Pr3+ Visible to Ultraviolet Upconversion for Antimicrobial Applications
Abstract
1. Introduction
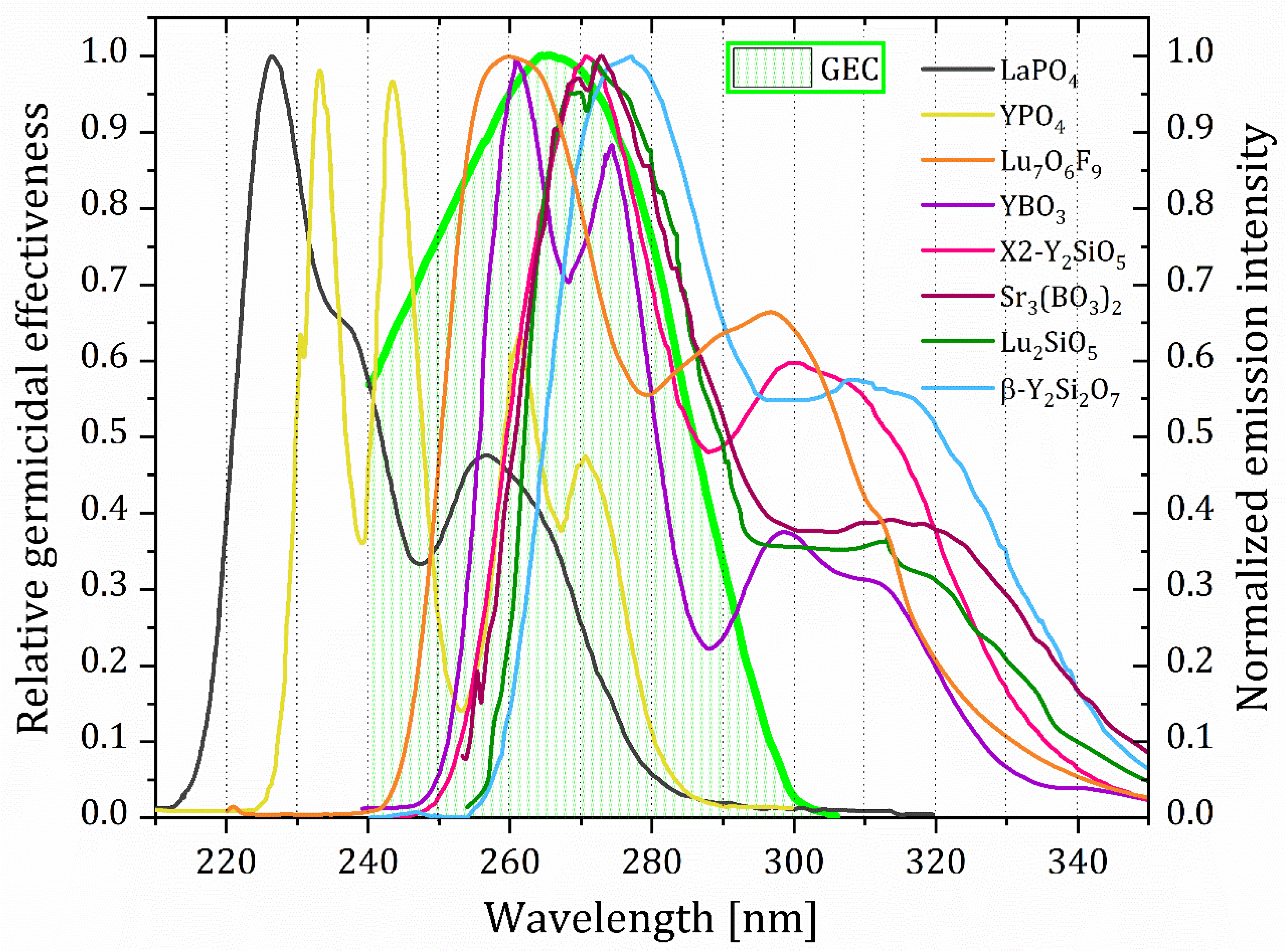
2. On UV Radiation’s Germicidal Effects
3. UVC Light Sources
4. Short Primer on Lanthanide-Mediated Upconversion
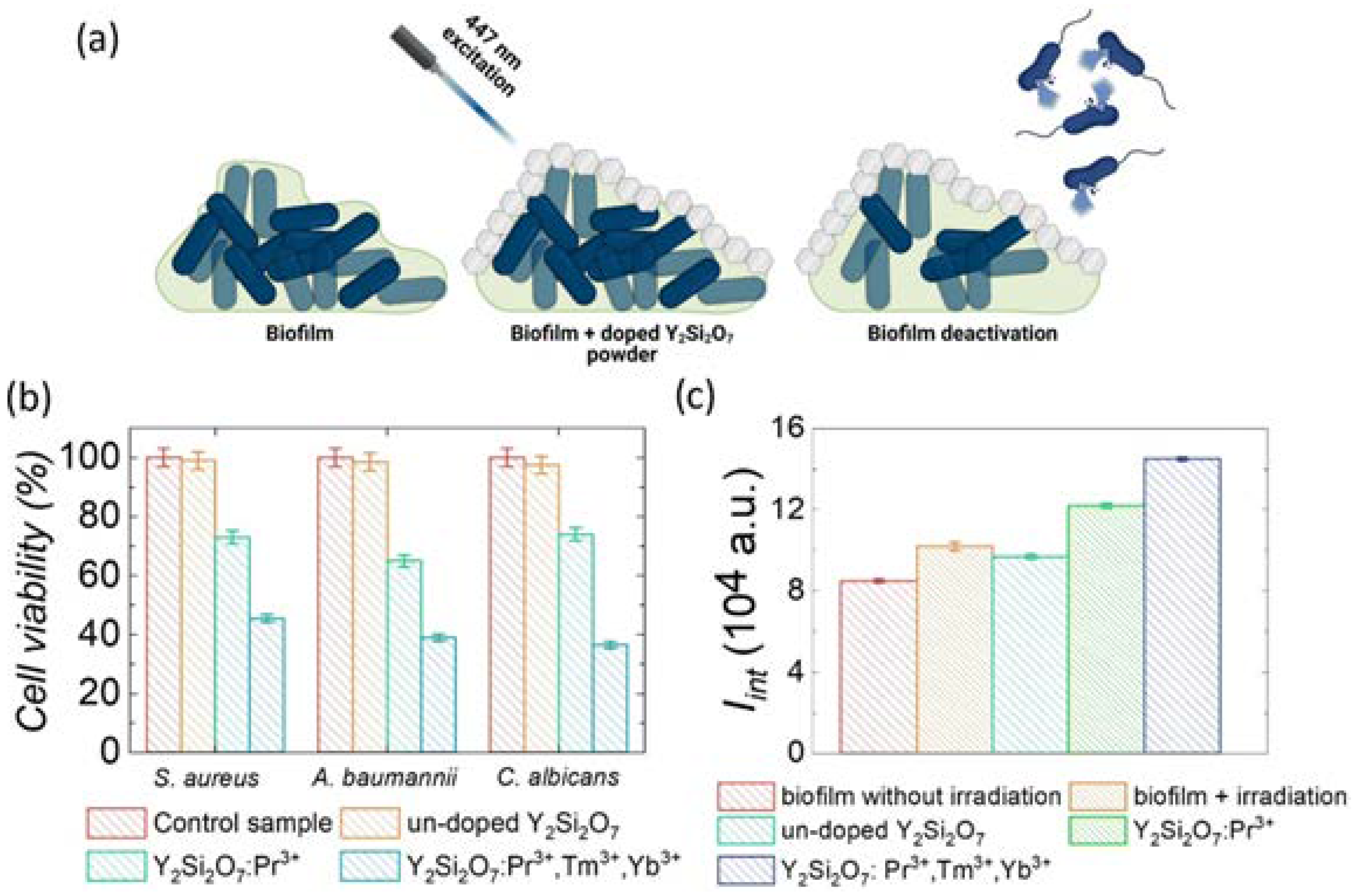
5. Antimicrobial Applications of Lanthanide-Facilitated UC
6. Lanthanide-Facilitated Near-Infrared-to-UV(C) and Visible-to-UV(C) UC
7. Electronic and Optical Properties of Pr3+ Ions
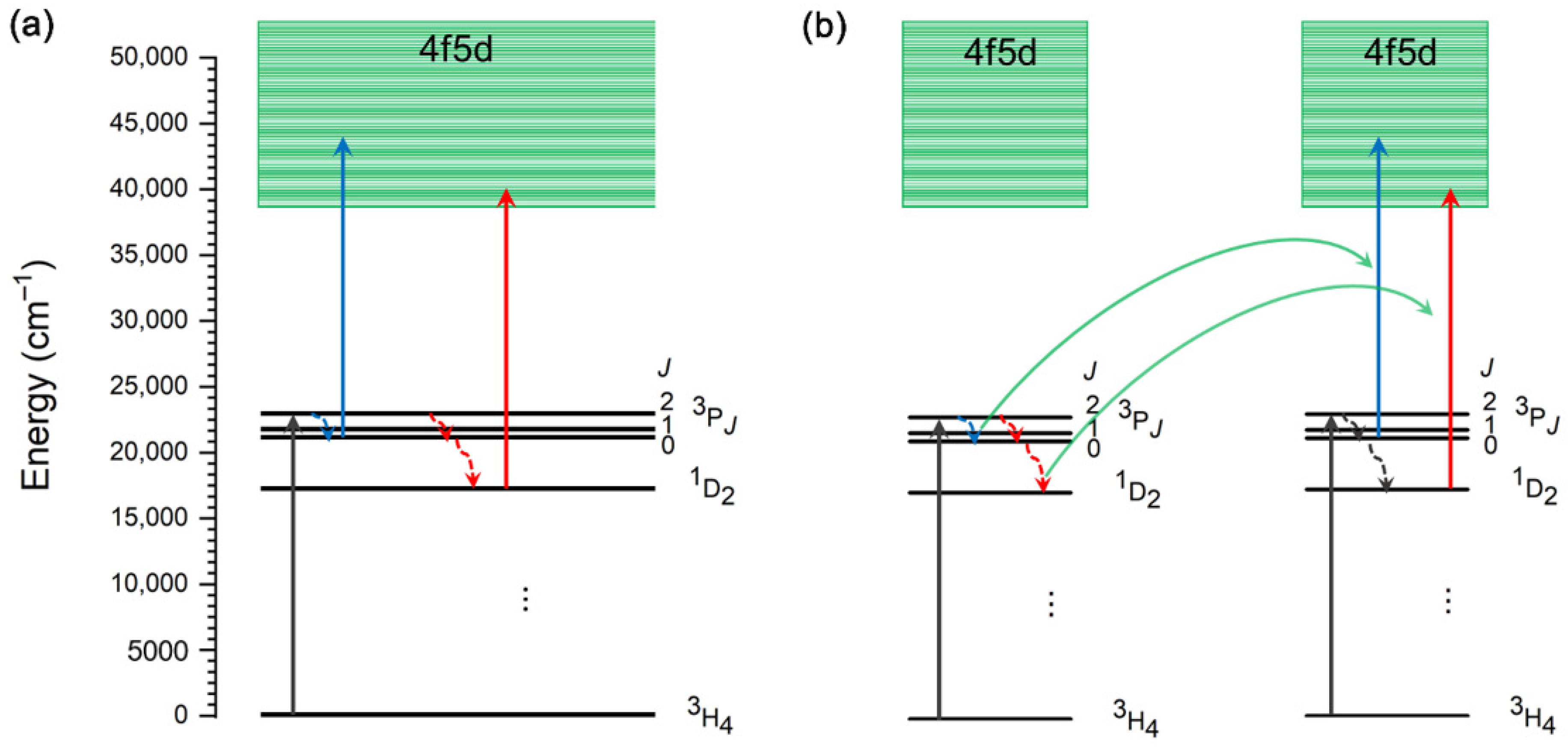
8. Mechanisms of Visible-to-UV Upconversion in Pr3+
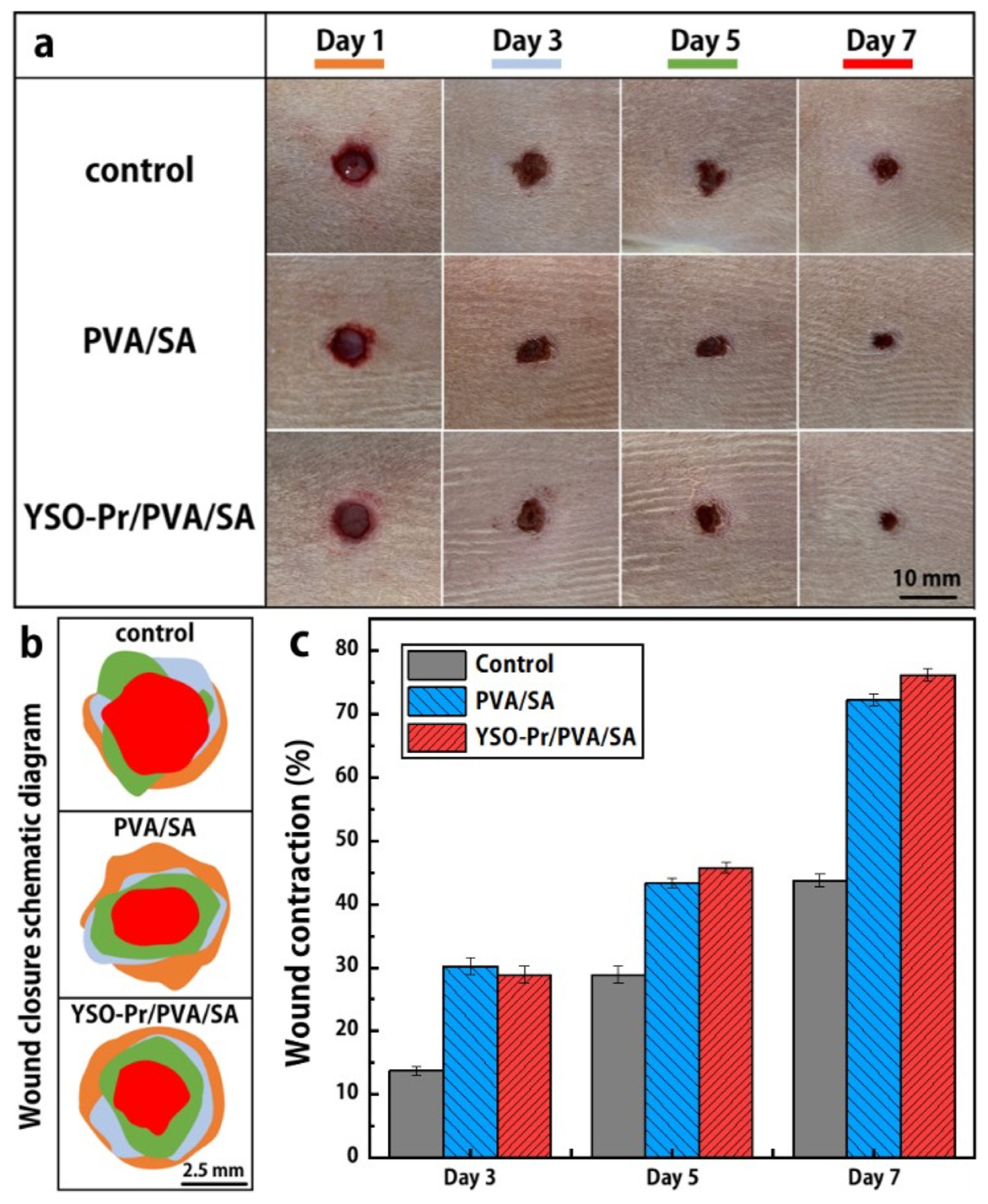
9. Antimicrobial Applications of Lanthanide-Facilitated Visible-to-UVC UC
10. The Toxicity of Some Elements Frequently Used for Lanthanide Upconversion Materials
10.1. Yttrium
10.2. Gadolinium
10.3. Erbium
10.4. Lutetium
10.5. Thulium
10.6. Praseodymium
10.7. The Toxicity of Fluorides
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-01998-2. [Google Scholar]
- Moore, G.; Ali, S.; Cloutman-Green, E.A.; Bradley, C.R.; Wilkinson, M.A.; Hartley, J.C.; Fraise, A.P.; Wilson, A.P.R. Use of UV-C Radiation to Disinfect Non-Critical Patient Care Items: A Laboratory Assessment of the Nanoclave Cabinet. BMC Infect. Dis. 2012, 12, 174. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Rezaie, A.; Leite, G.G.S.; Melmed, G.Y.; Mathur, R.; Villanueva-Millan, M.J.; Parodi, G.; Sin, J.; Germano, J.F.; Morales, W.; Weitsman, S.; et al. Ultraviolet A Light Effectively Reduces Bacteria and Viruses Including Coronavirus. PLoS ONE 2020, 15, e0236199. [Google Scholar] [CrossRef]
- Arrage, A.A.; Phelps, T.J.; Benoit, R.E.; White, D.C. Survival of Subsurface Microorganisms Exposed to UV Radiation and Hydrogen Peroxide. Appl. Environ. Microbiol. 1993, 59, 3545–3550. [Google Scholar] [CrossRef] [PubMed]
- Gates, F.L. A Study of the Bactericidal Action of Ultra Violet Light. J. Gen. Physiol. 1930, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Fondriest Environmental, Inc. Solar Radiation and Photosynethically Active Radiation. Fundamentals of Environmental Measurements. Available online: https://www.fondriest.com/environmental-measurements/parameters/weather/photosynthetically-active-radiation/ (accessed on 3 March 2025).
- Du, Y.; Ai, X.; Li, Z.; Sun, T.; Huang, Y.; Zeng, X.; Chen, X.; Rao, F.; Wang, F. Visible-to-Ultraviolet Light Conversion: Materials and Applications. Adv. Photonics Res. 2021, 2, 2000213. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Y.; Wang, X.; Mao, Y. Recent Advances in Pr3+-Activated Persistent Phosphors. J. Mater. Chem. C Mater. 2022, 10, 3626–3646. [Google Scholar] [CrossRef]
- Li, L.; Zi, L.; Yang, F.; Feng, S.; Wang, C.; Yang, Y. Pr3+-Based Visible-to-Ultraviolet Upconversion. A Minireview. Adv. Phys. Res. 2025, 4, 2400097. [Google Scholar] [CrossRef]
- Besaratinia, A.; Yoon, J.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength Dependence of Ultraviolet Radiation-induced DNA Damage as Determined by Laser Irradiation Suggests That Cyclobutane Pyrimidine Dimers Are the Principal DNA Lesions Produced by Terrestrial Sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC Light (222 Nm) Efficiently and Safely Inactivates Airborne Human Coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C Irradiation: An Alternative Antimicrobial Approach to Localized Infections? Expert Rev. Anti Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED Disinfection Performance and Investigating Potential Dual-Wavelength Synergy; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 109, ISBN 3034924798. [Google Scholar]
