Optical and Transport Properties of ZnO Thin Films Prepared by Reactive Pulsed Mid-Frequency Sputtering Combined with RF ECWR Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hybrid Mid-Frequency Sputtering Assisted by an Electron Cyclotron Wave Resonance Plasma (MFS and ECWR)
2.2. Optical Transmittance, Reflectance, and Photothermal Deflection Spectroscopy (PDS)
2.3. Steady-State Photoluminecence at Low Temperature (Steady-State PL)
2.4. Frequency-Resolved Photoluminecence at Room Temperature (f-Resolved PL)
2.5. Hall and Photo-Hall Measurements
3. Results
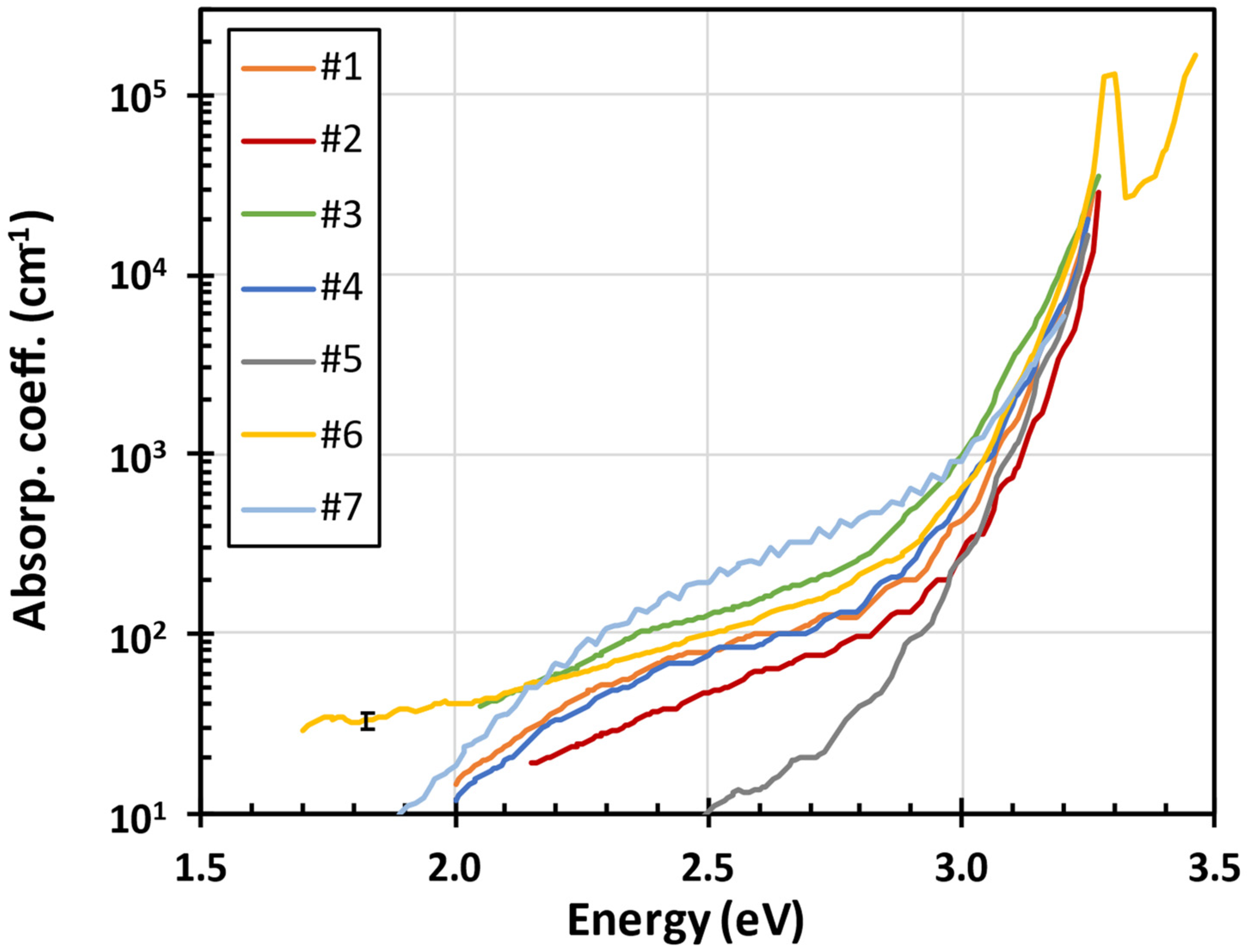
3.1. Optical Transmittance, Reflectance and Absorption Spectra
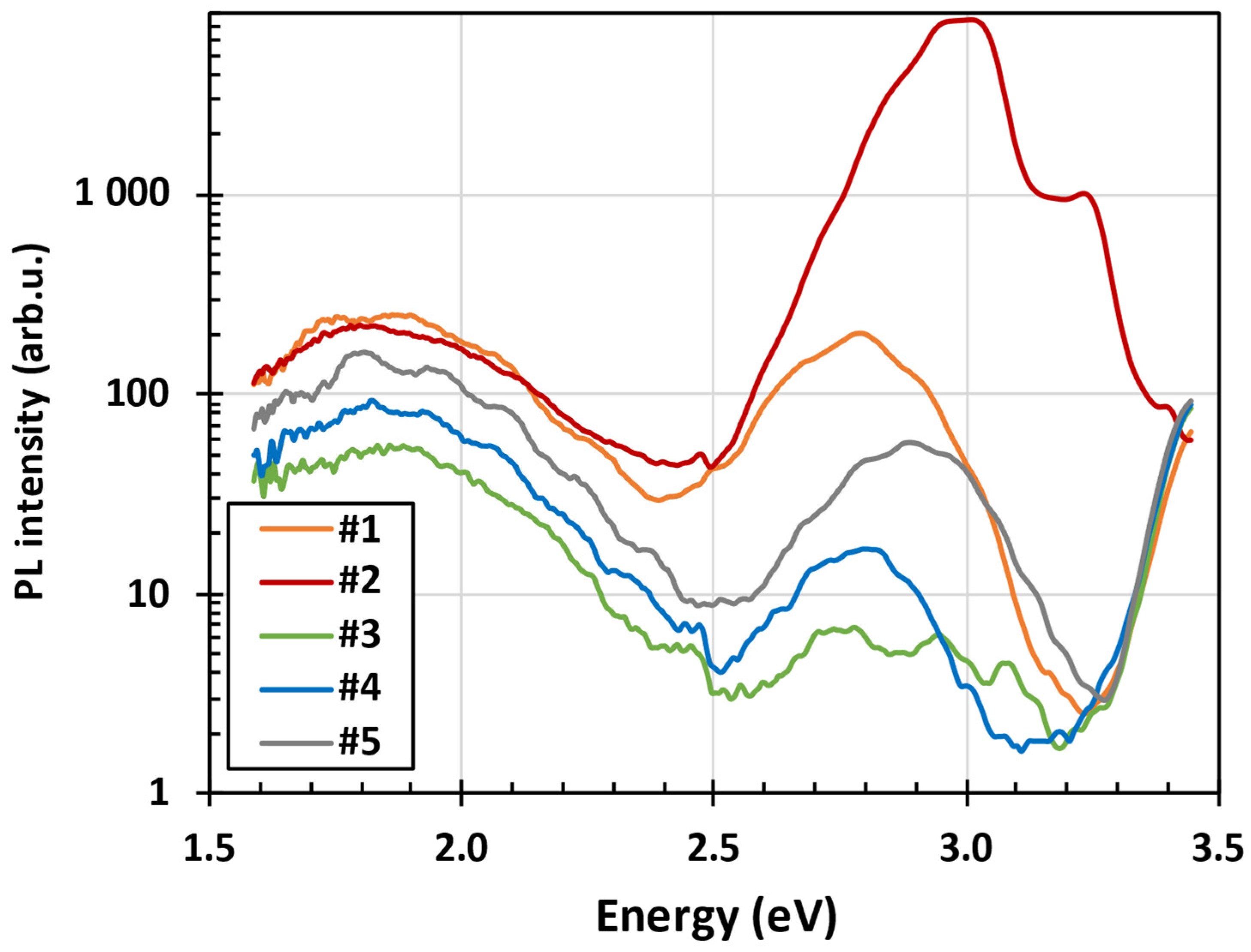
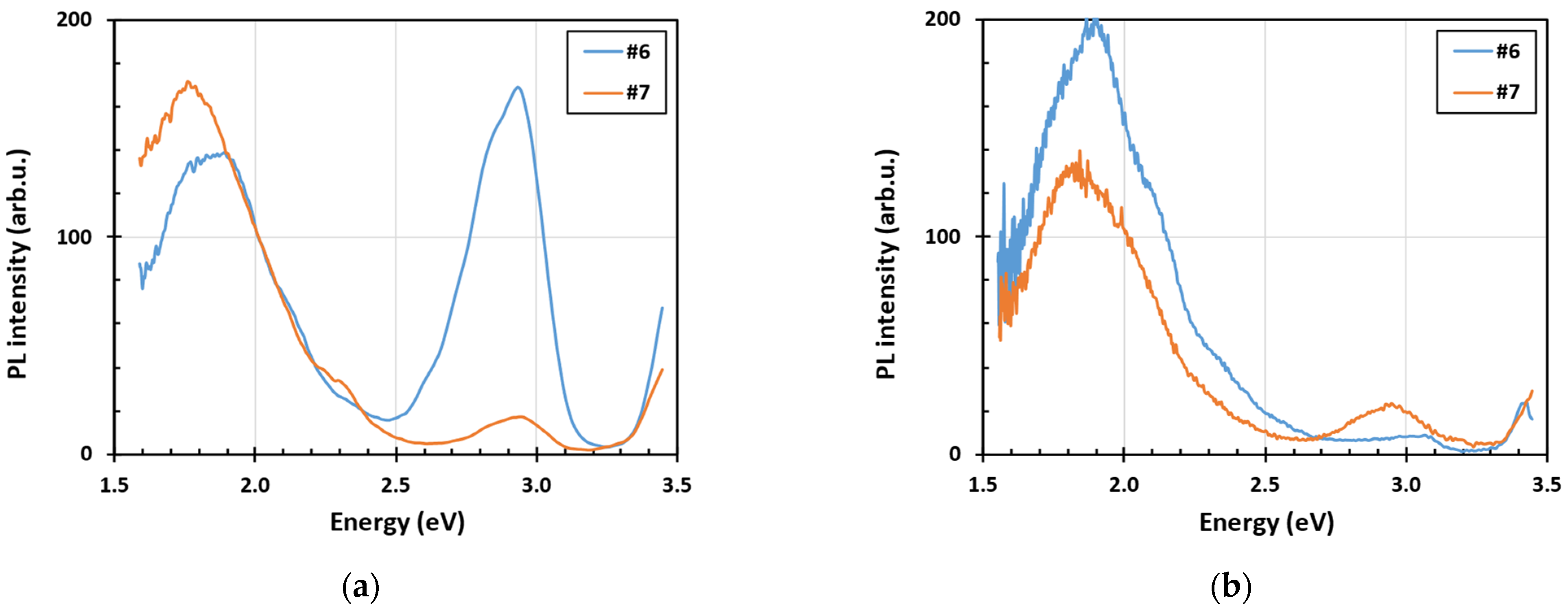
3.2. Steady State Photoluminescence Spectra at Low Temperature
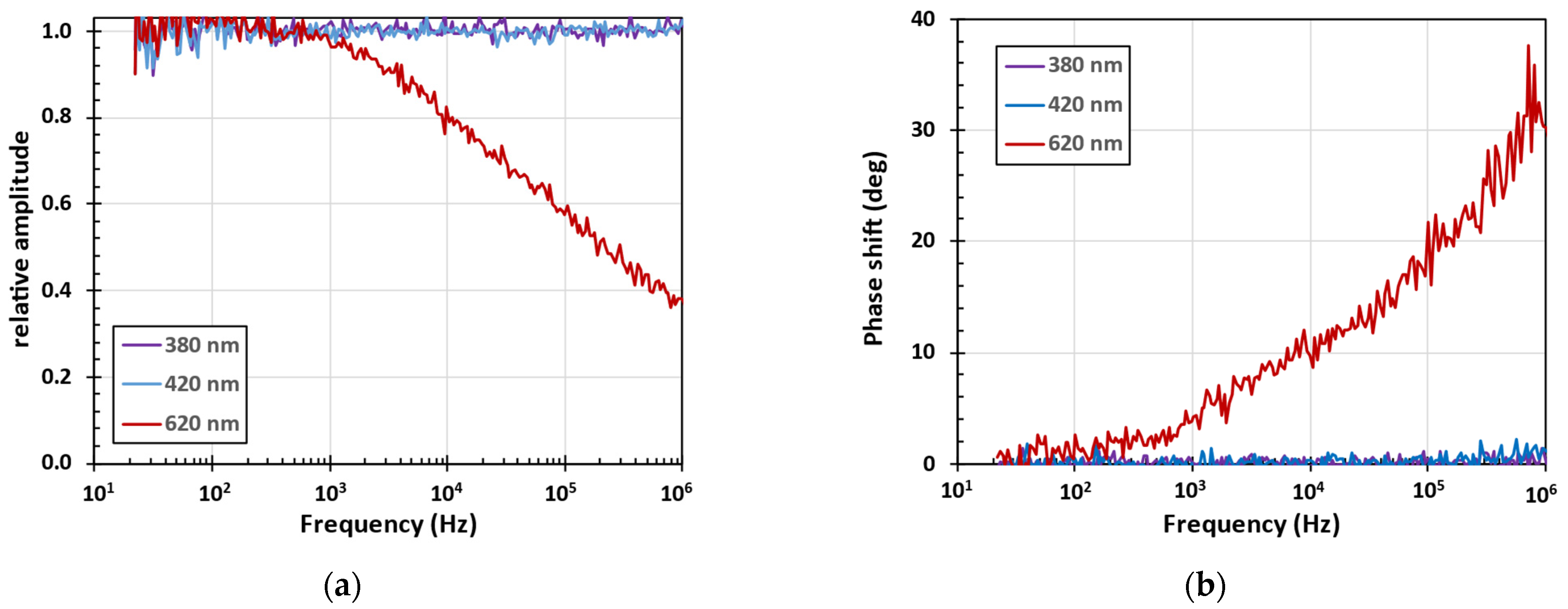
3.3. Frequency Resolved Photoluminescence Spectra
3.4. Hall and Photo-Hall Effect Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECWR | Electron Cyclotron Wave Resonance |
| FWHM | Full Width at Half Maximum |
| ICP | Inductively Coupled Plasma |
| LED | Light emitting diode |
| MFS | Mid-frequency Sputtering |
| PDS | Photothermal Deflection Spectroscopy |
| PL | Photoluminescence |
| PMT | PhotoMultiplier Tube |
| RF | Radio Frequency |
| UV | Ultraviolet |
References
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 Years of ZnO Research—Old and Very Recent Results. Phys. Stat. Sol. (B) 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, Physics and Applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef]
- Klingshirn, C.F. (Ed.) Zinc Oxide: From Fundamental Properties Towards Novel Applications; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-10576-0. [Google Scholar]
