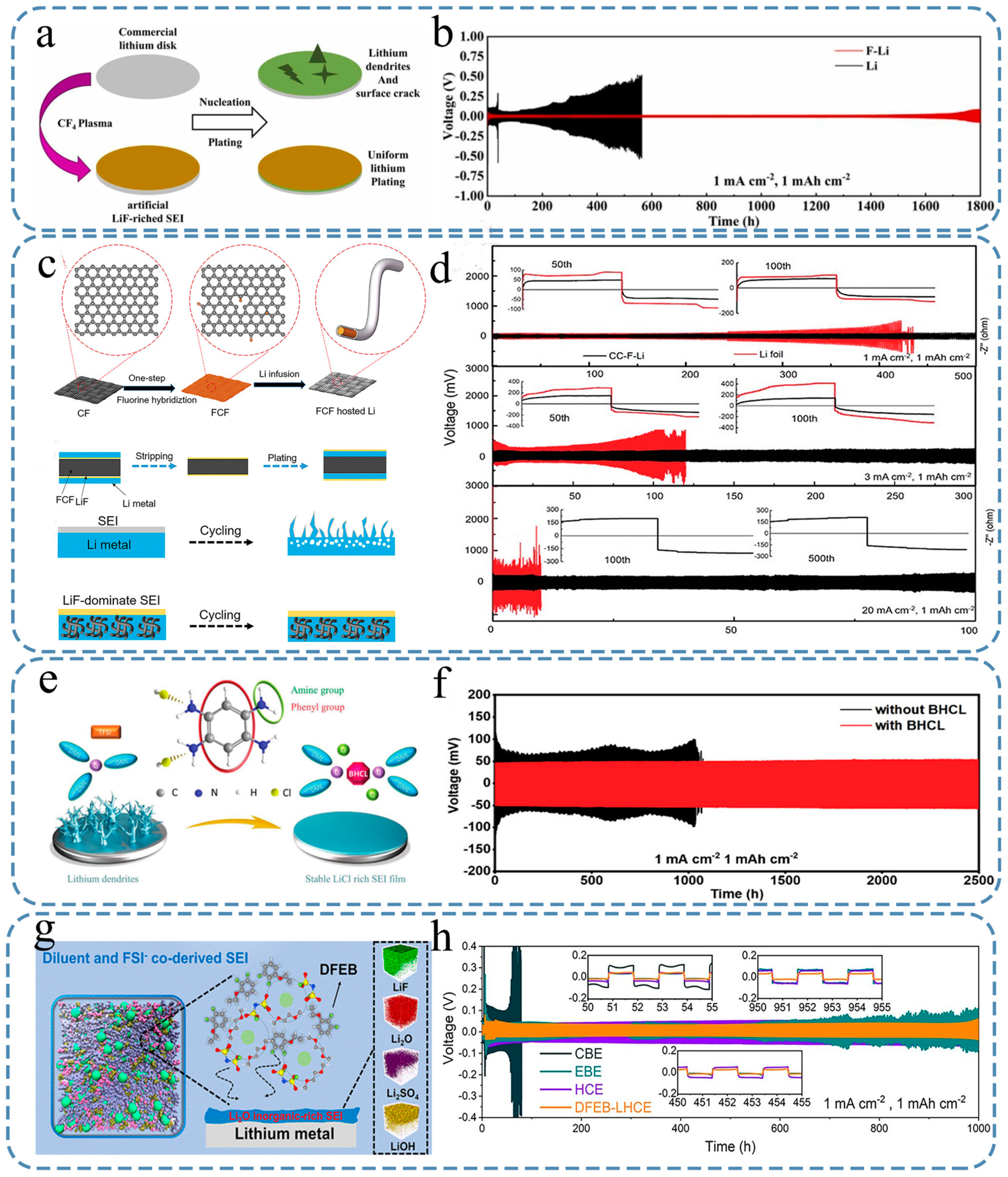
4.2. Artificial Organic SEI

4.3. Artificial Inorganic/Organic Composite SEI
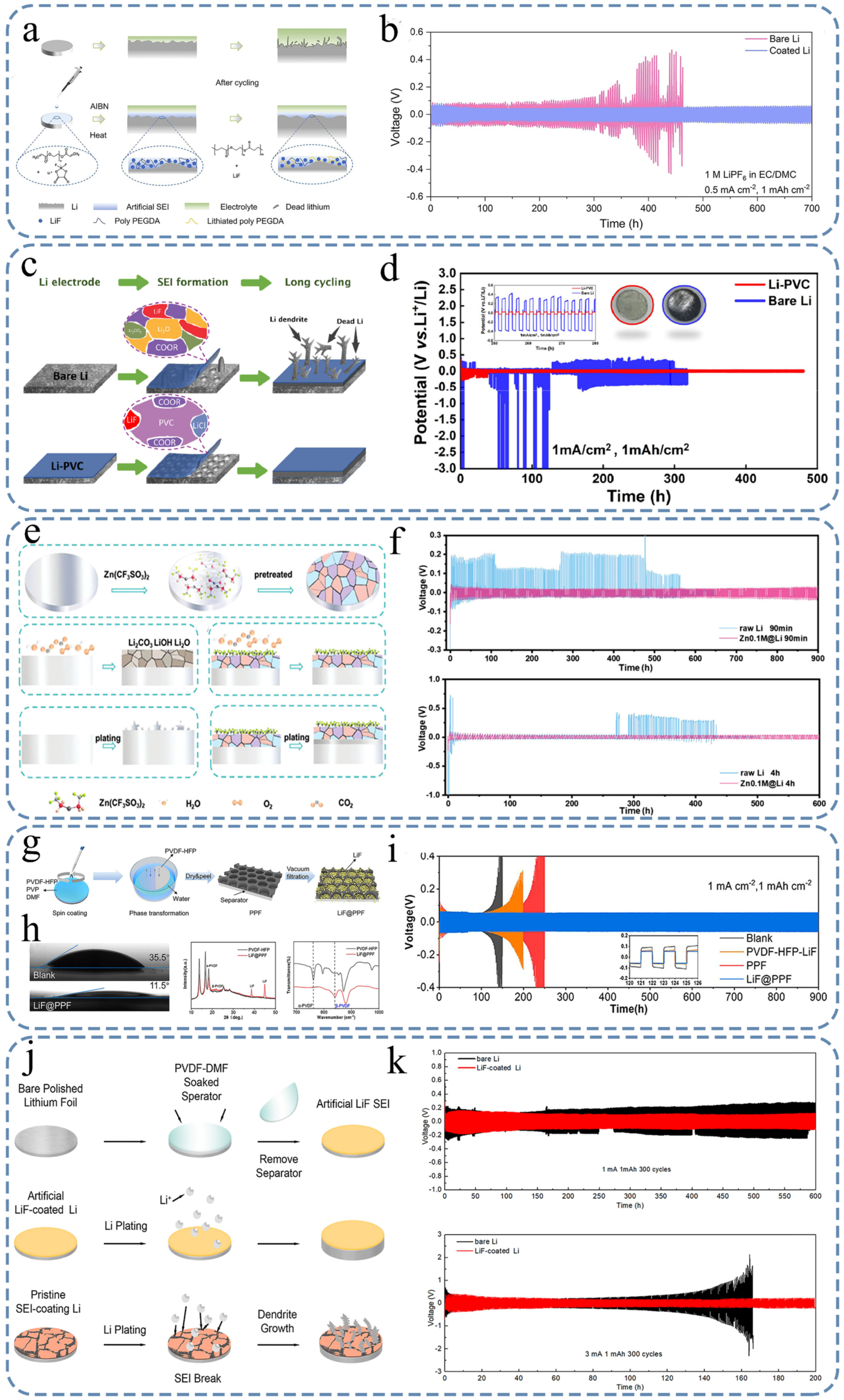
4.4. Alloy Interphase Layer
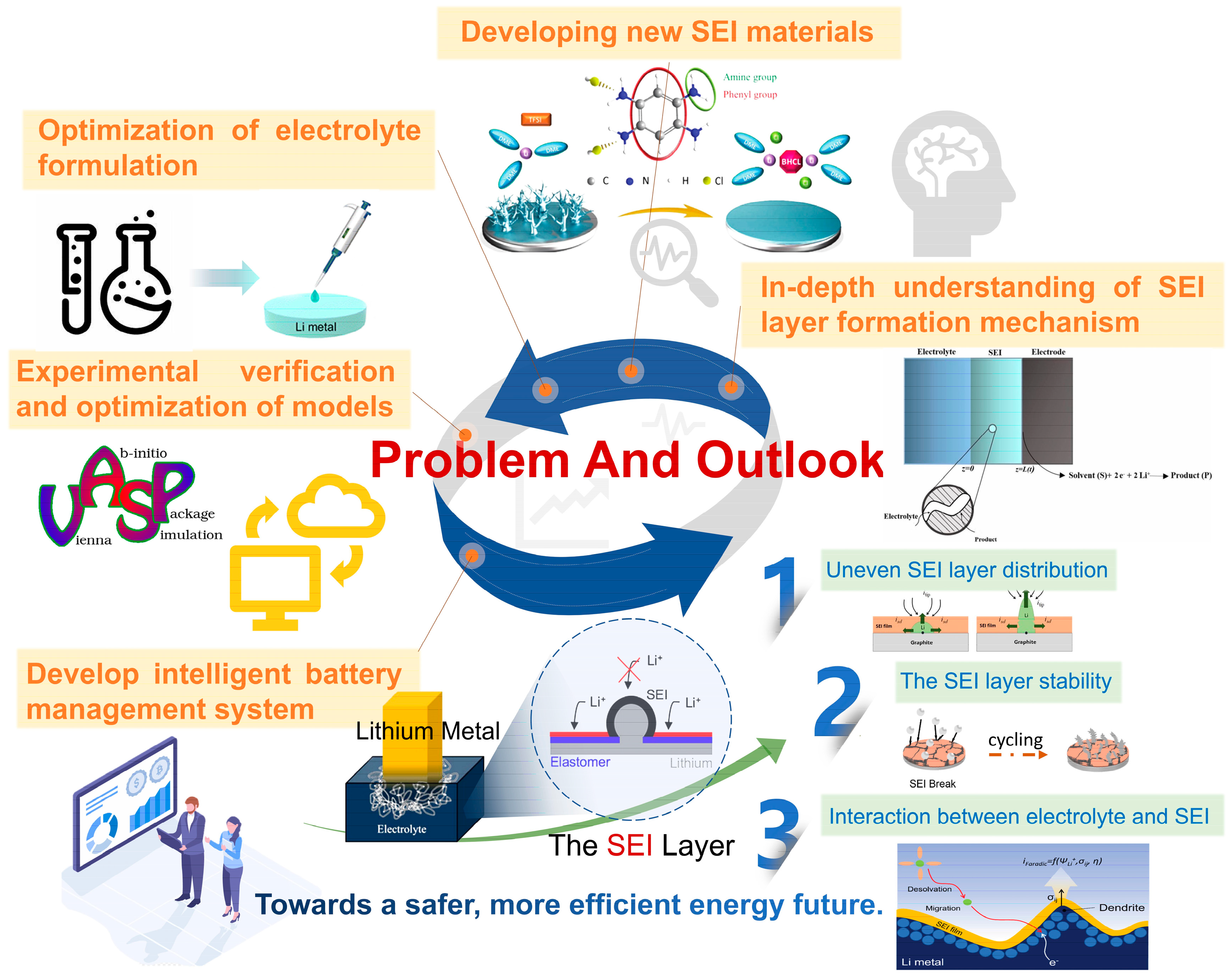
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SEI | solid electrolyte interphase |
| LMBs | lithium metal batteries |
| LMA | lithium metal anode |
| CES | Consumer Electronics Show |
| Si | silicon |
| LUMO | lowest unoccupied molecular orbital |
| EC | ethylene carbonate |
| DMC | dimethyl carbonate |
| FTIR | Fourier transform infrared spectrometer |
| XPS | X-ray photoelectron spectroscopy |
| TEM | transmission electron microscopy |
| EQCM | electrochemical quartz crystal microbalance |
| ToF-SIMS | time-of-flight secondary ion mass spectrometry |
| DFT | density functional theory |
| MD | molecular dynamics |
| PFM | phase-field models |
| MC | Monte Carlo |
| EC | ethylene carbonate |
| LiEC | lithium ethyl carbonate |
| Li2BDC | dilithium butylene dicarbonate |
| LiPC | lithium phthalocyanine |
| UFF | universal force field |
| COMPASS | Condensed-phase Optimized Molecular Potentials for Atomistic Simulation Studies |
| ReaxFF | Reactive Force Field |
| VC | vinylene carbonate |
| FEC | fluoroethylene carbonate |
| AFM | atomic force microscope |
| SIMS | secondary ion mass spectrometry |
| FIB | focused ion beam |
| cryo-TEM | cryogenic transmission electron microscopy |
| kMC | kinetic Monte Carlo |
| LiFSI | lithium bis(fluorosulfonyl)imide |
| F5DEE | 1-(2,2-difluoroethoxy)-2-(2,2,2-trifluoroethoxy)ethane |
| LIBs | lithium-ion batteries |
| ASBs | all-solid-state batteries |
| AI | artificial intelligence |
| LiPF6 | lithium hexafluorophosphate |
| ROCO2Li | lithium semicarbonates |
| FES | finite element simulations |
| DOL | 1,3-dioxolane |
| BHCL | 1,2,4,5-benzenetetramine with high amine content |
| DFEB | 2, 3-difluoroethoxy-benzene |
| LHCEs | localized high-concentration electrolytes |
| SPAN | sulfurized polyacrylonitrile |
| CE | coulombic efficiency |
| PAMPSLi | PVDF-lithium poly (acrylamide-2-methyl-1-propane-sulfonate) |
| HNT | halloysite nanotubes |
| PEO | polyethylene oxide |
| LiPAA | Li polyacrylic acid |
| AIBN | azodiisobutyronitrile |
| PEGDA | poly (ethylene glycol) diacrylate |
| YF3-PAN-CNFs | YF3-doped PAN-based carbon nanofibers |
| PCNFs | as-prepared porous CNFs |
| PVC | polyvinyl chloride |
| EP | electrochemical polymerization |
| ALD | atomic layer deposition |
| PVDF | polyvinylidene fluoride |
| DMF | dimethyl formamide |
| Al(MMP)3 | aluminum coordination compound |
References
- Ju, Z.; Lu, G.; Sheng, O.; Yuan, H.; Zhou, S.; Liu, T.; Liu, Y.; Wang, Y.; Nai, J.; Zhang, W.; et al. Soybean Protein Fiber Enabled Controllable Li Deposition and a LiF-Nanocrystal-Enriched Interface for Stable Li Metal Batteries. Nano Lett. 2022, 22, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Kapitanova, O.O.; Evdokimov, P.V.; Song, Z.; Matic, A.; Xiong, S. Electro-Chemo-Mechanical Modeling of Artificial Solid Electrolyte Interphase to Enable Uniform Electrodeposition of Lithium Metal Anodes. Adv. Energy Mater. 2022, 12, 2103589. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Chen, L.; Yan, F.; Lin, Z.; Wang, J.; Qiu, J.; Cao, G.; Wang, B.; Zhang, H. Expanding the reversibility of graphite-Li metal hybrid anodes by interface and inner-structure modifications. Energy Storage Mater. 2022, 53, 621–628. [Google Scholar] [CrossRef]
- Li, J.; Su, H.; Li, M.; Xiang, J.; Wu, X.; Liu, S.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Fluorinated Interface Layer with Embedded Zinc Nanoparticles for Stable Lithium-Metal Anodes. ACS Appl. Mater. 2021, 13, 17690–17698. [Google Scholar] [CrossRef]
- Ju, Z.; Jin, C.; Cai, X.; Sheng, O.; Wang, J.; Luo, J.; Yuan, H.; Lu, G.; Tao, X.; Liang, Z. Cationic Interfacial Layer toward a LiF-Enriched Interphase for Stable Li Metal Batteries. ACS Energy Lett. 2022, 8, 486–493. [Google Scholar] [CrossRef]
- Wang, Q.; Wan, J.; Cao, X.; Wen, R.; Guo, Y.; Liu, W.; Zhou, H. Organophosphorus Hybrid Solid Electrolyte Interphase Layer Based on LixPO4 Enables Uniform Lithium Deposition for High-Performance Lithium Metal Batteries. Adv. Funct. Mater. 2021, 32, 2107923. [Google Scholar] [CrossRef]
- Yuan, H.; Ding, X.; Liu, T.; Nai, J.; Wang, Y.; Liu, Y.; Liu, C.; Tao, X. A review of concepts and contributions in lithium metal anode development. Mater. Today 2022, 53, 173–196. [Google Scholar] [CrossRef]
- Kim, M.S.; Zhang, Z.; Wang, J.; Oyakhire, S.T.; Kim, S.C.; Yu, Z.; Chen, Y.; Boyle, D.T.; Ye, Y.; Huang, Z.; et al. Revealing the Multifunctions of Li3N in the Suspension Electrolyte for Lithium Metal Batteries. ACS Nano. 2023, 17, 3168–3180. [Google Scholar] [CrossRef]
- Xu, S.; Duan, H.; Shi, J.; Zuo, T.; Hu, X.; Lang, S.; Ya, M.; Liang, J.; Yang, Y.; Kong, Q.; et al. In situ fluorinated solid electrolyte interphase towards long-life lithium metal anodes. Nano Res. 2020, 13, 430–436. [Google Scholar] [CrossRef]
- Lu, G.; Nai, J.; Yuan, H.; Wang, J.; Zheng, J.; Ju, Z.; Jin, C.; Wang, Y.; Liu, T.; Liu, Y.; et al. In-Situ Electrodeposition of Nanostructured Carbon Strengthened Interface for Stabilizing Lithium Metal Anode. ACS Nano. 2022, 16, 9883–9893. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Wang, J.; Yuan, H.; Liu, Y.; Liu, T.; Luo, J.; Nai, J.; Tao, X. Toward Understanding the Effect of Fluoride Ions on the Solvation Structure in Lithium Metal Batteries: Insights from First-Principles Simulations. ACS Appl. Mater. 2022, 14, 48762–48769. [Google Scholar] [CrossRef]