- Brash, D.E. UV Signature Mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption Measurements of a Cell Monolayer Relevant to Phototherapy: Reduction of Cytochrome c Oxidase under near IR Radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Zhang, Z.S.; Feng, P.; Liu, W.L. Quantitative Assessment of the Effects of Human Activities on Phytoplankton Communities in Lakes and Reservoirs. Sci. Total Environ. 2019, 665, 213–225. [Google Scholar] [CrossRef]
- Oguma, K.; Katayama, H.; Ohgaki, S. Photoreactivation of Escherichia coli after Low- or Medium-Pressure UV Disinfection Determined by an Endonuclease Sensitive Site Assay. Appl. Environ. Microbiol. 2002, 68, 6029–6035. [Google Scholar] [CrossRef]
- Fan, X.; Huang, R.; Chen, H. Application of Ultraviolet C Technology for Surface Decontamination of Fresh Produce. Trends Food Sci. Technol. 2017, 70, 9–19. [Google Scholar] [CrossRef]
- Liltved, H.; Landfald, B. Effects of High Intensity Light on Ultraviolet-Irradiated and Non-Irradiated Fish Pathogenic Bacteria. Water Res. 2000, 34, 481–486. [Google Scholar] [CrossRef]
- Chevrefils, G.; Caron, É.; Wright, H.; Sakamoto, G. UV Dose Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa and Viruses. IUVA News 2006, 8, 38–45. [Google Scholar]
- Martínez-Hernández, G.B.; Huertas, J.P.; Navarro-Rico, J.; Gómez, P.A.; Artés, F.; Palop, A.; Artés-Hernández, F. Inactivation Kinetics of Foodborne Pathogens by UV-C Radiation and Its Subsequent Growth in Fresh-Cut Kailan-Hybrid Broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Harrison, M.A. Effectiveness of UV light as a means to reduce Salmonella contamination on tomatoes and food contact surfaces. Food Control 2016, 66, 166–173. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Chen, H. Inactivation of Escherichia coli O157: H7 and Salmonella Enterica on Blueberries in Water Using Ultraviolet Light. J. Food Sci. 2015, 80, M1532–M1537. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Lhotsky, M.; Haider, T.; Cabaj, A. UV Inactivation, Liquid-Holding Recovery, and Photoreactivation of Escherichia coli O157 and Other Pathogenic Escherichia coli Strains in Water. J. Food Prot. 2000, 63, 1015–1020. [Google Scholar] [CrossRef]
- Chun, H.H.; Kim, J.Y.; Song, K. Bin Inactivation of Foodborne Pathogens in Ready-to-Eat Salad Using UV-C Irradiation. Food Sci. Biotechnol. 2010, 19, 547–551. [Google Scholar] [CrossRef]
- Allende, A.; McEvoy, J.L.; Luo, Y.; Artes, F.; Wang, C.Y. Effectiveness of Two-Sided UV-C Treatments in Inhibiting Natural Microflora and Extending the Shelf-Life of Minimally Processed “Red Oak Leaf” Lettuce. Food Microbiol. 2006, 23, 241–249. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, S.G.; Back, K.H.; Park, K.H.; Chung, M.S.; Kang, D.H. Effect of Various Conditions on Inactivation of Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes in Fresh-Cut Lettuce Using Ultraviolet Radiation. Int. J. Food Microbiol. 2013, 166, 349–355. [Google Scholar] [CrossRef]
- Chang, J.C.; Ossoff, S.F.; Lobe, D.C.; Dorfman, M.H.; Dumais, C.M.; Qualls, R.G.; Johnson, J.D. UV Inactivation of Pathogenic and Indicator Microorganisms. Appl. Environ. Microbiol. 1985, 49, 1361–1365. [Google Scholar] [CrossRef]
- Gryko, Ł.; Błaszczak, U.J.; Zajkowski, M. The Impact of Time and Temperature of Operation on the Characteristics of High-Power UVC LEDs and Their Disinfection Efficiency. Appl. Sci. 2023, 13, 12886. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Rolz-Cruz, G.; Cumbie, W.E.; Hamblin, M.R. Ultraviolet C Inactivation of Dermatophytes: Implications for Treatment of Onychomycosis. Br. J. Dermatol. 2008, 158, 1239–1246. [Google Scholar] [CrossRef]
- Alcantara-Diaz, D. Divergent Adaptation of Escherichia coli to Cyclic Ultraviolet Light Exposures. Mutagenesis 2004, 19, 349–354. [Google Scholar] [CrossRef]
- DIN 5031-10:2018-03; Optical Radiation Physics and Illuminating Engineering—Part 10: Photobiologically Effective Radiation, Quantities, Symbols and Action Spectra. European Standard: Berlin, Germany, 2018.