- Erhart, P.; Juslin, N.; Goy, O.; Nordlund, K.; Müller, R.; Albe, K. Analytic Bond-Order Potential for Atomistic Simulations of Zinc Oxide. J. Phys. Condens. Matter 2006, 18, 6585–6605. [Google Scholar] [CrossRef]
- Schulz, H.; Thiemann, K.H. Structure Parameters and Polarity of the Wurtzite Type Compounds Sic—2H and ZnO. Solid State Commun. 1979, 32, 783–785. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Chertopalov, S.; Děcká, K.; Dominec, F.; Mičová, J.; Neykova, N. Free-Standing ZnO:Mo Nanorods Exposed to Hydrogen or Oxygen Plasma: Influence on the Intrinsic and Extrinsic Defect States. Materials 2022, 15, 2261. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.A.; Segura, A.; Mollar, M.; Marí, B. Optical Properties of Thin Films of ZnO Prepared by Pulsed Laser Deposition. Thin Solid Film. 2004, 453–454, 251–255. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Kohan, A.F.; Ceder, G.; Morgan, D.; Van De Walle, C.G. First-Principles Study of Native Point Defects in ZnO. Phys. Rev. B—Condens. Matter Mater. Phys. 2000, 61, 15019–15027. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Morkoç, H. Similarities in the Bandedge and Deep-Centre Photoluminescence Mechanisms of ZnO and GaN. Solid State Commun. 1997, 101, 643–646. [Google Scholar] [CrossRef]
- Li, M.; Xing, G.; Xing, G.; Wu, B.; Wu, T.; Zhang, X.; Sum, T.C. Origin of Green Emission and Charge Trapping Dynamics in ZnO Nanowires. Phys. Rev. B 2013, 87, 115309. [Google Scholar] [CrossRef]
- Kodama, K.; Uchino, T. Thermally Activated Below-Band-Gap Excitation behind Green Photoluminescence in ZnO. J. Appl. Phys. 2012, 111, 093525. [Google Scholar] [CrossRef]