- Wan, J.; Chen, W.; Liu, G.; Shi, Y.; Xin, S.; Guo, Y.; Wen, R.; Wan, L. Insights into the nitride-regulated processes at the electrolyte/electrode interface in quasi-solid-state lithium-metal batteries. J. Energy Chem. 2021, 67, 780–786. [Google Scholar] [CrossRef]
- Dong, K.; Xu, Y.; Tan, J.; Osenberg, M.; Sun, F.; Kochovski, Z.; Pham, D.T.; Mei, S.; Hilger, A.; Ryan, E.; et al. Unravelling the Mechanism of Lithium Nucleation and Growth and the Interaction with the Solid Electrolyte Interface. ACS Energy Lett. 2021, 6, 1719–1728. [Google Scholar] [CrossRef]
- Bao, W.; Wang, R.; Sun, K.; Qian, C.; Zhang, Y.; Li, J. Interface Crystallographic Optimization of Crystal Plane for Stable Metallic Lithium Anode. ACS Appl. Mater. 2022, 14, 38696–38705. [Google Scholar] [CrossRef]
- Fang, R.; Han, Z.; Li, J.; Yu, Z.; Pan, J.; Cheong, S.; Tilley, R.D.; Trujillo, F.; Wang, D.-W. Rationalized design of hyperbranched trans-scale graphene arrays for enduring high-energy lithium metal batteries. Sci. Adv. 2022, 8, eadc9961. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, K.; Jie, Y.; Adelhelm, P.; Chen, Y.; Xu, L.; Yu, P.; Kim, J.; Kochovski, Z.; Yu, Z.; et al. Promoting Mechanistic Understanding of Lithium Deposition and Solid-Electrolyte Interphase (SEI) Formation Using Advanced Characterization and Simulation Methods: Recent Progress, Limitations, and Future Perspectives. Adv. Energy Mater. 2022, 12, 2200398. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, S.; Oh, K.; Kang, K. Challenges and Strategies towards Practically Feasible Solid-State Lithium Metal Batteries. Adv. Mater. 2021, 34, 2104666. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Zhang, J.; Li, L.; Jia, L.; Guan, Q.; Hu, H.; Liu, H.; Jia, Y.; Zhuang, Q.; et al. Hydrophobic lithium diffusion-accelerating layers enables long-life moisture-resistant metallic lithium anodes in practical harsh environments. Energy Storage Mater. 2022, 52, 210–219. [Google Scholar] [CrossRef]
- Kang, T.; Wang, Y.; Guo, F.; Liu, C.; Zhao, J.; Yang, J.; Lin, H.; Qiu, Y.; Shen, Y.; Lu, W.; et al. Self-Assembled Monolayer Enables Slurry-Coating of Li Anode. ACS Cent. Sci. 2019, 5, 468–476. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Kevin, L.; Susan, B.R.; Michael, E.F.; Yuguang, M.; Julibeth, M.M.d.l.H.; Na, S.; Perla, B.B. Modeling Electrochemical Decomposition of Fluoroethylene Carbonate on Silicon Anode Surfaces in Lithium Ion Batteries. J. Electrochem. Soc. 2013, 161, A213. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J. Power Sources 1999, 81, 95–111. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Spotte-Smith, E.W.C.; Kam, R.L.; Barter, D.; Xie, X.; Hou, T.; Dwaraknath, S.; Blau, S.M.; Persson, K.A. Toward a Mechanistic Model of Solid–Electrolyte Interphase Formation and Evolution in Lithium-Ion Batteries. ACS Energy Lett. 2022, 7, 1446–1453. [Google Scholar] [CrossRef]
- Ujwal Shreenag, M.; Libin, L.; Sushantha, M.; Paridhi, G. Solid Electrolyte Interphase (SEI), a boon or a bane for lithium batteries: A review on the recent advances. J. Energy Storage 2021, 47, 103564. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta. 1999, 45, 67–86. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Su, Y.-C.; Zhang, X.-G.; Xia, B.-J. A novel perspective on the formation of the solid electrolyte interphase on the graphite electrode for lithium-ion batteries. Electrochim. Acta 2009, 55, 1785–1794. [Google Scholar] [CrossRef]
- Steinhauer, M.; Diemant, T.; Heim, C.; Jürgen Behm, R.; Wagner, N.; Andreas Friedrich, K. Insights into solid electrolyte interphase formation on alternative anode materials in lithium-ion batteries. J. Appl. Electrochem. 2016, 47, 249–259. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes. J. Electrochem. 1997, 144, L208. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Li, J.; Wang, C.; Dong, S.; Cui, G. Unveiling the dynamic Li+–solvent interaction evolution in lithium metal batteries. Chem. Commun. 2023, 59, 2023–2026. [Google Scholar] [CrossRef]
- Grissa, R.; Fernandez, V.; Fairley, N.; Hamon, J.; Stephant, N.; Rolland, J.; Bouchet, R.; Lecuyer, M.; Deschamps, M.; Guyomard, D.; et al. XPS and SEM-EDX Study of Electrolyte Nature Effect on Li Electrode in Lithium Metal Batteries. ACS Appl. Energy Mater. 2018, 1, 5694–5702. [Google Scholar] [CrossRef]
- Jungjohann, K.; Harrison, K.; Goriparti, S.; Mook, W.; Leenheer, A.; Zavadil, K. In-Situ S/TEM of Li-Ion Batteries: Lithium Metal and Sn Anode Interfacial Processes. Microsc. Microanal. 2018, 24, 1484–1485. [Google Scholar] [CrossRef][Green Version]
- Uchino, Y.; Nishihara, K.; Araki, Y.; Kondo, T. EQCM and EIS Investigation of Lithium Anode Reaction in Various Electrolyte Solutions. ECS Meet. Abstr. 2020, MA2020-02, 3475. [Google Scholar] [CrossRef]