- Madronich, S. The Atmosphere and UV-B Radiation at Ground Level. In Environmental UV Photobiology; Young, A.R., Moan, J., Björn, L.O., Nultsch, W., Eds.; Springer: Boston, MA, USA, 1993; pp. 1–39. ISBN 978-1-4899-2408-7. [Google Scholar]
- Sharma, V.K.; Demir, H.V. Bright Future of Deep-Ultraviolet Photonics: Emerging UVC Chip-Scale Light-Source Technology Platforms, Benchmarking, Challenges, and Outlook for UV Disinfection. ACS Photonics 2022, 9, 1513–1521. [Google Scholar] [CrossRef]
- Paul, J.; Kaneda, Y.; Wang, T.-L.; Lytle, C.; Moloney, J.V.; Jones, R.J. Doppler-Free Spectroscopy of Mercury at 2537 nm Using a High-Power, Frequency-Quadrupled, Optically Pumped External-Cavity Semiconductor Laser. Opt. Lett. 2011, 36, 61. [Google Scholar] [CrossRef] [PubMed]
- Obileke, K.; Onyeaka, H.; Miri, T.; Nwabor, O.F.; Hart, A.; Al-Sharify, Z.T.; Al-Najjar, S.; Anumudu, C. Recent Advances in Radio Frequency, Pulsed Light, and Cold Plasma Technologies for Food Safety. J. Food Process Eng. 2022, 45, e14138. [Google Scholar] [CrossRef]
- Bergman, R.S. Germicidal UV Sources and Systems. Photochem. Photobiol. 2021, 97, 466–470. [Google Scholar] [CrossRef] [PubMed]
- UN Environment. Minamata Convention on Mercury Text and Annexes, 2024 ed.; UN Environment: Nairobi, Kenya, 2024. [Google Scholar]
- Mildren, R.P.; Carman, R.J. Enhanced Performance of a Dielectric Barrier Discharge Lamp Using Short-Pulsed Excitation. J. Phys. D Appl. Phys. 2001, 34, L1–L6. [Google Scholar] [CrossRef]
- Kogelschatz, U. Excimer Lamps: History, Discharge Physics, and Industrial Applications; Tarasenko, V.F., Ed.; SPIE: Bellingham, WA, USA, 2004; pp. 272–286. [Google Scholar]
- Masoud, N.M.; Murnick, D.E. High Efficiency Fluorescent Excimer Lamps: An Alternative to Mercury Based UVC Lamps. Rev. Sci. Instrum. 2013, 84, 123108. [Google Scholar] [CrossRef]
- Susilo, N.; Hagedorn, S.; Jaeger, D.; Miyake, H.; Zeimer, U.; Reich, C.; Neuschulz, B.; Sulmoni, L.; Guttmann, M.; Mehnke, F.; et al. AlGaN-Based Deep UV LEDs Grown on Sputtered and High Temperature Annealed AlN/Sapphire. Appl. Phys. Lett. 2018, 112, 041110. [Google Scholar] [CrossRef]
- Kneissl, M.; Seong, T.-Y.; Han, J.; Amano, H. The Emergence and Prospects of Deep-Ultraviolet Light-Emitting Diode Technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Takano, T.; Mino, T.; Sakai, J.; Noguchi, N.; Tsubaki, K.; Hirayama, H. Deep-Ultraviolet Light-Emitting Diodes with External Quantum Efficiency Higher than 20% at 275 Nm Achieved by Improving Light-Extraction Efficiency. Appl. Phys. Express 2017, 10, 031002. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Jmerik, V.N.; Nechaev, D.V.; Kozlovsky, V.I.; Tiberi, M.D. E-beam Pumped Mid-UV Sources Based on MBE-grown AlGaN MQW. Phys. Status Solidi (A) 2015, 212, 1011–1016. [Google Scholar] [CrossRef]
- Kang, Y.; Zhao, J.; Wu, J.; Zhang, L.; Zhao, J.; Zhang, Y.; Zhao, Y.; Wang, X. Superior Deep-Ultraviolet Source Pumped by an Electron Beam for NLOS Communication. IEEE Trans. Electron. Devices 2020, 67, 3391–3394. [Google Scholar] [CrossRef]
- Li, D.; Jiang, K.; Sun, X.; Guo, C. AlGaN Photonics: Recent Advances in Materials and Ultraviolet Devices. Adv. Opt. Photonics 2018, 10, 43. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Niiyama, T.; Miya, K.; Taniguchi, M. Far-Ultraviolet Plane-Emission Handheld Device Based on Hexagonal Boron Nitride. Nat. Photonics 2009, 3, 591–594. [Google Scholar] [CrossRef]
- Moffatt, J.E.; Tsiminis, G.; Klantsataya, E.; de Prinse, T.J.; Ottaway, D.; Spooner, N.A. A Practical Review of Shorter than Excitation Wavelength Light Emission Processes. Appl. Spectrosc. Rev. 2020, 55, 327–349. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.; Li, J.; Gao, W.; Deng, Y.; Dong, W.; Zhao, C.; Lu, G. Visible-to-Ultraviolet Upconvertion: Energy Transfer, Material Matrix, and Synthesis Strategies. Appl. Catal. B 2017, 206, 89–103. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion Processes in Coupled Ion Systems. J. Lumin. 1990, 45, 341–345. [Google Scholar] [CrossRef]
- van der Ende, B.M.; Aarts, L.; Meijerink, A. Lanthanide Ions as Spectral Converters for Solar Cells. Phys. Chem. Chem. Phys. 2009, 11, 11081. [Google Scholar] [CrossRef]
- Auzel, F. History of Upconversion Discovery and Its Evolution. J. Lumin. 2020, 223, 116900. [Google Scholar] [CrossRef]
- Auzel, F. Compteur Quantique Par Transfert d’energie Entre Deux Ions de Terres Rares Dans Un Tungstate Mixte et Dans Un Verre. CR Acad. Sci. Paris 1966, 262, 1016–1019. [Google Scholar]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy Transfer in Lanthanide Upconversion Studies for Extended Optical Applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef]
- Fumes, A.C.; da Silva Telles, P.D.; Corona, S.A.M.; Borsatto, M.C. Effect of APDT on Streptococcus Mutans and Candida Albicans Present in the Dental Biofilm: Systematic Review. Photodiagnosis. Photodyn. Ther. 2018, 21, 363–366. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic Therapy in the Control of Oral Biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M. The Evolution of Clinical Guidelines for Antimicrobial Photodynamic Therapy of Skin. Photochem. Photobiol. Sci. 2022, 21, 385–395. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Zhou, B.; Chen, Y.; Liu, M.; Wang, L.; Qi, M.; Liu, B.; Dong, B. Near-Infrared Light Triggered Upconversion Nanocomposites with Multifunction of Enhanced Antimicrobial Photodynamic Therapy and Gas Therapy for Inflammation Regulation. J. Colloid Interface Sci. 2024, 663, 834–846. [Google Scholar] [CrossRef]
- Li, T.; Xue, C.; Wang, P.; Li, Y.; Wu, L. Photon Penetration Depth in Human Brain for Light Stimulation and Treatment: A Realistic Monte Carlo Simulation Study. J. Innov. Opt. Health Sci. 2017, 10, 1743002. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in Photodynamic Therapy: Plumbing the Depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef]
- Liao, J.; Yang, L.; Wu, S.; Yang, Z.; Zhou, J.; Jin, D.; Guan, M. NIR-II Emissive Properties of 808 Nm-Excited Lanthanide-Doped Nanoparticles for Multiplexed in Vivo Imaging. J. Lumin. 2022, 242, 118597. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Ju, E.; Wang, Z.; Dong, K.; Ren, J.; Qu, X. Multifunctional Upconverting Nanoparticles for Near-Infrared Triggered and Synergistic Antibacterial Resistance Therapy. Chem. Commun. 2014, 50, 10488–10490. [Google Scholar] [CrossRef]
- Grüner, M.C.; Arai, M.S.; Carreira, M.; Inada, N.; de Camargo, A.S.S. Functionalizing the Mesoporous Silica Shell of Upconversion Nanoparticles To Enhance Bacterial Targeting and Killing via Photosensitizer-Induced Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Ju, E.; Gao, N.; Wang, Z.; Ren, J.; Qu, X. Synergistic Eradication of Antibiotic-Resistant Bacteria Based Biofilms in Vivo Using a NIR-Sensitive Nanoplatform. Chem. Commun. 2016, 52, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual Antibacterial Activities of a Chitosan-Modified Upconversion Photodynamic Therapy System against Drug-Resistant Bacteria in Deep Tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; You, W.; Su, J.; Yu, S.; Dai, T.; Huang, Y.; Chen, X.; Song, X.; Chen, Z. Near-Infrared-Excited Upconversion Photodynamic Therapy of Extensively Drug-Resistant Acinetobacter baumannii Based on Lanthanide Nanoparticles. Nanoscale 2020, 12, 13948–13957. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, Y.; Hu, M.; Zhang, P.; Kong, N.; Liu, R.; Liu, C.; Choi, S.K. Lanthanide-Doped Core–Shell Nanoparticles as a Multimodality Platform for Imaging and Photodynamic Therapy. Chem. Commun. 2018, 54, 9525–9528. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Wu, D.; Gao, Z.; Song, Y.-Y.; Schmuki, P. Upconversion Nanoparticle-Assisted Payload Delivery from TiO2 under Near-Infrared Light Irradiation for Bacterial Inactivation. ACS Nano 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Nsubuga, A.; Morice, K.; Fayad, N.; Pini, F.; Josserand, V.; Le Guével, X.; Alhabi, A.; Henry, M.; Puchán Sánchez, D.; Plassais, N.; et al. Sub 20 nm Upconversion Photosensitizers for Near-Infrared Photodynamic Theranostics. Adv. Funct. Mater. 2025, 35, 2410077. [Google Scholar] [CrossRef]
- Karami, A.; Farivar, F.; de Prinse, T.J.; Rabiee, H.; Kidd, S.; Sumby, C.J.; Bi, J. Facile Multistep Synthesis of ZnO-Coated β-NaYF4:Yb/Tm Upconversion Nanoparticles as an Antimicrobial Photodynamic Therapy for Persistent Staphylococcus Aureus Small Colony Variants. ACS Appl. Bio Mater. 2021, 4, 6125–6136. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning Upconversion through Energy Migration in Core–Shell Nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef]
- Fu, X.; Fu, S.; Lu, Q.; Zhang, J.; Wan, P.; Liu, J.; Zhang, Y.; Chen, C.-H.; Li, W.; Wang, H.; et al. Excitation Energy Mediated Cross-Relaxation for Tunable Upconversion Luminescence from a Single Lanthanide Ion. Nat. Commun. 2022, 13, 4741. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, L.; Sun, T.; Kong, W.; Yu, S.F.; Wang, F. Energy Migration Upconversion in Ce(III)-Doped Heterogeneous Core−Shell−Shell Nanoparticles. Small 2017, 13, 1701479. [Google Scholar] [CrossRef]
- Su, Q.; Wei, H.-L.; Liu, Y.; Chen, C.; Guan, M.; Wang, S.; Su, Y.; Wang, H.; Chen, Z.; Jin, D. Six-Photon Upconverted Excitation Energy Lock-in for Ultraviolet-C Enhancement. Nat. Commun. 2021, 12, 4367. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.-G. Theoretical Spectroscopy of Transition Metal and Rare Earth Ions: From Free State to Crystal Field; Jenny Stanford Publishing: Singapore, 2020; ISBN 9780429278754. [Google Scholar]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R.S. A Systematic Analysis of the Spectra of the Lanthanides Doped into Single Crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Sugar, J. Analysis of the Spectrum of Triply Ionized Praseodymium (Pr Iv). J. Opt. Soc. Am. 1965, 55, 1058. [Google Scholar] [CrossRef]
- Crosswhite, H.M.; Dieke, G.H.; Carter, W.J. Free-Ion and Crystalline Spectra of Pr3+ (Pr IV). J. Chem. Phys. 1965, 43, 2047–2054. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J. NIST ASD Team NIST Atomic Spectra Database (Ver. 5.12). Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 5 March 2025).