- Marotti, R.E.; Badán, J.A.; Quagliata, E.; Dalchiele, E.A. Red Photoluminescence and Band Edge Shift from ZnO Thin Films. Phys. B Condens. Matter 2007, 398, 337–340. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chen, X.Y.; Ng, A.M.C.; Guo, M.Y.; Liu, F.Z.; Djurišić, A.B.; Chan, W.K.; Shi, X.Q.; Van Hove, M.A. Green Emission in ZnO Nanostructures—Examination of the Roles of Oxygen and Zinc Vacancies. Appl. Surf. Sci. 2013, 271, 202–209. [Google Scholar] [CrossRef]
- Erhart, P.; Albe, K. Diffusion of Zinc Vacancies and Interstitials in Zinc Oxide. Appl. Phys. Lett. 2006, 88, 201918. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of Defects for Enhancing Their Technological Potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Defect Analysis and Engineering in ZnO. Phys. B Condens. Matter 2001, 308–310, 899–903. [Google Scholar] [CrossRef]
- Galazka, Z.; Irmscher, K.; Pietsch, M.; Ganschow, S.; Schulz, D.; Klimm, D.; Hanke, I.M.; Schroeder, T.; Bickermann, M. Experimental Hall Electron Mobility of Bulk Single Crystals of Transparent Semiconducting Oxides. J. Mater. Res. 2021, 36, 4746–4755. [Google Scholar] [CrossRef]
- Jayah, N.A.; Yahaya, H.; Mahmood, M.R.; Terasako, T.; Yasui, K.; Hashim, A.M. High Electron Mobility and Low Carrier Concentration of Hydrothermally Grown ZnO Thin Films on Seeded A-Plane Sapphire at Low Temperature. Nanoscale Res. Lett. 2015, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.M.; Hofstaetter, A.; Leiter, F.; Zhou, H.; Henecker, F.; Meyer, B.K.; Orlinskii, S.B.; Schmidt, J.; Baranov, P.G. Hydrogen: A Relevant Shallow Donor in Zinc Oxide. Phys. Rev. Lett. 2002, 88, 045504. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef]