- Mense, M.; Bela, M.M.; Kühn, S.P.; Cekic-Laskovic, I.; Börner, M.; Wiemers-Meyer, S.; Winter, M.; Nowak, S. ToF-SIMS sputter depth profiling of interphases and coatings on lithium metal surfaces. Commun. Chem. 2025, 8, 31. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Xie, W.; Zhao, Q. Exploring Chlorinated Solvents as Electrolytes for Lithium Metal Batteries: A DFT and MD Study. Int. J. Quantum Chem. 2024, 124, e27515. [Google Scholar] [CrossRef]
- Yurkiv, V.; Foroozan, T.; Ramasubramanian, A.; Shahbazian-Yassar, R.; Mashayek, F. Phase-field modeling of solid electrolyte interface (SEI) influence on Li dendritic behavior. Electrochim. Acta 2018, 256, 609–619. [Google Scholar] [CrossRef]
- Takenaka, N.; Suzuki, Y.; Sakai, H.; Nagaoka, M. On Electrolyte-Dependent Formation of Solid Electrolyte Interphase Film in Lithium-Ion Batteries: Strong Sensitivity to Small Structural Difference of Electrolyte Molecules. J. Phys. Chem. C. 2014, 118, 10874–10882. [Google Scholar] [CrossRef]
- Nagaoka, M.; Suzuki, Y.; Okamoto, T.; Takenaka, N. A hybrid MC/MD reaction method with rare event-driving mechanism: Atomistic realization of 2-chlorobutane racemization process in DMF solution. Chem. Phys. Lett. 2013, 538, 80–86. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Casewit, C.J.; Colwell, K.S.; Rappe, A.K. Application of a universal force field to organic molecules. J. Am. Chem. Soc. 1992, 114, 10035–10046. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Weismiller, M.R.; van Duin, A.C.T.; Lee, J.; Yetter, R.A. ReaxFF Reactive Force Field Development and Applications for Molecular Dynamics Simulations of Ammonia Borane Dehydrogenation and Combustion. J. Phys. Chem. A 2010, 114, 5485–5492. [Google Scholar] [CrossRef]
- Bedrov, D.; Smith, G.D.; van Duin, A.C.T. Reactions of Singly-Reduced Ethylene Carbonate in Lithium Battery Electrolytes: A Molecular Dynamics Simulation Study Using the ReaxFF. J. Phys. Chem. A 2012, 116, 2978–2985. [Google Scholar] [CrossRef]
- Islam, M.M.; van Duin, A.C.T. Reductive Decomposition Reactions of Ethylene Carbonate by Explicit Electron Transfer from Lithium: An eReaxFF Molecular Dynamics Study. J. Phys. Chem. C 2016, 120, 27128–27134. [Google Scholar] [CrossRef]
- Kim, S.-P.; Duin ACTv Shenoy, V.B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: A molecular dynamics study. J. Power Sources 2011, 196, 8590–8597. [Google Scholar] [CrossRef]
- Yu, J.; Perla, B.B.; Joanne, B.; Kevin, L. Hybrid DFT Functional-Based Static and Molecular Dynamics Studies of Excess Electron in Liquid Ethylene Carbonate. J. Electrochem. Soc. 2011, 158, A400. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Theoretical Insights into the Reductive Decompositions of Propylene Carbonate and Vinylene Carbonate: Density Functional Theory Studies. J. Phys. Chem. B 2002, 106, 4486–4495. [Google Scholar] [CrossRef]
- Ushirogata, K.; Sodeyama, K.; Okuno, Y.; Tateyama, Y. Additive Effect on Reductive Decomposition and Binding of Carbonate-Based Solvent toward Solid Electrolyte Interphase Formation in Lithium-Ion Battery. J. Am. Chem. Soc. 2013, 135, 11967–11974. [Google Scholar] [CrossRef] [PubMed]
- Cresce Av Russell, S.M.; Baker, D.R.; Gaskell, K.J.; Xu, K. In Situ and Quantitative Characterization of Solid Electrolyte Interphases. Nano Lett. 2014, 14, 1405–1412. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Li, F.; Liu, C.; Tan, J.; Cheng, H.-M. New insight into the solid electrolyte interphase with use of a focused ion beam. J. Phys. Chem. B 2005, 109, 22205–22211. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.A.; Nishioka, K.; Sato, T.; Yamaguchi, S.; Mori, S. Investigation of graphite composite anode surfaces by atomic force microscopy and related techniques. J. Power Sources 1997, 69, 97–102. [Google Scholar] [CrossRef]
- Alliata, D. Electrochemical SPM investigation of the solid electrolyte interphase film formed on HOPG electrodes. Electrochem. Commun. 2000, 2, 436–440. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Wang, R.; Dong, W.; Lu, W.; Wu, X.; Wang, X.; Li, H.; Chen, L. Influences of Additives on the Formation of a Solid Electrolyte Interphase on MnO Electrode Studied by Atomic Force Microscopy and Force Spectroscopy. J. Phys. Chem. C 2014, 118, 20756–20762. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Aurbach, D.; Zaban, A.; Gofer, Y.; Ely, Y.E.; Weissman, I.; Chusid, O.; Abramson, O. Recent studies of the lithium-liquid electrolyte interface Electrochemical, morphological and spectral studies of a few important systems. J. Power Sources 1995, 54, 76–84. [Google Scholar] [CrossRef]
- Edström, K.; Herstedt, M.; Abraham, D.P. A new look at the solid electrolyte interphase on graphite anodes in Li-ion batteries. J. Power Sources 2006, 153, 380–384. [Google Scholar] [CrossRef]
- Andersson, A.M.; Henningson, A.; Siegbahn, H.; Jansson, U.; Edström, K. Electrochemically lithiated graphite characterised by photoelectron spectroscopy. J. Power Sources 2003, 119, 522–527. [Google Scholar] [CrossRef]
- Lu, P.; Harris, S.J. Lithium transport within the solid electrolyte interphase. Electrochem. Commun. 2011, 13, 1035–1037. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, M.; Yu, X.; Zhang, Y.; Wang, J.-G.; Ren, X.; Cao, R.; Xu, W.; Baer, D.R.; Du, Y.; et al. Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery. Nat. Nanotechnol. 2020, 15, 224–230. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; He, Y.; Chen, Q.; Zhang, J.-G.; Xu, W.; Wang, C. Atomic to Nanoscale Origin of Vinylene Carbonate Enhanced Cycling Stability of Lithium Metal Anode Revealed by Cryo-Transmission Electron Microscopy. Nano Lett. 2019, 20, 418–425. [Google Scholar] [CrossRef]