- Srivastava, A.M.; Jennings, M.; Collins, J. The Interconfigurational (4f15d1 → 4f2) Luminescence of Pr3+ in LuPO4, K3Lu(PO4)2 and LiLuSiO4. Opt. Mater. 2012, 34, 1347–1352. [Google Scholar] [CrossRef]
- Antić, Ž.; Racu, A.V.; Medić, M.; Alodhayb, A.N.; Kuzman, S.; Brik, M.G.; Dramićanin, M.D. Concentration and Temperature Dependence of Pr3+ F-f Emissions in La(PO3)3. Opt. Mater. 2024, 150, 115226. [Google Scholar] [CrossRef]
- Jüstel, T.; Mayr, W.; Schmidt, P.J.; Wiechert, D. On the Host Lattice Dependence of the 4fn−15d → 4fn Emission of Pr3+ and Nd3+. Available online: https://www.fh-muenster.de/ciw/downloads/personal/juestel/juestel/On_the_Host_Lattice_Dependence_of_the_4fn-15d1_Emission_of_Pr3__and_Nd3___November_2001_.pdf (accessed on 5 March 2025).
- Dorenbos, P. The 5d Level Positions of the Trivalent Lanthanides in Inorganic Compounds. J. Lumin. 2000, 91, 155–176. [Google Scholar] [CrossRef]
- Dorenbos, P. The 4f ↔ 4f − 15d Transitions of the Trivalent Lanthanides in Halogenides and Chalcogenides. J. Lumin. 2000, 91, 91–106. [Google Scholar] [CrossRef]
- Laroche, M.; Bettinelli, M.; Girard, S.; Moncorgé, R. F–d Luminescence of Pr3+ and Ce3+ in the Chloro-Elpasolite Cs2NaYCl6. Chem. Phys. Lett. 1999, 311, 167–172. [Google Scholar] [CrossRef]
- Tanner, P.A. Spectra, Energy Levels and Energy Transfer in High Symmetry Lanthanide Compounds. In Transition Metal and Rare Earth Compounds III Excited States, Transitions, Interactions; Yersin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 241, pp. 167–278. [Google Scholar]
- Li, Y.; Lu, H.; Li, J.; Miao, X. Luminescence of Several Rare Earth Ions in LaOI. J. Lumin. 1986, 35, 107–109. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhang, E.; Chao, X.; Yu, L.; Luo, J.; Zhang, W.; Yin, M. Synthesis and NIR-to-Violet, Blue, Green, Red Upconversion Fluorescence of Er3+:LaOBr. J. Alloys Compd. 2005, 397, 1–4. [Google Scholar] [CrossRef]
- Rebrova, N.; Grippa, A.; Zdeb, P.; Dereń, P.J. Blue to UV Upconversion Properties of Pr3+ Doped ACaF3 (A = K, Rb, Cs) Phosphors. Scr. Mater. 2025, 255, 116395. [Google Scholar] [CrossRef]
- Daniel, P.; Rousseau, M.; Toulouse, J. Raman Scattering Study of Potassium Calcium Fluoride KCaF3. Phys. Status Solidi (B) 1997, 203, 327–335. [Google Scholar] [CrossRef]
- Ridou, C.; Rousseau, M.; Gervais, F. The Temperature Dependence of the Infrared Reflection Spectra in the Fluoperovskites RbCaF3, CsCaF3 and KZnF3. J. Phys. C Solid State Phys. 1986, 19, 5757–5767. [Google Scholar] [CrossRef]
- Sarantopoulou, E.; Abdulsabirov, R.Y.; Korableva, S.L.; Cefalas, A.C.; Dubinskii, M.A.; Naumov, A.K.; Semashko, V.V.; Nicolaides, C.A. VUV and UV Fluorescence and Absorption Studies of Pr3+-Doped LiLuF4 Single Crystals. Opt. Lett. 1994, 19, 499. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, T.; Liu, K.; Jiang, D.; Zhao, M.-H.; Lin, C.; Wang, Y. Pressure-Induced Structural Phase Transition, Irreversible Amorphization and Upconversion Luminescence Enhancement in Ln3+-Codoped LiYF4 and LiLuF4. J. Mater. Chem. C Mater. 2023, 11, 6588–6596. [Google Scholar] [CrossRef]
- Vink, A.P.; van der Kolk, E.; Dorenbos, P.; van Eijk, C.W.E. Opposite Parity 4f−15d1 States of Ce3+ and Pr3+ in MSO4 (M = Ca, Sr, Ba). J. Alloys Compd. 2002, 341, 338–341. [Google Scholar] [CrossRef]
- Smith, D.H.; Seshadri, K.S. Infrared Spectra of Mg2Ca(SO4)3, MgSO4, Hexagonal CaSO4, and Orthorhombic CaSO4. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999, 55, 795–805. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Li, Z.-Y.; Zhang, J.-Y.; Lu, Y.; Guo, S.-Q.; Zhao, Q.; Wang, X.; Yong, Z.-J.; Li, H.; Ma, J.-P.; et al. X-Ray-Activated Long Persistent Phosphors Featuring Strong UVC Afterglow Emissions. Light Sci. Appl. 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.G.; Nigam, S.; Sudarsan, V.; Vatsa, R.K.; Jain, V.K. YBO3 versus Y3BO6 Host on Tb3+ Luminescence. J. Lumin. 2018, 195, 271–277. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Liang, H.; Tian, Z.; Su, Q.; Zhang, G. Luminescence of Pr3+ in La2CaB10O19: Simultaneous Observation PCE and f–d Emission in a Single Host. Opt. Mater. 2009, 32, 115–120. [Google Scholar] [CrossRef]
- Szymborska-Małek, K.; Ptak, M.; Tomaszewski, P.E.; Majchrowski, A. Raman and IR Spectroscopic Study of a Nonlinear Optical Crystal, La2CaB10O19. Vib. Spectrosc. 2016, 82, 53–59. [Google Scholar] [CrossRef]
- Zeler, J.; Sulollari, M.; Meijerink, A.; Bettinelli, M.; Zych, E. Chemical Stabilization of Eu2+ in LuPO4 and YPO4 Hosts and Its Peculiar Sharp Line Luminescence. J. Alloys Compd. 2020, 844, 156096. [Google Scholar] [CrossRef]
- Kappelhoff, J.; Keil, J.-N.; Kirm, M.; Makhov, V.N.; Chernenko, K.; Möller, S.; Jüstel, T. Spectroscopic Studies on Pr3+ Doped YPO4 and LuPO4 upon Vacuum Ultraviolet (VUV) and Synchrotron Radiation Excitation. Chem. Phys. 2022, 562, 111646. [Google Scholar] [CrossRef]
- Keil, J.-N.; Jenneboer, H.; Jüstel, T. Temperature Dependent Luminescence of Pr3+ Doped NaCaPO4. J. Lumin. 2021, 238, 118307. [Google Scholar] [CrossRef]
- Jastrzębski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared Spectroscopy of Different Phosphates Structures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar] [CrossRef]
- Ivanovskikh, K.V.; Pustovarov, V.A.; Omelkov, S.; Kirm, M.; Piccinelli, F.; Bettinelli, M. Phase Transition, Radio- and Photoluminescence of K3Lu(PO4)2 Doped with Pr3+ Ions. J. Lumin. 2021, 230, 117749. [Google Scholar] [CrossRef]
- Pelczarska, A.; Watras, A.; Godlewska, P.; Radomińska, E.; Macalik, L.; Szczygieł, I.; Hanuza, J.; Dereń, P.J. Structural, Raman, FT-IR and Optical Properties of RbY2(PO4)3 and Rb3La(PO4)2 Doped with Eu3+ Ions. New J. Chem. 2015, 39, 8474–8483. [Google Scholar] [CrossRef]
- Keil, J.-N.; Lindfeld, E.; Jüstel, T. Synthesis and Characterization of Sr3(PO4)2:Pr3+, Si4+. J. Lumin. 2020, 225, 117376. [Google Scholar] [CrossRef]
- Zhai, S.; Lin, C.-C.; Xue, W. Raman Spectra of Sr3(PO4)2 and Ba3(PO4)2 Orthophosphates at Various Temperatures. Vib. Spectrosc. 2014, 70, 6–11. [Google Scholar] [CrossRef]
- Rebrova, N.; Lisiecki, R.; Zdeb-Stańczykowska, P.; Zorenko, Y.; Voloshinovskii, A.; Pushak, A.; Dereń, P.J. Optical and Upconversion Properties of AY(PO4)3:Pr3+ (A = Sr, Ba) Phosphors. J. Phys. Chem. C 2025, 129, 1873–1884. [Google Scholar] [CrossRef]
- Guan, A.; Chen, P.; Zhou, L.; Wang, G.; Zhang, X.; Tang, J. Color-Tunable Emission and Energy Transfer Investigation in Sr3Y(PO4)3:Ce3+, Tb3+ Phosphors for White LEDs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shang, M.; Lian, H.; Lin, J. Recent Development in Phosphors with Different Emitting Colors via Energy Transfer. J. Mater. Chem. C Mater. 2016, 4, 5507–5530. [Google Scholar] [CrossRef]
- Lemański, K.; Bezkrovna, O.; Rebrova, N.; Lisiecki, R.; Zdeb, P.; Dereń, P.J. UVC Stokes and Anti-Stokes Emission of Ca9Y(PO4)7 Polycrystals Doped with Pr3+ Ions. Molecules 2024, 29, 2084. [Google Scholar] [CrossRef]