- Remes, Z.; Chang, Y.Y.; Stuchlík, J.; Neykova, N.; Soucek, J.; Hsu, H.S. Enhanced Room Temperature Exciton Photoluminescence of Plasma Hydrogenated ZnO Nanocolumns. IOP Conf. Ser. Mater. Sci. Eng. 2019, 465, 012008. [Google Scholar] [CrossRef]
- Cadatal-Raduban, M.; Olejníček, J.; Hibino, K.; Maruyama, Y.; Písaříková, A.; Shinohara, K.; Asaka, T.; Lebedová Volfová, L.; Kohout, M.; Jiaqi, Z.; et al. Ultrafast UV Luminescence of ZnO Films: Sub-30 Ps Decay Time with Suppressed Visible Component. Adv. Opt. Mater. 2024, 12, 2400377. [Google Scholar] [CrossRef]
- Kavan, L.; Krýsová, H.; Zukalová, M.; Tarábková, H.; Hubička, Z. Peculiar Photoelectrochemical Activity of Zinc Oxide and Tin Dioxide. J. Photochem. Photobiol. A Chem. 2025, 458, 115929. [Google Scholar] [CrossRef]
- Hibino, K.; Olejníček, J.; Yamanoi, K.; Ponseca, C.S.; Shuaib, A.; Maruyama, Y.; Písaříková, A.; Kohout, M.; Čada, M.; Kapran, A.; et al. Impact of Electron Cyclotron Wave Resonance Plasma on Defect Reduction in ZnO Thin Films. Sci. Rep. 2025, 15, 5555. [Google Scholar] [CrossRef]
- Krysova, H.; Mansfeldova, V.; Tarabkova, H.; Pisarikova, A.; Hubicka, Z.; Kavan, L. High-Quality Dense ZnO Thin Films: Work Function and Photo/Electrochemical Properties. J. Solid State Electrochem. 2024, 28, 2531–2546. [Google Scholar] [CrossRef]
- Remes, Z.; Babchenko, O.; Varga, M.; Stuchlik, J.; Jirasek, V.; Prajzler, V.; Nekvindova, P.; Kromka, A. Preparation and Optical Properties of Nanocrystalline Diamond Coatings for Infrared Planar Waveguides. Thin Solid Film. 2016, 618, 130–133. [Google Scholar] [CrossRef]
- Jackson, W.B.; Amer, N.M.; Boccara, A.C.; Fournier, D. Photothermal Deflection Spectroscopy and Detection. Appl. Opt. 1981, 20, 1333–1344. [Google Scholar] [CrossRef]