- Xu, Y.; He, Y.; Wu, H.; Xu, W.; Wang, C. Atomic Structure of Electrochemically Deposited Lithium Metal and Its Solid Electrolyte Interphases Revealed by Cryo–electron Microscopy. Microsc. Microanal. 2019, 25, 2220–2221. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent Progress in Understanding Solid Electrolyte Interphase on Lithium Metal Anodes. Adv. Energy Mater. 2020, 11, 2003092. [Google Scholar] [CrossRef]
- Liu, G.; Lu, W. A Model of Concurrent Lithium Dendrite Growth, SEI Growth, SEI Penetration and Regrowth. J. Electrochem. Soc. 2017, 164, A1826. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Fan, G.; Qiu, X.; Liu, J.; Yan, Z.; Zhang, K.; Cheng, F.; Chen, J. Electroless Formation of a Fluorinated Li/Na Hybrid Interphase for Robust Lithium Anodes. J. Am. Chem. Soc. 2021, 143, 2829–2837. [Google Scholar] [CrossRef]
- Hu, F.; Li, Z.; Wang, S.; Tenhaeff, W.E. Mirror-Like Electrodeposition of Lithium Metal under a Low-Resistance Artificial Solid Electrolyte Interphase Layer. ACS Appl. Mater. Interfaces 2020, 12, 39674–39684. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Matios, E.; Li, W. Critical Role of Ultrathin Graphene Films with Tunable Thickness in Enabling Highly Stable Sodium Metal Anodes. Nano Lett. 2017, 17, 6808–6815. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Rainey, C.; Lu, W. Piezoelectric Mechanism and a Compliant Film to Effectively Suppress Dendrite Growth. ACS Appl. Mater. Interfaces 2020, 12, 51448–51458. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Cheng, Z.; Zhao, Y.; Zhang, B.; Yuan, L.; Shen, Y.; Guo, Z.; Zhang, Y.; Jiang, J.; Huang, Y. A Lithium-Ion Pump Based on Piezoelectric Effect for Improved Rechargeability of Lithium Metal Anode. Adv. Sci. 2019, 6, 1901120. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Hao, H.; Basu, S.; Feng, X.; Xu, Y.; Boscoboinik, J.A.; Nanda, J.; Watt, J.; Mitlin, D. Stable Potassium Metal Anodes with an All-Aluminum Current Collector through Improved Electrolyte Wetting. Adv. Mater. 2020, 32, 2002908. [Google Scholar] [CrossRef]
- Li, R.; Zou, X.; Xu, T.; Wang, H.; Li, B.; Jiao, F.; Chen, F.; Ma, C. Thermal distribution evolution model of SEI in lithium metal anodes. J. Energy Storage 2024, 88, 111664. [Google Scholar] [CrossRef]
- Saul, P.-B.; Dacheng, K.; Perla, B.B. SEI Formation and Lithium-Ion Electrodeposition Dynamics in Lithium Metal Batteries via First-Principles Kinetic Monte Carlo Modeling. ACS Energy Lett. 2024, 9, 5268–5278. [Google Scholar] [CrossRef]
- Kamyab, N.; Weidner, J.W.; White, R.E. Mixed Mode Growth Model for the Solid Electrolyte Interface (SEI). J. Energy Chem. 2019, 166, A334. [Google Scholar] [CrossRef]
- Wagner-Henke, J.; Kuai, D.; Gerasimov, M.; Röder, F.; Balbuena, P.B.; Krewer, U. Knowledge-driven design of solid-electrolyte interphases on lithium metal via multiscale modelling. Nat. Commun. 2023, 14, 6823. [Google Scholar] [CrossRef]
- Phul, S.; Deshpande, A.; Krishnamurthy, B. A Mathematical model to study the effect of potential drop across the SEI layer on the capacity fading of a lithium ion battery. Electrochim. Acta 2015, 164, 281–287. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Tan, J.; Ma, L.; Li, Z.; Wang, Y.; Ye, M.; Shen, J. Structural insights into solid electrolyte interphase (SEI) on lithium metal anode: From design strategies to the stability evaluation. Mater. Today 2023, 69, 287–332. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A Growing Appreciation for the Role of LiF in the Solid Electrolyte Interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Witt, D.; Röder, F.; Krewer, U. Analysis of Lithium-Ion Battery State and Degradation via Physicochemical Cell and SEI Modeling. Batter. Supercaps 2022, 5, e202200067. [Google Scholar] [CrossRef]
- Qi, Y. (Invited) Modeling the Charge Transfer Reactions at Li/SEI/Electrolyte Interfaces in Lithium-Ion Batteries. ECS Meet. Abstr. 2023, MA2023-01, 2452. [Google Scholar] [CrossRef]
- Jagger, B.; Pasta, M. Solid electrolyte interphases in lithium metal batteries. Joule 2023, 7, 2228–2244. [Google Scholar] [CrossRef]
- Li, W.; Tchelepi, H.A.; Ju, Y.; Tartakovsky, D.M. Stability-Guided Strategies to Mitigate Dendritic Growth in Lithium-Metal Batteries. J. Electrochem. Soc. 2022, 169, 060536. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, Y.; Qi, Z.; He, X. Dynamically Reversible Gelation of Electrolyte for Efficient Wide-Temperature Adaptable Energy Storage. Adv. Funct. Mater. 2024, 34, 2316813. [Google Scholar] [CrossRef]
- Ren, F.; Wu, Y.; Zuo, W.; Zhao, W.; Pan, S.; Lin, H.; Yu, H.; Lin, J.; Lin, M.; Yao, X.; et al. Visualizing the SEI formation between lithium metal and solid-state electrolyte. Energy Environ. Sci. 2024, 17, 2743–2752. [Google Scholar] [CrossRef]
- Chen, B.-R.; Kunz, M.R.; Tanim, T.R.; Dufek, E.J. A machine learning framework for early detection of lithium plating combining multiple physics-based electrochemical signatures. Cell Rep. Phys. Sci. 2021, 2, 100352. [Google Scholar] [CrossRef]