- Su, C.; Ao, L.; Zhang, Z.; Zhai, Y.; Chen, J.; Tang, Y.; Liu, L.; Fang, L. Crystal Structure, Raman Spectra and Microwave Dielectric Properties of Novel Temperature-Stable LiYbSiO4 Ceramics. Ceram. Int. 2020, 46, 19996–20003. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, P.; Zhu, Z.; Li, T.; Yang, Y. Pr3+ Doped Li2SrSiO4: An Efficient Visible-Ultraviolet C up-Conversion Phosphor. Ceram. Int. 2021, 47, 4858–4863. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, B.; Yu, H.; Hu, Z.; Wang, J.; Wu, Y.; Halasyamani, P.S. Designing Silicates as Deep-UV Nonlinear Optical (NLO) Materials Using Edge-Sharing Tetrahedra. Angew. Chem. 2020, 132, 9007–9011. [Google Scholar] [CrossRef]
- Rebrova, N.; Zdeb, P.; Dereń, P.J. Synthesis and Upconversion Luminescence of LiY(SiO)6O2 Phosphor Doped with Pr3+. J. Phys. Chem. C 2024, 128, 9090–9098. [Google Scholar] [CrossRef]
- Li, Y.; Liang, D.; Zhang, X.; Xiong, Z.; Tang, B.; Si, F.; Fang, Z.; Shi, Z.; Chen, J.; Wang, F.; et al. Sintering Behavior, Crystal Structure, and Microwave Dielectric Properties of a Novel NaY9Si6O26 Ceramic. J. Am. Ceram. Soc. 2024, 107, 4077–4085. [Google Scholar] [CrossRef]
- Yan, S.; Liang, Y.; Chen, Y.; Liu, J.; Chen, D.; Pan, Z. Ultraviolet-C Persistent Luminescence from the Lu2SiO5:Pr3+ Persistent Phosphor for Solar-Blind Optical Tagging. Dalton Trans. 2021, 50, 8457–8466. [Google Scholar] [CrossRef]
- Han, L.; Song, F.; Chen, S.-Q.; Zou, C.-G.; Yu, X.-C.; Tian, J.-G.; Xu, J.; Xu, X.; Zhao, G. Intense Upconversion and Infrared Emissions in Er3+–Yb3+ Codoped Lu2SiO5 and (Lu0.5Gd0.5)2SiO5 Crystals. Appl. Phys. Lett. 2008, 93, 011110. [Google Scholar] [CrossRef]
- Cates, E.L.; Wilkinson, A.P.; Kim, J.-H. Delineating Mechanisms of Upconversion Enhancement by Li+ Codoping in Y2SiO5:Pr3+. J. Phys. Chem. C 2012, 116, 12772–12778. [Google Scholar] [CrossRef]
- Denoyer, A.; Lévesque, Y.; Jandl, S.; Guillot-Noël, O.; Goldner, P.; Viana, B.; Thibault, F.; Pelenc, D. Crystal Field Study of Ytterbium Doped Lu2SiO5 and Y2SiO5 under a Magnetic Field. J. Phys. Condens. Matter 2008, 20, 125227. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Remillieux, A.; Jacquier, B. IR-to-Visible up-Conversion Mechanisms in Pr3+-Doped ZBLAN Fluoride Glasses and Fibers. J. Lumin. 1996, 68, 279–289. [Google Scholar] [CrossRef]
- Balda, R.; Fernández, J.; Saéz de Ocáriz, I.; Voda, M.; García, A.J.; Khaidukov, N. Laser Spectroscopy of Pr3+ Ions in LiKY1−xPrxF5 Single Crystals. Phys. Rev. B 1999, 59, 9972–9980. [Google Scholar] [CrossRef]
- Dereń, P.J.; Mahiou, R.; Stręk, W.; Bednarkiewicz, A.; Bertrand, G. Up-Conversion in KYb(WO4)2:Pr3+ Crystal. Opt. Mater. 2002, 19, 145–148. [Google Scholar] [CrossRef]
- Schröder, F.; Pues, P.; Enseling, D.; Jüstel, T. On the Quantum Yield Determination of UV Emitting Up-Converters. Luminescence 2023, 38, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Zalucha, D.J.; Wright, J.C.; Fong, F.K. Energy Transfer Upconversion in LaF3:Pr3+. J. Chem. Phys. 1973, 59, 997–1001. [Google Scholar] [CrossRef]
- Zalucha, D.J.; Sell, J.A.; Fong, F.K. Infrared and Visible Photon Upconversion in LaCl3:Pr3+ (Nd3+). J. Chem. Phys. 1974, 60, 1660–1667. [Google Scholar] [CrossRef]
- Auzel, F.E. Materials and Devices Using Double-Pumped-Phosphors with Energy Transfer. Proc. IEEE 1973, 61, 758–786. [Google Scholar] [CrossRef]
- Brown, M.R.; Whiting, J.S.S.; Shand, W.A. Ion—Ion Interactions in Rare-Earth-Doped LaF3. J. Chem. Phys. 1965, 43, 1–9. [Google Scholar] [CrossRef]
- Wright, J.C.; Zalucha, D.J.; Lauer, H.V.; Cox, D.E.; Fong, F.K. Laser Optical Double Resonance and Efficient Infrared Quantum Counter Upconversion in LaCl3:Pr3+ and LaF3:Pr3+. J. Appl. Phys. 1973, 44, 781–786. [Google Scholar] [CrossRef]
- Sun, C.L.; Li, J.F.; Hu, C.H.; Jiang, H.M.; Jiang, Z.K. Ultraviolet Upconversion in Pr3+:Y2SiO5 Crystal by Ar+ Laser (488 nm) Excitation. Eur. Phys. J. D 2006, 39, 303–306. [Google Scholar] [CrossRef]
- Jones, C.M.S.; Gakamsky, A.; Marques-Hueso, J. The Upconversion Quantum Yield (UCQY): A Review to Standardize the Measurement Methodology, Improve Comparability, and Define Efficiency Standards. Sci. Technol. Adv. Mater. 2021, 22, 810–848. [Google Scholar] [CrossRef]
- Kaiser, M.; Würth, C.; Kraft, M.; Hyppänen, I.; Soukka, T.; Resch-Genger, U. Power-Dependent Upconversion Quantum Yield of NaYF4:Yb3+,Er3+ Nano- and Micrometer-Sized Particles—Measurements and Simulations. Nanoscale 2017, 9, 10051–10058. [Google Scholar] [CrossRef]
- Homann, C.; Krukewitt, L.; Frenzel, F.; Grauel, B.; Würth, C.; Resch-Genger, U.; Haase, M. NaYF4:Yb, Er/NaYF4 Core/Shell Nanocrystals with High Upconversion Luminescence Quantum Yield. Angew. Chem. Int. Ed. 2018, 57, 8765–8769. [Google Scholar] [CrossRef]
- Cates, E.L.; Cho, M.; Kim, J.-H. Converting Visible Light into UVC: Microbial Inactivation by Pr3+-Activated Upconversion Materials. Environ. Sci. Technol. 2011, 45, 3680–3686. [Google Scholar] [CrossRef] [PubMed]
- Cates, E.L.; Li, F. Balancing Intermediate State Decay Rates for Efficient Pr3+ Visible-to-UVC Upconversion: The Case of β-Y2Si2O7:Pr3+. RSC Adv. 2016, 6, 22791–22796. [Google Scholar] [CrossRef]
- Du, Y.; Jin, Z.; Li, Z.; Sun, T.; Meng, H.; Jiang, X.; Wang, Y.; Peng, D.; Li, J.; Wang, A.; et al. Tuning the 5d State of Pr3+ in Oxyhalides for Efficient Deep Ultraviolet Upconversion. Adv. Opt. Mater. 2024, 12, 2400971. [Google Scholar] [CrossRef]
- Zdeb, P.; Rebrova, N.; Dereń, P.J. Discovering the Potential of High Phonon Energy Hosts in the Field of Visible-to-Ultraviolet C Upconversion. J. Phys. Chem. Lett. 2024, 15, 9356–9360. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, G.; Peng, M.; Qiu, J. Comparative Investigation on the Spectroscopic Properties of Pr3+-Doped Boro-Phosphate, Boro-Germo-Silicate and Tellurite Glasses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 93, 223–227. [Google Scholar] [CrossRef]
- Naresh, V.; Ham, B.S. Influence of Multiphonon and Cross Relaxations on 3P0 and 1D2 Emission Levels of Pr3+ Doped Borosilicate Glasses for Broad Band Signal Amplification. J. Alloys Compd. 2016, 664, 321–330. [Google Scholar] [CrossRef]
- van Dijk, J.M.F.; Schuurmans, M.F.H. On the Nonradiative and Radiative Decay Rates and a Modified Exponential Energy Gap Law for 4 f–4 f Transitions in Rare-Earth Ions. J. Chem. Phys. 1983, 78, 5317–5323. [Google Scholar] [CrossRef]
- Kumar, M.V.V.; Gopal, K.R.; Reddy, R.R.; Reddy, G.V.L.; Hussain, N.S.; Jamalaiah, B.C. Application of Modified Judd–Ofelt Theory and the Evaluation of Radiative Properties of Pr3+-Doped Lead Telluroborate Glasses for Laser Applications. J. Non. Cryst. Solids 2013, 364, 20–27. [Google Scholar] [CrossRef]