- Mooney, J.; Kambhampati, P. Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Pelant, I. Luminescence Spectroscopy of Semiconductors; Oxford University Press: Oxford, UK; New York, NY, USA, 2012; ISBN 978-0-19-958833-6. [Google Scholar]
- Tompkins, H.G. Spectroscopic Ellipsometry and Reflectometry: A User’s Guide; Wiley: New York, NY, USA, 1999; ISBN 0-471-18172-2. [Google Scholar]
- Remes, Z.; Stuchlik, J.; Purkrt, A.; Chang, Y.-Y.; Jirasek, V.; Stenclova, P.; Prajzler, V.; Nekvindova, P. The Intrinsic Submicron ZnO Thin Films Prepared by Reactive Magnetron Sputtering. In Proceedings of the NANOCON 2016—Conference Proceedings, Brno, Czech Republic, 19–21 October 2016; TANGER Ltd.: Ostrava, Czech Republic, 2016; pp. 36–41. [Google Scholar]
- Aspnes, D.E.; Theeten, J.B.; Hottier, F. Investigation of Effective-Medium Models of Microscopic Surface Roughness by Spectroscopic Ellipsometry. Phys. Rev. B 1979, 20, 3292–3302. [Google Scholar] [CrossRef]
- Franta, D.; Ohlídal, I. Influence of Lateral Dimensions of the Irregularities on the Optical Quantities of Rough Surfaces. J. Opt. A Pure Appl. Opt. 2006, 8, 763–774. [Google Scholar] [CrossRef]
- Tikhonravov, A.V.; Trubetskov, M.K.; Tikhonravov, A.A.; Duparré, A. Effects of Interface Roughness on the Spectral Properties of Thin Films and Multilayers. Appl. Opt. 2003, 42, 5140. [Google Scholar] [CrossRef]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Artemenko, A.; Vaněček, V.; Aubrechtová Dragounová, K.; Landová, L.; Kučerková, R.; Mičová, J. Transformation of Free-Standing ZnO Nanorods upon Er Doping. Appl. Surf. Sci. 2021, 562, 150217. [Google Scholar] [CrossRef]