- Diddens, D.; Appiah, W.A.; Mabrouk, Y.; Heuer, A.; Vegge, T.; Bhowmik, A. Modeling the Solid Electrolyte Interphase: Machine Learning as a Game Changer? Adv. Mater. 2022, 9, 2101734. [Google Scholar] [CrossRef]
- Sara, K.; Xu, Y.; Zheng, Z.; Wang, P. Physics-informed machine learning model for battery state of health prognostics using partial charging segments. Mech. Syst. Sig. Process. 2022, 172, 109002. [Google Scholar] [CrossRef]
- Sun, Q.; Xiang, Y.; Liu, Y.; Xu, L.; Leng, T.; Ye, Y.; Fortunelli, A.; Goddard, W.A.; Cheng, T. Machine Learning Predicts the X-ray Photoelectron Spectroscopy of the Solid Electrolyte Interface of Lithium Metal Battery. J. Phys. Chem. Lett. 2022, 13, 8047–8054. [Google Scholar] [CrossRef] [PubMed]
- Nan, Q.; Kang, Y.; Yajuan, Y.; Rui, L.; Rong, H.; Lai, C.; Yuefeng, S. Machine learning and neural network supported state of health simulation and forecasting model for lithium-ion battery. Front. Eenergy 2023, 18, 223–240. [Google Scholar] [CrossRef]
- Lu, B.; Xia, Y.; Ren, Y.; Xie, M.; Zhou, L.; Vinai, G.; Morton, S.A.; Wee, A.T.S.; van der Wiel, W.G.; Zhang, W.; et al. When Machine Learning Meets 2D Materials: A Review. Adv. Sci. 2024, 11, 2305277. [Google Scholar] [CrossRef]
- Shi, S.; Gao, J.; Liu, Y.; Zhao, Y.; Wu, Q.; Ju, W.; Ouyang, C.; Xiao, R. Multi-scale computation methods: Their applications in lithium-ion battery research and development. Chin. Phys. B 2016, 25, 018212. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Song, Y.; Wang, Y. Machine learning-guided discovery of ionic polymer electrolytes for lithium metal batteries. Nat. Commun. 2023, 14, 2789. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zou, X.; Ma, S.; Liu, D.; Avdeev, M.; Shi, S. Data quantity governance for machine learning in materials science. Natl. Sci. Rev. 2023, 10, nwad125. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Yu, Z.; Liu, Z.; Liu, D.; Lin, H.; Li, M.; Ma, S.; Avdeev, M.; Shi, S. Generative artificial intelligence and its applications in materials science: Current situation and future perspectives. J. Mater. 2023, 9, 798–816. [Google Scholar] [CrossRef]
- Lou, X.; Zhong, J.; Cheng, D.; Han, Q.; Wang, F.; Ji, S.; Sha, W.; Wang, F.; Tian, J.; Zhang, W.; et al. Solvent-free quasi-solid polymer electrolyte with a high dielectric constant for stable lithium metal anodes. Chem. Eng. J. 2023, 468, 143681. [Google Scholar] [CrossRef]
- Xiao, P.; Yun, X.; Chen, Y.; Guo, X.; Gao, P.; Zhou, G.; Zheng, C. Insights into the solvation chemistry in liquid electrolytes for lithium-based rechargeable batteries. Chem. Soc. Rev. 2023, 52, 5255–5316. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Luo, Y.; Shi, S. Computational physics in lithium batteries. Physics 2022, 51, 384–396. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, J.; Xie, Y.; Wei, D.; Jinbiao, C.; Shi, Z. Solid Electrolyte Interphase on Lithium Metal Anodes. ChemSusChem 2024, 17, e202301777. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Liu, Z.; Weng, S.; Li, X.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Phase Diagram Determined Lithium Plating/Stripping Behaviors on Lithiophilic Substrates. ACS Energy Lett. 2021, 6, 4118–4126. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Eli, Y.; Markovsky, B.; Zaban, A.; Luski, S.; Carmeli, Y.; Yamin, H. The Study of Electrolyte Solutions Based on Ethylene and Diethyl Carbonates for Rechargeable Li Batteries. J. Electrochem. 1995, 142, 2873. [Google Scholar] [CrossRef]
- Swastik, B.; Gyeong, S.H. Amorphization of Inorganic Solid Electrolyte Interphase Components and Its Impact on Enhancing Their Transport and Mechanical Properties. ACS Appl. Mater. 2023, 15, 59494–59501. [Google Scholar] [CrossRef]
- Zhou, H.P.; Zhang, H.; Wang, H.M.; Zhang, S.; Feng, T.T.; Xu, Z.Q.; Fang, Z.X.; Wu, M.Q. Plasma Grown Fluoride-rich Artificial SEI for Stabilizing Li Metal Anodes. J. Alloys Compd. 2022, 935, 168081. [Google Scholar] [CrossRef]
- Han, Y.; Fang, R.; Lu, C.; Wang, K.; Zhang, J.; Xia, X.; He, X.; Gan, Y.; Huang, H.; Zhang, W.; et al. LiF-Rich Interfacial Protective Layer Enables Air-Stable Lithium Metal Anodes for Dendrite-Free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 31543–31551. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Luo, L.; Pang, Y.; Yang, J.; Huang, Y.; Zheng, S. Highly Stable and Ultrahigh-Rate Li Metal Anode Enabled by Fluorinated Carbon Fibers. Small 2020, 17, 2006002. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Liu, Y.; Zheng, J.; Xia, Y.; Tao, X.; Wang, Y.; Xia, X.; Huang, H.; Gan, Y.; et al. High-Performance Solid Lithium Metal Batteries Enabled by LiF/LiCl/LiIn Hybrid SEI via InCl3-Driven In Situ Polymerization of 1,3-Dioxolane. Small 2023, 19, 2303210. [Google Scholar] [CrossRef]
- Lin, Z.; Frederik, B.; Li, T.; Sreeja, K.S.; Liu, Y.; Zhang, C.; Ding, F.; Zhang, L. structing LiCl-Rich Solid Electrolyte Interphase by High Amine-Containing 1,2,4,5-Benzenetetramine Tetrahydrochloride Additive. Adv. Electron. Mater. 2023, 10, 2300772. [Google Scholar] [CrossRef]