- Malyukin, Y.V.; Masalov, A.A.; Zhmurin, P.N.; Znamenskii, N.V.; Petrenko, E.A.; Yukina, T.G. Two Mechanisms of 1D2 Fluorescence Quenching of Pr3+-doped Y2SiO5 Crystal. Phys. Status Solidi (B) 2003, 240, 655–662. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Schuck, P.J.; Suh, Y.D.; Schmidt, T.W.; Jin, D. Future and Challenges for Hybrid Upconversion Nanosystems. Nat. Photonics 2019, 13, 828–838. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L.; Lu, J.; Chai, S.; Wei, J.; Yu, Y.; Miao, R.; Zhong, L. Visible-UVC Upconversion Polymer Films for Prevention of Microbial Infection. J. Mater. Chem. B 2023, 11, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Fu, S.; Lv, X.; He, Q.; Ji, F.; Xu, X. Visible-to-UVC Driven Upconversion Photocatalyst Sterilization Efficiency and Mechanisms of β-NaYF4: Pr3+, Li+@BiOCl with a Core-Shell Structure. J. Environ. Manag. 2021, 288, 112394. [Google Scholar] [CrossRef] [PubMed]
- Cates, E.L.; Wilkinson, A.P.; Kim, J.H. Visible-to-UVC Upconversion Efficiency and Mechanisms of Lu7O6F9:Pr3+ and Y2SiO5:Pr3+ Ceramics. J. Lumin. 2015, 160, 202–209. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Fałat, P.; Antoniak, M.A.; Ziniuk, R.; Zelewski, S.J.; Samoć, M.; Nyk, M.; Qu, J.; Ohulchanskyy, T.Y.; Wawrzyńczyk, D. Pr3+ Doped NaYF4 and LiYF4 Nanocrystals Combining Visible-to-UVC Upconversion and NIR-to-NIR-II Downconversion Luminescence Emissions for Biomedical Applications. Nanoscale 2022, 14, 14770–14778. [Google Scholar] [CrossRef]
- Lv, P.; Li, L.; Yin, Z.; Wang, C.; Yang, Y. Visible-to-Ultraviolet-C Upconverted Photon for Multifunction via Ca2SiO4:Pr3+. Opt. Lett. 2022, 47, 4435. [Google Scholar] [CrossRef]
- Fałat, P.; Tsang, M.Y.; Maliszewska, I.; Zelewski, S.J.; Cichy, B.; Ohulchanskyy, T.Y.; Samoć, M.; Nyk, M.; Wawrzyńczyk, D. Enhanced Biocidal Activity of Pr3+ Doped Yttrium Silicates by Tm3+ and Yb3+ Co-Doping. Mater. Adv. 2023, 4, 5827–5837. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Zhao, Y.; Han, K.; Xia, Z. Multi-Responsive Deep-Ultraviolet Emission in Praseodymium-Doped Phosphors for Microbial Sterilization. Sci. China Mater. 2022, 65, 1103–1111. [Google Scholar] [CrossRef]
- Zi, L.; Li, L.; Wang, C.; Yang, F.; Feng, S.; Lv, P.; Yang, Y. Triple-Responsive Visible-To-Ultraviolet-C Upconverted Photons for Multifunctional Applications. Adv. Opt. Mater. 2024, 12, 2301881. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, S.; Xiao, F.; Shan, X.; Lv, X.; Wang, W.; Liang, Y. Long-Persistent Far-UVC Light Emission in Pr3+-Doped Sr2P2O7 Phosphor for Microbial Sterilization. Inorg. Chem. Front. 2023, 10, 5958–5968. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Y.; Pu, G.; Chen, W.; Deng, M.; Wang, J. Realizing Golden Ultraviolet C Emission of 265 nm by Oxygen Vacancies Engineering for 100% Sterilization Efficiency. Ceram. Int. 2024, 50, 30579–30586. [Google Scholar] [CrossRef]
- Rim, K.-T. Effects of Rare Earth Elements on the Environment and Human Health: A Literature Review. Toxicol. Environ. Health Sci. 2016, 8, 189–200. [Google Scholar] [CrossRef]
- Constantin, M.; Chifiriuc, M.C.; Vrancianu, C.O.; Petrescu, L.; Cristian, R.-E.; Crunteanu, I.; Grigore, G.A.; Chioncel, M.F. Insights into the Effects of Lanthanides on Mammalian Systems and Potential Applications. Environ. Res. 2024, 263, 120235. [Google Scholar] [CrossRef] [PubMed]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting Nanoparticles: Assessing the Toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Watts, D.J. Particle Surface Characteristics May Play an Important Role in Phytotoxicity of Alumina Nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Donaldson, K.; Brown, D.; Clouter, A.; Duffin, R.; MacNee, W.; Renwick, L.; Tran, L.; Stone, V. The Pulmonary Toxicology of Ultrafine Particles. J. Aerosol. Med. 2002, 15, 213–220. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Lovreglio, P.; D’Errico, M.N.; De Pasquale, P.; Gilberti, M.E.; Drago, I.; Panuzzo, L.; Lepera, A.; Serra, R.; Ferrara, F.; Basso, A.; et al. Environmental Factors Affecting the Urinary Excretion of Inorganic Arsenic in the General Population. Med. Lav. 2012, 103, 372–381. [Google Scholar]
- Selvaraj, V.; Bodapati, S.; Murray, E.; Rice, K.M.; Winston, N.; Shokuhfar, T.; Zhao, Y.; Blough, E. Cytotoxicity and Genotoxicity Caused by Yttrium Oxide Nanoparticles in HEK293 Cells. Int. J. Nanomed. 2014, 9, 1379–1391. [Google Scholar] [CrossRef]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of Inflammation in Vascular Endothelial Cells by Metal Oxide Nanoparticles: Effect of Particle Composition. Environ. Health Perspect 2007, 115, 403–409. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, Q.; Zhang, L.; Li, J.; Wu, Y. Occurrence of Rare Earth Elements in Umbilical Cord Serum and Association with Thyroid Hormones and Birth Outcomes in Newborns. Chemosphere 2024, 359, 142321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ding, Y.; Xie, S.; Hu, Y.; Xiao, H.; Liu, X.; Fan, X. Chronic Exposure to Yttrium Induced Cell Apoptosis in the Testis by Mediating Ca2+/IP3R1/CaMKII Signaling. Front. Public Health 2023, 11, 1104195. [Google Scholar] [CrossRef]
- Turyanskaya, A.; Rauwolf, M.; Pichler, V.; Simon, R.; Burghammer, M.; Fox, O.J.L.; Sawhney, K.; Hofstaetter, J.G.; Roschger, A.; Roschger, P.; et al. Detection and Imaging of Gadolinium Accumulation in Human Bone Tissue by Micro- and Submicro-XRF. Sci. Rep. 2020, 10, 6301. [Google Scholar] [CrossRef]
- Ergün, I.; Keven, K.; Uruç, I.; Ekmekçi, Y.; Canbakan, B.; Erden, I.; Karatan, O. The Safety of Gadolinium in Patients with Stage 3 and 4 Renal Failure. Nephrol. Dial. Transplant. 2006, 21, 697–700. [Google Scholar] [CrossRef]
- Grobner, T. Gadolinium—A Specific Trigger for the Development of Nephrogenic Fibrosing Dermopathy and Nephrogenic Systemic Fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Rydahl, C.; Thomsen, H.S.; Marckmann, P. High Prevalence of Nephrogenic Systemic Fibrosis in Chronic Renal Failure Patients Exposed to Gadodiamide, a Gadolinium-Containing Magnetic Resonance Contrast Agent. Investig. Radiol. 2008, 43, 141–144. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Gadolinium Oxide Nanoparticles Induce Toxicity in Human Endothelial Huvecs via Lipid Peroxidation, Mitochondrial Dysfunction and Autophagy Modulation. Nanomaterials 2020, 10, 1675. [Google Scholar] [CrossRef]
- Lattanzio, S.M.; Imbesi, F. Fibromyalgia Associated with Repeated Gadolinium Contrast-Enhanced MRI Examinations. Radiol. Case Rep. 2020, 15, 534–541. [Google Scholar] [CrossRef]
- Bilgin, B.; Adam, M.; Hekim, M.G.; Bulut, F.; Ozcan, M. Gadolinium-Based Contrast Agents Aggravate Mechanical and Thermal Hyperalgesia in a Nitroglycerine-Induced Migraine Model in Male Mice. Magn. Reson. Imaging 2024, 111, 67–73. [Google Scholar] [CrossRef]
- Edward, M.; Quinn, J.A.; Burden, A.D.; Newton, B.B.; Jardine, A.G. Effect of Different Classes of Gadolinium-Based Contrast Agents on Control and Nephrogenic Systemic Fibrosis-Derived Fibroblast Proliferation. Radiology 2010, 256, 735–743. [Google Scholar] [CrossRef]
- Becker, P.C.N.; Olsson, A.; Simpson, J.R. Erbium-Doped Fiber Amplifiers: Fundamentals and technology; Academic Press: San Diego, CA, USA, 1999; Volume 102, ISBN 0120845903. [Google Scholar]
- Cieślik, I.; Płocińska, M.; Płociński, T.; Zdunek, J.; Woźniak, M.J.; Bil, M.; Hirano, S. Influence of Polymeric Precursors on the Viability of Human Cells of Yttrium Aluminum Borates Nanoparticles Doped with Ytterbium Ions. Appl. Surf. Sci. 2019, 488, 874–886. [Google Scholar] [CrossRef]