- Remeš, Z.; Neyková, N.; Remeš, Š.; Novák, R.; Kučerková, R.; Jarý, V.; Mičová, J. Nanosecond Photoluminescence Decay in Zinc Molybdate Oxide Nanocrystals Observed by TCSPC and Phase Shift Methods. J. Phys. Conf. Ser. 2024, 2931, 012011. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Laczko, G.; Cherek, H.; Gratton, E.; Limkeman, M. Analysis of Fluorescence Decay Kinetics from Variable-Frequency Phase Shift and Modulation Data. Biophys. J. 1984, 46, 463–477. [Google Scholar] [CrossRef]
- Gao, S.-L.; Qiu, L.-P.; Zhang, J.; Han, W.-P.; Ramakrishna, S.; Long, Y.-Z. Persistent Photoconductivity of Metal Oxide Semiconductors. ACS Appl. Electron. Mater. 2024, 6, 1542–1561. [Google Scholar] [CrossRef]
- Devore, J.L. Probability and Statistics for Engineering and the Sciences, 8th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2012; ISBN 978-0-538-73352-6. [Google Scholar]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 978-0-471-14323-9. [Google Scholar]
- Sheetz, R.M.; Ponomareva, I.; Richter, E.; Andriotis, A.N.; Menon, M. Defect-Induced Optical Absorption in the Visible Range in ZnO Nanowires. Phys. Rev. B 2009, 80, 195314. [Google Scholar] [CrossRef]
- Teke, A.; Özgür, Ü.; Doğan, S.; Gu, X.; Morkoç, H.; Nemeth, B.; Nause, J.; Everitt, H.O. Excitonic Fine Structure and Recombination Dynamics in Single-Crystalline ZnO. Phys. Rev. B 2004, 70, 195207. [Google Scholar] [CrossRef]
- Buryi, M.; Babin, V.; Chang, Y.-Y.; Remeš, Z.; Mičová, J.; Šimek, D. Influence of Precursor Age on Defect States in ZnO Nanorods. Appl. Surf. Sci. 2020, 525, 146448. [Google Scholar] [CrossRef]
- Thomas, D.G. Interstitial Zinc in Zinc Oxide. J. Phys. Chem. Solids 1957, 3, 229–237. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, X.; Lei, B.; Liu, P.; Mai, W. Novel Blue-Violet Photoluminescence from Sputtered ZnO Thin Films. J. Alloys Compd. 2011, 509, 5437–5440. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors; Unabridged Republication, with Slight Corr.; Dover: Mineola, NY, USA, 1975; ISBN 978-0-486-60275-2. [Google Scholar]