- Beichel, W.; Skrotzki, J.; Klose, P.; Njel, C.; Butschke, B.; Burger, S.; Liu, L.; Thomann, R.; Thomann, Y.; Biro, D.; et al. An Artificial SEI Layer Based on an Inorganic Coordination Polymer with Self-Healing Ability for Long-Lived Rechargeable Lithium-Metal Batteries. Batter. Supercaps 2021, 5, e202100347. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, K.; Li, J.; Yuan, M.; Wang, J.; Wang, Q.; Lai, A.; Jiang, Y.; Yan, X.; Zhang, G.; et al. Beyond LiF: Tailoring Li2O-Dominated Solid Electrolyte Interphase for Stable Lithium Metal Batteries. ACS Nano 2024, 18, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, W.; Hou, J.; Yan, F.; Hu, R.; Zhao, J.; Fang, F.; Ruan, J.; Song, Y. Separator-SEI interactions: Unlocking new pathways for lithium metal battery stability. Chem. Eng. J. 2025, 508, 160998. [Google Scholar] [CrossRef]
- Liu, J.; Fan, L.-W. Atomistic insights into the thermal transport properties of inorganic components of solid electrolyte interphase (SEI) in lithium-ion batteries. Int. J. Heat Mass Transf. 2023, 221, 125069. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Sun, Y.; Fang, H.; Jiang, Y.; Zhao, B. Theoretical calculation study on the electrochemical properties of lithium halide-based artificial SEI films for lithium metal anodes. Surf. Interfaces 2023, 44, 103768. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Wei, Z.; Xie, T.; Zhu, H.; He, J.; Yang, J.; Tan, Z.; Yan, K.; Huang, S. Enabling Superior Lithium Metal Anodes via the In-Situ Construction of a Li–Sn Alloy/Lithium Halide Biphasic Interface Layer. ACS Appl. Energy Mater. 2024, 7, 9327–9335. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tan, S.; Yang, Y.; Zhong, H.; Ghose, S.; Waluyo, I.; Hunt, A.; Ma, L.; Yang, X.-Q.; Hu, E. An inorganic-rich but LiF-free interphase for fast charging and long cycle life lithium metal batteries. Nat. Commun. 2023, 14, 8414. [Google Scholar] [CrossRef]
- Hobold, G.M.; Wang, C.; Steinberg, K.; Li, Y.; Gallant, B.M. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high Coulombic efficiency. Nat. Energy 2024, 9, 580–591. [Google Scholar] [CrossRef]
- Zheng, M.-S.; Dong, Q.-F.; Cai, H.-Q.; Jin, M.-G.; Lin, Z.-G.; Sun, S.-G. Formation and Influence Factors of Solid Electrolyte Interphase Film on the Negative Electrode Surface in Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2207. [Google Scholar] [CrossRef]
- Wu, S.; Li, G.; Liu, H.; Duan, H. Porous polymer membrane with uniform lithium-ion transport via phase separation for stable lithium metal batteries. J. Power Sources 2022, 547, 232018. [Google Scholar] [CrossRef]
- Kang, D.; Sardar, S.; Zhang, R.; Noam, H.; Chen, J.; Ma, L.; Liang, W.; Shi, C.; Lemmon, J.P. In-Situ Organic SEI Layer for Dendrite-Free Lithium Metal Anode. Energy Storage Mater. 2020, 27, 69–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, W.; Huang, Z.; Zhou, G.; Deng, Y.; Zhang, J.; Zhang, C.; Hao, B.; Qi, Q.; He, Y.-B.; et al. An air-stable and waterproof lithium metal anode enabled by wax composite packaging. Sci. Bull. 2019, 64, 910–917. [Google Scholar] [CrossRef]
- Liu, H.; Tao, R.; Guo, C.; Zhang, W.; Liu, X.; Guo, P.; Zhang, T.; Liang, J. Lithiated halloysite nanotube/cross-linked network polymer composite artificial solid electrolyte interface layer for high-performance lithium metal batteries. Chem. Eng. J. 2021, 429, 132239. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Z.; Zhuang, J.; Li, G.; Ding, X.; Liu, M.; Zhang, Q.; Liao, Y.; Zhang, Y.; Li, W. Simultaneously Regulating Lithium Ion Flux and Surface Activity for Dendrite-Free Lithium Metal Anodes. ACS Appl. Mater. 2019, 11, 5159–5167. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Wan, J.; Liang, N.-Y.; Yan, C.; Wen, R.; Zhang, Q. Nucleation and Growth Mode of Solid Electrolyte Interphase in Li-Ion Batteries. J. Am. Chem. Soc. 2023, 145, 8001–8006. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Huang, X.; Zhai, Z.; Tang, J.; You, J.; Shi, C.; Li, W.; Dai, P.; Zheng, W.; et al. Rigid and Flexible SEI Layer Formed Over a Cross-Linked Polymer for Enhanced Ultrathin Li Metal Anode Performance. Adv. Energy Mater. 2022, 12, 2103972. [Google Scholar] [CrossRef]
- He, D.; Lu, J.; He, G.; Chen, H. Recent Advances in Solid-Electrolyte Interphase for Li Metal Anode. Front. Chem. 2022, 10, 916132. [Google Scholar] [CrossRef]
- Gao, S.; Sun, F.; Liu, N.; Yang, H.; Cao, P.-F. Ionic conductive polymers as artificial solid electrolyte interphase films in Li metal batteries—A review. Mater. Today. 2020, 40, 140–159. [Google Scholar] [CrossRef]
- Cao, W.; Lu, J.; Zhou, K.; Sun, G.; Zheng, J.; Geng, Z.; Li, H. Organic-inorganic composite SEI for a stable Li metal anode by in-situ polymerization. Nano Energy. 2022, 95, 106983. [Google Scholar] [CrossRef]
- Yang, H.; Guo, C.; Naveed, A.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Deng, N.; Peng, Z.; Tian, X.; Li, Y.; Yan, J.; Liu, Y.; Kang, W. Yttrium trifluoride doped polyacrylonitrile based carbon nanofibers as separator coating layer for high performance lithium-metal batteries. J. Colloid Interface Sci. 2022, 634, 949–962. [Google Scholar] [CrossRef]
- Li, L.; Yang, K.; Xi, C.; Li, M.; Li, B.; Xu, G.; Xiao, Y.; Cui, X.; Liu, Z.; Li, L.; et al. Highly-chlorinated inert and robust interphase without mineralization of oxide enhancing high-rate Li metal batteries. Chin. Chem. Lett. 2023, 35, 108814. [Google Scholar] [CrossRef]
- Lang, J.; Long, Y.; Qu, J.; Luo, X.; Wei, H.; Huang, K.; Zhang, H.; Qi, L.; Zhang, Q.; Li, Z.; et al. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2018, 16, 85–90. [Google Scholar] [CrossRef]