- Antinori, S.; Versaci, C.; Fuhrberg, P.; Panci, C.; Caffa, B.; Gholami, G.H. Andrology: Seventeen Live Births after the Use of an Erbium-Yytrium Aluminium Garnet Laser in the Treatment of Male Factor Infertility. Hum. Reprod. 1994, 9, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of Short-Term Effects of Rare Earth and Other Elements Used in Magnesium Alloys on Primary Cells and Cell Lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef]
- Weltje, L.; Verhoof, L.R.C.W.; Verweij, W.; Hamers, T. Lutetium Speciation and Toxicity in a Microbial Bioassay: Testing the Free-Ion Model for Lanthanides. Environ. Sci. Technol. 2004, 38, 6597–6604. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Dolgikh, O.V.; Alekseev, V.B.; Dianova, D.G.; Vdovina, N.A. Features of the Immune Profile of Workers of a Non-Ferrous Metallurgy Enterprise in Conditions of Contamination of Biological Media with Rare Earth Elements (Using the Example of Thulium). Russ. J. Occup. Heralth Indust. Ecol. 2024, 64, 525–530. [Google Scholar] [CrossRef]
- Perry, J.; Minaei, E.; Engels, E.; Ashford, B.G.; McAlary, L.; Clark, J.R.; Gupta, R.; Tehei, M.; Corde, S.; Carolan, M.; et al. Thulium Oxide Nanoparticles as Radioenhancers for the Treatment of Metastatic Cutaneous Squamous Cell Carcinoma. Phys. Med. Biol. 2020, 65, 215018. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium Use, Toxicity, and Detoxification Therapy: An Overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Singh, A.; Raj, A.; Shah, P.; Agrawal, N. Nanoparticles: An Activator of Oxidative Stress. In Toxicology of Nanoparticles: Insights from Drosophila; Agrawal, N., Shah, P., Eds.; Springer: Singapore, 2020; pp. 89–106. ISBN 978-981-15-5522-0. [Google Scholar]
- Koedrith, P.; Boonprasert, R.; Kwon, J.Y.; Kim, I.-S.; Seo, Y.R. Recent Toxicological Investigations of Metal or Metal Oxide Nanoparticles in Mammalian Models in Vitro and in Vivo: DNA Damaging Potential, and Relevant Physicochemical Characteristics. Mol. Cell Toxicol. 2014, 10, 107–126. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef]
- Yang, N.; Yang, J.; Liu, Y.; Fan, H.; Ji, L.; Wu, T.; Jia, D.; Ye, Q.; Wu, G. Impaired Learning and Memory in Mice Induced by Nano Neodymium Oxide and Possible Mechanisms. Environ. Toxicol. 2021, 36, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Bonni, A. Modeling Oxidative Stress in the Central Nervous System. Curr. Mol. Med. 2006, 6, 871–881. [Google Scholar] [CrossRef]
- Peng, L.; Weiying, Z.; Xi, L.; Yi, L. Structural Basis for the Biological Effects of Pr(III) Ions: Alteration of Cell Membrane Permeability. Biol. Trace Elem. Res. 2007, 120, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Rahman, M.M. Editorial: Exposure Pathways, Characterization and Risk Assessment of Chemical Contaminants in the Food Chain. Front. Environ. Sci. 2022, 10, 881334. [Google Scholar]
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmäe, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio Fischeri and Tetrahymena Thermophila. Materials 2017, 10, 754. [Google Scholar] [CrossRef]
- Whitford, G.M. The Metabolism and Toxicity of Fluoride. Monogr. Oral Sci. 1996, 16, 151–153. [Google Scholar]
- Centers for Disease Control and Prevention CDC. Recommendations for Using Fluoride to Prevent and Control Dental Caries in the United States. MMWR Recomm. Rep. 2001, 50, 41–42. [Google Scholar]
- WHO (World Health Organization). Fluoride in Drinking Water. In Environmental Health Criteria Monograph; WHO: Geneva, Switzerland, 2004; Volume 227. [Google Scholar]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Fluoride, Developmental Fluoride Neurotoxicity. Environ. Health Perspect. 2012, 120, 1362–1369. [Google Scholar] [CrossRef]
- NRC (National Research Council). Fluoride in Drinking Water: A Scientific Review of EPA’s Standards; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular Mechanisms of Fluoride Toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef]
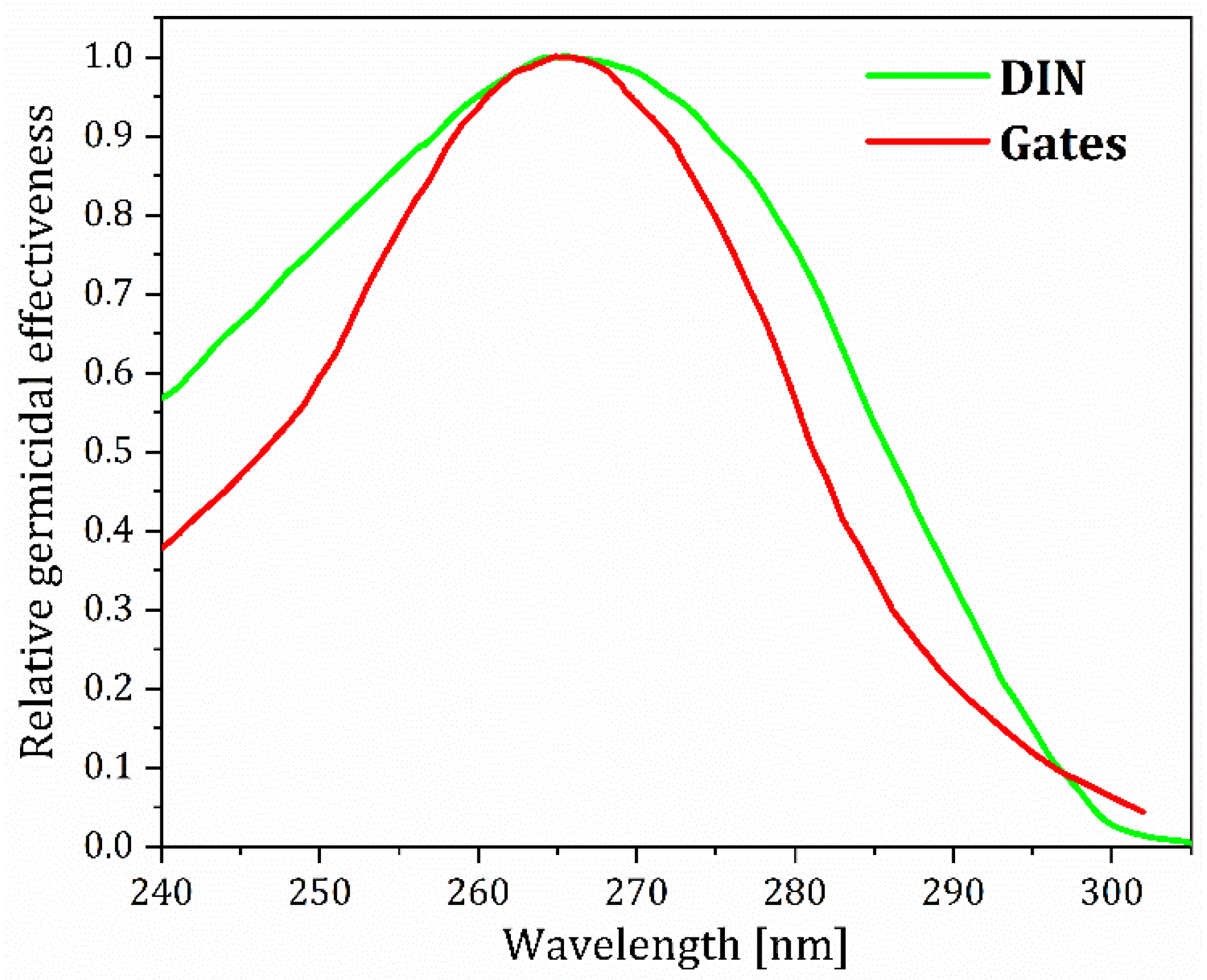

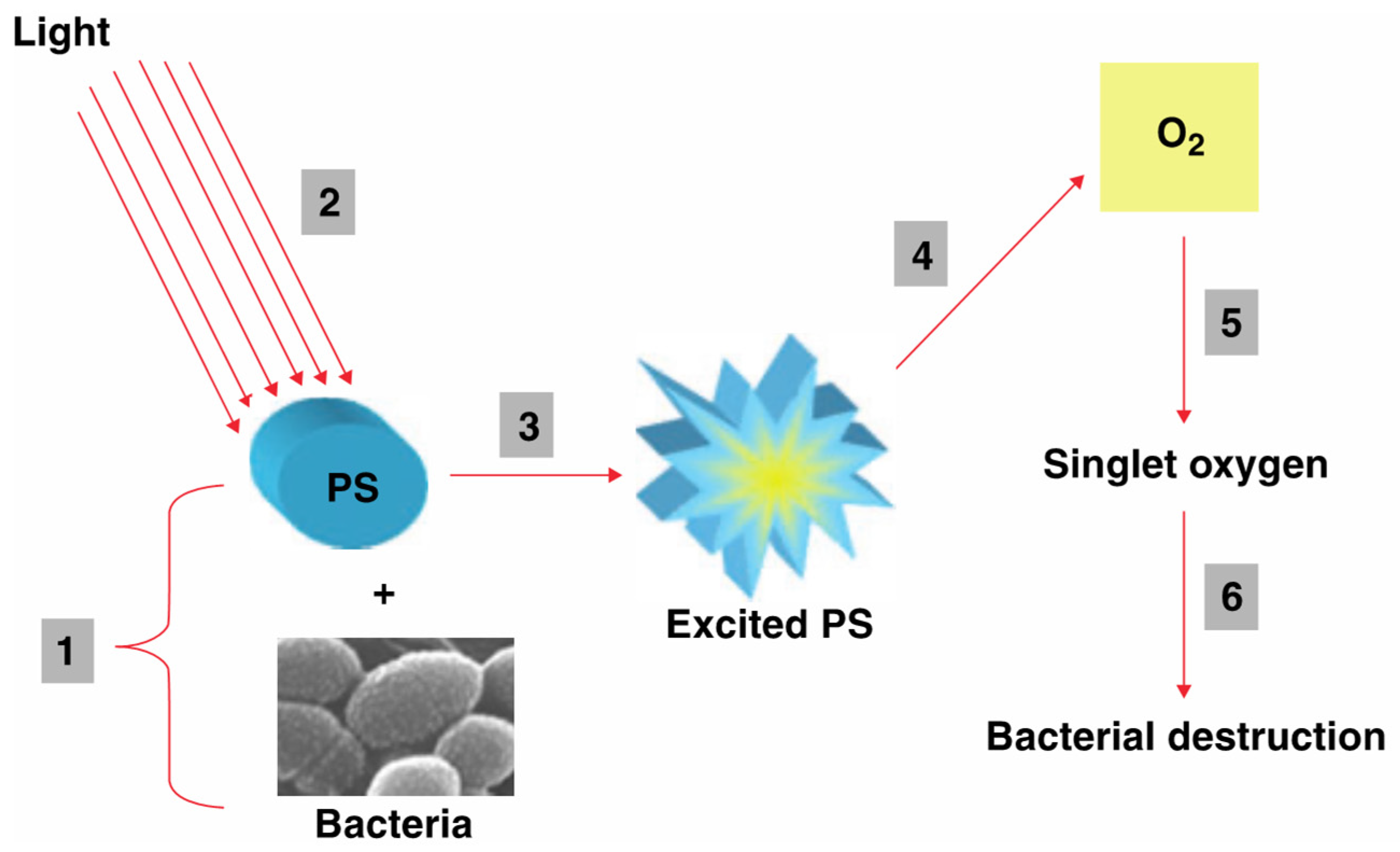

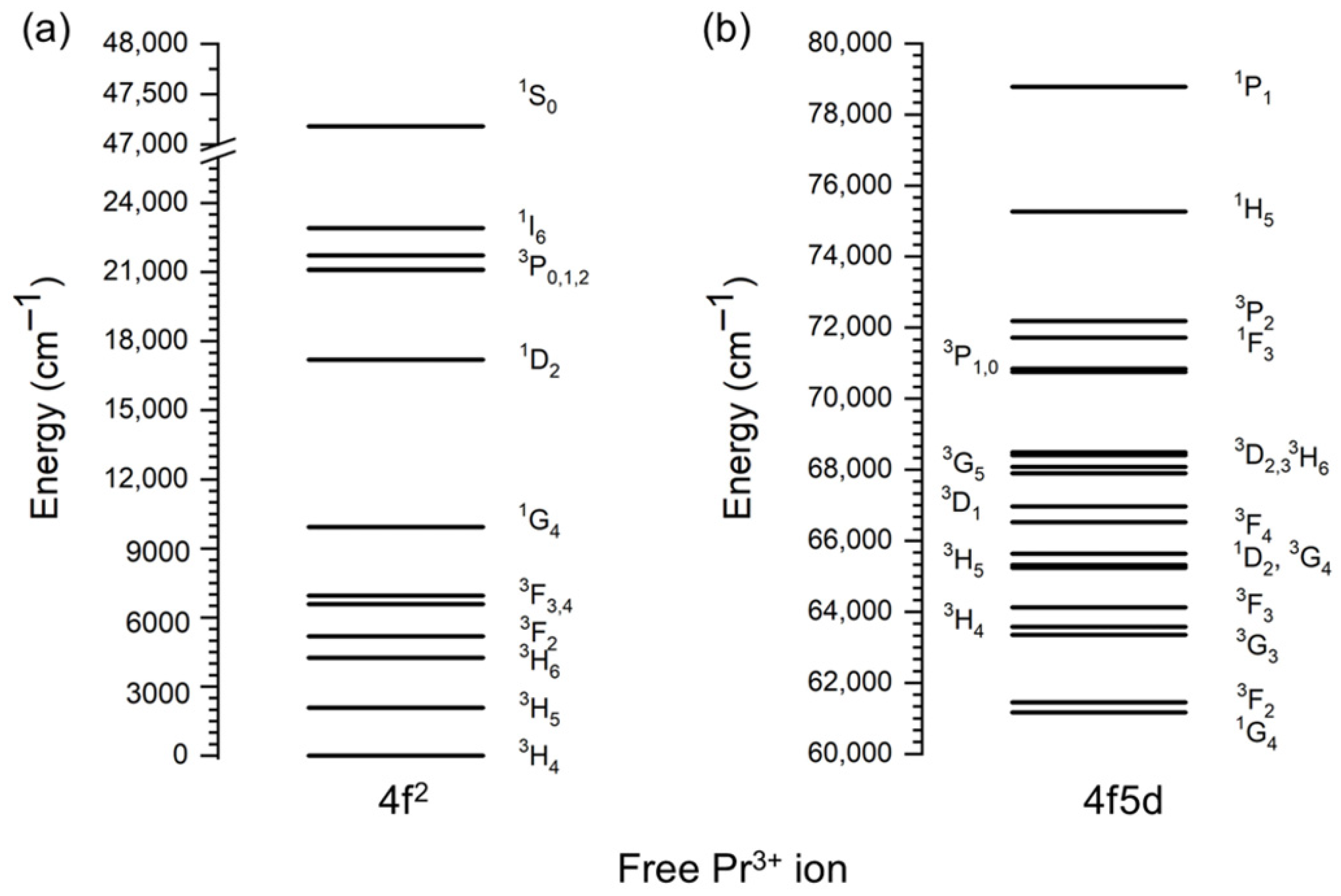
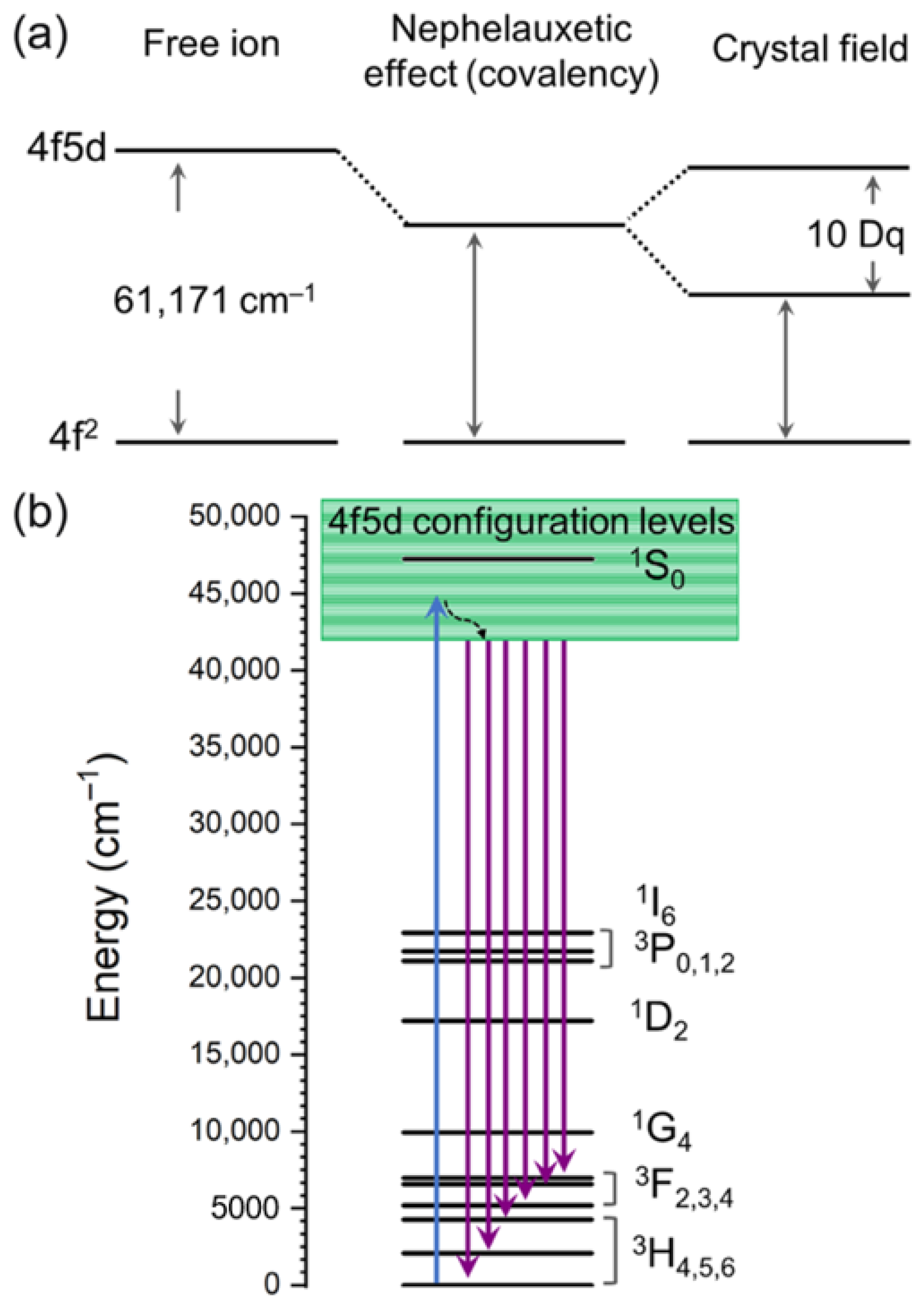
| Material | UC Host Doping Ions (Concentrations) | Excitation and Emission | Antimicrobial Effect | Ref. |
|---|---|---|---|---|
| CuS-decorated NaYF4 nanoparticles coated with methylene blue doped silica and grafted with chitosan | NaYF4 Mn2+/Yb3+/Er3+ (30/18/2 mol%) | Exc. 980 nm emission in red (651 nm) | Synergistic photothermal and photodynamic therapy effective against Gram-positive S. aureus and Gram-negative E. coli. | [69] |
| NaYF4@mSiO2 mSiO2 (mesoporous silica) shell loaded with hydrophobic photosensitizer SiPc (silicon 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine dihydroxide) | Cubic NaYF4 Yb3+/Er3+ (20/2 mol%) | Exc. 976 (power density of 2 W/cm2) emission in green (520–560 nm) and red (640–680 nm) | Complete eradication of E. coli and seven-order-of-magnitude decrease in colony-forming units of S. aureus. | [70] |
| Roussin’s black salt (RBS)-loaded UCNPs NaGdF4@mSiO2@qC (qC—quaternized ammonium chitosan) | NaGdF4 Yb3+/Tm3+ (25/0.3 mol%) | Exc. 980 nm (1 W) emission in UV (290, 345, and 362 nm), blue (450 nm and 474 nm), red (574 nm and 643 nm), and near-infrared (807 nm) | Nitric oxide triggered antibacterial activity against methicillin-resistant S. aureus (MRSA) and E. coli in vitro and in vivo. | [71] |
| N-octyl chitosan-coated NaYF4:Yb,Er@NaYF4 core–shell nanoparticles loaded with the zinc phthalocyanine photosensitizer | hexagonal NaYF4 Yb3+/Er3+ (concentrations are not provided) | Exc. 980 nm emission in green (520–560 nm) and red (640–680 nm) | Effective against methicillin-resistant S. aureus (MRSA) and E. coli. Effective treatment of the MRSA-infected abscesses in deep tissue (1 cm). | [72] |
| Rose Bengal (photosensitizer)-loaded LiYF4 capped with polyvinylpyrrolidone | LiYF4 Yb3+/Er3+ (concentrations are not provided) | Exc. 980 nm (power density of 1 W/cm2) Emission in green (520–560 nm) and red (640–680 nm) | Effective in deep tissue infections; used with methylene blue for aPDT. Decline of 4.72 log10 in viability of drug-resistant Acinetobacter baumannii at a dose of 50 μg mL−1 UCNPs-PVP-RB. | [73] |
| Rose Bengal (photosensitizer)-loaded NaYF4:Yb,Er@NaGdF4:Nd@SiO2 core–shell nanoparticles | NaYF4 Yb3+/Er3+ (18/2 mol%) | Exc. 980 nm (power density of 1 W/cm2); emission in green (520–560 nm) and red (640–680) nm | Effective against methicillin-resistant S. aureus (MRSA) and E. coli. | [74] |
| D-TiO2/Au@SiO2@Y2O3:Yb3+,Er3+ with an antibiotic drug Ampicillin sodium covalently linked to the nanoparticles by a (3- glycidyloxypropyl)trimethoxysilane monolayer linker | Y2O3 Yb3+/Er3+ (concentrations are not provided) | Exc. 980 nm (power density of 0.68 W/cm2); emission in green (520–560 nm) and red (640–680 nm) | Effective against methicillin-resistant S. aureus (MRSA) and E. coli. | [75] |