| Sample | Subst. | P (W) | d (nm) | rms (nm) | Dep. Rate (nm/min) | EU (meV) | α (cm−1) | ρ (Ω.cm) |
|---|---|---|---|---|---|---|---|---|
| #1 | SLG | 0 | 1580 | 12 | 48 | 65 | 77 | |
| #2 | SLG | 100 | 2316 | 50 | 100 | 58 | 48 | |
| #3 | SLG | 200 | 1068 | 2 | 63 | 79 | 127 | |
| #4 | SLG | 300 | 2254 | 15 | 138 | 72 | 77 | |
| #5 | SLG | 380 | 2086 | 18 | 98 | 58 | 10 | |
| #6 | FSG | 200 | 1320 | 160 | 44 | 64 | 100 | 4.6 × 105 |
| #7 | FSG | 200 | 4700 | 300 | 130 | 109 | 200 | 1.1 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remeš, Z.; Hubička, Z.; Hubík, P. Optical and Transport Properties of ZnO Thin Films Prepared by Reactive Pulsed Mid-Frequency Sputtering Combined with RF ECWR Plasma. Nanomaterials 2025, 15, 590. https://doi.org/10.3390/nano15080590
Remeš Z, Hubička Z, Hubík P. Optical and Transport Properties of ZnO Thin Films Prepared by Reactive Pulsed Mid-Frequency Sputtering Combined with RF ECWR Plasma. Nanomaterials. 2025; 15(8):590. https://doi.org/10.3390/nano15080590
Chicago/Turabian StyleRemeš, Zdeněk, Zdeněk Hubička, and Pavel Hubík. 2025. "Optical and Transport Properties of ZnO Thin Films Prepared by Reactive Pulsed Mid-Frequency Sputtering Combined with RF ECWR Plasma" Nanomaterials 15, no. 8: 590. https://doi.org/10.3390/nano15080590
APA StyleRemeš, Z., Hubička, Z., & Hubík, P. (2025). Optical and Transport Properties of ZnO Thin Films Prepared by Reactive Pulsed Mid-Frequency Sputtering Combined with RF ECWR Plasma. Nanomaterials, 15(8), 590. https://doi.org/10.3390/nano15080590