- Li, H.; Zhang, F.; Wei, W.; Zhao, X.; Dong, H.; Yan, C.; Jiang, H.; Sang, Y.; Chen, H.; Liu, H.; et al. Promoting Air Stability of Li Anode via an Artificial Organic/Inorganic Hybrid Layer for Dendrite-Free Lithium Batteries. Adv. Energy Mater. 2023, 13, 2301023. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Zhao, O.; Lee, S.B.; Rubloff, G.W.; Noked, M. Stabilization of Lithium Metal Anodes by Hybrid Artificial Solid Electrolyte Interphase. Chem. Mater. 2017, 29, 6298–6307. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, W.; Li, X.; Fang, D.; Teo, S.L.; Li, Y.; Seh, Z.W.; Tian, B.; Shi, Y.; Yang, H.Y. In-situ construction of a hybrid interfacial protective layer for highly stable Li metal anodes. Mater. Today Energy. 2023, 38, 101444. [Google Scholar] [CrossRef]
- Li, N.; Wei, W.; Xie, K.; Tan, J.; Zhang, L.; Luo, X.; Yuan, K.; Song, Q.; Li, H.; Shen, C.; et al. Suppressing Dendritic Lithium Formation Using Porous Media in Lithium Metal-Based Batteries. Nano Lett. 2018, 18, 2067–2073. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, T.; Zhang, N.; Xue, T.; Chen, Y.; Ye, Y.; Wu, F.; Chen, R. Spatially Confined LiF Nanoparticles in an Aligned Polymer Matrix as the Artificial SEI Layer for Lithium Metal Anodes. Nano Lett. 2023, 23, 276–282. [Google Scholar] [CrossRef]
- Luo, J.; Fang, C.C.; Wu, N.L. High Polarity Poly(vinylidene difluoride) Thin Coating for Dendrite-Free and High-Performance Lithium Metal Anodes. Adv. Energy Mater. 2017, 8, 1701482. [Google Scholar] [CrossRef]
- Sun, S.; Myeong, S.; Kim, J.; Lee, D.; Kim, J.; Park, K.; Kim, J.; Kwon, J.; Paik, U.; Song, T. Design of inorganic/organic bi-layered Li protection layer enabled dendrite-free practical Li metal battery. Chem. Eng. J. 2022, 450, 137993. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, X.-B.; Yan, C.; Zhang, X.-Q.; Xiao, Y.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. Artificial Interphases for Highly Stable Lithium Metal Anode. Matter 2019, 1, 317–344. [Google Scholar] [CrossRef]
- McDowell, M.T.; Xia, S.; Zhu, T. The mechanics of large-volume-change transformations in high-capacity battery materials. Extrem. Mech. Lett. 2016, 9, 480–494. [Google Scholar] [CrossRef]
- Zhang, W.-J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2010, 196, 877–885. [Google Scholar] [CrossRef]
- Cui, J.; Yao, S.; Guerfi, A.; Kim, C.; John, B.G.; Khani, H. Melt-quenching of artificial metallic interlayer enables a resistance-free garnet/lithium interface for all-solid-state lithium-metal batteries. Energy Storage Mater. 2022, 53, 899–908. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, F.; Chen, N.; Yang, T.; Liang, Y.; Sun, Z.; Luo, G.; Du, J.; Shang, Y.; Feng, M.; et al. A Dual Functional Artificial SEI Layer Based on a Facile Surface Chemistry for Stable Lithium Metal Anode. Molecules 2022, 27, 5199. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Zhao, T.; Qian, J.; Chen, N.; Li, L.; Wu, F.; Chen, R. In situ formation of a LiF and Li–Al alloy anode protected layer on a Li metal anode with enhanced cycle life. J. Mater. Chem. 2019, 8, 1247–1253. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K.; et al. Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries. Chem 2017, 4, 174–185. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, L.; Shi, F.; Lei, T.; Chen, G.; Pei, A.; Sun, J.; Yan, K.; Zhou, G.; Xie, J.; et al. Surface Fluorination of Reactive Battery Anode Materials for Enhanced Stability. J. Am. Chem. Soc. 2017, 139, 11550–11558. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.T.; Lee, D.-C.; Oschatz, M.; Cho, W.I.; Kaskel, S.; Yushin, G. Enhancing performance of Li–S cells using a Li–Al alloy anode coating. Electrochem. Commun. 2013, 36, 38–41. [Google Scholar] [CrossRef]
- Bang, H.J.; Kim, S.; Prakash, J. Electrochemical investigations of lithium-aluminum alloy anode in Li/polymer cells. J. Power Sources 2001, 92, 45–49. [Google Scholar] [CrossRef]
- Zhao, F.; Deng, W.; Dong, D.; Zhou, X.; Liu, Z. Seamlessly integrated alloy-polymer interphase for high-rate and long-life lithium metal anodes. Mater. Today Energy 2022, 26, 100988. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Z.; Liu, Y.; Xing, J.; Wei, C.; Zou, W.; Li, J. Stabilization of the Li metal anode through constructing a LiZn alloy/polymer hybrid protective layer towards uniform Li deposition. Phys. Chem. Chem. Phys. 2022, 25, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, C.; Zhang, Y.; Zhang, X.; Zhao, Z.; Song, T.; Wu, B.; Mu, D. A dual lithiated alloy interphase layer for high-energy–density lithium metal batteries. Chem. Eng. J. 2022, 434, 134637. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 2018, 3, 310–316. [Google Scholar] [CrossRef]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable Artificial Solid Electrolyte Interphases for Lithium Batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Liang, X.; Pang, Q.; Kochetkov, I.R.; Sempere, M.S.; Huang, H.; Sun, X.; Nazar, L.F. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2017, 2, 17119. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, S.; Tang, C.; Zhai, Q.; Zhang, X.; Wang, R. Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries. Energies 2018, 11, 2512. [Google Scholar] [CrossRef]