| NaErF4:Tm3+@NaYF4:Yb3+-Chlorin e6-Mn(CO)5Br@Silane | NaErF4:Tm3+ Er3+/Tm3+ (concentrations are not provided) NaYF4 Yb3+ (concentration is not provided) | Exc. 980 nm (power density of 1 W/cm2). Emission in red (660 nm) | At 150 μg/mL, the therapy results in inhibition of over 70% of E. coli and S. aureus, while at 200 μg/mL, it inhibits approximately 90% of both bacteria strains. Additionally, an anti-inflammatory effect is observed. | [65] |
| Heavy metal-free organic photosensitizer attached to the NaGdF4:Nd3+/Tm3+/Yb3+@NaGdF4 core–shell nanoparticles coated with a phospholipid bilayer | NaGdF4 Yb3+/Nd3+/Tm3+ (25/1/0.5 mol%) | Exc. 808 nm (power density of 140 mW/cm2 or 3.2 W/cm2). Emission in UV (340, and 360 nm) and blue (450 nm and 480 nm). | HeLa cells are efficiently destroyed via 808 nm laser irradiance of 140 mW/cm2 for 3 min (<30% cell viability) or via 3.2 W/cm2 for 6 min (<10% cell viability). | [76] |
| (CTAB-coated NaYF4:Yb/Tm)@ZnO | β-NaYF4 Yb3+/Tm3+ (18/5 mol%) | Exc. LED 970 nm (power of 12 mW/cm2). Emission in UVA (345 and 362 nm) and blue (451 nm and 475 nm) | CFU reduction of S. aureus WCH-SK2-SCV of 82.6% and S. aureus WCH-SK2 of 78.8% is demonstrated. | [77] |
| Host | Crystal Structure | Space Group | Main Emission Peaks (nm) | Reference | (cm−1) with [Reference] |
|---|---|---|---|---|---|
| Cs2NaYCl6 | Cubic | Fm3m | 263, 277, 301, 314 | [92] | 284 [93] |
| LaOI | Tetragonal | P4nmm | 300 | [94] | <430 [95] * |
| KCaF3 | Hexagonal | Pnma | 257 | [96] | 412 [97] |
| RbCaF3 | Cubic | Pm-3m | 261 | [96] | 486 [98] |
| CsCaF3 | Cubic | Pm-3m | 250, 273 | [96] | 449 [98] |
| LiLuF4 | Tetragonal | I41/amd | 223–281 | [99] | 445 [100] |
| CaSO4 | Orthorhombic | Amma | 223, 234, 250, 255 | [101] | 1185 [102] |
| Cs2NaYF6 | Cubic | Fm-3m | 250, 270 | [103] | 467 [93] |
| YBO3 | Hexagonal | P63/m | 263, 275 | [89] | 1368 [104] |
| La2CaB10O19 | Monoclinic | C2 | 279, 334 | [105] | 1493 [106] |
| LuPO4 | Tetragonal | I41/amd | 235, 246, 263, 274 | [87] | 1161 [107] |
| YPO4 | Tetragonal | I41/amd | 232, 244.5, 261.6, 271 | [108] | 1149 [107] |
| NaCaPO4 | Orthorhombic | Pn21a | 251, 261, 282 | [109] | 1080 [110] |
| K3Lu(PO4)2 | Trigonal | P-3 | 253, 282, 315 | [87,111] | 1147 [112] ** |
| Sr3(PO4)2 | Trigonal | R3-m | 231, 269 | [113] | 1072 [114] |
| Sr3Y(PO4)3 | Cubic | I-43d | 248, 278 | [115] | 1080 [116] |
| Ba3Y(PO4)3 | Cubic | I-43d | 250, 280 | [115] | 1044 [117] |
| Ca9Y(PO4)7 | Trigonal | R-3c | 240, 275 | [118] | 1125 [118] |
| LiLuSiO4 | Orthorhombic | Pnma | 268, 283, 316 | [87] | 980 [119] *** |
| Li2SrSiO4 | Hexagonal | P3121 | 265, 315 | [120] | 884 [121] |
| LiY9(SiO4)6O2 | Hexagonal | P63/m | 268 | [122] | 958 [123] |
| Lu2SiO5 | Monoclinic | C2/c | 275, 313 | [124] | 970 [125] |
| X2-Y2SiO5 | Monoclinic | C2/c | 270, 282, 308 | [126] | 971 [127] |
| UC Material | Emission | Excitation | Antimicrobial Effect | Ref. |
|---|---|---|---|---|
| β-NaYF4:Pr3+/Li+ and β-NaYF4: Pr3+/Li+@BiOCl composite (dopant concentrations are not provided) | UVC (253 nm, 259 nm, 284 nm) | 444 nm | Antimicrobial effect of β-NaYF4:Pr3+/Li+ under 444 nm excitation demonstrated. With the β-NaYF4: Pr3+/Li+@BiOCl composite, the effect is significantly improved (visible light excitation ≥ 420 nm kills 99.99% of E. coli in 180 min—aPDT effect). | [153] |
| Lu7O6F9:Pr3+ (1 mol%) | UVC 260 nm | 447 nm | Inactivation of E. coli implicated by the authors. | [154] |
| NaYF4:Pr3+/Yb3+ (2/10 mol%) LiYF4:Pr3+/Yb3+ (1/10 mol%) | UVC 275 nm | 447 nm | Significant denaturation of a double strand DNA after exposure to 447 nm radiation for 20 to 40 min. Material’s additional functionality is luminescence imaging in the NIR-II spectral region. | [155] |
| Cs2NaYF6:Pr3+ (1 mol%) | UVC afterglow at 250 nm | X-ray | Following 16 min of X-ray irradiation, the sample is placed near to a plate containing a colony of the Gram-negative bacteria Pseudomonas aeruginosa. The viability of around 40% of bacteria is seen under the UVC afterglow of this material. | [103] |
| Y2SiO5:Pr3+ (1 mol%) Y2SiO5:Pr3+/Gd3+ (1/1 mol%) Y2SiO5:Pr3+/Gd3+/Li+ (1.2/1.2/7.2 mol%) Y2SiO5:Pr3+/Li+ (1.2/7.2 mol%) | UVC 280 nm, additional emission at 318 nm with samples containing Gd3+ | “daylight” fluorescent lighting | Inactivation of B. subtilis spores on dry phosphor-coated surfaces (best results with Pr3+/Gd3+/Li+-doped material). Inhibition of P. aeruginosa biofilms grown on the coated surfaces. | [142] |
| Ca2SiO4:Pr3+ (dopant concentration is not provided) | UVC 247 nm | 450 nm laser | Inactivation of B. subtilis. | [156] |
| Y2SiO5:Pr3+ composite film with polyvinyl alcohol (PVA) and sodium alginate (SA) | UVC 280 nm | 455 nm and white LED | Inhibition of Gram-positive S. aureus and Gram-negative E. coli Pseudomonas aeruginosa bacteria. | [152] |
| Y2Si2O7:Pr3+/Tm3+/Yb3+ (1.2/0.5/5 mol%) | UVC (278 nm) + UVB (308 nm) + UVA (370 nm) | 447 nm laser, 800 mW/cm2 | The viability of planktonic cultures of A. baumannii, S. aureus, and C. albicans. | [157] |
| Li2SrSiO4:Pr3+ (dopant concentration is not provided) | Two broad peaks (265 nm and 320 nm). | 450 nm laser, 1 W | Inactivation of Bacillus subtilis: after 300 s of irradiation, the mortality rate reached 90%, and after 600 s of irradiation, almost all bacteria died. | [120] |
| Li2CaGeO4:Pr3+ (1 mol%) | UVC+UVB (~240–330 nm) | 450 nm laser, 1 W | Complete inactivation of S. aureus bacteria in 30 min. | [158] |
| Li2SrGeO4 (1 mol%) | UVC+UVB (~240–330 nm) | 450 nm laser diode, 0.6 W | Inactivation of Staphylococcus aureus, Salmonella enterica, Klebsiella pneumoniae, and Escherichia coli in 40 to 80 min. | [159] |
| LaPO4 | YPO4 | Lu7O6F9 | YBO3 | X2-Y2SiO5 | Sr3(BO3)2 | Lu2SiO5 | β-Y2Si2O7 | |
|---|---|---|---|---|---|---|---|---|
| SOC | 0.282 | 0.357 | 0.674 | 0.529 | 0.533 | 0.535 | 0.504 | 0.477 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dramićanin, M.D.; Brik, M.G.; Antić, Ž.; Bănică, R.; Mosoarca, C.; Dramićanin, T.; Ristić, Z.; Dima, G.D.; Förster, T.; Suta, M. Pr3+ Visible to Ultraviolet Upconversion for Antimicrobial Applications. Nanomaterials 2025, 15, 562. https://doi.org/10.3390/nano15070562
Dramićanin MD, Brik MG, Antić Ž, Bănică R, Mosoarca C, Dramićanin T, Ristić Z, Dima GD, Förster T, Suta M. Pr3+ Visible to Ultraviolet Upconversion for Antimicrobial Applications. Nanomaterials. 2025; 15(7):562. https://doi.org/10.3390/nano15070562
Chicago/Turabian StyleDramićanin, Miroslav D., Mikhail G. Brik, Željka Antić, Radu Bănică, Cristina Mosoarca, Tatjana Dramićanin, Zoran Ristić, George Daniel Dima, Tom Förster, and Markus Suta. 2025. "Pr3+ Visible to Ultraviolet Upconversion for Antimicrobial Applications" Nanomaterials 15, no. 7: 562. https://doi.org/10.3390/nano15070562
APA StyleDramićanin, M. D., Brik, M. G., Antić, Ž., Bănică, R., Mosoarca, C., Dramićanin, T., Ristić, Z., Dima, G. D., Förster, T., & Suta, M. (2025). Pr3+ Visible to Ultraviolet Upconversion for Antimicrobial Applications. Nanomaterials, 15(7), 562. https://doi.org/10.3390/nano15070562