- Song, C.-L.; Li, Z.-H.; Ma, L.-Y.; Li, M.-Z.; Huang, S.; Hong, X.-J.; Cai, Y.-P.; Lan, Y.-Q. Single-Atom Zinc and Anionic Framework as Janus Separator Coatings for Efficient Inhibition of Lithium Dendrites and Shuttle Effect. ACS Nano 2021, 15, 13436–13443. [Google Scholar] [CrossRef]
- Li, F.; Tan, Y.-H.; Yin, Y.-C.; Zhang, T.-W.; Lu, L.-L.; Song, Y.-H.; Tian, T.; Shen, B.; Zhu, Z.-X.; Yao, H.-B. A fluorinated alloy-type interfacial layer enabled by metal fluoride nanoparticle modification for stabilizing Li metal anodes. Chem. Sci. 2019, 10, 9735–9739. [Google Scholar] [CrossRef]
- Ishikawa, M.; Morita, M.; Matsuda, Y. In situ scanning vibrating electrode technique for lithium metal anodes. J. Power Sources 1997, 68, 501–505. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Lu, H.; Zhang, Y.; Yang, J.; Nuli, Y.; Wang, J. Artificial Alloy/Li3N Double-Layer Enabling Stable High-Capacity Lithium Metal Anodes. ACS Appl. Energy Mater. 2021, 4, 13132–13139. [Google Scholar] [CrossRef]
- Zhan, Y.-T.; Wu, L.-T.; Teng, H.; Jiang, J.-C. Unveiling multi-component hybrid organic-inorganic SEI formation in gel polymer electrolytes: A computational study. Electrochim. Acta. 2024, 512, 145513. [Google Scholar] [CrossRef]
- Yin, X.; Li, B.; Liu, H.; Wen, B.; Liu, J.; Bai, M.; Zhang, Y.; Zhao, Y.; Cui, X.; Su, Y.; et al. Solvent-derived organic-rich SEI enables capacity enhancement for low-temperature lithium metal batteries. Joule 2025, 4, 101823. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, L.; Dai, D.; Huang, Z.; Han, Y.; Huang, J.; Yang, Y.; Zou, Y.; Guo, S.; Zhao, X.; et al. LiF-induced in-situ engineering of a dense inorganic SEI for superior lithium storage in black phosphorus anode. J. Colloid Interface Sci. 2024, 680, 364–372. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, W.; Liang, T.; Shi, W.; Peng, G. Synergistic optimization of a compound electrolyte additive for the solid electrolyte interface in lithium metal batteries. J. Power Sources 2024, 630, 236100. [Google Scholar] [CrossRef]

| Type of SEI Film | Ionconductivity | Mechanical Strength | High Stability | Electrochemical Performance | Testing Condition | Ref. |
|---|---|---|---|---|---|---|
| LiF-rich | √ | √ | over 1700 h | 1 mA cm−2/1 mAh cm−2 | [107] | |
| over 1400 h | 2 mA cm−2/2 mAh cm−2 | |||||
| FCF | √ | over 500 h | 1 mA cm−2/1 mAh cm−2 | [109] | ||
| over 300 h | 3 mA cm−2/1 mAh cm−2 | |||||
| LiF/LiCl/LiIn | √ | √ | over 1000 h | 1 mA cm−2/1 mAh cm−2 | [110] | |
| LiCl-rich | √ | √ | over 2500 h | 1 mAhcm−2/1 mAh cm−2 | [111] | |
| over 1000 h | 5 mA cm−2/5 mAh cm−2 | |||||
| LiBFEP-enriched | √ | over 600 h | 0.1 mA cm−2/1 mAh cm−2 | [112] | ||
| Li2O-rich | √ | over 400 h | 0.5 mA cm−2/1 mAh cm−2 | [113] | ||
| over 300 h | 1 mA cm−2/1 mAh cm−2 | |||||
| PVDF/PAMPSLi | √ | over 350 h | 2 mA cm−2/1 mAh cm−2 | [121] | ||
| over 150 h | 5 mA cm−2/1 mAh cm−2 | |||||
| Carboxylate | √ | over 1000 h | 0.5 mA cm−2/1 mAh cm−2 | [122] | ||
| PEO | √ | over 500 h | 1 mA cm−2/1 mAh cm−2 | [123] | ||
| NCL | √ | √ | √ | over 1000 h | 1 mA cm−2/1 mAh cm−2 | [124] |
| over 1100 h | 1 mA cm−2/3 mAh cm−2 | |||||
| Terpolymer | √ | over 800 h | 0.5 mA cm−2/1 mAh cm−2 | [125] | ||
| AIBN-PEGDA | √ | over 700 h | 0.5 mA cm−2/1 mAh cm−2 | [130] | ||
| PVDF-HFP/Ag-LixAgy | √ | √ | over 1000 h | 0.25 mA cm−2/0.5 mA h cm−2 | [137] | |
| over 1400 h | 1 mA cm−2/1 mAh cm−2 | |||||
| YF3-PAN-CNFs | √ | √ | over 500 h | 1 mA cm−2/1 mAh cm−2 | [132] | |
| LiCl-rich (PVC) | √ | over 500 h | 1 mA cm−2/1 mAh cm−2 | [133] | ||
| Zn(CF3SO3)2 | √ | √ | over 700 h | 1 mA cm−2/2 mAh cm−2 | [135] | |
| LiF@PPF | √ | √ | over 900 h | 1 mA cm−2/1 mAh cm−2 | [139] | |
| EP-ALD | √ | over 550 h | 1 mA cm−2/1 mAh cm−2 | [136] | ||
| over 220 h | 2 mA cm−2/2 mAh cm−2 | |||||
| PVDF/DMF/LiF | √ | over 600 h | 1 mA cm−2/1 mAh cm−2 | [134] | ||
| over 200 h | 3 mA cm−2/1 mAh cm−2 | |||||
| CuzSnyOx | √ | √ | over 1600 h | 4 mA cm−2/2 mA cm−2 | [145] | |
| Li-Mg | √ | √ | over 2000 h | 1 mA cm−2/1 mAh cm−2 | [146] | |
| PVDF-HFP/AlF3 | √ | √ | over 600 h | 3 mA cm−2/1 mAh cm−2 | [147] | |
| over 400 h | 3 mA cm−2/3 mAh cm−2 | |||||
| ISPI | √ | over 700 h | 3 mA cm−2/1 mAh cm−2 | [152] | ||
| over 1000 h | 1 mA cm−2/5 mAh cm−2 | |||||
| Li-Zn/PEO | √ | √ | over 1000 h | 1 mA cm−2/1 mAh cm−2 | [153] | |
| TGD/Al(MMP)3 | √ | √ | √ | over 2000 h | 1 mA cm−2/1 mAh cm−2 | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Zheng, W.; Li, Y.; Huang, S. Progress in Modeling and Applications of Solid Electrolyte Interphase Layers for Lithium Metal Anodes. Nanomaterials 2025, 15, 554. https://doi.org/10.3390/nano15070554
Wei Z, Zheng W, Li Y, Huang S. Progress in Modeling and Applications of Solid Electrolyte Interphase Layers for Lithium Metal Anodes. Nanomaterials. 2025; 15(7):554. https://doi.org/10.3390/nano15070554
Chicago/Turabian StyleWei, Zhicong, Weitao Zheng, Yijuan Li, and Shaoming Huang. 2025. "Progress in Modeling and Applications of Solid Electrolyte Interphase Layers for Lithium Metal Anodes" Nanomaterials 15, no. 7: 554. https://doi.org/10.3390/nano15070554
APA StyleWei, Z., Zheng, W., Li, Y., & Huang, S. (2025). Progress in Modeling and Applications of Solid Electrolyte Interphase Layers for Lithium Metal Anodes. Nanomaterials, 15(7), 554. https://doi.org/10.3390/nano15070554






